Abstract
Present study has been carried out to identify the processes controlling groundwater quality of Moradabad city, Uttar Pradesh. A total 188 groundwater samples were collected and analyzed for major ions in pre- and post-monsoon seasons of years 2012 and 2013. Analysis results showed higher concentration of Na, K, Cl, SO4 and NO3. LL diagram showed dominance of alkalis and Cl + SO4, in three sampling seasons except in post-monsoon 2012, when Na + K–Cl + SO4 facies is recorded in 50% samples. There is dearth of HCO3 complexes in the pre-monsoon season of 2012, and then, their appearance in the post-monsoon season with alkali bicarbonates being 50%. This trend is reversed in 2013. Groundwater composition showed potential impact of varying rainfall pattern suggesting that chemistry of groundwater is intimately related to meteorological parameters. To predict minerals and their reactivity in groundwater, saturation index (SI) was calculated for pre- and post-monsoon of 2012. Groundwater is highly under-saturated with respect to halite, sulphates of Ca and fluorite and super-saturated with respect to chalcedony, quartz and talc, and these minerals may possibly be precipitated out. Relationship of SiO2 with Cl and TDS gives unequivocal evidence of anthropogenic factors having significant effect in determining the chemical characteristics of groundwater. Groundwater chemistry of the area is sum total of factors, such as, meteorological, hydrological and lithological and a set of processes related to various domains of anthropogenic activities, where variation in intensity and distribution of rainfall seems to be one of the driving factors responsible for overall temporal variations in groundwater chemistry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Groundwater constitutes an important part of water resources necessary for the existence of life. It is indispensable for multiple purposes like supporting agriculture, escalating economic expansion and urban growth (Schlager 2006). Changing environmental conditions and human activities imposes significant impacts on groundwater environment (Peiyue 2016). Unrestrained release of various toxic effluents from different industries find their way into the water system and have caused irreversible damage to chemical and biogeochemical quality of both fresh water and groundwater. This, in turn, is a cause of many health problems, particularly, water-borne diseases. Quality of groundwater is getting worse and worse in densely populated areas, thickly industrialized belts and shallow water table regions (Sajjad et al. 2014). Agriculture is the largest livelihood provider fulfilling the basic needs of humans and animals. At the same time, it is the largest abstractor and predominant consumer of groundwater resources. The result is decline in water table and most of the aquifers experience seasonal fluctuations in response to the abstraction (Foster et al. 2000; Foster and Candela 2008).
Urban development has also a profound impact on the hydrology of the area and is the leading cause in the depletion and deterioration of water quantity and quality by altering the parameters governing the hydrologic cycle, such as, the climate; factors related to quantity, quality and regime of surface water and groundwater and various aspects linked to the land surface and subsurface conditions (Changnon 1976; Bornstein and Lin 2000). Prior to urbanization in a given area, recharge of the groundwater resource occurs through the pervious surfaces and is relatively less contaminated. Urbanization, resulting in concrete structures, causes the desiccation and reduction in the surface area available earlier for groundwater recharge. The bulk of the surface, thus, becomes untenable for hosting groundwater recharge zones and there is increased runoff. The routes of infiltration also change. Leakages from septic tanks, landfills and municipal waste water beneath the ground provide large volumes of infiltration. As cities become larger, the water infrastructure may increasingly be dependent on surface water or groundwater brought in from outside the urban area itself (Morris et al. 2003).
Besides the overexploitation of groundwater and declining water table, the quality of groundwater is also a major concern which once degraded cannot be restored. Whereas some regions are being over exploited, other regions of the country are facing the problem of water logging leading to salinity and alkalinity. Except for the dissolution of saline and alkaline patches on the surface by the meteoric water recharging groundwater aquifers, precipitation of carbonates, silica and silicates and cation exchange process, which affect the groundwater chemistry, most obvious chemical changes causing deterioration of water quality are caused due to anthropogenic influences.
Several studies have been carried out in the Ganga plain on hydrogeochemical characterization of aquifers. A detailed investigation has been carried out on spatial variations in groundwater quality by employing GIS-based water quality indices in the Rajgir–Nalanda area of Southern Gangetic Plain by Sethy et al. (2017) with emphasis on the utilization of groundwater for drinking purposes. The investigation reveals that the groundwater of the area needs some level of treatment prior to consumption. A study was conducted by Raju (2012a) in Varanasi to deduce the source of arsenic in groundwater. This study indicates high concentration of As in the Holocene sandy aquifer sediments of Varanasi environs. The probable mechanism for high concentration of As in groundwater might be attributed to the reductive dissolution of Fe oxyhydroxide which is present as coatings on altered mica content as well as sand grains. Verma and Kumar (2014), carried out a study in Amroha district to assess the groundwater quality and concluded that groundwater is highly alkaline, very hard, highly polluted with reference to all physico-chemical parameters studied and is not suitable for drinking, domestic and industrial as well as irrigation purposes. Various other important studies have also been conducted by various researchers in the Ganga plain on groundwater quality (Saba et al. 2016; Saba and Umar 2016; Khan et al. 2015; Alam and Saha 2014; Misra 2011; Bhardwaj et al. 2010).
It is always very important to carry out groundwater related studies in and around major urban centres so as to know what quality of water is consumed by the local populace. Study area i.e. Moradabad city is a major industrial city and export hub, famous for its huge export of brass handicrafts (http//:www.uhi-india.org). However, primarily the economy of the Moradabad district as a whole is agrarian based. Agricultural activities in the vicinity of urban centres are confined to peripheral areas.
