Abstract
Presently, campaign for potable water supply is on the increase while the need to harness groundwater to alleviate the shortage of potable water is very crucial. This study investigated the groundwater potential of the local geologic formations and to determine the extent and distribution of the saline zones at Eha-Amufu. Integrated approach using geological, geoelectrical and hydrogeochemical investigations was adopted. Twenty-seven (27) vertical electrical sounding (VES) were performed at different locations within the area, using the Schlumberger electrode configuration with a maximum electrode separation of 600 m. Results from the VES revealed that the study area is made up of 3–6 geoelectrical layers, and are predominantly of QH and H curve types. The formations were observed to be characterized by low permeability zones/intervals, suggesting that Eha-Amufu could only possess perched aquifers table which supports the use of hand-dug wells. Hydro-chemical analysis of the ten (10) groundwater samples collected from different hand-dug wells indicated that the groundwater is characterized by Calcium (Ca)—Magnesium (Mg)—Chloride (Cl) water type, and could be classified as moderately hard to very hard. Water samples showed evidence of contamination with heavy metal such as Pb2+ beyond WHO standard, thereby making it unfit for domestic uses and consumption. Further result probe revealed that the local geology is the major process controlling the fate of groundwater chemistry in the area through weathering and rock–water interaction. Therefore, the present study proposes that other forms of portable water provisions should be explored such as harvesting and treatment of the various river resources in the area using purification plants and development of regional water conduits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
1.1 Background of study
Water is an indispensable resource and is a primary driver of man’s socioeconomic development but its availability in a potable form across places especially in rural areas remains a gigantic problem which needs to be overcome (Bob-Duru 2001). Landmark in Nigeria’s legislation is the provision of potable water by government, under the auspices of Government Owned Public Water Utilities (GPWUs), taking its supplies from artificially designed water reservoirs, surface water, viable boreholes and other standing water bodies. In spite of this bold step which aimed at keeping the nation wet with potable water, the GPWUs could not meet the water demand and supply of the masses (Jalali 2007; Okogbue et al. 2012; Aghamelu et al. 2013). This is because regions have witnessed numerical growth and industrial development leading to a global drive towards sustainability (World Commission on Environment and Development 1987).
The populace of Eha-Amufu and environs has decried the acute scarcity of potable water. The situation has been aggravated by lack of institutionalized water supply scheme to support the teeming population of Federal College of Education in Eha-Amufu. Compounding the onerous task of sourcing potable water in Eha-Amufu and its environs is the effect of geology. The low permeable Nkporo and Awgu Shales underlay a vast section of the area, thereby presenting Engineers with a lot of difficulties which are hydro-geologically oriented (Nganje et al. 2015) sequel to the exorbitant cost incurred while drilling boreholes. Unfortunately, in the present study area, land spaces are dotted with numerous perched aquifers which dry up after a short service. Potable water is both a costly and a rare commodity to come by mostly during the dry season when there are limited alternatives such as the rains and streams.
Prominent in most groundwater investigation based researches within the basement and sedimentary complex is the electrical resistivity method via; the vertical electrical sounding (VES) (Olayinka 1992; Vchery and Hobbs 2003; Egwebe et al. 2004; Olaleye 2005; Akaolisa 2006; Alile et al. 2008; Tizro et al. 2010; Arabi et al. 2008; Joshua et al. 2011). VES provides an excellent prediction of the groundwater potential of an area (Olayinka 1992; Selemo et al. 1995; Nwankwoala and Shalokpe 2008; Okogbue and Omonona 2013). Ultimately, VES is very useful in ascertaining and validating the index of resistivity log similar to the induction log of a well without the actual well drilling (Hamill and Bell 1986).
Uma (1998) explained that the natural hydrochemistry of surface and groundwater is principally controlled by the rocks and sediments they are in contact with. Prominent outcrops (which commonly support local salt industries) are found in the Southern and Central Benue Trough which is associated to tectonic elements (intrusive and mineralized vein) together with weathering, precipitation, ion exchange reactions and anthropogenic processes (Nganje et al. 2015). Against this background, the occurrence of saline polluted groundwater in Eha-Amufu is undisputable. Saline groundwater constitutes a serious hydro-geological problem and is the most common of all the pollutants in freshwater (Adeoti et al. 2010) which can be checked through a background evaluation of its hydrochemistry for effective planning, monitoring and protection of water quality (Cocker 1995; Hook 2005; Pazand et al. 2011). To this end, we have placed in high premium unraveling-cum-mapping of the zones of salinity which is considered very essential for the proper management of the groundwater in Eha-Amufu and its environs. Therefore, the trust of this research orient towards undertaking a hydro-geological survey within the town and its environment using advanced geophysical equipment. This will provide a vista for the location of viable aquifers (groundwater source) and sustainable boreholes to forestall problems.
2 Methods of study
2.1 Study area
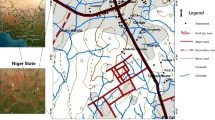
Eha-Amufu, Southeast Nigeria, is bounded within latitude 6°37′ and 6°45′ and longitudes 7°40′ and 7°50′ (Fig. 1a). The area is accessible by railway and a network of major and minor roads such as Obollo-Nkalagu Express Road connecting Eha-Amufu and Nkalagu.
