Abstract
Landfill leachate has an adverse impact on groundwater quality as well as on living being. It contain high levels of organic, inorganic, heavy metal, and xenobiotics, which percolates through the subsoil and contaminate the groundwater. To assess the effect of landfills on groundwater, various physicochemical parameters including heavy metals, and microbiological examination of leachate and groundwater samples was conducted. The results obtained were compared with Bureau of India Standards and World Health Organization guidelines. The results of the study shows that the majority of the sample do not lie in the permissible limits. According to the results, the concenteration of ammoniacal nitrogen (9.8 mg/L), chemical oxygen demand (128 mg/L), chloride (115 mg/L), sodium (98 mg/L) and potassium (42.2 mg/L) was found relatively higher in water samples that have lower depth (30 ft) and distance (1 km) from the landfill. The concentration of measured parameters decreases with increase in depth and distance confirming that the leachate is the potential source of groundwater contamination. Hazard index of Chandigarh, Mohali, and Panchkula landfill site was 0.61, 0.53, and 0.01 mg/kg/day in pre-monsoon and 0.38, 0.24, and 0.01 mg/kg/day in post-monsoon indicating non-carcinogenic health risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water is important for sustaining life, growth, and the environment. It is one of the key determinants of economic development and social prosperity of a nation (Sarvajayakesavalu et al. 2018). It can be obtained from two different sources, i.e., surface water and groundwater, but both the sources no longer exist in pure form (Siraj and Kitte 2013). Last few decades have witnessed the threats to the sustainability of clean water resources due to rapid urbanization, increasing irrigation demand, addition of contaminates, and climate change impact (Shivaprasad et al. 2014). Uneven distribution of surface water and increasing demand of safe water lead to excess use of groundwater. Approximately 2 billion people and about one-third of the world’s population rely upon groundwater (UNESCO 2012). In a developing country like India, about 59% of the irrigation and 80% of the potable water supplies are dependent on the groundwater (CGWB 2017). In spite of high enslavement of underground water, many instances of groundwater contamination have increased from a variety of sources such as infiltration of irrigation water, septic tank, spillages, disposal of industrial effluent, and leaching of intricate parameters from dumpsites (Rajasekhar et al. 2018).
Leaching of toxic chemicals from unlined landfill site is one of the major environment impacts in most of the developing countries like India (Mor et al. 2006a). Most of the landfills not follow the principle of sanitary landfill (leachate treatment and gas collection system) and thus associated with surface and groundwater contamination (Mangimbulude et al. 2009). The leachate generated from the landfill takes up organic, inorganic, COD, BOD, xenobiotics, and heavy metals as a constitute by means of physical, chemical, and fermentative processes (Yaqout-Al and Hamoda 2003). Further, constituent of leachate from uncontrolled dumpsite percolate from subsoil and make groundwater unfit for human use. Interaction between unconfined aquifer with open dump and its effect on the hydrogeological chemistry of groundwater was reported by many studies (Mor et al. 2018; Alexandre Bogas and Gomes 2015; Magombeyi and Nyengera 2012; Raju 2012; Akinbile et al. 2011; Mor et al. 2006b). Once the groundwater becomes polluted, contamination persists and becomes difficult to treat due to physical inaccessibility, long residence times, and large storage (Wang et al. 2012).
According to WHO, about 80% of all the diseases in mankind are caused by polluted water (Rao et al. 2013). The leachate mixed with the unconfined aquifer forms a plume that can extend up to several hundred of meters and affect the hydro geological system of aquifer (Al-Tarazi et al. 2008; Mor et al. 2016). Multiple indicators, including major hydrochemistry, stable isotope (δ2H), and tracer element (ammoniacal nitrogen, chloride, sodium, potassium) can be used to understand the movement of contaminated plume. Nagarajan et al. (2012) reported the high percentage of the trace elements such as chloride and ammoniacal nitrogen in the groundwater found near the Semur landfill site. Maiti et al. (2016) conducted a study in the Dhapa landfill site (Kolkata) to access the impact on health due to leachate plume and reported health problem such as diarrhea, vomiting, abdominal pain, hepatitis, and dysentery in the population residing adjacent to Dhapa landfill site. Similar observations were also reported by Ravindra et al. (2016) in MSW workers in Chandigarh, India.
Availability of safe water quality is a vital concern for the mankind as it is directly related to the human health condition. Thus, effectively preventing or reducing ground pollution is necessary for the growth and sustainability (Ravindra et al. 2015; Kaur et al. 2015). Considering this, the study aim to assess the environmental impact of the leachate on underground water resources in the surrounding area of landfill sites in tricity (Chandigarh, Mohali, and Panchkula).