In spite of preponderance of the literature on groundwater studies, there is relative dearth of material covering aspects related to hydrogeochemical studies and identification of pollutants in and around the main city. Therefore, present study is aimed to understand the geometry of aquifer system and delineation of its behaviour which is discussed in hydrogeological section. Secondly, it aims to study the chemistry of groundwater in terms of major ions and silica concentration to explain the mechanism below the surface such as water–rock interaction and/or anthropogenic effects responsible for resulting chemistry of groundwater. Structure of the paper and methodology adopted is illustrated in Fig. 1.
2 Study area
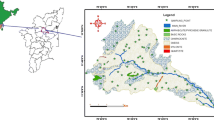
Present study is carried out in Moradabad city which forms the north-western part of Moradabad district of Uttar Pradesh. The area is bounded by Ramganga on its north-eastern side and Gagan to its southern side (Fig. 2). The city is dominated by small-scale industries. However, primarily the economy of the Moradabad district as a whole is agrarian based. The area lies in the sub tropical region with tropical to sub tropical climate. It receives about 86% of rainfall from June to September. Monsoon periods allow deep percolation to groundwater due to surplus water availability. The average annual rainfall of the district is 900 mm (Saba 2016). Geologically, study area forms a part of the Ganga plain. Thick alluvial deposits of more than 1000 m of Quaternary age which are categorized as Older and Newer Alluviums covers the area. Silt, clay and calcareous nodules and different grades of sand and gravel are the major compositional units of alluvium. Calcareous nodules occur as thin beds and lenses and are indicative of sedimentation gaps. The sedimentations are dominantly arenaceous.
3 Hydrogeological settings
The study area forms a part of Central Ganga Plain (CGP). Some of the most productive aquifer systems in India lie in CGP (CGWB 2011). The thickness aquifer is variable at different places ranging from few metres (m) to 300 m (Verma 2009). Fence diagram prepared from lithological logs of boreholes describe the extent and geometry of aquifer. Top clay layer persists throughout the area ranging in thickness from 2 to 10 m bgl. The top clay bed is followed by a granular zone which is subdivided at places into two to three sub-groups by the occurrence of sub-regional clay beds. The granular zone forms 76% of the total thickness in the northern part of the area, whereas in the southern part the granular zone constitutes as much as 91% of the total thickness. Aquifer material is composed of fine, medium to coarse sand (Fig. 3). In pre-monsoon season 2012, depth to water level, ranges from 3.2 to 13.7 m bgl. Central part of the study area encounters deeper water level conditions. Water table elevation, as evident from water table contour maps of pre-monsoon season 2012, ranges from 187 m above mean sea level (AMSL) in the north-west to 190 m in the south-east. Major groundwater flow direction is from south-east to north-west (Fig. 4). A groundwater trough is developed due to over pumping in the central part of the study area, a phenomenon frequently observed in urban area distorts the flow direction (Saba 2016).
4 Methodology
Appropriate criterion for selection of sampling sites was used for groundwater quality assessment. Groundwater samples were collected from hand pumps which were in use for drinking and domestic purpose from main city representing urbanized and industrial area and from north-eastern and south-western margin representing agricultural area. Depth of sampled wells varies from about 15 m to 35 m, the average being 20 m. A network of sampling wells was established in such a way that the entire area is covered by representative samples. A total number of 188 samples 47 in each season (pre- and post-monsoon) were collected in 2012 and 2013 (Fig. 5) in a well rinsed 1-L polyethylene bottles after pumping the well for about 5–10 min to remove the stagnant water, if any, in the well assembly from hand pumps. Samples were filtered and analyzed for various physico-chemical parameters including pH, EC, TDS, major ions (Ca, Mg, Na and K, Cl, SO4, HCO3, NO3, F) and SiO2 as per the standard methodologies (APHA 1992) (Table 1). Concentration of major cations (Ca, Mg, Na and K) was determined by volumetric method. Hardness and calcium were determined by titration method using EDTA; bicarbonate was determined by titration using HCl, chloride was determined using AgNO3 solution in titration method, and flame photometry was used for the determination of sodium and potassium. Sulphate was analyzed by gravimetric method, and nitrate was determined by colorimetric method using phenol disulphonic acid. Fluoride was measured by the spectrophotometer using SPADNS (sodium 2-(parasulfophenylazo)-1, 8-dihydroxy-3, 6-naphthalene disulfonate), and silica was analyzed by molybdenum silicate method. Saturation Indices (SI) of minerals were calculated using PHREEQC. This programme computes SI of all possible minerals that can be formed from the given solution according to the data entered. The data were obtained from the major ion and trace elements analysis of groundwater. To determine the effect of rainfall on chemical variations in groundwater, Rainfall data of Moradabad rain gauge station were collected for the period of 51 years from 1964 to 2015.
5 Results and discussion
5.1 Total dissolved solids and its spatial distribution
The concentration levels of major ions can be summed up for the calculation of Total Dissolved Solids (TDS). These values have been considered more reliable compared with those estimated by EC (Table 2, Fig. 6). It has been generally observed in the Gangetic plain that post-monsoon concentration of major ions and consequently of TDS is relatively high in shallow groundwater for obvious reasons that during this season, descending meteoric recharge is capable of taking the pollutants and water soluble constituents from surface and soil zone downwards to relatively elevated water table (Rao 2008). Similar observation was made by Singh et al. (2014) in a study conducted in Ghaziabad.