The climate of the study area falls within wet and dry (Aw) climate of the Koppen classification. The area experiences seven months of effective rainfall, normally from April to October and five months of dry season (November—March). During the rainy season, the intensity of rainfall in the area is about 49 mm per hour at the beginning of the season and the end is often marked by violent thunderstorms (Eze 1998). The total annual rainfall ranges from 2000 to 2500 mm and mean annual temperature range of the study area is 27–28 °C (Monanu 1975a). The area is moderately humid, about 60–80% (Monanu 1975b; Iloeje 1981) and pressure ranges from 1010 to 1012.9 mbar (Monanu and Inyang 1975).
Igbozuruike (1975), Phil-Eze (2001) and Anyadike (2002) associated the vegetation unit in the study area with extensive savanna and rain forest vegetation in varying densities from one area to another occasioned by relief, soil pattern and the prevailing climatic condition.
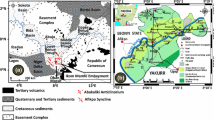
The study area lies within the Anambra Basin in the western part of the Lower Benue Trough of Nigeria. Anambra Basin originated during the Santonian stage by contemporaneous subsidence of the Anambra platform and the uplift of the Abakiliki–Benue Anticlinorium (Mamah et al. 2014). This basin is peacock headed in its northern limits, where the basin is thought of as a playa lake model within the Benue Trough, but filled with texturally and mineralogical mature sediment of the Cretaceous age. Murat (1972) recorded that the stratigraphic packaging of the Anambra Basin consists of the Nkporo Group, Mamu Formation (Lower Coal Measure) Ajali Formation and Nsukka Formation (Upper Coal Measure). The Npkoro Group consists of Nkporo Shale, Enugu Shale and Owelli Sandstone (Fig. 2).
The vast section of the study area is underlain by Agwu Formation on the southeastern part while the other areas are covered by Nkporo Formation (Fig. 2). Drainage of the area is typically dendritic (Fig. 1b). The Nkporo and Agwu Shale Formations are described as aquicludes due to their low permeability (Offodile 2002). There are no boreholes penetrating these formations in the research area for an independent confirmation of their permeability. Outcrop of the Nkporo Shale is scarce in the study area but cuttings (sample) from hand-dug wells showed that the formation consists of mudstone and black shale with sparse intercalation of sandstones (Fig. 3). Lithological characteristic and faunal content of the Awgu Shale indicate that the sediments were laid in a shallow water environment consisting of gray shale with calcareous sandstone and limestone (Nwachukwu 1972). Shale of Agwu Formation is deposited in a marine setting with closed playa lake model and evaporate depositions, thus explaining the source of salinity/brackish and water hardness in Eha-Amufu (Desborough 1978).
A playa lake Model of deposition implies an increase in magnesium–calcium ratio in the groundwater towards the lake margin. Deposition of dolomitic limestone and brine evolution is dominant at the margins also. Transgressions would mark a reflux of saline water to a playa lake while regressions, especially when evaporation exceeds precipitations and runoff, would lead to settlement and high salinity brines at the deeper portions of the basin. Extreme arid conditions concentrates evaporates and oolites as seen in deepest parts of the basin adjacent to the study area and super salinity may lead to deposition of nachcolite or rock salt (Hite 1972). Due to the salty taste and hardness of the water in Eha-Amufu, the water is not good for domestic purposes. These playa lake concepts underscore the importance of prospecting for groundwater in Eha-Amufu and its environs.
2.2 Geophysical investigation
Vertical electrical sounding survey and hydrogeochemical principles were employed in this work. In line with the custody of practice in geophysical researches, electrical resistivity technique of Vertical Electrical Sounding (VES) was adopted for mapping the saline zones due to its ability to detect increase in conductivity trends or zones within an aquifer that results from increased salinity of pore-water (Loke 1999). It is also useful in determining the tabular nature, geometry and thickness of geological formations in saline zones within formations (Telford et al. 1977). In this research, a total of twenty-seven (27) VES were utilized (Fig. 4), and Schlumberger configuration of a half current electrode spread (AB/2) of 600 m and half potential electrode separation (MN/2) were maintained between 0.5 and 45 m.
The VES curves were quantitatively interpreted by partial curve matching and computer interaction techniques based on linear filter theory using 1P12win computer software. Vertical electrical sounding (VES) was carried out by extending the electrode system (four electrodes) on a straight line, to produce vertical changes in the electrical resistivity of the subsurface; relative to the various strata encountered. Spatial distribution of resistivity within each stratum would delineate conductive zones and would be salinity conduits. Resistivity survey and water analysis complements each other and has been used extensively (Sherif et al. 2006; Srinivas et al. 2013; Akpan et al. 2013).
2.3 Hydro-chemical investigation
In addition to the resistivity survey, groundwater sample was collected from (10) hand-dug well during dry season (Fig. 4). The electrical conductivity and temperature of the sample were measured in the field. Total dissolved solid (TDS) where measured using EDTA (Ethylene-Diaminetetra Acetate trimetric method) complex metric method. Cations (Na+, K+, Pb2+, Fe2+, Mg2+ and Ca2+) and anions (NO3−, SO42−, Cl− and HCO3−) were analyzed. The pH was determined using a Pye-Unican 290MK PH meter. The reliability of the water quality data was determined using the ionic balance or electric neutrality formula (Hounslow 1995) as shown in Eq. 1.
From the computation of the ionic balance, the water quality data were found to be 88% reliable.
