Abstract
Uttarakhand has the maximum potential of pine of about 20 lakh tonnes/year including reserved forests and van panchayat which makes pine needle an abundant resource. The analysis of the pine needles reveals it a potential biomass feedstock for gasification to produce electricity. This manuscript deals with the combustion study of Pine needles (Pinusroxburghii) biomass using a thermogravimetric analyzer to investigate the thermal degradation behavior and kinetic parameters. The pine needles were heated in the presence of air at four different heating rates 5, 10, 25 and 50 °C/min, and the degradation phenomenon was studied. From the proximate and ultimate analysis of pine needles, it was observed that the biomass could be a potential feedstock for gasification. The TG-DTG curves revealed that the main decomposition was between 190 and 450 °C with the release of 80–84% volatiles. It was observed that as the heating rate increases, the maximum decomposition temperature also increases and the peak shifts to the right. The obtained thermal data were used to calculate the kinetic parameters using Kissinger–Akharia–Sunose, Ozawa-Flynn-Wall, Friedman and Kissinger. The average values obtained from the above methods are 190.74, 190.75, 199.48 and 172 kJ/mol and 2.749 × 1022, 5.13 × 1022, 4.21 × 1021 and 4.14 × 106/min, respectively. The model fitting method and Coats–Redfern method were used to determine the kinetic triplet (A, E and n). The above model-free methods and model fitting methods predicts the progress of gasification at different positions along the reactor. Considering proximate analysis and heating value, pine needles could be considered as a potential feedstock for energy production through gasification process. The estimated results help as a source to understand the thermal degradation of biomass during the gasification process and be used to design the systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
According to IEA, 2017, Biomass is the only largest source of renewable energy throughout the world which can supply approximately 9% of the total global primary energy demand (Saravanan et al. 2018). The Ministry of New and Renewable Energy (MNRE) report says that about 500 millions metric tonnes per year biomass is available in India and has the potential to generate about 18,000 MW electricity. The primary focus of India is on biofuels and electricity generation from agricultural and forest waste (Saravanan et al. 2018; Perlack 2005). A total of 2665 MW capacity biomass power generation projects has been installed in the country and around 30 biomass power projects aggregating to about 350 MW are under various stages of implementation.
The Indian Himalayan region comprises of Greater Himalaya, Lesser Himalaya and Outer Himalaya extending from northwest to southeast. The Siwalik hills covering Jammu, Himachal, Uttarakhand and Sikkim are extremely rich in flora and fauna (Joshi et al. 2015). In the Indo-Pacific region, the Himalayan subtropical pine forests are the largest. They spread over most of the world’s youngest and highest mountain range of 3000 km length. The subtropical forests of the Himalayas are the largest subtropical forest of conifers covering parts of Bhutan, India, Nepal and Pakistan. The Indian portion of the Himalayas encompasses an area of approximately 5 Lakh km2 (about 10.2% of the total geographic area of the nation) and forms the northern boundary of the country. In the forests of Uttarakhand, the overall area of Chir Pine (Pinusroxburghii) woods is approximately 3.43 Lakh hectares (Chauhan and Saini 2015). The total pine needle availability in the reserved forest of 11 districts of Uttarakhand is around 16,73,408 tonnes/year and (Table 1). Uttarkashi has the maximum potential of pine i.e., 24% followed by Tehri Garhwal, Nainital, while Almora, Bageshwar and Champawat has equal share of 9% each. The family’s sole genus Pinus (Pinaceae) is one of the mostly spread genera of trees including 100 species (Wang and Szmidt 1993). Pine trees have leaves in the shape of a needle and woody cones with seeds. These trees were commercially essential and grown for their timber, wood, pulp, carpentry products such as furniture and the resin of certain species that produce turpentine (Kurose et al. 2007; Xu et al. 2012). The major problem associated with the pine forest is that the area becomes highly vulnerable to fire due to the large amount of pine needles. During the summer season, forest fires are prevalent in as pine needles are highly inflammable. These fires ruin local ecology by replacing the other species, damaging the soil’s fertile top layer and destroying cattle’s grazing grounds. The accumulating carpet of pine needles on the forest floor under these trees makes it unfavorable for many common plants and trees thus losing biodiversity and becoming a threat to Flora and Fauna. Where pine trees the waters rushed down the slopes carrying away soil and debris and leading to floods (Bisht et al. 2014). Based on the proximate, ultimate and energy value analysis, it seems that the pine needles could be the potential source to generate electricity through pine needle gasification to meet the energy requirements of the hilly districts of Uttarakhand. Biomass gasification is highly appropriate for decentralized energy systems to provide power in rural areas with the highest efficiency. In Uttarakhand state, as per the 2011 census, there are 123 villages and 1966 village hamlets that needs to be connected to the electric grid (Chauhan and Saini 2015; Katre and Gupta 2011).