2 Materials and method
2.1 Study area
The study was conducted at the three landfill sites located in Chandigarh, Panchkula, and Mohali (Sahibzada Ajit Singh Nagar) as shown in supplementary data Fig. 1 and known as tricity. Chandigarh and Mohali landfill sites are located in low lying semiarid area in Dadumajra and near the industrial area (sector 74), respectively and both sites are also surrounded by agriculture fields and residential areas. On the other hand, the Panchkula landfill site is located in moderator steep semiarid area in sector 23 across the Ghaggar River near the National Highway with coordinats 73 30°40′54.18″N, 76°52′50.19″E and 30°40′44.73″N, 76°52′56.06″E. Chandigarh (Dadumajra) landfill site is 30 years old and stretched up to an area of 45 acres, whereas Mohali and Panchkula are 17 and 15 years old, respectively, and covers 15 acres and 10 acre area, respectively. Chandigarh city generates 450 TPD (ton per day) of waste and dump site is located near the village Dadumajra site and lies between north latitudes 30°43′16.55″N, 76°41′21.43″E and 30°43′12.66″N, 76°41′24.49″E. The residents nearby area of the landfill is highly dependent on the groundwater for meeting their daily needs and draw either through hand pumps or bore wells. Residential area and agricultural land are located in its vicinity and a seasonal rivulet (Patiala-Ki- Rao) is flowing near the dumpsite. Mohali landfill lies 30°43′16.55″N, 76°41′21.43″E and 30°43′12.66″N, 76°41′24.49″E. Mohali city generates approximately 130 TPD of waste and is in close vicinity to the industrial unit, slum and agriculture field and wetland (marshy). A wetland near landfill site receives leachate. A slum and agriculture field in the vicinity of Mohali landfill site use groundwater for their daily needs and irrigation. Mohali and Chandigarh predominated by alluvial soil with shallow aquifer. In contrast, soil in the Panchkula is loam with deep aquifer. Panchkula city generates around 100 TPD ton of waste.
2.2 Sample collection
The groundwater samples were collected from vicinity area of the landfill during pre-monsoon (May 2014) and post-monsoon (October 2014) in tricity. Garmin (Montana 650) Global Position System (GPS) was used to locate the sampling points. Leachate sample of tricity were collected from the base of the waste heap. Latitude and longitude of the sampling sites of Chandigarh, Mohali, and Panchkula are shown in supplementary data (Table S1). Total 140 samples were collected from the study area and include 18 leachate samples and 122 water samples during pre-monsoon as well as in post-monsoon. A 100-mL microbiological bottle and 2-L plastic bottles were used to collect the water sample for microbiological and physicochemical analysis. Further, 50-mL bottles were used to collect samples for heavy metals. To reduce error involved during collection of samples, the water source (tube well, submersible pump, and hand pump) was pumped initially for 2–3 min, and bottles were rinsed thoroughly. 1% sodium thiosulfate was added in the 100-mL microbiological bottle before collecting the microbiological sample. The samples of heavy metal were preserved by adding few drops of H2SO4.
2.3 Analytical method
After the sampling, the collected samples were stored at 4 °C until analysis. Different analytic methods were adopted for the analysis of physicochemical, microbiological, and heavy metals. Some parameter like, temperature, electrical conductivity (EC), pH, and total dissolve solids (TDS) content were recorded at the time of sampling by digital water sampling kit (Elico digtal meter). Chemical Oxygen Demand (COD) was carried out using reflux titrimetry method. Calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), carbonate (CO32−), bicarbonate (HCO3−), total alkalinity (TA), and total hardness (TH) were analyzed by titrimetry. Phosphate (PO42−), sulfate (SO42−), nitrate (NO32−), ammoniacal nitrogen (NH4+-N) measured by UV–visible spectrophotometer (Shimadzu1800) method, and fluoride (F−) by SPADNS method. Flame photometer (systronic flame photometer, 128µc) was used to determined sodium (Na+) and potassium (K+). Biochemical oxygen demand (BOD) was calculated by Winkler titration method. Microbiology parameter was done by the most probable number (MPN) method with the help of laminar flow (Microfilt). Heavy metals like lead (Pb2+), chromium,(Cr IV, VI), copper (Cu2+), zinc (Zn2+), cadmium (Cd2+), iron (Fe2−) were estimated by atomic absorption spectrophotometer (Contra AA700, Analytic Jena AG, Germany).
2.4 Human health risk assessment
It is the process of estimating the detrimental health consequences of human exposure to environment hazard. In the present study, health risk assessment was done using EPA (2014) methodology including three steps
- 1.
Hazard identification
- 2.
Exposure assessment
- 3.