3 Results
The VES exhibited wide range of variability in curve characteristics at different locations. It recorded curves ranging from AK, QH, Q, KQHK, H, HK, QHA, KHK, KQH, QQH and QQ as shown in Table 1. Resistivity in the area ranges from 0.117 to 2697 Ω while the layer thickness ranges between 0.367 m and infinity (Table 2). Eha-Amufu groundwater recorded pH (5.96–8.04), electrical conductivity (EC) (121.78–1155.36ug/cm), temperature (27.0–28.5 °C), total dissolved solids (TDS) (198–1702 mg/l), total hardness (TH) (44.0–404.0 mg/l), calcium hardness (16.0–320.0 mg/l), Magnesium hardness (10.0–84.0 mg/l). Hydrochemical analysis of the groundwater revealed the presence of Na+ (7.0–55.0 mg/l), K+ (2.1–11.4 mg/l), Mg2+ (2.4–65.5 mg/l), Ca2+ (5.4–127.0 mg/l), NO3− (0.9–16.7 mg/l), HCO3− (10.0–160.0 mg/l), Cl− (122.1–401.7 mg/l), SO42− (11.0–36.1 mg/l), Fe2+ (0.03–0.45 mg/l), Pb2+ (0.03–0.08 mg/l) as shown in Table 3.
4 Discussion of results
4.1 Geoelectrical delineation of saline zone
Three to six geoelectrical layers were detected within the study area and were predominantly of QH and H curve types (Table 1). According to Telford et al. (1977), electrical resistivity contrast existing between lithological sequences in subsurface are often adequate to enable geo-electric layers delineation and identification of saline and non-saline layers. In this study, all the depth sounding curves interpreted as saline layer are characterized by a steep descent from the non-saline to the saline layer. This decrease could be attributed to increase in porosity, hydraulic conductivity, fluid content, and most possibly a high conductivity arising from saline water. Such steep descents occurred at the study area in VES 4 (55.2–6.54 Ωm), VES 5 (130–1.25 Ωm) and VES 8 (20.98–2.53 Ωm). The thickness of the saline layer/zones and their corresponding resistivity are presented in Table 2.
Figure 5, 6 and 7shows the spatial distribution maps of the element and their accompanying variograms. From the spatial distribution maps, all the elements have similar distribution pattern with the highest concentration found around the northeast while the lowest falls within the North West (Fig. 5a, c, 6a, c, 7a, c). Since the northeast is mostly underlain by shale (Fig. 2), the higher concentration of these element could be attributed to weathering of the shale.
In a variogram, the sill is the net variance where empirical variogram appears to level off. When the variogram point (nugget) plots above the sill it implies a negative spatial correlation but signifies positive spatial correlation when plotted below the sill. However, it is zero when the nuggets are on the sill. From the plotted variograms (Fig. 5b, d, 6b, d, 7b, d), it can be observed that most of the element concentration fall below the sill, indicating a close relationship of element concentration with distance. However some concentration plotted above the sill signifying high concentration with no correlation with other locations.
Integration of hydrogeological and geophysical results with hydrogeochemical data obtained from monitoring wells revealed an empirical relationship between earth resistivity and total dissolved solids (TDS). It was used to delineate the fresh and saline-water zones (Sherif et al. 2006; Srinivas et al. 2013; Akpan et al. 2013). The spatial distributions of the saline and non-saline zones generated using well control in the study area are illustrated in Fig. 8. The figure showed the distribution of saline zone to be on the Northwest (NW) and Southeast (SE) spread. The spatial distribution map of Na and Cl (Fig. 6) agrees with the salinity map (Fig. 8). Both maps were observed to correspond with the VES. Areas of low VES were observed to correspond with high salinity (See Fig. 8; Table 2).
High ratings of conductive zones are southeast and northwest of the study area. The conductive zone on the southeast is a topographic low zone. Thus, the higher the chemical concentration may be mostly attributed to the shale lithology in the area. It also appears to be the discharge area for all streams in the study. The streams define salinity gradients and are trending dominantly northwest–southeast. Geologically, this is the area underlain by the marine and brackish Awgu Shale.
4.2 Hydrogeochemical analysis result and interpretation
The result of hydro-geochemical analysis is presented in Table 3. The sizes of the plots in Fig. 9 show that total dissolved solid (TDS) values do not differ significantly for the entire samples, except for sample Nos. 4, 8, and 9 (Table. 3). Charts and graphs were used to present the hydrogeochemical results for easy assessment of water composition.
4.2.1 Stiff and piper classification scheme
The Stiff and Piper plots are relatively distinctive methods of showing water composition differences and similarities (Hem 1985; Kumar et al. 2014). Both charts are often used by hydrogeologists and geochemists to display the major ion composition of a water sample. The Stiff patterns are helpful in giving prompt illustrative comparison between water from different sources (Salehi and Zeinivand 2016). The size of the pattern is approximately equal to the total ionic content (Hounslow 1995). On the other hand, Piper diagram is broadly used to understand problems relating to geochemical evolution of groundwater (Kumar et al. 2014). It is applicable in determining water type, hydro-chemical facies and ionic exchange (Freeze and Cherry 1979; Hounslow 1995). Furthermore, piper plot can also reveal the degree of mixing between waters (Sidi et al. 2016).