Biomass gasification is a thermochemical method and is evolving as a promising energy recovery technology due to its higher combustion efficiency with the higher heating value per unit mass. Biomass gasification in a gasifier produces volatiles, a mixture of combustible gas such as carbon monoxide (CO), hydrogen (H2) and Methane (CH4) and non-combustible gas such as carbon dioxide (CO2) and Nitrogen (N2), using air as a medium of gasification under controlled temperature and pressure (Susastriawan and Saptoadi 2017). These gases are called producer gas or synthesis gas which can be used for cooking or to generate electricity.
Knowledge and understanding of chemical kinetics of combustion and thermal degradation are essential and also helpful in gasification systems design and evaluation. Before using the biomass as the feedstock for any conversion process, it is basically important to have the information about the physical, chemical and morphological properties of the biomass, because these properties have significant impacts on the quality of the products.
Thermogravimetric analysis (TGA) is a contemporary method normally employed to study thermal degradation in which a sparse sample of the fuel is measured as a function of temperature or time as the sample is subjected to a controlled program of temperature in a controlled atmosphere of either inert gas or air in a thermogravimetric device (Wilburn 1989). The analysis also offers quantitative methods for thorough observations of combustion behaviors owing to its capacity to evaluate various types of materials such as coal and biomass with high precision. For the design of the reactor, the kinetic parameters namely apparent activation energy E, (kJ/mol), pre-exponential factor A (min−1), and the reaction order n are important. The TGA data are fed into kinetic models which employ a rate law that obeys the fundamental Arrhenius rate expression (White et al. 2011). There are two fundamental kinetic techniques, model fitting and model-free approaches to derive the kinetic parameters for non-iosthermal data (Vyazovkin and Wight 1999).
In the literature review, it was observed that there are combustion studies on different biomass materials like acorn shells, almond shells, cornstalk, rice straw, rice hull, rice husk, sawdust, olive leaves and olive wood, and few studies also reported the degradation behavior of pine needles under atmospheric conditions. Safi et al. (2004) performed the thermal degradation analysis of pine needles in air and observed four distinct degradation zones. They suggested apparent activation energy in the range of 34.60–85.34 kJ/mol and pre-exponential factor of 3.29 × 104 to 5.98 × 106 mg/min. The dehydration kinetics of pine needles from DSC curves in the air was determined by Leoni et al. (2003). Senecca (2007) suggested values for activation energy, pre-exponential factor and reaction order by the pyrolysis kinetics of pine seed shells. Concerning the degradation kinetics of pine needles, only a few studies are present with the oxidation of biomass. In determining kinetic parameters of pine needles, extensive literature was not accessible using model-free techniques and model fitting techniques.
The objective of this study is to utilize the pine needles for setting up gasification-based power plant so that it provides the increased electricity access to households which can help children in education and rural cottage industries. The study of combustion and kinetic parameter estimation of pine needles using TGA will enable a clear understanding of the gasification process. The pine needles are characterized in terms of its composition, morphology and crystallinity with the help of elemental analyzer, SEM and XRD techniques. The data acquired from TGA were used to obtain the activation energy and pre-exponential factor using the iso-conversional methods such as Kissinger–Akharia–Sunose (KAS), Ozawa-Flynn-Wall (FWO), Friedman, Kissinger and Coats Redfern Method to assess the kinetic parameters and the order of the reaction at distinct heating rates (Vyazovkin and Wight 1999). The presented methodology helps in deeper analysis of the studied biomass and could be helpful in the design on the gasification systems.
2 Materials and methods
2.1 Sample preparation
Samples of pine needle as shown in Fig. 1 used in this research were collected from various areas of Uttarakhand state, of North India. Pine needle samples chosen for study were collected from Khetikhan, Saur (Uttarkashi), Chakrata (Dehradun), Naitwar (Uttarkashi), Jakhol (Uttarkashi), Rudraprayag of Uttarakhand state. As the needles were collected from the ground they were cleaned to remove twigs and debris manually. Almost 10 kg of pine needles were sun-dried for a few days so that the surface moisture can be removed.
2.2 Particle size distribution
The sieving process is the basic separation of particles by shapes and sizes. The quality and performance are affected by particle size and particle size distribution (PSD). Particle size distribution plays a significant role in flowability characteristics and changes in the particle size can result in modifications in flowability (Guo et al. 2012). Biomass particles are characterized by a diverse range of sizes and shapes; hence, it is important to determine and control the distribution of the particle size. Mechanical Sieve analysis is the generally used technique for PSD of ground biomass (Gil et al. 2014). The pine needles (Pinusroxburghii) were chipped, dried naturally and were milled at a lab-scale milling facility by a ball mill (Williams et al. 2016) with model No. (KCMO-004). A horizontal vibrating sieve shaker comprising eleven sieves with opening sizes of 0.71, 0.85, 1.00, 1.18, 1.40, 1.70, 2.00, 2.36, 2.80, 3.35, 4.00 mm and a bottom pan (< 0.71) was used. For each test, a representative weighed sample was poured into the top sieve with the largest screen opening size and 30-min sieve shaking time and amplitude was applied. After shaking, each sieve with the material retained was weighted and then divided by the total weight to give a percentage of material retained. Using MS Excel, the data were processed, and the results were tabulated and graphically plotted.