In Moradabad city, this trend is seen for the year 2012 but in the succeeding year the trend has reversed and pre-monsoon average value of 1151 mg/l is followed by the post-monsoon average of 970 mg/l. The reason for this observed trend reversal probably lies in large fluctuations in the average rainfall from 1159 mm in 2011 and 801 mm in 2012 to 980 mm in 2013, recording a deficiency of 31% in 2012 compared with 2011 rainfall and increase in rainfall by over 23% in 2013 compared with 2012 data. It appears, therefore, that as a relatively lean period of 2012 was followed by a wetter year in 2013, there were some dilution effects, causing a marginal decrease in groundwater TDS. A study conducted by Venugopal et al. (2009) in Chennai, India shows the similar results. In the spatial distribution map for pre-monsoon 2012 (Fig. 7a), two high TDS zones with values of > 1000 mg/l were observed extending southwards from the central part of the study area. The first zone encompasses densely populated areas, and the other zone covers the area under cropland and plantation. During the post-monsoon season 2012 (Fig. 7b), the two high TDS zones of pre-monsoon season merge into one elongated N–S trending zone with values of 1400 mg/l and above. This zone has northwards extension across the river Ramganga. Values, as high as 1600 mg/l, are recorded in areas on the bank of the river. In the pre-monsoon season 2013 (Fig. 7c), three TDS highs encompassed by 1200 mg/l contour are observed. Two of these highs have NNE–SSW trend as seen in post-monsoon 2012. One of these highs is in the eastern part of the area along river Ramganga, the other in the south-central part. The third high is in the north-western part of the area. In the succeeding post-monsoon season (Fig. 7d), a major high, with values of > 1000 mg/l, is observed in almost the central part of the area and the other, relatively minor one, is in the north-west.
6 Temporal variations of major ions
Chemical characteristics of groundwater in terms of temporal variations are given below.
-
1.
Consistently high concentration of Na and occasionally anomalously high K values were observed. In post-monsoon season 2012, both the ions show increasing concentration as compared with pre-monsoon season. The trend gets reversed in 2013.
-
2.
Occurrence of Ca– deficient and Mg-enriched water is one of the characteristic features of the shallow groundwater system of the area. Temporal behaviour of these two ions, which under ideal conditions tend to be together, is different. While post-monsoon seasons show Ca enrichment as compared to the preceding pre-monsoon values, Mg is relatively less sensitive temporally or tends to show a decreasing concentration in post-monsoon period.
-
3.
A very substantial increase in HCO3 concentration is observed in post-monsoon season of 2012 but this trend gets reversed in 2013. As HCO3 content in groundwater is dependent on uptake of CO2 by meteoric recharge, it is possible that it would depend on rainfall parameters. Low HCO3 content of groundwater in post-monsoon 2013 may be due to the fact that August–September rainfall in 2013, preceding the post-monsoon sampling, was 30 to 70% less than that during the same months in 2012.
-
4.
Chloride shows an increase in concentration during post-monsoon seasons with some anomalously high values ranging from 310 to 537 mg/l. This may be related to the meteoric recharge water carrying Cl pollutants to deeper levels after monsoonal rainfall.
-
5.
SO4 shows no significant temporal variation and its average concentration always remains > 200 mg/l, except for few samples where anomalously high SO4 values of > 400 mg/l are reported. Bulk of this ion may have an anthropogenic origin with contributions from industrial and agricultural domains mainly. Similar observation was made by Porowski et al. (2019) in a study carried in Poland.
-
6.
Although many samples show high and anomalous values of NO3 exceeding 100 mg/l, this ion shows no significant variations during pre- and post-monsoon seasons.
-
7.
Anthropogenic factors affect the concentration levels of major ions viz. Na, K, Cl, SO4 and NO3 to variable extents. In addition to anthropogenic factors, some external non-geogenic sources also play an important role in affecting the concentration of Ca and Mg. The possible cause of variation in the observed concentrations of major ions is given in the succeeding section.
7 Possible causes of observed variations in major ion concentration
Seasonal or temporal variations in groundwater chemistry may be due to deviations in meteorological factors, groundwater recharge, land use and management of groundwater resource (Guo et al. 2018). In general, rainfall recharge is one of the sources for variation in groundwater chemistry (Sakakibara et al. 2017). Rainfall recharge occurs during the monsoon period from July to September, and evaporation is very high in April to June when temperature is always in excess of 40 °C.
There may be two types of temporal variations in groundwater ionic concentrations. The first type of variation occurs during the rainfall recharge as the concentration of ions decreases in groundwater and the water level increase. The decreasing concentrations of major ions are due to the dilution of groundwater. The reverse is true in the second type of variations. During dry periods, when the groundwater level decreases, the major ion concentration increases indicating evaporation. Further, during the beginning of monsoonal rainfall recharge, the recharging water dissolves the salts deposited during the preceding dry months in the soil zone and takes them downwards to the groundwater table resulting in an increase in the major ions concentration in groundwater. If the rainfall continues for more times, the concentration of major ions may decrease due to dilution effects (Elango 1992; Rajmohan and Elango 2006; Rajesh et al. 2012).
