Abstract
The study area Tamabil, Sylhet, is an important area in importing coal and its storing for supplying to different industrial uses in Bangladesh. This area is located in the north-eastern part of Bangladesh. Currently, the water environment in the study area is threatened by the processing of high sulphur coal, agricultural activities, and other anthropogenic effects. Hence, the protection of water resources in terms of quality and quantity from such contamination should be considered a serious issue. From this view point, this study intends to evaluate the hydrogeochemical characteristics and sulphate contamination of water bodies in the area. The hydrochemical evaluation of water for all samples has been carried out under standard laboratory methods with various diagrams, charts, and statistical methods. From the results of the laboratory testing, it has been observed that the water in the wells close to the coal stock pile is highly acidic (pH = 3.41), whereas the water shows the standard pH values as its distance increases from the stock area. The hydrogeochemical evaluation of water under the Piper chart implied that mixed type of water facies such as (i) Ca–Mg–Cl (38.7%), (ii) CaHCO3 (38.7%), (iii) NaCl (16.12%), and (iv) CaCl (6.4%) are dominant in the area. The Gibbs diagram showed the rock- and evaporation-dominant mixed environment, while the Durov Diagram reflected mixing–dissolution state with few in reverse ion-exchange condition. These results suggest that the major geochemical facies and evolution of water are prevailed by the carbonate rock dissolution and the weathering process. In the case of sulphate contamination, the overall concentration of sulphate falls within the standard limit of WHO and EQS, but this study observed that the intensity of SO4 is high in the samples adjacent to the coal stock pile. These scenarios entailed that the stored coal is primarily responsible for it while the sulphur content ranges from 1.42 to 7.26 Wt% (average 3.73 Wt%) of this coal. In addition, the leaching experiment of this coal shows that the pH values of the water reduce from 7 to 1.2, consequently increasing the acidity of the water environment around the area. In conclusion, this study recommends that (i) more cautious and watchful activity has to be taken to import, store, and use this coal; (ii) more precautions have to be considered to protect the water from such contamination to save the green environment as a whole for the present and future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water is the most essential element among resources existing in nature. Water is present everywhere, but its availability has always been limited in terms of quantity and quality, especially in coal mining as well as coal stock pile areas (Cutruneo et al. 2014; Dias et al. 2014; Howladar 2012, 2017; Howladar et al. 2014; Martinello et al. 2014; Sanchís et al. 2015; Silva et al. 2012). Water is used for different purposes for the advantage of mankind such as drinking, irrigation, municipal and industrial uses, hydropower generation, flood management, fish farming, navigation, ecological needs, and recreation. Unfortunately, this valuable resource is contaminated through various ways causing different life-threatening diseases and environmental pollution all over the world. In the case of Bangladesh, it is one of the most populous countries in the world, located in the eastern part of the Asia, importing a large quantity of coal for the power generation sector and its dependent industries (Imam 2005). However, the imported coal is primarily stored in the specific coal stock pile in Tamabil; then, it distributes to different industries in the country. This coal contains high levels of ash, sulphur, and arsenic (Pronab and Mrinal 1996; Hashan et al. 2013, 2016). Also, it contains many heavy metals such as lead, mercury, nickel, tin, cadmium, antimony, thorium, and strontium. In fact, many of these heavy metals are mixed with soil and water by leaching at the time of rain, causing acute or chronic toxicity in the soil and water environs (Finkelman et al. 1990; Gutta 1999). Moreover, the leached water from coal stock piles can be acidic and contains toxic trace elements (Hower et al. 2013; Ribeiro et al. 2013). As a result, the surface and subsurface water is turning unsafe for agriculture, human consumption, bathing, or other household uses (Tiwary 2001). Thus, from these features, it can be noted that water as well as soil environs close to the coal stock pile is polluted day by day, especially through the leaching of sulphate, arsenic, iron, etc. (Arenas-Lago et al. 2013; Cerqueira et al. 2011, 2012). Therefore, a detailed research for understanding the quality of water bodies, their interaction with the surface and subsurface environment, the possible impact as well as a sustainable management plan is very much needed. From these points of view, this study aims at (i) evaluating the chemical characteristics and hydrogeochemical facies of water bodies; (ii) assessing the intensity of sulphate, possible sources, and the contamination of water environs. At the end, it illustrates the necessary recommendations for managing water environmental contamination around the coal stock pile.