2.3 Proximate analysis
The moisture content of the pine needles was estimated using ASTM D-871-82, the standard test technique for particulate wood fuels. To estimate moisture content, a sample weight of 50 g is taken, and after heating constantly at 103 °C in an oven for approximately 30 min till constant, weight is obtained. The difference between the wet sample and dried sample gives the moisture content. In a laboratory muffle furnace (Ambassador F01) as per ASTM E872-82, the volatile matter in the sample was determined. 1.0 g of the sample was taken from 50 g of the test sample ground to a size of less than 1 mm and placed in a covered crucible to prevent contact with air during devolatilization and placed in a muffle furnace at 950 °C and heated for 7 min. The crucible was then removed, cooled in a desiccator, and the weight loss in the pine needles yields the volatile matter. In a muffle furnace, the ash content was also determined as per ASTM D-1102. A sample of 2.0 g was collected and placed in a muffle furnace, removing the crucible lid. The temperature of the furnace is slowly increased from 580 to 600 °C to prevent any flame. The sample is cooled and weighed when all the carbon is burned. The fixed carbon content was then calculated by the difference such as:
2.4 Ultimate analysis
The ultimate analysis provides the elemental composition of biomass samples. The major elements present in the biomass carbon, hydrogen, oxygen, nitrogen and a trace amount of sulfur. The CHNS(O) analyzer is used in estimating the percentage weight content of the elements. As the determination of oxygen is not possible with the analyzer, this element is determined by difference (Demirbaş 1997), as given in the equation below.
2.5 Calorific value
The heating value of any biomass is the amount of energy produced per unit of mass per unit volume of the fuel when it is wholly burned. The higher heating value of biomass is calculated using an empirical correlation developed by Channiwala and Parikh (2002) as shown below representing the carbon, hydrogen, oxygen and nitrogen in the percentage basis.
2.6 Scanning electron microscopy (SEM)
The structure of biomass was investigated by scanning electron microscopy (SEM). SEM is an effective method to characterize the surface morphology of the biomass (Claoston et al. 2014). It is among the powerful tools widely used to investigate the lignocelluloses surfaces. This method helps in investigating any kind of pores, anomalies present over the sample surface. These morphological natures are valuable in choice of biomass for various purposes. The fine sample was dried in an oven at 105 °C for 24 h. The sample was analyzed by scanning electron microscope instrument in IIT Roorkee (LEO435VP) at an electron acceleration voltage of 15 kV.
2.7 XRD analysis
Cellulose crystal structure plays an important role in the mechanical strength of plant cell. Instrumental technique such as X-ray diffraction method (XRD) is a valuable tool used to study the crystallinity of the biomass based on the cellulose fraction (Smidt and Schwanninger 2005). It is also useful for visual examination of surface morphology of the biomass. To perform the XRD experiment an analytical XRD diffractometer (Make: Bruker, Model: D8 Advance Eco) was used. The pine needle XRD analysis was investigated using Cu radiation source with voltage 40 kV and 25 mA. The scan speed of angle 2θ ranging from the angle of 10°–80° at a scanning speed of 0.5°min−1.
2.8 Thermal analysis
Thermal analysis is an important method for studying fuel properties which is related to thermal stability. Thermogravimetric analysis (TGA) is a procedure where the sample weight change is continuously recorded at a fixed heating rate as a function of time or temperature and is plotted against temperature (Cerc Korošec et al. 2009). As weight and temperature data are continuously recorded, the reporting of data with the first derivative of weight versus time or temperature emphasizing the zones of reaction is called differential thermogravimetry (DTG). It is possible to characterize the behavior of a sample with this method over a temperature range of 25–1000 °C, thus giving an overview of thermal behavior at all temperatures. The kinetic parameters of the reaction zones can be estimated using TGA data by varying the heating rate. The thermogravimetric analysis was performed in an analyzer EXSTAR DSC/TGA 6300 instrument. This equipment has a horizontal differential balance with 0.2 μg balance sensitivity, a temperature range of up to 1500 °C. The reference sample used as the standard in this equipment is thermally inactive alumina powder. The studies were conducted with the flow rate maintained at 200 ml/min in the atmosphere of air. The biomass (pine needle) were grounded coarsely and about 10.49 mg of sample was taken from the bulk and placed in an alumina pan and the pan resides in a furnace and the heating was programmed. To estimate kinetic parameters using model-free and model fitting techniques, a series of dynamic experimental runs were performed at distinct heating rates of 5, 10, 25 and 50 °C min−1 from room temperature to 660 °C. The sample mass is monitored during heating. The instrument gives the weight loss of the sample with continuous data of TG (%), DTG (mg/min) at different temperatures.