It is considered imperative to give a succinct account of the processes responsible for acquisition of major ions and their variation patterns and the same are discussed here. In general, high Ca values are observed in post-monsoon seasons as descending recharging water gets fresh inputs of CO2 for absorption. This CO2–charged water may dissolve Ca– carbonate from soil zone to enhance concentrations of Ca and HCO3 (Shanyengana et al. 2004). In addition, there may be some likelihood of dissolution of minerals, such as, gypsum and anhydrite to provide Ca ions to groundwater. Addition of Ca may also be possible from fertilizers and other anthropogenic sources (Khatri and Tyagi 2015). As far as Mg concentration levels are concerned, high Mg contents, as high as 162 mg/l, are enigmatic as so much of Mg is difficult to be conceived as the product of water–rock interaction unless there are some water soluble Mg-minerals, such as, dolomite. Cation exchange processes could have played a role but Mg divalent and one of the smallest ions would tend to be sorbed on clay surfaces rather than being released (http://www.deq.state.or.us/wq/groundwater/docs/lubgwma/hydrogeologyrpt/appx4a.pdf).
There may be possibility of some Mg addition through agricultural activities. It is relevant to mention here that Mg concentration levels were even higher, averaging 52 mg/l, about 4 to 5 years ago (Pathak et al. 2008). There has been a tendency, therefore, of the groundwater chemistry having been characterized by Mg > Ca (Table 3). Distinctly high Na and K values, averaging 140 to 239 mg/l and 13 to 33 mg/l, respectively, for the two pre-monsoon and two post-monsoon seasons of 2012 and 2013, with the highest values being > 400 mg/l and 96 mg/l (Table 3) cannot be accounted for silicate weathering alone and evidently there had been a major inputs of these ions to the system as a result of anthropogenic activities related to domestic, industrial and agricultural domains. A study conducted by Panno et al. (2002) had also revealed that anthropogenic sources may be an important contributor of Na and Cl in groundwater.
As far as anions are concerned, HCO3 concentrations are the result of dissociation of H2CO3 which is formed due to absorption of CO2 from organic matter and plant roots respiration zones. Conducive conditions for these processes would be the post-monsoon season when descending recharge waters traverse these zones (Shanyengana et al. 2004).
Chloride values range from 9 to > 500 mg/l with relatively consistent average values of 87 to 118 mg/l and seem to have a considerable input from anthropogenic sources (Panno et al. 2002). Samples from the abandoned bone mill site giving values of > 500 mg/l provide unequivocal evidence of external anthropogenic source of Cl. Sulphate values too are anomalously high to be explained by water–rock interaction or dissolution of sulphate minerals, such as, gypsum, as value as high as 537 mg/l has been reported and the average concentration level is in excess of 200 mg/l. A study conducted by Zereg et al. (2018), in Algeria also reveals that high concentration of Na, Cl and SO4 may results from water–rock interaction and dissolution of halite, pyrite and related salt minerals.
Nitrate too has occasionally anomalous concentration exceeding 200 mg/l but, in general, the average concentration levels are relatively moderate at 23 to 33 mg/l. Nitrate could have its source either in agricultural activities or in human and animal wastes. Similar conclusions have also been made by Shaji et al. (2018) in Chittur block, Plakkad, Kerela and Agrawal et al. (1999) in India, where they revealed that nitrate levels in groundwater can be correlated with intensive irrigated agriculture alongwith use of nitrogenous fertilizers. Concentration of F is within the prescribed limits. A conceptual model (Fig. 8) shows various processes in operation for the acquisition of chemical species from both geogenic and anthropogenic processes.
8 Classification of groundwater on the basis of L–L Diagram
L–L diagram shows in pre-monsoon 2012, > 90% samples in the upper half of the plot implying overwhelming dominance of Cl + SO4 over HCO3. About 72% samples are of Na–K–Cl–SO4 type, falling in the top right quarter, and 17% of the samples are Ca–Mg–Cl–SO4 type and plot in the top left quadrant. This implies the dominance of alkalis and Cl + SO4 in pre-monsoon season (Fig. 9a).
In post-monsoon 2012, no samples plot in Ca–Mg–HCO3 and Ca–Mg–Cl–SO4 fields (bottom left and top left quadrants, respectively). All the samples show dominance of Na + K over Ca + Mg and plot in top right and bottom right quarters. About 50% samples each plot in Na–K–Cl–SO4 and Na–K–HCO3 fields (Fig. 9b) indicating dominance of alkali bicarbonates.
In pre-monsoon season 2013, as many as 32 samples (68%) form the largest group plotting in the top right quarter characterizing Na–K–Cl–SO4 type water. The second largest group, comprising 13 samples (28%), is also characterized by high concentrations of Na and K but the dominant anion is HCO3. This group with Na + K–HCO3 signatures is the typical alkali bicarbonate type (Fig. 9c). Post-monsoon season 2013 shows somewhat different pictures though the principal chemical character remains the same, that is, the dominance of Na–K–Cl-SO4 aqueous species. About 70% of the samples show these chemical signatures.
Another 20% samples lies in Ca–Mg–Cl-SO4 field and the rest 10% in Na–K–HCO3 and Ca–Mg–HCO3 fields. One very obvious change with respect to pre-monsoon scenario is that relative abundance of HCO3– facies has greatly reduced. Almost all the samples cluster in the upper half of the plot suggesting an approach of the groundwater system towards attaining relative homogeneity in its chemical characteristics with relative paucity of HCO3 complexes in this season (Fig. 9d).