2 Geographical setting of the study area
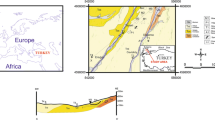
The study area is located in the north-eastern corner of Bangladesh, about 55 km away from the district headquarters of Sylhet and about 500 m south from the border of India (Fig. 1). It adjoins with three northeast Indian states, namely Assam, Meghalaya, and Tripura. The geographical settings around the area are awfully nice and prospective. The investigated area is bounded from west to east by the Khasi–Jaintia hill range, which is bordered on the northeast by the abrupt scarp of Shillong plateau of 4000 to 6000 feet high. The region is almost hilly. The high plains of the region are higher land than the Haor basin, and the streams are rather deep-rooted. The hill tracts are anti-clinally folded, recognized as the remnant of Pleistocene Terraces. A variety of small-to-moderately elevated hillocks are present here. The elevation of the area ranges from 60 to 340 feet. The area exhibits moderately hilly topography. The hills that characterize low-to-moderate elevation are east–west trending (Shaha 2014). The maximum and minimum temperatures of the region range from 10 to 32 degree celsius, and the annual rainfall is about 5000 mm. Also the maximum humidity and fog are common in winter. The groundwater in the study area occurs at a shallow depth. In the hilly area, the Pliocene Tipam sands serve as aquifer. The water table lies close to the surface and fluctuates with the annual recharge and discharge condition in the area. The recharge to the aquifers is mainly from the vertical percolation of rainwater and floodwater (Shamsudduha et al. 2009). The biggest group of the people is Bangali, and other includes the Khasia, Tipra, and Garos. They still live in their primitive way in the hills. The other remarkable tribes found here are the Oriya people who were brought to this region in the first quarter of the last century.
3 Sampling and analytical methods
This study firstly reviews the available physical resources on the geographic and hydrologic setting, imported coal quality, and water environs. Secondly, the intensive field investigation programs were conducted to recognize different sources such as ponds, canals, artesian sources, shallow wells, deep wells, and dug wells near and far from the coal stock pile from which samples were collected. During the field investigation and sample collection, all samples were collected in 1500-ml plastic bottles from the selected wells. The latitude, longitude, and elevation were recorded at that time with the help of a GPS (Global Positioning System) Metre. The details information about samples and the place was also collected. The dry, clean, and sterilized plastic bottles were used to maintain natural condition of water for laboratory analysis. Moreover, cautious care was taken for all times during the sample collection to assure samples were not contaminated. Immediately after sampling, pH and EC values were measured in the field by a portable pH and an EC/TDS metre. The temperature was measured with a normal mercury thermometer. Evaluation of chemical parameters of the collected water was analysed in the water treatment laboratory in the Barapukuria Thermal Power Station, Dinajpur. Different standard methods were used to evaluate the specific parameters of the water within a month after the sampling time. The summary of the laboratory analysis results is shown in Tables 1 and 2. The electrical conductivity (EC), pH, and temperature (T) of the samples were measured in situ condition. In fact, a 0.45-lm cellulose acetate syringe filter was used to filter the cation and the anion samples. The cation samples were acidified to a pH value of 2 using nitric acid. In particular, the sodium (Na+) and potassium (K+) were measured using the flame photometer (Model No. PEP 7 and PEP 7/C). The calcium (Ca2+) and magnesium (Mg2+) were determined titrimetrically using the standard EDTA. The chloride (Cl−) values were determined with an ion-selective electrode method (Cole-Parmer iodine electrode, model no. 27502-13). Bicarbonate (HCO3−) concentrations were determined by a potentiometric titration method. Sulphate (SO42−), ortho-phosphate (PO43−), and dissolved silica (SiO2) analyses of samples were carried out under the UV–visible spectrophotometer. Nitrate (NO3−) was measured with an ion-selective electrode method (Cole-Parmer iodine electrode, model no. 27502-19). The high-purity analytical reagents were used throughout the whole study, and the chemical standards were prepared separately for each element. Arsenic (As) was tested by using the Hach EZ Arsenic test kit (calt. No. 28228-00). The total dissolved solids (TDS) were estimated from cation and anion contents by the calculation method (Hem 1991). However, the similar physical and analytical procedures were previously reported by many researchers such as Howladar et al. 2013, 2017a, b; Howladar and Rahman 2016; Martinello et al. 2014; Ramos et al. 2015; Tezza et al. 2015. At the end, the hydrogeochemical facies and water type have been interpreted with the help of different established charts and diagrams (e.g. Gibbs diagram, Piper chart, and expanded Durov diagram) in the research. Subsequently, a leaching experiment was conducted under SW 486, EPA method 1312 (Synthetic Precipitation Leaching Procedure, SPLP). The experiment was started by preparing the mixed acid solution. The subsequent stages of this analysis and the state of pH values were monitored and noted for different times.