2.9 Kinetic modeling
Conventional analysis methods for model fitting require a kinetic model either known or assumed. The kinetic parameters namely activation energy and frequency factor can be estimated by fitting the model with the experimental data. Iso-conversional kinetics evaluates the reliance of efficient activation energy on conversion or temperature and through this dependence kinetic predictions have been made to find the mechanisms for thermal processes. Iso-conversional does not involve a reaction mechanism and reaction model to assess the activation energy. A number of tests were carried out to assess the parameters of the reaction at distinct heating rates. The principle of iso-conversion states that at different temperatures with the same conversion, \(f\left( \alpha \right)\) is equal. The current work describes the assessment of activation energy and frequency factor by non-isothermal methods and model-free iso-conversional methods such as Kissinger, Friedman, Kissinger–Akharia–Sunose (KAS) and Ozawa-Flynn-wall (OFW). Not much literature was available for kinetic parameter estimation for pine needle using the above-said methods.
The global kinetics of the thermal degradation of biomass can be assumed to be single-step reaction kinetics, and change in the extent of reaction α is given by
where \(m_{o} ,m_{t, } m_{\infty }\) are initial sample mass, sample mass at time t and sample mass at the end of the reaction. The rate of reaction can be expressed as
where \(f\left( \alpha \right)\) is the mechanism function.
The dependence of k follows the Arrhenius equation \(k\left( T \right) = A { \exp }\left( {\frac{ - E}{RT} } \right)\) k is the reaction rate, A is the (pre-exponential factor) frequency factor, E is the activation energy (kJ/mol). R the universal gas constant and T is the absolute temperature in K. Substituting the value of k (T) and if combined with a linear heating rate \(\beta = \frac{{{\text{d}}T}}{{{\text{d}}t}}\) the above Eq. (2) becomes
And rewriting the above equation,
where \(g\left( \alpha \right)\) is the integral reaction model.
The exact solution of the above integral is not possible and can be done by numerical solution or expressing \(g\left( \alpha \right)\) by some approximate equations.
The model-free iso-conversional methods of Friedman method, Kissinger–Akharia–Sunose (KAS) methods and Ozawa-Flynn-wall (OFW) and also model fitting Coats–Redfern method were chosen due to their simplicity to estimate the kinetic parameters.
The Coats–Redfern method (Coats and Redfern 1964) is a versatile tool to compute the kinetic parameters for the degradation process. The solution of the integral in Eq. (7) using the Coats–Redfern approximation is
For n = 1,
For n \(\ne\) 1,
The above equation enables to determine the dependence of the activation energy from experimental data performed at different temperatures. By assuming an approximation that \(\frac{2RT}{E}\) is very much less than 1, the intercept can be adjusted to \(\ln \left[ {\frac{AR}{\beta E}} \right]\) and the method is known as the modified Coats–Redfern method (MCR). In Kissinger–Akahira–Sunose method (KAS) method, \(\ln \left[ {\frac{\beta }{{T^{2} }}} \right]\) is plotted for each value of conversion \(\alpha\) against 1/T and the slope of the line is − E/R and intercept gives the frequency factor.
The use of Doyle’s approximation (Doyle 1962) for the integral in Eq. (7) gives the below equation on simplification which is the base of another iso-conversional method by Flynn and Wall (Chan and Balke 1997) and Ozawa (Ozawa 1965).
Thus, for a constant extent of reaction, ln \(\beta\) is plotted against \(1/T\) which is obtained by thermogravimetric curves at different heating rates. The activation energy is calculated through the slope of the plot.
The Friedman method (Friedman 1964) is a differential iso-conversional method that provides correct values of kinetic parameters, and is expressed by Eq. (13).
where \(\ln \left( {\beta \cdot \frac{{{\text{d}}\alpha }}{{{\text{d}}T}}} \right)\) is plotted against 1/T at diverse values of conversion. For convenience, all the plots were plotted against 1000/T.
3 Results and discussion
3.1 Characteristics of biomass
To quantify the composition of biomass, proximate analysis and ultimate analysis are usually applied. Proximate analysis, ultimate analysis and calorific value are significant parameters for determining the performance of biomass as a fuel, efficiency and the environmental friendliness level (García et al. 2013).
3.1.1 Particle size distribution
A particle’s morphological properties have a major impact on the physical properties of granular biomass materials. The standard sieving technique is usually used for the particle size distribution of crushed biomass (Vigneault et al. 1992). In this test, the pine needle sample was grounded by the ball mill and separated into different size groups by mechanical sieving. The particle size distribution of pine needle dust was uniform and the decreased screen opening size resulted in decreased particle sizes (Fig. 2). The particle size distribution of pine needles is shown in Table 2. From Table 2, it is clear that the almost 56% of the material was captured by the last three sieves with apertures 0.85,0.71 and < 0.71 mm, respectively. The maximum mass which is 17 g was retained by the sieve with aperture 1.4 mm and on the other side, the screens with the largest apertures i.e., 4, 3.35, 2.80 mm retained the less amount of material due to the spherical shape of the particle. However, it was clear that the sieving of fine materials requires special techniques, as the standards indicate a lower limit.