9 Influence of meteorological factors (rainfall) on groundwater chemistry
Since chemistry of shallow groundwater is so intimately related to meteorological parameters, particularly, intensity and month wise distribution of rainfall, it is logical to evaluate observed chemical variations in the light of the available rainfall data. Rainfall particularly monsoonal rainfall (15th June–15th October) had been quite uneven for the years 2011, 2012 and 2013, being 1086, 755 and 855 mm, respectively. Monsoonal rainfall in 2012 was more than 300 mm deficient compared with that in 2011 and 78% of it was during the period August to October. There was 100 mm additional monsoonal rainfall in 2013 compared with that in 2012 but only 38% of it was in the period August to October, immediately preceding post-monsoon sampling. As mentioned below, rainfall related parameters may be one of the driving factors responsible for overall temporal variations in groundwater chemistry.
-
1.
Pre-monsoon 2012 chemistry would depend on rainfall data for the preceding year 2011 when there was luxuriant rainfall, the bulk of which (60%) was during the months of August and September. Availability of relatively large quantities of recharging water would result in dilution, and accordingly, the TDS for pre-monsoon 2012 has a relatively low average value of 781 mg/l (Table 3). Availability of Ca and Mg and their association in Cl + SO4 rather than HCO3 aqueous facies (Fig. 9a) implies that these have been derived either as a result of cation exchange or from anthropogenic sources. Abundance of Cl + SO4 over HCO3 suggests that the process of dissolution and uptake of pollutants in water was more dominant than absorption of CO2 from organic matter and root respiration zone.
-
2.
Post-monsoon samples of 2012 (Fig. 9b) would carry the effects of the preceding monsoonal rainfall which had been rather scanty with the bulk falling in August and September. Relatively low overall availability of recharging water would tend to increase ionic concentration and TDS (Table 3). Lower abundances of Ca and Mg may suggest precipitation of Ca and Mg carbonates during the hot summer with temperature in excess of 44 °C. Bicarbonate still left, under this scenario, would form aqueous species with Na and K. However, as mentioned above, the observed trend of blank Ca + Mg fields (top left and bottom left quadrants) may also be due to large scale induction of alkalis rather than the removal of Ca + Mg through deposition of carbonates.
-
3.
In difference that in the former, the samples with Na–K–Cl–SO4 and Na–K–HCO3 attribute are 68% and 28%, respectively, whereas in the latter, about 50% samples each occur in the two fields mentioned above. Average TDS values of the two sets of samples are also comparable. The same processes of deposition of Ca and Mg carbonates or large scale flux of alkalis, most likely from anthropogenic sources, may be responsible for pre-monsoon 2013 trend (Fig. 9c).
-
4.
Post-monsoon 2013 (Fig. 9d) samples may certainly be affected by the pattern of rainfall that year. Of the total annual average rainfall of 989 mm, the area received 855 mm during the monsoon period extending from 15th June to 15th October. There had been torrential rains during June to August, cumulatively accounting for 93% of the total monsoonal rainfall in the area. This was followed by a very lean period during September and October, just preceding the sampling, during which only 7% of the annual monsoonal rainfall occurred in the area. The first spell of torrential rains could have resulted in more of runoff and less of infiltration. Another effect would have been that bulk of surface spread of pollutants, including industrial effluents, would have been washed rather than carried downwards by descending waters. Yet another effect would be that sewage discharges and leakages from septic tanks and landfills could get substantially diluted. All these factors would result in substantial dilution of groundwater. As depicted in Table 3, pre-monsoon 2013 TDS value is 1151 mg/l and that of the post-monsoon season is 970 mg/l. The lean period succeeding the period of heavy rains provides an explanation for the observed clustered nature of samples (Fig. 9c). It seems that the lean period preceding the post-monsoon sampling in November has given time for groundwater to attain a kind of steady state through processes such as attaining equilibrium with the surroundings, precipitation and dissolution of minerals and lateral mixing of groundwater.
10 Saturation index (SI)
The quantitative deviation of water from equilibrium with respect to dissolved minerals is described by the Saturation Indices (Sandow et al. 2011; Raju 2012b). Rainfall, climate, evaporation and minerals present in geological formations and hydrogeochemical processes like precipitation, dissolution, sorption and ion-exchange and anthropogenic activities controls the chemical composition variation of groundwater in an area (Raju et al. 2009, 2011).
The SI was calculated using the PHREEQC programme. The summary of results is given in Table 4. It needs to be mentioned here that the data on saturation indices corresponds to the pre- and post-monsoon seasons of the year 2012 only. It is seen from Table 4 that groundwater of the area show a range of SI values for the common minerals known to occur in groundwater systems.
Groundwater of the area is highly under-saturated with respect to halite. There is also distinct under-saturation with respect to sulphate minerals gypsum and anhydrite. This implies that these minerals, if present in the aquifer, would be dissolved in the groundwater.
For carbonates, such as, aragonite, calcite and dolomite, the trends are variable implying that these minerals may be dissolved or precipitated depending upon the prevailing physico- chemical conditions like temperature, pH and dissolved CO2 content (CO2 partial pressure) in the groundwater. There may be a possibility that carbonates, which have a decrease in their solubility with increasing temperature (http://comp.uark.edu/), may be precipitated during high temperatures occurring in pre-monsoon period and may be dissolved during the cooler post-monsoon period. The possibility, therefore, cannot be ruled out that carbonates of both Ca and Mg may be precipitating and getting dissolved in the groundwater system of the area influencing the concentration levels of Ca, Mg and HCO3 in groundwater.