4 Results and discussions
4.1 Hydrochemical evaluation
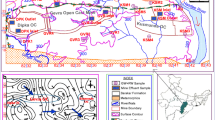
Hydrochemical evaluation is an important factor to understand the suitability and standard of water for drinking, domestic, agricultural, and industrial purposes (Subramani et al. 2005). The tested chemical compositions of the samples collected from the studied area are shown in Tables 1 and 2. In particular, the analytical results of pH value ranges from 3.41 to 7.86 are shown in Fig. 2a. The intensity of contour map shows that the low pH value (3.41, sample no 2) of water is very close to the coal stock pile that reflects the acidic water environment. It is well known that if the pH value is 7, it is considered as neutral state (Reddy 2012). The overall observation of the analysis shows that maximum water samples are in acidic state, while some samples are in alkaline. The pH value of water is controlled by the amount of dissolved CO2, carbonate, and bicarbonate concentrations (Zhou et al. 2012). From the human consumption point of view, all the samples should not be considered as potable, as they show strongly acidic state for some cases. Electrical conductivity is a measure of water capacity to convey electric current. It was observed that the electrical conductance values of water sample range from 2210 µS/cm to 130 µS/cm. The maximum value of EC was recorded as 2210 µS/cm in the surface water samples (Fig. 2) which is close to coal stock pile. These values indicate the presence of some dissolved inorganic substances in the ionized form in the water. The EC values of dug well range from 1959 µS/cm to 186µS/cm. At a glance, the EC values of canals, ponds, and field water are higher than that of dug wells water. The turbidity of samples ranges from 3.9 to 97 NTU besides the mean total dissolved solids value is 270.73 mg/l. However, the ranges of TDS should be from 500 to 1500 mg/l (WHO 2011). The TDS was found to be within limit, while the maximum value of the TDS of 695 mg/l (sample no 2, Fig. 2b) was recorded near the coal pile. The minimum value 48 mg/l (sample no 19) was found in the dug well stands far from the stock pile. The hardness of the water shows variable values. The hardness has been calculated as CaCO3 in mg/l. Hardness results from the presence of divalent metallic cations, of which calcium and magnesium are the most abundant one. The total hardness in water samples ranges from 21 to 100 mg/l which thoroughly belongs to the limit (500 mg/l) proposed by WHO (2011). The iron (Fe3+) concentration in the area varies from 0.015 mg/l to 0.34 mg/l. The dug well ranges from 0.01 mg/l to 0.34 mg/l. Fe in the water is acquired from the weathering process of minerals. Under the reduction condition, the ferric oxides and oxyhydroxides such as haematite (Fe2O3) or goethite (FeOOH) could possibly leach out as dissolved Fe (Zhou 2012).
The mean magnesium concentration of water shows the natural condition. The concentration of magnesium of a large number of samples falls between 1 mg/l to 40 mg/l. Chloride can be used as an important index to assess the influence of seawater intrusion. The chloride (Fig. 2c), potassium, and sodium concentrations in water vary from 1.28 mg/l to 25.56 mg/l, 1.4 mg/l to 11.7 mg/l, and 6 mg/l to 49.8 mg/l, respectively. The measured highest intensity of arsenic in the study area is 0.038 mg/l (sample 2), found close to the coal pile. In fact, the standard permissible limit of the arsenic in drinking water is 0.05 mg/l (WHO 2011). The mean value of phosphate (Fig. 2) in water is in permissible limit ranges from 0.25 mg/l to 8.3 mg/l. The highest value of sulphate (200 mg/l, sample no 2) was found in the canal water passed through the coal stock pile. The minimum value (2.5 mg/l, sample no 19) was recorded in filed water that is far from the coal pile. The ranges of ammonia content in water samples are recorded from 0.053 mg/l to 0.53 mg/l. The copper concentration of the study area was below the detection level. The nitrate, calcium, carbonate, bicarbonate, suspended solid, and soluble silica concentrations are in the allowable limit for different uses (WHO 2011).
Hydrochemical characteristics of the water are influenced by the source materials such as a variety of physical, chemical, and biological processes in the area. Moreover, for understanding the various hydrochemical mechanisms of the water chemistry and quality around the area, the 1:1 ratio plots and cross-plots for different elements were constructed considering the major and minor ions concentration of water (Fig. 3). In particular, the plot of Ca + Mg and HCO3, the SO4 versus Ca + Mg, the HCO3 and Cl, the Cl against Na plot, Cl and SO4, Cl against K, Ca + Mg against Na + K, Ca with Cl, and Cl + SO4 versus Mg + Ca (Fig. 3) display that the sample points are aligned to positive linear trend but are spread above and below the unit line. In fact, the low abundance of HCO3 and SO4 than Ca + Mg and Cl (Fig. 3a and b) suggests the influence of mixing chemical environments such as partly the marine environment and partly the anthropogenic activities on the major ion of the water (Rao and Rao 2009). The relative abundance of Na, K, and Ca is higher than that of the Cl ion shown in Figs. 3d, f, and h. However, in Figs. 3c and e Cl concentration is more dominant than that of the HCO3 and SO4. The ionic loads relationship between some elements, namely Na + K>Ca + Mg and Cl + SO4 < Mg + Ca, was also observed from Figs. 3g and i, respectively. Moreover, the overall ionic distribution and the relative abundance of water bodies suggest that these ions enrichment contributes to several processes such as the natural with multiple anthropogenic sources, the leaching of stock coal, the existing agriculture activity, and the organic decompositions (Kumar and Ramanathan 2008 and Kumar et al. 2009).