The shapes of the stiff diagrams (Fig. 10) are varied distinctively suggesting that the water sample is of different sources. The variation in shape pattern can be attributed to fluctuation in the relative concentration of Cl, Ca and Mg in the samples. The diamond part of the piper diagram may be used to characterize water of different types (Hounslow 1995). Water plotted at the upper corner of the diamond is primarily composed of Calcium, Magnesium Chloride type (Fig. 11). Therefore, the groundwater in Eha-Amufu area can be classified as Calcium–Magnesium–Chloride (Appelo and Postma 1993; Bahar and Reza 2010). This water type denotes permanent hardness in water that can only be softened via ion exchange processes.
4.2.2 Gibbs system of classification
Major ion concentrations in groundwater can be explained largely by mixing and water–rock interactions. Basic processes that add to groundwater salinity are controlled by reaction with host rocks, which is influenced by the network of flow paths and the time each path spends in contact with various minerals and mixing with older waters at depth (Marandi and Shand 2018). It has been reported that along extensive flow path with increasing time, groundwater tends to increase salinity, thus changing its type, which often is accompanied by changes in the dominant cation (Chebotarev 1955). Hence, the Gibbs plot could qualitatively be used to determine flow directions and ages of groundwater in wells, and may require isotope geochemistry for improved understanding of hydrogeochemical processes (Marandi and Shand 2018).
The origin of the hydro-chemical ions in the study area is well understood using Gibbs (1970) graphical diagram. Gibbs diagram reveals three groundwater chemical dominances used to establish the relationship of water composition and aquifer lithological characteristics. The chemical dominances are evaporation-crystallization, rock weathering and precipitation (Fig. 12). From the graphical plot, it was found that majority of the samples fall in rock weathering dominance, indicating that the major source of dissolved salts in the aquifer is supplied from the host rocks; that is, rock-derived dissolved salts.
4.3 Assessment of groundwater of the study area
Magnesium and Calcium constitute the most prevailing carbonate mineral found in the study area. Enrichment in Mg2+, Cl2− and Ca2+ in the groundwater of the study area yields Ca–Mg–Cl as the common water type in the study area. These ions indicate precipitation of halite and dolomite during dry phases in the history of a playa lake.
Magnesium and Calcium are the component of hard water (Neri et al. 1975). Total hardness CaCO3 is an important criterion for ascertaining the suitability of water for domestic, drinking and many industrial uses (Karanth 1994). Hardness of water for domestic uses relates mainly to its reaction with soap, since soap is precipitated principally by Ca2+ and Mg2+. Hardness of water is defined as the sum of the concentration of these ions expressed as mg/l of CaCO3.Water with hardness ranging from 0 to 60 mg/l, 61 to 120 mg/l, 121to 180 mg/l and > 180 mg/l are regarded as soft, moderately hard, hard and very hard, respectively (Hem 1970).
Groundwater of Eha-Amufu and environs varies in total hardness (Table 3). The wells range between moderately hard to very hard, with total hardness ranging from 76.0 to 404.0 mg/l. The hardness is also evidenced by the mineral saturation of the area—Calcite and Aragonite/dolomite CaCO3. Two hand-dug wells were found to be soft water with total hardness of 40 mg/l and 44 mg/l. This is because the aquifer is highly permeable, and as a result, groundwater flows are active and have less chemical reaction with its surrounding sediment. Variation in hardness in the water is a function of the hydrodynamic dispersion and absorption of the contaminant in the fine grain of the aquifer (Todd 1980). The chemical composition of water in Eha-Amufu environs showed that the water is generally hard. Thus, use of the groundwater for domestic purpose may lead to soap wastage, furring of kettles and boilers together with discoloration and stiffness of textiles. To overcome such occurrences, water should be treated temporally by boiling or permanently by addition of caustic soda or lime, or by filtration through some naturally occurring minerals such as zeolite (Sivasankar and Ramachandramoorthy 2011; Abdolahnejad et al. 2014).
Heavy metals were also analyzed in order to determine their concentration in the groundwater. Iron and lead were detected in the groundwater from the wells, with concentration ranging between 0.03–0.06 and 0.03–0.08 mg/l, respectively (Table 3). The possible sources of the iron could be the iron minerals (Pyrite, Siderite and Marcasite) which originated from sandstone and shale deposits in the area. Sources of the lead could be from the black Nkporo Shale, reflecting the affinity of Pb for organic matter and clay fractions associated with kaolinite and mica (Heinrichs et al. 1980) or through erosion and leaching of the highly mined limestone deposit abandoned at closely located Nkalagu cement site, which could be mineralized. The high values could also be as a result of anthropogenic factors.
The concentrations were noted to be above the World Health Organization (WHO 2011) standard limits for Iron (0.03 mg/l) and lead (0.01 mg/l) in water. Concentration of lead in the groundwater also failed the Nigerian Standard for Drinking Water Quality (NSDWQ) limit set at 0.01 mg/l while iron was within the NSDWQ limit of 0.3 mg/l. Therefore, the shallow groundwater within Eha-Amufu and its environs is not good for drinking purpose with regard to the WHO stipulated guideline for drinking water. However, a number of techniques have been used to remove heavy metals from contaminated water. They include, chemical precipitation (Fu and Wang 2011); ion exchange (Lai et al. 2016); adsorption (Davarnejad and Panahi 2016); membrane filtration (Rahmanian et al. 2011); reverse osmosis (Yoon et al. 2009); solvent extraction (Lertlapwasin et al. 2010); and electrochemical treatment (Dharnaik and Ghosh 2014). The aforementioned techniques are useful, but has a high-cost implication (Wołowiec et al. 2019). Consequently, treatments may require government and other stakeholders’ intervention through the provision of purification plants and regional water conduits.