There is under-saturation with respect to fluorite implying that this mineral, if available in the system is likely to be taken in solution. Interestingly, there are indications of super-saturation with respect to talc, a hydrated silicate of Mg. As far as silica minerals quartz and chalcedony are concerned, both show super-saturation, which is particularly more pronounced for the former. Silica tends to deposit when there is a decrease in temperature and therefore, the possibility of some silica precipitation during the post-monsoon period cannot be ruled out. However, such precipitation would only be trivial in terms of its influence on the composition of groundwater. Variations in Saturation Indices of various minerals during pre- and post-monsoon seasons of 2012 are depicted in Figs. 10 and 11.
11 Dissolved silica and its role in evaluating groundwater pollution
Present study includes the analysis for dissolved silica in four sets of samples collected from pre- and post-monsoon seasons of 2012 and 2013 from all the 47 groundwater sampling sites, as normally dissolved silica determinations are not part of the conventional chemical analysis protocol of groundwater systems.
The concentration of Silica in groundwater samples ranges from 16 to 68 mg/l and 10 to 68 mg/l in pre- and post-monsoon seasons of 2012, respectively. During pre- and post-monsoon seasons of 2013, concentration levels are between 19 and 60 mg/l and 20 and 60 mg/l, respectively. In pre- and post-monsoon seasons of 2012, as many as 9 and 10 samples have recorded values of 60 mg/l and above, whereas values of 60 mg/l and above in the two respective seasons in 2013 have been recorded in two and one samples.
Silica in groundwater is exclusively derived from water–rock interaction. The circulating groundwater dissolves the silica derived from the chemical weathering of silicate minerals in rocks and sediments (Hem 1959). Chloride acts as an inert element in all natural water–rock environments, i.e. once in solution, it stays there both at ambient and elevated temperatures (Ellis 1970). It usually does not enter common rock-forming minerals due to the large size of its ion and hence does not participate in ion exchange in groundwater (Hem 1959).
Its concentration may therefore be viewed in relation to Cl and TDS to evaluate the effects of pollution caused as a result of anthropogenic activities. A similar study has also been conducted by Khan and Umar 2010 in parts of Central Ganga Plain, U.P., India, yielded valuable information on relative roles of geogenic and anthropogenic solute acquisition processes.
Silica-Cl relationship for post-monsoon season 2012 (Fig. 12) clearly elucidates this. For two distinct populations with average silica content of about 25 and 60 mg/l, variation in Cl is from < 50 to > 300 mg/l clearly indicating that Cl variations are of anthropogenic rather than geogenic origin in samples with silica < 25 mg/l. This aspect is further strengthened by SiO2–TDS plot for pre- and post-monsoon seasons of 2012 and 2013 (Fig. 13). In systems with water–rock interaction as the dominant process, one would normally expect a positive correlation between SiO2 and TDS as both would tend to increase with increasing interaction between rock and water and it is observed in hard-rock hosted groundwater systems of deeper origin. Again the same two populations with average SiO2 values of 25 and 60 mg/l have TDS values showing wide range of variations from about 400 to > 1600 mg/l clearly implying that the bulk of TDS are of anthropogenic origin.
12 Conclusions
Present study is done to assess the role of various processes (geogenic and/or anthropogenic) on the groundwater system of the area. The impact on the quality of groundwater can be seen from the chemical analysis results.
Moradabad city possess highest K, Na, SO4, NO3 and Cl values of 96 mg/l, 440 mg/l, 537 mg/l, 244 mg/l and 537 mg/l, respectively. Average TDS values lie between 781 and 1292 mg/l, the highest values being in excess of 1900 mg/l, a clear indicative of anthropogenic influences. One of the characteristic features of the shallow groundwater system of the area as far as variations in major ions are concerned is that it is Ca-deficit and Mg-enriched. HCO3 displays very substantial increase in its concentration in post-monsoon season of 2012 but this trend gets reversed in 2013. This is evidently related to rainfall parameters. Some anomalously high chloride values of 310 to 537 mg/l shows an increase in its concentration during post-monsoon seasons probably as a consequence of the meteoric recharge water carrying Cl pollutants to deeper levels. Saturation indices for various minerals suggest that groundwater is highly under-saturated with respect to halite, amorphous silica, sulphates of Ca and fluorite and super-saturated for chalcedony, quartz and talc, and these minerals may possibly be precipitated by groundwater of the area. For carbonates, the possibility cannot be ruled out that these may be precipitated as well as dissolved depending upon temperature, pH and dissolved CO2 in groundwater. Correlation of silica with Cl and TDS suggest the influence of anthropogenic processes on groundwater. From the anthropogenic activities point of view, Moradabad offers a good example of Amalgamated Activities such as application of fertilizers, manures, insecticides and pesticides in the peripheral agricultural land is responsible for addition of Na, K, Ca, NO3 and SO4. Other sources of pollution are human and animal wastes, leakages from septic tanks and drains, industrial effluents and landfill leachates. The results of the study showed that the groundwater of the study area is contaminated with respect to major ions, therefore to keep a check on this contamination, few suggestions are given here. The areas in the city devoid of sewage disposal lines should be provided sewage lines. The entire city should have piped water supply from tubewells tapping the deeper aquifer at levels below 180 m. There has to be strict monitoring of effluent disposal systems used by industrial units under the supervision of Central and State Pollution Control Boards and if there is need, these units have to be relocated outside the populated area. Effluent discharges into rivers, which are known to be some of the most polluted rivers of Ganga basin, needs to be monitored as the influent nature of these rivers tends to pour the pollutants into the groundwater system.