4.2 The application of Gibbs, Piper, and expanded Durov diagram to evaluate the hydrogeochemical facies and water types
The Gibbs (1970), Piper (1953) and an expanded Durov (1948) diagram have been applied for assessing the hydrogeochemical facies and water types. The drawing of this chart and graphic has been completed with the help of the Rock Ware, the surfer 9, and the AquaChem Software. The Gibbs (1970) diagram is an essential method for estimating the geochemical processes of various chemical parameters of water bodies. It is constructed based on the ratio of some anions and cations that can be expressed with the following relations:
where the unit of all the ions is meq/L. In this study, the ratio of different ions belongs to the rock-dominant region (Fig. 4). This attribute replicates that all the water of the area have long time interface with the rocks. In Fig. 4, some ions are spread around the boundary between the rock and the evaporation-dominant fields. This situation indicates that the evolution of water chemistry is partly influenced by the evaporation process. However, it is known that the marine processes have a direct role to raise the Na and Cl− ions in water environment, consequently increasing the quantity of TDS in water (Rao 2007 and Reddy 2012). As a result, some ions shift towards the boundary between the rock and the evaporation zone (Rao and Rao 2009; Reddy 2012). This study also adopts the Piper diagram (1953) to recognize and classify the hydrogeochemical facies and the groundwater types around the area. The samples have been ploted on the Piper diagram with the help of Aquachem software which are shown in Fig. 5. The samples were plotted using piper diagram which is shown in Fig. 5. This figure shows four different groups of ionic concentration of the water sample that are summarized in Table 3. In general, the sample can be classified into six fields in the piper diagram, namely CaHCO3 type, NaCl type, CaMgCl type, CaNaHCO3 type, CaCl type, and NaHCO3 type (Ghoraba and Khan 2013; Kumar 2013). In this diagram, about 38.7% of samples are occupied in the middle upper side of the diamond shape. This group indicates mixed water (chloride-calcium and chloride magnesium water type). On the other hand, another 38.7% (Table 3) of samples covered in the upper corner of the diamond shape implied that these samples belong to the calcium bicarbonate water type of the hydrogeochemical facies (Hossain et al. 2010). As a result, the major ion chemistry of the area is dominated by the carbonate rock weathering, i.e. calcite and dolomite are responsible for water chemistry (Dalai et al. 2002). The rest of the facies are NaCl and CaCl types (Table 3), and are covered about 16.12% and 6.4% samples, respectively.
In addition, the Durov (1948) diagram is applied to provide more information on the hydrogeochemical facies with their possible geochemical processes. The construction of this diagram is based on the percentage of major ions that are expressed in mg/L. For this calculation, the total volume of cations and anions together would be the 100 per cent (Burden and Mazloum 1965; Howladar et al. 2014). The major cations and anions were plotted in the Durov diagram under the AquaChem software. The results are shown in Fig. 6. It indicates that most of the samples belong to the mixing–dissolution phase with a few in the reverse ion-exchange condition. Thus, these results implicate the mixed type of water facies such as calcium bicarbonate, calcium–magnesium-chloride, and calcium-chloride types of hydrogeochemical facies around the coal stock area. It indicates the shallow recently recharged young water in the area (Davis and Exley 1992; Ahmed et al. 2000; Hossain et al. 2010; Howladar 2012, 2014).
4.3 The intensity of sulphate concentration with its possible source of origin
The concentrations of sulphate ranges from 2.5 to 200 mg/l were found in the study area. In fact, the sulphate intensity contour map (Fig. 7) shows that the maximum sulphate concentration (200 mg/l, sample number 2) is very close to coal pile and the minimum (2.5 mg/l, sample number 18) is far from the coal pile. The mean concentration is 37.54, whereas the overall sulphate concentrations gradually decrease in both the surface and subsurface water as the distance increases from the pile. Based on these concentrations, the water in the area can be classified into two categories such as low sulphate (2.5 mg/l) and moderate-to-high sulphate (200 mg/l) water. Usually, the water from the nearest sampling points of coal pile reflects high sulphate concentrations. The intensity of sulphate is low in left top side high elevated area (123 ft) of contour map and close to the coal stock pile. Besides, the low elevated area of right side (35 ft) shows comparatively the higher concentration. These typical characteristics indicate that the uplifted area is less vulnerable than the basinal area as coal washing/dressing or leaching water directly deposits in this depressed area. On the whole, when the water contains high concentration of sulphate, this scenario reflects a good affinity between sulphate, calcium, magnesium, and bicarbonate. This feature also implies the similar sources for sulphate and these ions. For example, mineral dissolution of carbonates and sulphate deposits or similar processes are responsible for the high intensity of SO4, whereas the low sulphur water does not have the well-built affinity to other solute concentrations in the water (Gráfias et al. 2010). In view of recognizing these relationships, Fig. 8 is constructed for all the surface and subsurface water. This figure shows that the correlation between sulphate with pH, TDS, Cl, Ca, Mg, and HCO3 is well analogous. Thus, these results pointed out that the sources of such ions are same as the high intensity of sulphur ions area that has the high intensity of these ions (Fig. 8). In addition to understanding the visible source of sulphate and other ions, this study reviewed and analysed 18 coal samples from these stock piles from 2011 to 2014. The summary of the results is shown in Table 4. The maximum, minimum, and average values of these samples are 7.26, 1.42, and 3.73 (Wt%), respectively, which implied that the imported stock coal is high sulphur coal (Ward 1984). As a result, this scenario reflects that this high sulphur coal is one of the sources of sulphate in the area. In addition, the other anthropogenic supplies of the pollutant in the water, namely industrial, agricultural and the municipal waste, are also responsible for it.