3.1.2 Proximate analysis
The results obtained from the proximate analysis and ultimate analysis are presented in Table 3. Under proximate analysis, the moisture content, volatiles, ash content and fixed carbon were examined using standard methods. The moisture content is the amount of water in the biomass per unit mass of the dry sample. The moisture content is a key parameter that affects the yield of the thermal systems like pyrolyzers and gasifiers. Most gasification systems use biomass with 10–20% moisture content for trouble-free and economical operation of the gasifier to achieve a higher heating value (Basu 2010). Higher moisture content reduces the combustion yield of the biomass, thermal efficiency of the gasifier and produces gas with low heating values. The results showed that the moisture content of different samples of pine needles is between 8.3 and 13.8 (wt%) which is acceptable and is well within the range of 5.58% and 20% as reported by Din and Zainal (2016) for some biomass materials.
The biomass volatile matter is the condensable and noncondensable vapor released when the fuel is heated. The amount of volatile matter determines how easily the biomass can be gasified which will influence the gasifier design and the fuel reactivity. The volatile matter in the selected pine needles is between 66.7 and 72.4 (wt%) which is somewhat higher due to high organic matter content. The volatile matter of some biomass materials varies between 60% and 80% as compared to bituminous coal with 35% as reported by Li et al. (2017).
The solid residue formed when the biomass endures a high-temperature treatment process is called ash. Ash comprises the mineral matter of the fuel and inorganic matter. With many, types of components, it is an essential component of plant material and differs from fuel to fuel. The ash content of biomass plays a major role if it contains components like chlorine, potassium and alkalis as they cause severe agglomeration, fouling and corrosion in gasifiers which is known as slagging. On the off chance that no measures are taken slagging prompts blockage of the gasifier. For the most part, slagging doesn’t happen with fuel having ash content below 5%. The ash content of biomass generally varies between 1 and 16% (Li et al. 2017). In the current research, the ash content of the pine needles ranged from 1.8 to 2.2 wt%, which is in excellent agreement with several authors who reported the proximate pine needle analysis.
Fixed carbon is the combustible residue remaining after heating biomass and expelling the volatile matter. It is estimated by subtracting from a sample the percentages of moisture, volatile matter and ash. The fixed carbon content in pine needles is 13.4–19.1(wt%). With less ash and moisture content, pine needles can be a potential biomass feedstock for thermochemical conversion.
3.1.3 Ultimate analysis
The ultimate analysis emphasizes the elements in the sample tested, and the results are shown in Table 3. It was carried out on the basis of the elemental composition of the sample. Mainly, the content of carbon, hydrogen, oxygen and nitrogen was estimated. The C and O content of pine needle is high with 53.29 and 31.2%. The hydrogen and nitrogen content was observed to be less compared to the carbon content in the pine needles. The amount of sulfur content in most of the biomass samples is very low and is 0.28 wt% in pine needles. This is an advantage from the environmental point of view in the energy conversion where SO2 emissions are considered. Biomass feedstocks with less nitrogen and sulfur content are viable for thermochemical conversion. Almost similar results were reported by Saifi et al. (2004).
3.1.4 Calorific value
The calorific value estimation of the biomass samples was determined by the empirical formula as outlined above on the basis of the elemental structure. The calorific value of pine needles in the present study i.e., 21 MJ/kg that particularly resembles 20 MJ/kg detailed by Demirbas (1997). The high calorific value of pine needles uncovers that these are comparatively the potential biomass for the production of bio-energy.
3.2 Biomass structural behavior
Using scanning electron microscopy (SEM) and X-ray diffraction (XRD), pine needle was investigated to understand its prospects as a gasification feedstock. The SEM analysis provides information about the surface morphology of any biomass sample and XRD about the crystallinity of biomass.
3.2.1 SEM
The raw samples of pine needle were collected and sent to SEM analysis to understand the surface topology of the fuel. The results of the analysis at different magnitudes are shown in Fig. 3 with the magnifications of 500×, 1000×, 4000× and 6000×. It can be seen from Fig. 3 that some regular long, flat and fibrous flakes are observed on the surface of the pine needles. There are several similar size grains along with few larger grains which explains that the surface morphology of the pine needle is homogeneous. The images of SEM has no pores on the pine needle morphology.
3.2.2 XRD
The XRD analysis of pine needles is presented in Fig. 4. The crystallinity was obtained using XRD from pine needles. The nature of the diffractogram shows that the biomass has amorphous nature and local crystalline structure. The peaks at 2θ = 16.6 and 22 indicate the cellulose structure in pine needles.