4.4 Correlation between earth resistivity (ρe) and groundwater quality
The integration of electrical resistivity method and geochemical analysis depicts a better representation of groundwater quality in an area (Sherif et al. 2006; Srinivas et al. 2013; Akpan et al. 2013). Hydrogeochemical and geophysical data obtained (Table 4) were statistically evaluated in order to understand the relationship between earth resistivity and hydrochemical properties of the groundwater. Such processes are vital in explaining empirical relationship (Sherif et al. 2006; Srinivas et al. 2013), together with the possibility of identifying a connection between earth resistivity (ρe) and the amount of TDS (Ebraheem et al. 1997; Sherif et al. 2006; Srinivas et al. 2013). These ions: Ca2+, Mg2+, total Fe, Na+, K+, HCO3–, SO42–, Cl–, and NO3– constitute 90% of the TDS in natural water (Sherif et al. 2006).
In processing the geophysical and hydrogeological data, the measured EC of groundwater samples were converted into water resistivity (Table 4) using the relation ( \(\rho_{{\text{w}}} = {\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {{\text{EC}}}}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${{\text{EC}}}$}}\)) as noted by other authors (Vouillamoz et al. 2007; Sherif et al. 2006; Srinivas et al. 2013), where ρw is the water resistivity and EC represents the electrical conductivity. In this study, water resistivity (ρw) was plotted as a function of earth resistivity (Fig. 13a) and the empirical relationship between them indicated:
where ρe is the earth resistivity and ρw is the water resistivity in Ohmmeters.
The attained best of fit line between earth resistivity and water resistivity (R2 = 0.901) shows that the earth resistivity of Eha Amufu which bears the aquifer is mostly of coarse sand and sandy clay with a very shallow water table. This implies that it is strongly affected by groundwater salinity (Sherif et al. 2006). This observation may provide justification for applying resistivity methods in delineating salinity distribution and groundwater contamination zones in recent studies (Sherif et al. 2006; Srinivas et al. 2013; Akpan et al. 2013; Bouderbala and Remini 2014).
From the plot shown in Fig. 13b, regression analysis established a strong positive relationship (R2 = 0.9511) between TDS against ρe., and the fitted line indicates the following empirical relationship:
where TDS is the TDS in milligram per liter and ρe is the earth resistivity in Ohms. This relation shows that two different groundwater types may be delineated, and these types are fresh (ρe > 22 Ωm) and saline groundwater (ρe < 22 Ωm) as shown in Table 2. There is also a possibility of plotting water EC (in μg/ms) against TDS (in mg/l), as shown in Fig. 13c.
5 Conclusion
The result of the VES studies revealed that Eha-Amufu and environs has a shallow groundwater, which explains the abundance of hand–dug wells. The absence of borehole in Eha-Amufu was attributed to low permeability of the shale formations underlying the area. The high salt concentration (low electrical resistivity) found in the study area was localized within the Agwu and Nkporo Shale units interpreted to have been deposited during alternating transgressions and regressions of the Coniacian and Campanian seas, respectively.
Empirical relationship exists between earth resistivity and TDS. It was vital in ascertaining the average TDS along the resistivity profile. Furthermore, it was useful in classifying the groundwater as fresh or saline. Thus, earth resistivity methods are viable and resourceful tools for quantitative and qualitative studies related to groundwater resources.
Based on Piper (1944) plot, result of hydro-chemical analysis revealed one water facie, namely: Ca–Mg–Cl. Also, the groundwater sample for all the locations was observed to have similar ionic content based on Stiff (1951) patterns. This may be an indication that the groundwater of the area came from the same source, with evaporation occurring in the deeper parts of the basin. From Gibbs plot, the groundwater of Eha-Amufu and its environs recharge from precipitation. The origin of ions that favor the accumulation of salts in the groundwater of Eha-Amufu is rock chemical interactions. The hydrochemical analysis also showed that the water is not suitable for drinking due to occurrence of Pb2+ in concentrations above the World Health Organization (WHO 2011) and Nigerian Standard for Drinking Water Quality (NSDWQ) limits for drinking water.
Conclusively, the authors suggest that other channels of portable water provision such as harvesting and treatment of river water using purification plants and development of regional water conduits be explored. Furthermore, it is recommended that water from hand-dug well in Eha-Amufu and its environs should be treated to remove heavy metals and reduce salinity before domestic uses in other to prevent outbreak of epidemics in the area.
References
Abdolahnejad, A., Ebrahimi, A., & Jafari, N. (2014). Application of Iranian natural zeolite and blast furnace slag as slow sand filters media for water softening. International Journal of Environmental Health and Engineering, 3, 26.
Adeoti, L., Alile, O. M., & Uchebgulam, O. (2010). Geophysical investigation of saline water intrusion into freshwater aquifers: A case study of Oniru, Lagos State. SRE Academic Journal, 5(3), 248–259.