4.4 Implication about the environmental contamination around the study area
Currently, the water environment as well as the overall environments is under threat and stress due to different natural and man-made activities. In particular, the modern urbanization and the industrialization are mentionable. In the case of expanding techno-based industries in the country, an uncut electricity or power supply is very much essential. In this situation, the country imports a large amount of coal through Tamabil border of Sylhet for every year. Primarily, it is stored in different open stock areas for about the whole year. Small portions of this coal such as coal fines, ash, and dust are mixed with environment through water, air, rain, and so on. Consequently, it contaminates the environment. The utilization of coal for producing energy can be considered as one of the vital parameters to contaminate the environment (Safiullah et al. 2011; Howladar and Islam 2016). The environmental impacts from coal-based industries might not be ignored, even if it plays an immense role in the development of social and economic status of a country (Ram and Masto 2010). High sulphur coals have a notable effect on the environments, especially when these coals are used as a fuel (Pronab et al. 1996). In weathered condition, coal sulphur percolates with groundwater making the water highly acidic and causing the problem of the acid mine drainage (Gupta et al. 1977). However, this study tested some coal samples from the coal stock pile and the results (Table 4) show that the imported stock coal in Tamabil is highly sulphurous and low-grade lignitic coal. On the other hand, the chemical characteristics of water around the coal pile are imbalanced for some cases. For example, the pH value is very low (pH value 3.41, sample number 2), EC value is very high (2210 µS/cm, sample number 1), TDS, Cl, Ca, NO3, PO4, SO4, and others that show the higher concentration in water samples (Fig. 2) are close to the coal pile. The case of low pH value of 3.41 reflects an acidic water environment around the area which might be due to the influence of leaching and weathering processes of this high sulphur coal. Moreover, the leaching experiments were performed to show the relationship between the pH and sulphur. The results of these experiments are shown in Fig. 9. It is observed that the pH values decreased in noticeable level ranges from 7 to 1.2 after leaching. The main reasons are that the concentration of sulphur is high in fresh coals that are responsible to increase the acidity of the water environment, the soil environment, and others around the area. These results have a good agreement with the former researches by Van der Sloot et al. (1997) and Jang et al. (2002). They explained that high sulphur has an inclination to leach more in an extremely acidic pH condition. In fact, under the course of leaching, some minor elements such as sulphides will be melting completely or partly and separate from the coal. These sulphide oxides react with the water and other environmental components, forming the toxic elements in environment. Consequently the water, soil, and others parts of environment are polluted which unswervingly distress the normal natural environment for lives and the growth of plants and living organisms. Therefore, it is noticeable that the water environment close to coal pile is highly acidic. It will be more and spreading for large areas with time. Accordingly, the water as well as the overall environment has considerable chances to distress and imbalance for the present and future.
5 Summary and conclusions
In this study, we performed the detailed hydrochemical investigation with the sulphate contamination and the environmental pollution around the area. In the case of chemical and statistical analysis, 31 surface and subsurface water samples were collected from the adjacent area of the coal stock pile. The chemical characteristics of water analysis are presented by the estimation of TDS, pH, EC, Ca2+, Mg2+, K+, Na+, Fe2+, As3+, Cu2+, CO32−, PO43− HCO3−, SO42−, NO3, SO4, NH3, Cl−, suspended solid, alkalinity, and acidity in laboratory.
The results of laboratory-tested water samples and intensity of different locations have been elucidated with the help of statistical analysis, Gibbs ratio plot, Piper diagram, expanded Durov diagram, and leaching experiments were performed to illustrate the chemical characters of water, hydrogeochemistry, and possible sulphate contamination in the environment around the study area. The concentrations of major ions in surface and groundwater are within the tolerable limit. The intensity of cations and anions concentrations is Ca2+ > Na+ > Mg2+ > K+ > Fe 3+ and SO2− > HCO3− > CO32− > Cl−, respectively. The concentration of all the cations and anions are high at close to the coal stock and gradually decreases with distance from the pile. The pH of the study area ranges from 3.41 to 7.86, where the standard pH value ranges of water are from 6.5 to 8.5. This result indicates that water closest to the coal pile is acidic, but alkaline nature exists far away from the stock pile. Various ions, namely the iron, sulphate, nitrate, phosphate, total hardness, and chloride, were found within the permissible limit of both the Bangladesh and the WHO standard value.
In case of hydrogeochemical evaluation, the Gibbs diagram states that the ratio of different ions belong to the rock-dominant region. And some ions are shifted towards the boundary between rock- and evaporation-dominant fields. It indicates the influence of rocks on the water bodies in the area. The Piper’s diagram reveals that two major and two minor hydrogeochemical facies prevail in the area, which is:
- (i)
Major facies: CaHCO3 and mixed Ca–Mg–Cl;
- (ii)
Minor facies: NaCl and CaCl.