3.3 Thermal analysis of biomass
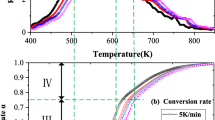
The thermal degradation of pine needles is carried out using TGA under an oxygen environment. The overall process of biomass oxidative degradation is consistent with the thermal decomposition of the various components of biomass (cellulose, hemicelluloses and lignin) (Fisher et al. 2002). This can be divided into five stages: (a) removal of moisture < 100 °C (b) the decomposition of extractives at 100–250 °C (c) decomposition of hemicellulose takes place at a temperature range of 250–350 °C (d) 350–500 °C mainly cellulose and lignin decomposition (e) > 500 °C the lignin decomposes which has relatively large carbon content compared to cellulose and hemicellulose and contributes to char formation. Figure 5 shows the TG and DTG evaluation of pine needles at various heating levels (5, 10, 25 and 50 °C/min). The TGA curves of pine needles indicated that in the first stage there is loss of weight due to moisture removal from initial temperature i.e., from 30 to 190 °C. The second stage corresponded to the combustion of volatiles of the low molecular weight gases from 190 to 450 °C where the maximum devolatilization occurs. This is ascribed to hemicellulose and cellulose decomposition. The third stage from 450 to 780 °C represents the degradation of lignin. Nailk et al. (2010) reported the thermogravimetric analysis of pinewood and for pine needles, similar results were reported by Statheropoulos et al. (1997). The percentage loss of weight of the samples at five temperature ranges is shown in Table 3. It was seen from DTG curves that the maximum peak is shifted to the right and that the maximum devolatilization rate is also increased as the heating rate increases. Figure 5 shows that the moisture removal is observed from 24.9 to 189 °C at 5 °C/min. As the heating rate is increased the temperature range for moisture removal also increased from 30 to 248 °C for heating rate 50 °C/min. It is because the rapid increase in temperature does not permit the entire moisture to be released. It is observed that as the heating rate is increased, the hemicellulose and cellulose decomposition temperatures also increased as shown in Tables 4 and 5.
3.4 Kinetic analysis of biomass
For the oxidative degradation of pine needles, the TG and DTG curves are used to estimate kinetic parameters (activation energy and frequency factor). The activation energy and frequency factor were estimated using model-free iso-conversional KAS, O–F–W and Friedman methods (Lu et al. 2009) at different heating rates 5, 10, 25 and 50 °C/min as shown in Fig. 7. The physical significance of A and E can be attributed according to collision theory that A denotes the number of collisions per unit time between the reactant molecules during the reaction and E represents the minimum amount of energy required for the molecules to participate in the chemical reaction (White et al. 2011). Hence, higher E means the slow reactions and lower E signifies the fast reactions. For all the above methods, Eqs. 8, 11 and 13 LHS are plotted against 1/T obtained from experimental data recorded at different heating rates which would give a straight line and whose slope is used to determine the activation energy and from the intercept the frequency factor is estimated at different conversions from 0.2 to 0.8. Since at the lower and higher levels of conversion, the experimental errors are larger, hence, α was chosen between 0.2 and 0.8. The plots of the differential and integral iso-conversional methods are shown in Figs. 5, 6 and 7. The estimated kinetic parameters and the regression coefficients R2 obtained using FWO, KAS, Friedman and Kissinger methods are shown in Table 6. It is clear from the iso-conversional plots that they show the same trend of lines which are parallel. From the results shown in Table 6, the activation energies calculated are following a trend that they are in good agreement at different conversion. It is observed that there can be three regions in the degradation of pine needles and assumed that the first process (0.02 < α<0.2) where there is removal of water from the sample and the second region (0.2 < α < 0.5) corresponding to the hemicellulose degradation of biomass and the third region (0.5 < α<0.8) where the cellulose degrades. The lignin present in the biomass degrades over the entire range of α from 0.5 to 0.8 and beyond it is not considered for the calculation of E. The values of E, A at α = 0.4 has regression coefficient in the range of 0.77, and the value of E is very higher and the set of values can be ignored for the reason that there can be some experimental noise. It is observed from Table 6 that in KAS method, the calculated activation energy was increased from 160.9 to 258.7 kJ/mol till α = 0.4 and then decreased to a minimum value of 129.7 kJ/mol at a conversion α = 0.8. The activation energy increased from 166.6 to 264 kJ/mol and then decreased to 135.8 kJ/mol in FWO method. In Freidman method, the E value increased from 166.3 to 264.5 kJ/mol and decreased to 135.1 kJ/mol. But the average values of activation energy are 190.74,190.74 and 199.4 kJ/mol at separate iso-conversional points from the above all techniques. The average values of E are very encouraging, suggesting that the activation energy E is almost identical through all the above techniques. This is a most of demonstration that E is independent of α. The values of E from KAS and FWO are similar but there is some variance in the Friedman method as it is a differential iso-conversional method and can contribute to some error due to differentiation. The Coats–Redfern method for the combustion degradation of pine needles for reaction orders n = 0, 0.5, 1.0, 1, 5, 2.0 and 3 are shown in Table 7 for all heating rates. The regression coefficients for Coats–Redfern method are higher for n = 1 compared to n = 2, and the first-order equation was found to fit better. Hence, it can be assumed that oxidative degradation follows first order and is the most feasible reaction. According to Coats–Redfern method, from Table 7, it was observed that there is a relationship between the order and the activation energy that as the order is increased from 0 to 3 the activation energy also increased from 8.11 to 60.81 kJ/mol. The average random squares of all the iso-conversional methods and model fitting methods show that the error is very minimum. The experimental data fitted well with the model data. The values obtained are in the range of the reported values of different authors in the literature (Arora et al. 2011; El-Sayed and Mostafa 2014; Ceylan et al. 2014; Garcia-Maraver et al. 2015; Kok and Özgür 2013; Font et al. 2009) for different biomass materials as shown in Table 7. In Fig. 7, it is noticed that apparent activation energy for the pine needle combustion was not the same for all conversions indicating the presence of a complex mechanism in the solid state.