References
Alam, F., & Saha, D. (2014). Groundwater vulnerability assessment using DRASTIC and pesticide DRASTIC models in intense agriculture area of the Gangetic plains, India. Environmental Monitoring and Assessment, 186, 8741–8763.
Agrawal, G. D. (1999). Diffuse agricultural water pollution in India. Water Science and Technology, 39(3), 33–47.
APHA, American Public Health Association, . (1992). Standard methods for the examination of water and waste water (16th ed.). Washington, D. C: American Public Health Association.
Bhardwaj, V., Singh, D. S., & Singh, A. K. (2010). Hydrogeochemistry of groundwater and anthropogenic control over dolomitization reactions in alluvial sediments of Deoria district: Ganga plain. India. Environmental Earth Sciences, 59(5), 1099–1109.
Bornstein, R., & Lin, Q. (2000). Urban heat islands and summertime convective thunderstorms in Atlanta: Tree case studies. Atmospheric Environment, 34, 507–526.
CGWB, Central Ground Water Board. (2011). Dynamic ground water resources of India. Ministry of Water Resources: Govt. of India.
Changnon, S. A. (1976). Inadvertent weather modification. Water Resources Bulletin, 12, 695–718.
Elango, L. (1992). Hydrogeochemistry and mass balance modelling of multilayered aquifers. Unpublished PhD thesis, Anna University, Chennai, India, p.181.
Ellis, A. J. (1970). Quantitative interpretation of chemical characteristics of hydrothermal systems. Geothermics, 2, 516–527.
Foster, S. S. D., & Candela, L. (2008). Diffuse groundwater quality impacts from agricultural land-use: Management and policy implications of scientific realities groundwater science & policy – an international overview. London: Royal Society of Chemistry Publishing.
Foster, S. S. D., Chilton, P. J., & Lawrence, A. R. (2000). Processes of diffuse groundwater pollution by agricultural land-use. In R. Fu & Q. Yi (Eds.), Groundwater Contamination and its Control in China. Beijing: Tsinghua University Press.
Guo, X., Zuo, R., Men, L., Wang, J., Teng, Y., Liu, X., & Chen, M. (2018). Seasonal and spatial variability of anthropogenic and natural factors influencing groundwater quality based on source apportionment. International Journal of Environmental Research and Public Health, 15(2), 279.
http://www.deq.state.or.us/wq/groundwater/docs/lubgwma/hydrogeologyrpt/appx4a.pdf
Hem, J. D. (1959). Study and interpretations of chemical characteristics of natural water. Water Supply, USGS, 2254, 263.
Khan, M. M. A., & Umar, R. (2010). Significance of silica analysis in groundwater in parts of Central Ganga Plain, Uttar Pradesh. India. Current Science, 98(9), 1237–1240.
Khan, A., Umar, R., & Khan, H. H. (2015). Hydrochemical characterization of groundwater in Lower Kali Watershed, Western Uttar Pradesh. Journal Geological Society of India., 86(2), 195–210.
Khatri, N., & Tyagi, S. (2015). Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Frontiers in Life Science, 8(1), 23–39. https://doi.org/10.1080/21553769.2014.933716.
Liu, X., Simunek, J., Lo, L., & He, J. (2013). Identification of sulfate sources in groundwater using isotope analysis and modeling of flood irrigation with waters of different quality in the Jinghuiqu district of China. Environmental Earth Science, 69, 1589–1600.
Misra, A. K. (2011). Impact of urbanization on the hydrology of Ganga Basin (India). Water Resource Management, 25(2), 705–719.
Morris, B.L., Lawrence, A.R., Chilton, P.J., Adams, B., Calow, R. and Klinck, B.A. (2003). Groundwater and its susceptibility to degradation: A global Assessment of the Problems and Options for Management. Early Warning and Assessment Report Series, RS, 03–3. United Nations Environment Programme, Nairobi, Kenya.
Pathak, J. K., Alam, M., & Sharma, S. (2008). Interpretation of groundwater quality using multivariate statistical technique in Moradabad city, Western Uttar Pradesh State. India. E Journal of Chemistry, 5(3), 607–619.
Panno S.V., Hackley K.C., Hwang H.H., Greenberg S.E., Krapac I.G., Landsberger S., O'Kelly D.J. (2002). Source identification of sodium and chloride contamination in natural waters: Preliminary results. Proceedings of the 12th annual conference of the illinois groundwater consortium. At Makanda, IL.
Peiyue, L., Jianhua, W., Hui, Q., Yuting, Z., Nuan, Y., Lijun, J., & Peiyuan, Y. (2016). Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger desert, Northwest China. Expo Health, 8, 331–348. https://doi.org/10.1007/s12403-016-0193-y.
Porowski, A., Porowska, D., & Halas, S. (2019). Identification of sulfate sources and biogeochemical processes in an aquifer affected by Peatland: Insights from monitoring the isotopic composition of groundwater sulfate in Kampinos national park Poland. Water, 11, 1388. https://doi.org/10.3390/w11071388.
Rajesh, R., Brindha, K., Murugan, R., & Elango, L. (2012). Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh, India. Environmental Earth Sciences, 65, 1203–1213.