The expanded Durov diagram implies that the majority of samples show the mixing–dissolution phase with a few in reverse ion-exchange state. These scenarios recommend that the evolution of hydrogeochemistry is habitually controlled by the carbonate rock dissolution and the weathering processes.
The average concentrations of sulphate are lower than the allowable limit of the WHO and EQS. The water samples closest to coal stock pile (sample number 1, 2, 3, and 4) show the maximum values of SO4. Interestingly, the pH value of water samples (2, 4, and others) adjacent to the coal pile as well as in the downstream area reflects an acidic water environment. This result is due to the influence of leaching and weathering processes of high sulphur coal of the stock pile. In fact, this observable fact was validated by the leaching of high sulphur coal. This experiment illustrates that the pH value of the solution reduced from 7 to 1.2 after leaching. Moreover, the interactions between rock and water, especially the chemical weathering of rocks for long time, is another source of enhancing the intensity of sulphate in the area for the present and future.
Therefore, this study suggests primarily that strong carefulness might be required to store this coal; secondly, the proper awareness and the monitoring programs might have to be taken to save the water and the environment as a whole in the area; and finally, the legal and the responsible authority might have a good environmental management plan to minimize such impacts on the green environment for the future.
References
Ahmed, S. A. et al. (2000). Arsenic: Water contamination and health hazard (pp. 7–62).
Arenas-Lago, D., Veja, F. A., Silva, L. S., & Andrade, L. (2013). Soil interaction and fractionation of added cadmium in some Galician soils. Microchemical Journal (Print),110(681), 690.
Burden, D. J. & Mazloum, S. (1965). Some chemical types of groundwater from Syria. In: UNESCO Symp. Tehran, UNESCO (pp. 73–90). Paris.
Cerqueira, B., Vega, F. A., Serra, C., Silva, L. F. O., & Andrade, M. L. (2011). Time of flight secondary ion mass spectrometry and high-resolution transmission electron microscopy/energy dispersive spectroscopy: A preliminary study of the distribution of Cu2+ and Cu2+/Pb2+ on a Bt horizon surfaces. Journal of Hazardous Materials, 195, 422–431.
Cerqueira, B., Vega, F. A., Silva, L. F. O., & Andrade, L. (2012). Effects of vegetation on chemical and mineralogical characteristics of soils developed on a decantation bank from a copper mine. Science of the Total Environment,421–422(220), 229.
Cutruneo, C. M. N. L., Oliveira, M. L. S., Ward, C. R., Hower, J. C., de Brum, I. A. S., Sampaio, C. H., et al. (2014). A mineralogical and geochemical study of three Brazilian coal cleaning rejects: Demonstration of electron beam applications. International Journal of Coal Geology,130(33), 52.
Dalai, T. K., Krishnaswami, S., & Sarin, M. M. (2002). Major ion chemistry in the headwaters of the Yamuna River system: chemical weathering, its temperature dependence and CO2 consumption in the Himalaya. Geochimica et Cosmochimica Acta,66(3397), 3416.
Davis, J. & Exley, C. (1992). Hydrochemical character of the main aquifer units of central and north-eastern Bangladesh and possible toxicity of groundwater to fish and humans. Final Report, British Geol Survey, Technical Report. WD/92/43R.
Dias, C. L., Oliveira, M. L. S., Hower, J. C., Taffarel, S. R., Kautzmann, R. M., & Silva, L. F. O. (2014). Nanominerals and ultrafine particles from coal fires from Santa Catarina, South Brazil. International Journal of Coal Geology,122(50), 60.
Durov, S. A. (1948). Natural water and graphic representation of their composition. Doklady Akademii Nauk SSSR,59(87), 90.
Finkelman, R. B., Palmer, C. A., & Krasnow, M. R. (1990). Combustion and leaching behavior of elements in the Argonne premium coal samples: Energy. Fuel,4(755), 766.
Gárfias, J., Arroyo, N., & Aravena, R. (2010). Hydrochemistry and origins of mineralized waters in the Puebla aquifer system. Mexico, Environ Earth Sci,59(1789), 1805. https://doi.org/10.1007/s12665-009-0161-y.
Ghoraba, S. M., & Khan, A. D. (2013). Hydrochemistry and groundwater quality assessment in balochistan province, pakistan. International Journal of Research and Reviews in Applied Sciences,2(17), 2–6.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. J Sci,170(1088), 1090.
Gupta, A., Saroj, K. K., & Thakur, D. N. (1977). Chemical Era,17, 238.
Gutta, D. C. (1999). Environmental aspects of selected trace elements associated with coal and natural waters of Pench Valley coalfield of India and their impact on human health. International Journal of Coal Geology,40(133), 149.
Hashan, M., Howladar, M. F., & Jahan, L. N. (2016). Sulfur concentration in imported coal around the Tamabil Area, Sylhet, Bangladesh. International Journal of Environmental Monitoring and Analysis,4(1), 27–30. https://doi.org/10.11648/j.ijema.20160401.15.