4 Conclusions
Pine needles acts as fuel in the forest fires as their foliage easily and catches fire and many districts are fire risk areas owing to the abundant availability of pine needles. The only measure to reduce the forest fire is the removal of pine needles from the forest floor. The collected pine needles can be used to generate electricity through gasification process. Thermogravimetric analysis is a fast and efficient method for evaluating combustion and kinetic parameters of pine needles. The weight loss and decomposition data from TGA were utilized to assess kinetic parameters using model-free and model fitting techniques. The thermogravimetric analysis of pine needles shown that the combustion degradation of pine needles can be understood in three stages: removal of moisture (< 200 °C), oxidation of volatiles (200–380 °C) and oxidation of char (400–680 °C). The heating rate influences the decomposition of biomass and as the heating rates increased, there is a heat transfer limitation in the thermogravimetric curves. The model-free techniques were applied to calculate the activation energy and frequency factor at different conversions. The high activation energies of biomass can be explained by the fact that the raw material reacts slowly. The E values are dependent on conversion, thus explaining that the combustion process involves a complex process. The KAS, FWO, Friedman and Kissinger methods gave the approximate same values for different stages studied. The activation energy estimated by KAS and FWO was similar and found to be 190.7 kJ/mol while Friedman methods showed 199.4 kJ/mol. The estimated kinetic parameters may be used to model the process of gasification and can be used as preliminary data for the design of gasification systems using pine needle as feedstock.
References
Arora, S., et al. (2011). Comparative degradation kinetic studies of three biopolymers: chitin, chitosan and cellulose. Arch Appl Sci Res, 3(3), 188–201.
Basu, P. (2010). Biomass gasification and pyrolysis: practical design and theory. Cambridge: Academic Press.
Bisht, A. S., Singh, S., & Kumar, M. (2014). Pine needles a source of energy for Himalayan Region. International Journal of Scientific & Technology Research, 3(12), 161–164.
Cerc Korošec, R., et al. (2009). Thermogravimetry as a possible tool for determining modification degree of thermally treated Norway spruce wood. Journal of Thermal Analysis and Calorimetry, 98(1), 189–195.
Ceylan, S., Topcu, Y., & Ceylan, Z. (2014). Thermal behaviour and kinetics of alga Polysiphonia elongata biomass during pyrolysis. Bioresource Technology, 171, 193–198.
Chan, J., & Balke, S. (1997). The thermal degradation kinetics of polypropylene: Part III. Thermogravimetric analyses. Polymer degradation and stability, 57(2), 135–149.
Channiwala, S., & Parikh, P. (2002). A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel, 81(8), 1051–1063.
Chauhan, A., & Saini, R. (2015). Renewable energy based off-grid rural electrification in Uttarakhand state of India: Technology options, modelling method, barriers and recommendations. Renewable and Sustainable Energy Reviews, 51, 662–681.
Claoston, N., et al. (2014). Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Management and Research, 32(4), 331–339.
Coats, A. W., & Redfern, J. (1964). Kinetic parameters from thermogravimetric data. Nature, 201(4914), 68.
Demirbaş, A. (1997). Calculation of higher heating values of biomass fuels. Fuel, 76(5), 431–434.
Din, Z. U., & Zainal, Z. (2016). Biomass integrated gasification–SOFC systems: Technology overview. Renewable and Sustainable Energy Reviews, 53, 1356–1376.
Doyle, C. (1962). Kinetic analysis of thermogravimetric data. Journal of Applied Polymer Science, 6(19), 120.
El-Sayed, S. A., & Mostafa, M. (2014). Pyrolysis characteristics and kinetic parameters determination of biomass fuel powders by differential thermal gravimetric analysis (TGA/DTG). Energy Conversion and Management, 85, 165–172.
Fisher, T., et al. (2002). Pyrolysis behavior and kinetics of biomass derived materials. Journal of Analytical and Applied Pyrolysis, 62(2), 331–349.
Font, R., et al. (2009). Kinetics of pyrolysis and combustion of pine needles and cones. Journal of Analytical and Applied Pyrolysis, 85(1–2), 276–286.
Friedman, H. L. (1964). Kinetics of thermal degradation of char‐forming plastics from thermogravimetry. Application to a phenolic plastic. Journal of Polymer Science Part C: Polymer Symposia. Wiley Online Library.