Aghamelu, O. P., Ezeh, H. N., & Obasi, A. I. (2013). Groundwater exploitation in the Abakaliki metropolis (southeastern Nigeria): Issues and challenges. African Journal of Environmental Science and Technology, 7(11), 1018–1027.
Akaolisa, C. (2006). Aquifer transmissivity and basement structure determination using resistivity sounding at Jos, Plateau state Nigeria. Environmental Monitoring and Assessment, 114, 27–34.
Akpan, A. E., Ugbaja, A. N., & George, N. J. (2013). Integrated geophysical, geochemical and hydrogeological investigation of shallow groundwater resources in parts of the Ikom–Mamfe embayment and the adjoining areas in Cross River State, Nigeria. Environmental Earth Sciences, 70(3), 1435–1456. https://doi.org/10.1007/s12665-013-2232-3.
Alile, M. O., Jegede, S. I., & Ehigiator, O. M. (2008). Underground water exploration using electrical resistivity method in Edo State, Nigeria. Asian Journal of Earth Sciences, 1, 38–43.
Anyadike, R. N. C. (2002). Climate and vegetation. In G. E. K. Ofomatu (Ed.), A survey of Igbo nation (pp. 73–82). Onitsha: Africa first publisher limited.
Appelo, C. A. J., & Postma, D. (1993). Geochemistry, groundwater and pollution. Rotterdam: Balkema Publishers.
Arabi, S. A., Dewu, B. B. M., Muhammad, A. M., Ibrahim, M. B., & Abafoni, J. D. (2008). Determination of weathered and fractured zones in part of the basement complex of North-Eastern Nigeria. Journal of Engineering and Technology Research, 2(11), 213–218.
Bahar, M., & Reza, S. (2010). Hydrochemical characteristics and quality assessment of shallow groundwater in a coastal area of southwestern Bangladesh. Environmental Earth Sciences, 61, 1065–1073.
Bob-Duru, R. C. (2001). Rural settlements in Nigeria: Survival or Demise? In G. E. K. Ofomata & P. O. Phil-Eze (Eds.), Geographical perspectives on environmental problems and management in Nigeria (pp. 96–115). Nigeria: Jamoe Publishers.
Bouderbala, A., & Remini, B. (2014). Geophysical approach for assessment of seawater intrusion in the coastal aquifer of Wadi Nador (Tipaza, Algeria). Acta Geophysica, 62(6), 1352–1372. https://doi.org/10.2478/s11600-014-0220-y.
Chebotarev, I. I. (1955). Metamorphism of natural waters in the crust of weathering-2. Geochimica et Cosmochimica Acta, 8, 137–170. https://doi.org/10.1016/0016-7037(55)90015-6.
Cocker, M. D. (1995). Geochemistry and hydrochemistry of the Oconee River Basin. In K. J. Hatcher (Ed.), Proceedings of 1995 Georgia water resources conference (pp. 67–70). University of Georgia
Davarnejad, R., & Panahi, P. (2016). Cu (II) removal from aqueous wastewaters by adsorption on the modified Henna with Fe3O4 nanoparticles using response surface methodology. Separation and Purification Technology, 158, 286–292.
Desborough, G. A. (1978). A biochemical stratified lake model for the origin of oil shale of the Green River Formation: An alternative to playa lake model. Geological Society of America Bulletin, 89, 961–971.
Dharnaik, A. S., & Ghosh, P. K. (2014). Hexavalent chromium Cr(VI) removal by the electrochemical ion-exchange process. Environmental Technology, 35, 2272–2279.
Ebraheem, A. M., Senosy, M. M., & Dahab, K. A. (1997). Geoelectrical and hydrogeochemical studies for delineating groundwater contamination due to saltwater intrusion in the northern part of the Nile Delta, Egypt. Ground Water, 35(2), 216–222.
Egwebe, O., Aigbedion, I., & Ifedili, S. O. (2004). A geo-electric investigation for groundwater at Ivbiaro Ebesse, Edo State, Nigeria. Journal of Applied Science, 22, 146–150.
Eze, H. I. (1998). The causes and environmental impact of soil erosion in Isi–Uzo Local Government Area. Research proposal in environmental management. Enugu: Enugu state university of technology.
Freeze, R. A., & Cherry, J. A. (1979). Groundwater (p. 604). Englewood Cliffs, New Jersey: Prentice-Hall Inc.
Fu, F., & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management, 92, 407–418.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 17, 1088–1090.
Hamill, L., & Bell, F. G. (1986). Groundwater resource development (pp. 119–128). London: Butterworths.
Heinrichs, H., Schulz-Dobrick, B., & Wedepohl, K. H. (1980). Terrestrial geochemistry of Cd, Bi, Tl, Pb, Zn and Rb. Geochimica Et Cosmochimica Acta, 44, 1519–1533.
Hem, J. D. (1970). Chemical behavior of mercury in aqueous media. In Mercury in the environment. Washington, D.C.: U.S. Geological Survey Professional Paper 713, U.S. Geological Survey.
Hem, J. D. (1985). Study and interpretation of the chemical characteristics of natural water. United States Geological Survey Water Supply Paper, 2254, 244.
Hite, R. J. (1972). Shelf carbonates sedimentation controlled by salinity in the Paradox basin southeast Utah. Mountain Geology, 27, 329–349.
Hook, Z. (2005). An assessment of the quality of drinking water in rural districts in Zimbabwe: The case of Gokwe South, NkayiLupene and Nwenezi districts. PhysChem Earth, 30, 859–866.