Hashan, M., Howladar, M. F., Jahan, L. N., & Deb, P. K. (2013). Ash content and its relevance with the coal grade and environment in Bangladesh. International Journal of Scientific and Engineering Research,4(4), 669–676.
Hem, J. D. (1991). Study and interpretation of the chemical characteristics of natural water: US Geol Surv Water Supply Paper-2254:264 (3rd ed.). Jodhpur: Scientific Publishers.
Hossain, G., Howladar, M. F., Nessa, L., Ahmed, S. S., & Quamruzzaman, C. (2010). Hydrochemistry and classification of groundwater resources of Ishwardi municipal area, Pabna district, Bangladesh. Geotechnical and Geological Engineering,28(5), 671–679.
Hower, J. C., O’Keefe, J. M. K., Henke, K. R., Wagner, N. J., Copley, G., Blake, D. R., et al. (2013). Gaseous emissions and sublimates from the Truman Shepherd coal fire, Floyd County, Kentucky: A re-investigation following attempted mitigation of the fire. International Journal of Coal Geology,116(63), 74.
Howladar, M. F. (2012). Coal mining impacts on water environs around the Barapukuria coal mining area, Dinajpur, Bangladesh. Environmental Earth Sciences,70, 215–226. https://doi.org/10.1007/s12665-012-2117-x.
Howladar, M. F. (2017). An Assessment of surface water chemistry with its possible sources of pollution around the Barapukuria Thermal Power Plant Impacted area, Dinajpur, Bangladesh. Groundwater for Sustainable Development,5, 38–48. https://doi.org/10.1016/j.gsd.2017.03.004.
Howladar, M. F., Deb, P. K., & Muzemder, A. T. M. S. H. (2017a). Monitoring the underground roadway water quantity and quality for irrigation use around the Barapukuria Coal Mining Industry, Dinajpur, Bangladesh. Groundwater for Sustainable Development,4, 23–34. https://doi.org/10.1016/j.gsd.2016.11.002.
Howladar, M. F., Deb, P. K., Muzemder, A. T. M. S. H., & Ahmed, M. (2014). Evaluation of Water Resources around Barapukuria Coal Mine Industrial Area, Dinajpur, Bangladesh. Applied Water Sciences. https://doi.org/10.1007/s13201-014-0207-5.
Howladar, M. F., Hasan, M. M., Islam, S., Shine, F. M. M., & Quamruzzaman, C. (2013). Gas blowout impacts on ground water environs around the tengratila gas field, Chattak, Bangladesh. Journal of Water Resources and Protection,5(164), 170. https://doi.org/10.4236/jwarp.2013.52018.
Howladar, M. F., Hashan, M., Rahman, M. M., Numanbakth, A. A., & Sohail, M. A. (2018). The quality of imported coal and its impact on environmental degradation. Environment, Development and Sustainability. https://doi.org/10.1007/s10668-018-0197-9.
Howladar, M. F., & Islam, R. (2016). A study on physico–chemical properties and uses of coal ash of Barapukuria coal fired thermal power plant, Dinajpur for Environmental Sustainability. Energy, Ecology and Environment,0, 1–15. https://doi.org/10.1007/s40974-016.0022-y.
Howladar, M. F., Numanbakth, M. A. A., & Faruque, M. O. (2017b). An application of water quality index (WQI) and multivariate statistics to evaluate water quality around maddhapara granite mining industrial area, Dinajpur, Bangladesh. Environmental Systems Research,6, 13. https://doi.org/10.1186/s40068-017-0090-9.
Howladar, M. F., & Rahman, M. M. (2016). Characterization of underground tunnel water hydrochemical system and uses through multivariate statistical methods: a case study from Maddhapara Granite Mine, Dinajpur, Bangladesh. Environmental Earth Sciences,75, 1501. https://doi.org/10.1007/s12665-016-6309-7.
Imam, B. (2005). Energy resources of Bangladesh (p. 277). Dhaka: University Grants Commission of Bangladesh.
Jang, Y., Townsend, T., Ward, M., & Bitton, G. (2002). Leaching of arsenic, chromium, and copper in a contaminated soil at a wood preserving site. Bulletin of Environment Contamination and Toxicology,69(808), 816.
Kumar, P. J. S. (2013). Interpretation of groundwater chemistry using piper and chadhás diagrams: a comparative study from perambalur taluk Brandenburgische Technische Universität, Cottbus-03046, Germany. Elixir Geoscience,54(12208), 12211.
Kumar, M., Kumari, K., Kumar Singh, U., & Ramanathan, A. L. (2009). Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environmental Geology,57(873), 884. https://doi.org/10.1007/s00254-008-1367-0.
Kumar Srivastava, S., & Ramanathan, A. L. (2008). Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environmental Geology,53(1509), 1528. https://doi.org/10.1007/s00254-007-0762-2.