García, R., et al. (2013). Biomass proximate analysis using thermogravimetry. Bioresource Technology, 139, 1–4.
Garcia-Maraver, A., et al. (2015). Determination and comparison of combustion kinetics parameters of agricultural biomass from olive trees. Renewable Energy, 83, 897–904.
Gil, M., Teruel, E., & Arauzo, I. (2014). Analysis of standard sieving method for milled biomass through image processing. Effects of particle shape and size for poplar and corn stover. Fuel, 116, 328–340.
Guo, Q., Chen, X., & Liu, H. (2012). Experimental research on shape and size distribution of biomass particle. Fuel, 94, 551–555.
Joshi, K., Sharma, V., & Mittal, S. (2015). Social entrepreneurship through forest bioresidue briquetting: An approach to mitigate forest fires in Pine areas of Western Himalaya, India. Renewable and Sustainable Energy Reviews, 51, 1338–1344.
Katre, D., & Gupta, M. (2011). Expert usability evaluation of 28 state government web portals of India. International Journal of Public Information Systems, 7(3), 1–16.
Kok, M. V., & Özgür, E. (2013). Thermal analysis and kinetics of biomass samples. Fuel Processing Technology, 106, 739–743.
Kurose, K., Okamura, D., & Yatagai, M. (2007). Composition of the essential oils from the leaves of nine Pinus species and the cones of three of Pinus species. Flavour and fragrance journal, 22(1), 10–20.
Leoni, E., et al. (2003). Thermal degradation of pinus pinaster needles by dsc, part 2: Kinetics of exothermic phenomena. Journal of Fire Sciences, 21(2), 117–130.
Li, Y., Zhou, L., & Wang, R. (2017). Urban biomass and methods of estimating municipal biomass resources. Renewable and Sustainable Energy Reviews, 80, 1017–1030.
Lu, C., Song, W., & Lin, W. (2009). Kinetics of biomass catalytic pyrolysis. Biotechnology Advances, 27(5), 583–587.
Naik, S., et al. (2010). Characterization of Canadian biomass for alternative renewable biofuel. Renewable Energy, 35(8), 1624–1631.
Ozawa, T. (1965). A new method of analyzing thermogravimetric data. Bulletin of the chemical society of Japan, 38(11), 1881–1886.
Perlack, R. D. (2005). Biomass as feedstock for a bioenergy and bioproducts industry: The technical feasibility of a billion-ton annual supply. Oak Ridge: Oak Ridge National Laboratory.
Safi, M., Mishra, I., & Prasad, B. (2004). Global degradation kinetics of pine needles in air. Thermochimica Acta, 412(1–2), 155–162.
Saravanan, A. P., et al. (2018). Biofuel policy in India: a review of policy barriers in sustainable marketing of biofuel. Journal of Cleaner Production, 193, 734–747.
Senneca, O. (2007). Kinetics of pyrolysis, combustion and gasification of three biomass fuels. Fuel Processing Technology, 88(1), 87–97.
Smidt, E., & Schwanninger, M. (2005). Characterization of waste materials using FTIR spectroscopy: Process monitoring and quality assessment. Spectroscopy Letters, 38(3), 247–270.
Statheropoulos, M., et al. (1997). Thermal degradation of Pinus halepensis pine-needles using various analytical methods. Journal of Analytical and Applied Pyrolysis, 43(2), 115–123.
Susastriawan, A., & Saptoadi, H. (2017). Small-scale downdraft gasifiers for biomass gasification: A review. Renewable and Sustainable Energy Reviews, 76, 989–1003.
Vigneault, C., Rothwell, T., & Bourgeois, G. (1992). Hammermill grinding rate and energy requirements for thin and conventional. Canadian Agricultural Engineering, 34(2), 203.
Vyazovkin, S., & Wight, C. A. (1999). Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochimica Acta, 340, 53–68.
Wang, X.-R., & Szmidt, A. E. (1993). Chloroplast DNA-based phylogeny of AsianPinus species (Pinaceae). Plant Systematics and Evolution, 188(3–4), 197–211.
White, J. E., Catallo, W. J., & Legendre, B. L. (2011). Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies. Journal of Analytical and Applied Pyrolysis, 91(1), 1–33.
Wilburn, F. (1989). Introduction to thermal analysis, techniques and applications: Michael E. Brown, published by Chapman and Hall, London, 1988, ISBN 0 412 30230 6, xx + 211 p.; price£ 17.50. Elsevier.
Williams, O., et al. (2016). Influence of mill type on densified biomass comminution. Applied Energy, 182, 219–231.
Xu, R.-B., et al. (2012). Chemical composition and antioxidant activities of three polysaccharide fractions from pine cones. International Journal of Molecular Sciences, 13(11), 14262–14277.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Havilah, P.R., Sharma, P.K. & Sharma, A.K. Characterization, thermal and kinetic analysis of Pinusroxburghii. Environ Dev Sustain 23, 8872–8894 (2021). https://doi.org/10.1007/s10668-020-01001-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-020-01001-8