Hounslow, A. W. (1995). Water quality data: Analysis and interpretation (p. 397). London: CRS Press Inc., Lewis Publishers.
Igbozuruike, M. U. (1975). Vegetation. In G. E. K. Ofomata (Ed.), Nigeria in maps (pp. 45–62). Benin City: Ethiope Publishing House.
Iloeje, N. P. (1981). New geography of Nigeria. Longman Nigeria Limited.
Jalali, M. (2007). Hydrochemical identification of groundwater resources and their changes under the impacts of human activity in the Chah Basin in western Iran. Environmental Monitoring and Assessment, 130, 347–364.
Joshua, E. O., Odeyemi, O. O., & Fawehinmi, O. O. (2011). Geoelectrical investigation of the groundwater potential of Moniya Area, Ibadan. Journal of Geology and Mining Research, 3(3), 54–62.
Karanth, K. R. (1994). Groundwater assessment development and management (p. 43). New Delhi: Tata Company limited.
Kumar, K. S., Logeshkumaran, A., Magesh, N. S., Godson, P. S., & Chandrasekar, N. (2015). Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Applied Water Science, 5, 335–343.
Lai, Y.-C., Chang, Y.-R., Chen, M.-L., Lo, Y.-K., Lai, J.-Y., & Lee, D.-J. (2016). Poly(vinyl alcohol) and alginate cross-linked matrix with immobilized Prussian blue and ion exchange resin for cesium removal from waters. Bioresource Technology, 214, 192–198.
Lertlapwasin, R., Bhawawet, N., Imyim, A., & Fuangswasdi, S. (2010). Ionic liquid extraction of heavy metal ions by 2-aminothiophenol in 1-butyl-3-methylimidazolium hexafluorophosphate and their association constants. Separation and Purification Technology, 72, 70–76.
Loke, M. H. (1999). Electrical imaging surveys for environmental and engineering studies: A practical guide to 2-D and 3-D surveys. Malaysia: Penang.
Mamah, L., Nwafor, C. G., Omada, J. I., Akpa, F., & Okogbue, C. (2014). Coal resource evaluation and models: A case study from parts of Okobo and Enjema in Kogi state within the lower coal measures of Mamu formation, Nigeria. International Journal of Science and Technology Research, 3(4), 108–115.
Marandi, A., & Shand, P. (2018). Groundwater chemistry and the Gibbs diagram. Applied Geochemistry, 97(2018), 209–212. https://doi.org/10.1016/j.apgeochem.2018.07.009.
Monanu, P. C. (1975a). Temperature and sunshine. In G. E. K. Ofomata (Ed.), Nigeria in maps (pp. 19–21). Benin City: Ethiope Publishing House.
Monanu, P. C. (1975b). Humdity. In G. E. K. Ofomata (Ed.), Nigeria in maps (pp. 19–21). Benin City: Ethiope Publishing House.
Monanu, S., & Inyang, F. (1975). Climatic regimes. In G. E. K. Ofomata (Ed.), Nigeria in maps (pp. 27–29). Benin City: Ethiope Publishing House.
Murat, R. C. (1972). Stratigraphy and paleography of the Cretaceous and Lower Tertiary in Southern Nigeria. In T. F. J. Dessauvagre & A. J. Whiteman (Eds.), African geology (pp. 251–256). Nigeria: University of Ibadan Press.
Neri, L. C., Hewitt, D., Schreiber, G. B., Anderson, T. W., Mandel, J. S., & Zdrojewski, A. (1975). Health aspects of hard and soft waters. Journal American Water Works Association, 67, 403–409.
Nganje, T. N., Hursthouse, A. S., Edet, A., Stirling, D., & Adamu, C. I. (2015). Hydrochemistry of surface water and groundwater in the shale bedrock, Cross River Basin and Niger Delta Region Nigeria. Applied Water Science. https://doi.org/10.1007/s13201-015-0308-9.
Nwachukwu, S. O. (1972). The tectonic evolution of the southern portion of the Benue Trough, Nigeria. Geological Magazine, 109, 411–419.
Nwankwoala, H. O., & Shalokpe, J. (2008). Groundwater resource evaluation of Amuwo Odofin, Lagos State, Nigeria. International Journal of Natural and Applied Science, 4(3), 330–336.
Offodile, E. M. (2002). Groundwater study and development in Nigeria (p. 453). Jos: Mecon Geology and Engineering Services Ltd.
Okogbue, C. O., & Omonona, O. V. (2013). Groundwater potential of the Egbe–Mopa basement area, central Nigeria. Hydrological Sciences Journal. https://doi.org/10.1080/02626667.2013.775445.
Okogbue, C. O., Omonona, O. V., & Aghamelu, O. P. (2012). Qualitative assessment of roundwater from Egbe–Mopa basement complex area, northcentral Nigeria. Environmental Earth Sciences, 67, 1069–1083.
Olaleye, B. M. (2005). Influence of oil structure on borehole depth determination in Osun State, Nigeria. International Research Journal in Engineering and Technology (IRJET), 2(1), 111–119.
Olayinka, A. I. (1992). Geophysical sitting of boreholes subsurface geo-electrical layers of various thicknesses in Crystalline Basement areas of Africa: Discriminated on the basis of their electrical resistivities. Journal of African Earth Sciences, 14(2), 197–207.