Dose response
2.4.1 Hazard identification
It is the initial step in exposure assessment. The population living in vicinity to the landfill site is exposed to hazard directly with ingestion of contaminated dust, foul smell, and indirectly by the drinking of groundwater. Most of the population depends on the groundwater for their daily needs. To exemplify the health hazard link with groundwater contamination, different parameters were measured such as heavy metal (Pb2+, Zn2+, Cu2+). For this, water sample were collected from nearby landfill sites and computed values were used to illustrate the health effect due to leachate. Information related to socioeconomic characteristics and population structure was also collected.
2.4.2 Exposure assessment
It estimates at what extent the population around the landfill is exposed to copper, lead and zinc through drinking water. Exposure assessment depends on the following factors.
2.4.2.1 Exposure time
The exposure time of the pollutant around the vicinity of the landfill site can be calculated by taking variation in time from the setting up of landfill site to the present, considering the population remains static (Bhupander and Mukherjee 2011). In this case, exposure frequency is 30 year (10,950 days) taken for Chandigarh, 17 years (6205 days) for Mohali, and 15 years (5475 days) for Panchkula.
2.4.2.2 Body weight
The average body weight, i.e., 56 kg of the Indian adult, was considered to calculate health risk as suggested by Shukla et al. (2002).
2.4.2.3 Water ingestion rate
According to the EPA (1996) Exposure Factor Handbook, water intake is 3 L/day in temperate climates and around 6–11 L in the tropical climate.
2.4.3 Average daily dose (ADD)
The assessment of frequency and duration of human exposure of lead, copper, zinc, and ammoniacal nitrogen with the help of average daily dose was calculated as:
where Ci represent the average value of pollutant, IR represent the rate of ingestion (L/day) whereas exposure duration (ED) in days/year and exposure frequency (EF) of hazard shows in days/year. Body weight (BW) of person express in kg and average time (AT) is in years.
The health risk due to consumption of leachate-effected water was evaluate by hazard quotient
where Rfd represent the reference dose obtained from HEAST (Health Effects Assessment Summary Tables) for Zn2+, Pb2+ and Cu2+ as 0.3 mg/kg, 3.5 × 10−3 mg/kg and 4 × 10−2 mg/kg, respectively (EPA 2005). If HQ more than 1, it indicates high risk of adverse non-carcinogen health effect; however, if HQ less than 1, it indicate acceptable risk (Sun et al. 2016).
The hazard index (HI) was calculated using Eq. 2
3 Result and discussion
3.1 Leachate characterization
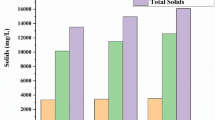
Leachate characteristics and rate of leachate generation depend upon various factors like waste composition, age of landfill, moisture content, ambient temperature, and available oxygen. The physiochemical analysis of tricity landfills leachate is given in Table 1. Leachate sample collected from tricity landfill site showed elevated levels of EC and TDS due to occurrence of high dissolved salt and minerals. Average range of EC and TDS was found 35,633 µohms−cm, 22,805 mg/L in Chandigarh, whereas 18,159 µohms−cm, 11,621, and 11,346 µohms−cm, 7216 mg/L in Mohali and Panchkula, respectively, during pre-monsoon. On the other hand, EC and TDS in post-monsoon in Chandigarh (12,263 µohms−cm, 12,392 mg/L), Mohali (12,263 µohms−cm, 6524 mg/L), and Panchkula (10,190 µohms−cm, 6524 mg/L) were lower than that of pre-monsoon. High values of EC and TDS in pre-monsoon than post-monsoon depict leachate plume diluted during rainy season. Result of present study are in agreement with the result obtained by Woldeyohans et al. (2014) reported 20,000 mg/L and 19,000 mg/L TDS in pre-monsoon and post-monsoon, respectively.
Concentration of COD, BOD, pH, and ammoniacal nitrogen in leachate provides the information regarding the biodegradability of a waste as well as stages of leachate (Bhatt et al. 2016). pH of tricity leachate ranges 7.7–7.8 and 6.5–6.7 in Chandigarh landfill site, 6.9–7 and 6.4–6.8 in Mohali landfill site, and 6.6–6.9 and 6.4–6.8 in Panchkula landfill site during pre- and post-monsoon repectively. COD value of Chandigarh landfill site, Mohali landfill site, and Panchkula landfill site during pre-monsoon varied from 14,560–17,920 mg/L, 5600–8960 mg/L, 8800–12000 mg/L, 4800–7200 mg/L, and post-monsoon 6500–8800 mg/L, 2880–5600 mg/L, respectively.