Rajmohan, N., & Elango, L. (2006). Hydrogeochemistry and its relation to groundwater level fluctuation in the Palar and Cheyyar river basins, southern India. Hydrological Processes, 20, 2415–2427.
Raju, N. J., Ram, P., & Dey, S. (2009). Groundwater quality in the lower Varuna River basin, Varanasi district, Uttar Pradesh, India. Journal Geological Society of India, 173, 279–300.
Raju, N. J., Shukla, U. K., & Ram, P. (2011). 1 Hydrogeochemistry for the assessment of groundwater quality in Varanasi: A fast urbanizing centre in Uttar Pradesh, India. Environmental Monitoring Assessment, 173, 279–300.
Raju, N. J. (2012a). Arsenic exposure through groundwater in the middle Ganga plain in the Varanasi environs, India: A future threat. Journal of the Geological Society of India, 79(3), 302–314.
Raju, N. J. (2012b). Evaluation of hydrogeochemical processes in the Pleistocene aquifers of middle Ganga Plain, Uttar Pradesh. India. Environmental Earth Sciences, 65(4), 1291–1308.
Rao, N. S. (2008). Factors controlling the salinity in groundwater in parts of Guntur district, Andhra Pradesh, India. Environment Monitoring and Assessment, 138, 327–341.
Saba, N., Umar, R., & Ahmed, S. (2016). Assessment of groundwater quality of major industrial city of Central Ganga plain, Western Uttar Pradesh, India through mass transport modeling using chloride as contaminant. Groundwater for Sustainable Development, 2–3(12), 154–168.
Saba, N., & Umar, R. (2016). Hydrogeochemical assessment of Moradabad city, an important industrial town of Uttar Pradesh. India. Sustainable Water Resources Management, 2(3), 217–236.
Saba, N. (2016). Segregation of various types of contamination in urban groundwater of Moradabad city and suggestion for suitable remediation. Unpublished Ph.D. Thesis, Aligarh Muslim University, Aligarh, pp. 49–61.
Sajjad, H., & Jyoti and Uddin, R. . (2014). Exploring water quality index and risk on quality of life in an industrial area: A Case from Ghaziabad City, India. International Journal of Environmental Monitoring and Analysis, 2(2), 65–72.
Sakakibara, K., Tsujimura, M., Song, X., & Zhang, J. (2017). Spatiotemporal variation of the surface water effect on the groundwater recharge in a low-precipitation region: Application of the multi-tracer approach to the Taihang Mountains, north China. Journal of Hydrology, 545, 132–144. https://doi.org/10.1016/j.jhydrol.2016.12.030.
Sandow, M. Y., Banoeng-Yakubo, B., & Akabzaa, T. (2011). Characterization of the groundwater flow regime and hydrochemistry of groundwater from the Buem formation, Eastern Ghana. Hydrological Processes, 25, 2288–2301.
Sethy, S. N., Syed, T. H., & Kumar, A. (2017). Evaluation of groundwater quality in parts of the Southern Gangetic Plain using water quality indices. Environ Earth Sci., 76, 116. https://doi.org/10.1007/s12665-017-6434-y.
Schlager, E. (2006). Challenges of governing groundwater in U.S. western states. Hydrogeology Journal, 14, 350–360. https://doi.org/10.1007/s10040-005-0012-1.
Shaji, E., Gomez-Alday, J. J., Hussein, S., Deepu, T. R., & Anilkumar, Y. (2018). Salinization and deterioration of groundwater quality by nitrate and fluoride in the Chittur block, Palakkad, Kerala. Journal of the Geological Society of India, 92, 337–345.
Shanyengana, M. K., Seely, M. K., & Sanderson, R. D. (2004). Major-ion chemistry and groundwater salinization in ephemeral floodplains in some arid regions of Namibia. Journal of Arid Environments, 57, 71–83.
Singh, U. V., Abhishek, A., Singh, K. P., Dhakate, R., & Singh, N. P. (2014). groundwater quality appraisal and its hydrochemical characterization in Ghaziabad (a region of indo-gangetic plain), Uttar Pradesh, India. Applied Water Science, 4, 145–157.
Verma, N. and Kumar, N. (2014). Quality characterization of groundwater at Amroha (Uttar Pradesh). International Journal of Pharmaceutical and Chemical Sciences. 3(1).
Verma, R. C. (2009). District brochure of Moradabad district (p. 7). India, U.P: Central Ground Water Board, CGWB.
Venugopal, T., Giridharan, L., Jayaprakash, M., & Periakali, P. (2009). Environmental impact assessment and seasonal variation study of the groundwater in the vicinity of River Adyar, Chennai. India. Enviornmental Monitoring and Assessment, 149(1–4), 81–97.
Zereg, S., Boudoukha, A., & Benaabidate, L. (2018). Impacts of natural conditions and anthropogenic activities on groundwater quality in Tebessa plain. Algeria. Sustainable Environment Research, 28(6), 340–349.
Acknowledgements
Financial assistance received by the first author in the form of Senior Research Fellowship (SRF) from UGC, New Delhi is acknowledged. The authors are thankful to the reviewers for their suggestions that improved the work. Thanks are due to Chairman, Department of Geology, Aligarh Muslim University, Aligarh for providing the basic facilities to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
us Saba, N., Umar, R. Identification of the processes controlling groundwater quality in shallow aquifers of Moradabad city, west Uttar Pradesh, India. Environ Dev Sustain 23, 12994–13015 (2021). https://doi.org/10.1007/s10668-020-01196-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-020-01196-w