Pazand, K., Gbanbari, Y., Aghavali, O., & Hezarkhani, A. (2011). Groundwater geochemistry in the middle Meshkinshahr basin of Ardabil province in Iran. Environmental Earth Sciences. https://doi.org/10.1007/s12665-011-1131-8.
Phil-Eze, P. O. (2001). Biodiversity and environmental problems in Nigeria. In G. E. K. Ofomata & P. O. Phil-Eze (Eds.), Geographical perspectives on environmental problems and management in Nigeria (pp. 33–52). Enugu, Nigeria: Jamoe Enterprises.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analysis. Transactions American Geographical Union, 25, 14–923.
Rahmanian, B., Pakizeh, M., Esfandyari, M., Heshmatnezhad, F., & Maskooki, A. (2011). Fuzzy modeling and simulation for lead removal using micellar-enhanced ultrafiltration (MEUF). Journal of Hazardous Materials, 192, 585–592.
Salehi, H., & Zeinivand, H. (2016). Evaluation and mapping of groundwater quality for irrigation and drinking purposes in Kuhdasht region, Iran. Environmental Resources Research, 4(1), 75–89.
Selemo, A. O., Okeke, P. O., & Nwankwor, G. I. (1995). An appraisal of the usefulness of vertical electrical sounding (VES) in groundwater exploration in Nigeria. Water Resources Journal, 6(2), 40–55.
Sherif, M., El Mahmoudi, A., Garamoon, H., Kacimov, A., Akram, S., Ebraheem, A., & Shetty, A. (2006). Geoelectrical and hydrogeochemical studies for delineating seawater intrusion in the outlet of Wadi Ham, UAE. Environmental Geology, 49, 536–551. https://doi.org/10.1007/s00254-005-0081-4.
Sidi, A. A., Waziri, N. M., Maji, A. T., Okunlola, I. A., Umar, A., & Waziri, S. H. (2016). Assessment of chemical quality of water from shallow alluvial aquifers in and around Badeggi, Central Bida Basin, Nigeria. Journal of Earth Sciences and Geotechnical Engineering, 6(3), 133–145.
Sivasankar, V., & Ramachandramoorthy, T. (2011). Water softening behaviour of sand materials-mimicking natural zeolites in some locations of Rameswaram Island, India. Chemical Engineering Journal, 171(1), 24–32.
Srinivas, Y., Muthuraj, D., Hudson, O. D., Stanley, R. A., & Chandrasekar, N. (2013). Environmental applications of geophysical and geochemical methods to map groundwater quality at Tuticorin, Tamilnadu, India. Environmental Earth Sciences. https://doi.org/10.1007/s12665-013-2502-0.
Stiff, H. A., Jr. (1951). The interpretation of chemical water analysis by means of pattern. Journal of Petroleum Technology, 3(10), 15–17.
Telford, W. H., Gilbert, L. P., & Sherif, R. E. (1977). Applied geophysics (p. 770). Cambridge, London: University Press.
Tizro, A. T., Voudouris, K. S., Salchzade, M., & Mashayekhi, H. (2010). Hydrogeological framework and estimation of aquifer hydraulic parameter using geoelectrical data: A case study from West Iran. Hydrogeology Journal, 18, 917–929.
Todd, D. K. (1980). Groundwater hydrology (p. 535). New York: Wiley.
Uma, K. O. (1998). The brine fields of Benue Trough, Nigeria: A comparative study of geomorphic, tectonic and hydrochemical properties. Journal of Africa Earth Sciences, 26(2), 261–275.
Vchery, A., & Hobbs, B. (2003). Resistivity imaging to determine clay cover and permeable units at an ex-industrial site. Near Surface Geophysics, 1, 21–30.
Vouillamoz, J. M., Chatenoux, B., Mathieu, F., Baltassat, J. M., & Legchenko, A. (2007). Efficiency of joint use of MRS and VES to characterize coastal aquifer in Myanmar. Journal of Applied Geophysics, 61, 142–154.
Wołowiec, M., Komorowska-Kaufman, M., Pruss, A., Rzepa, G., & Bajda, T. (2019). Removal of heavy metals and metalloids fromwater using drinking water treatment residuals as adsorbents: A review. Minerals, 9(487), 1–17. https://doi.org/10.3390/min9080487.
World Commission on Environment and Development. (1987). Our Common Future. Oxford: Oxford University Press.
World Health Organization. (2011). Guideline for drinking water quality (4th edn.).
Yoon, J., Amy, G., Chung, J., Sohn, J., & Yoon, Y. (2009). Removal of toxic ions (chromate, arsenate, and perchlorate) using reverse osmosis, nanofiltration, and ultrafiltration membranes. Chemosphere, 77(2), 228–235.
Acknowledgement
The authors acknowledge all the lecturers of the Department of Geology, University of Nigeria, Nsukka, for their advice during the course of this research. The authors will also like to appreciate members of the University of Nigeria Water Resources Management Laboratory Limited for their help in hydro-chemical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ayogu, N.O., Mamah, L.I., Ayogu, C.N. et al. Assessment of groundwater quality using geoelectrical potential and hydrogeochemical analysis in Eha-Amufu and environs, Enugu state, Nigeria. Environ Dev Sustain 23, 11025–11049 (2021). https://doi.org/10.1007/s10668-020-01103-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-020-01103-3