Martinello, K., Oliveira, M. L. S., Molossi, F. A., Ramos, C. G., Teixeira, E. C., Kautzmann, R. M., et al. (2014). Direct identification of hazardous elements in ultra-fine and nanominerals from coal fly ash produced during diesel co-firing. Science of the Total Environment,470–471(444), 452.
Piper, A. M. (1953). A graphic procedure in the geochemical interpretation of water analyses. Am Geophys Union Trans,25(914), 923.
Pronab, K. B., & Mrinal, K. B. (1996). Sulphur in Assam coal. Journal of Fuel Processing Technology,46(83), 97.
Ram, L. C., & Masto, R. E. (2010). An appraisal of the potential use of fly ash for reclaiming coal mine spoil. Journal of Environmental Management,91(3), 603–617.
Ramos, C. G., Querol, X., Oliveira, M. L. S., Pires, K., Kautzmann, R. M., & Oliveira, L. F. S. (2015). A preliminary evaluation of volcanic rock powder for application in agriculture as soil a remineralizer. Science of the Total Environment,512–513(371), 380.
Rao, N. S. (2007). Groundwater quality as a factor for identification of recharge zones. Environmental Geosciences,14(79), 90.
Rao, N. S., & Rao, P. S. (2009). Major ion chemistry of groundwater in a river basin: A study from India. Environmental Earth Sciences. https://doi.org/10.1007/s12665-009-0389-6.
Reddy, A. G. S. (2012). Evaluation of hydrogeochemical characteristics of phreatic alluvial aquifers in southeastern coastal belt of Prakasam district, South India. Environmental Earth Sciences. https://doi.org/10.1007/s12665-012-1752-6.
Ribeiro, J., Taffarel, S. R., Sampaio, C. H., Flores, D., & Silva, L. F. O. (2013). Mineral speciation and fate of some hazardous contaminants in coal waste pile from anthracite mining in Portugal. International Journal of Coal Geology,109–110(15), 23.
Safiullah, S., Khan, M. R. R., & Sabur, M. A. (2011). Comparative study of Bangladesh Barapukuria coal with those of various other countries. Journal of the Bangladesh Chemical Society,24(2), 221–225.
Sanchís, J., Oliveira, L. F. S., De Leão, F. B., Farrè, M., & Barcelò, D. (2015). Liquid chromatography-atmospheric pressure photoionization-Orbitrap analysis of fullerene aggregates on surface soils and river sediments from Santa Catarina (Brazil). Science of the Total Environment,505(172), 179.
Shaha, P. (2014). Geological field report on Jaintapur-tamabil Area, Sylhet. Department of Geology, University of Dhaka. http://www.scribd.com/doc/230841345/Geological-Field-Report-on-Jaintiapur-Tamabil-Area-Sylhet#scribd). Accessed 15 Jan 2018.
Shamsudduha, M., Chandler, R. E., Taylor, R. G., & Ahmed, K. M. (2009). Recent trends in groundwater levels in a highly seasonal hydrological system: the Ganges-Brahmaputra-Meghna Delta. Hydrology and Earth System Sciences,13(2373), 2385.
Silva, L. F. O., Sampaio, C. H., Guedes, A., Fdez-Ortiz de Vallejuelo, S., & Madariaga, J. M. (2012). Multianalytical approaches to the characterisation of minerals associated with coals and the diagnosis of their potential risk by using combined instrumental microspectroscopic techniques and thermodynamic speciation. Fuel,94(52), 63.
Subramani, T., Elango, L., & Damodarasamy, S. R. (2005). Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environmental Geology,24(194), 202.
Tezza, V. B., Scarpato, M., Oliveira, L. F. S., & Bernardin, A. M. (2015). Effect of firing temperature on the photocatalytic activity of anatase ceramic glazes. Powder Technology,276(60), 65.
Tiwary, R. K. (2001). Environmental impact of coal mining on water regime and its management. Water, Air, and Soil Pollution,132(1-2), 185–199. https://doi.org/10.1023/a:1012083519667.
Van der Sloot, H., Heasman, L., & Quevauiller, P. (1997). Harmonization of leaching/extraction tests. Amsterdam: Elsevier.
Ward, C. R. (1984). Coal geology and coal technology (p. 345). Oxford: Blackwell Scientific Publication.
WHO. (2011). Guidelines for drinking water quality, 4th edn. In Org World Health Organization, Geneva.
Zhou, Y., Wang, Y., Li, Y., Zwahlen, F. O., & Boillat, J. (2012). Hydrogeochemical characteristics of central Jianghan Plain, China. Environmental Earth Sciences. https://doi.org/10.1007/s12665-012-1778-9.
Acknowledgements
The authors would like to thank the Ministry of Education and Ministry of Science and Technology, Bangladesh, for the partial financial support to the successful completion of the research work; otherwise it was beyond our reach.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Howladar, M.F., Hossen, M., Jahan, L.N. et al. Hydrogeochemistry and sulphate contamination of water environment around the coal stock pile in Tamabil, Sylhet. Environ Dev Sustain 22, 3599–3619 (2020). https://doi.org/10.1007/s10668-019-00358-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-019-00358-9