Ammoniacal nitrogen is one of the best parameters to reveal leachate contamination potential. Ammoniacal nitrogen was found highest in Mohali landfill site 2067–2198 mg/L, 1300–1920 mg/L followed by Chandigarh landfill site 1899–3750 mg/L, 1960–2150 mg/L, and Panchkula landfill site 1530–1940 mg/L, 1420–1670 mg/L in pre- and post-monsoon. Results of COD, ammoniacal nitrogen, and pH represent leachate lies in the intermediate stage (Renou et al. 2008).
Total hardness and total alkalinity were very high in Chandigarh 11,760–13,800 mg/L, 3900–6900 mg/L followed by Mohali 6400–8600 mg/L, 2520–5600 mg/L, and Panchkula 1980–21,650 mg/L, 987–1950 mg/L during pre-monsoon as compared to post-monsoon where concentration of above-mentioned parameters varies from 2000–4720 mg/L, 1800–3360 mg/L in Chandigarh landfill site 7700–9800 mg/L, 2100–3800 mg/L in Mohali landfill site, 700–1320 mg/L, 1400–1760 mg/L in Panchkula landfill site respectively. Alkalinity of leachate in tricity was found significantly elevated due to biochemical decomposition as well as dissolution process occurring within the waste heap in the landfill site. The biodegradation of the organic matter produce huge load of bicarbonate, which indicate the presence of dissolved carbon dioxide which is also the key constituent of alkalinity as reported by Naveen et al. (2017).
Sodium and potassium not affected by the microbial degradation and often considered as a tracer for water pollution. The occurring of these ions in the leachate is a result of decomposition of vegetables and fruits residue present in the waste heap of the landfill. Sodium and potassium in Chandigarh, Mohali, and Panchkula were in the range of 1540–2006 mg/L, and 630–917 mg/L; 950–1450 mg/L, and 560–755 mg/L; 825–980 mg/L, and 375–760 mg/L, respectively, in pre-monsoon. Likewise, sodium and potassium during post-monsoon were 600–986 mg/L, 500–875 mg/L; 810–986 mg/L, 250–579 mg/L; 450–611 mg/L, 250–408 mg/L in Chandigarh, Mohali, and Panchkula.
Chlorine concentration was noticeably high in tricity landfills, and this was mainly due to dumping of alloy and industrial waste (Alexandre Bogas and Gomes 2015). Chloride values of tricity (Chandigarh, Mohali and Panchkula) ranges from 4898–6997 mg/L, 1499–3198 mg/L; 2799–3498 mg/L, 1799–3498 mg/L; 1052–1399 mg/L, 599–949 mg/L during pre-monsoon and post-monsoon respectively. Sulfate in Chandigarh landfill site ranges from 1461–2126 mg/L in pre-monsoon and 676–1144 mg/L in post-monsoon, whereas in Mohali landfill site and Panchkula landfill site, it ranges from 967–1498 mg/L, 876–1892 mg/L; 700–778 mg/L, 364–745 mg/L in pre- and post-monsoon. Similarly, average value of sulfate 118 mg/L and 65 mg/L in pre- and post-monsoon was recorded by (Aluko et al. 2003) in Ibadan landfill. Phosphate degraded in the early stage of landfill and concentration decreased in the intermediate stage as reported by Khai (2012). Phosphate was found quite low in leachate and varies 16–122 mg/L, 16–31 mg/L; 16.64–31.55 mg/L, 10–17 mg/L; 23–25 mg/L, 10–18 mg/L in Chandigarh landfill site, Mohali landfill site, and Panchkula landfill site in pre-monsoon and post-monsoon.
Metal ions generally low in leachate of municipal landfill as compared to secure landfill side but the dumping of used batteries, switches, and paint container were the reason behind these metal ions (Abdul et al. 2014). Concentrations of metal ions were not high in tricity landfill leachate. Lead (0.03–0.83, 0.09–0.48 mg/L), copper 1.22–5.01, 0.16–1.6 mg/L, zinc (7.89–10.1, 3.03–6.01 mg/L), and cadmium (0.03–1.06, 0.01–0.04 mg/L) in Mohali landfill site during pre- and post-monsoon respectively were higher than Chandigarh and Panchkula landfill site. Similarly, zinc (7.72–10.21 mg/L, 3.1–5.42 mg/L) was higher than lead (0.06–0.29 mg/L, 0.03–0.09 mg/L) followed by copper (2.05–4.01 mg/L, 0.02–1.05 mg/L) and cadmium (0.03–1.07–0.001–0.04 mg/L) in Chandigarh landfill site during pre- and post-monsoon. However, cadmium was absent in Panchkula landfill site and lead, copper, and zinc were 0.04–0.12, 0.02–0.05 mg/L; (0.08–1.94, 0.01–0.92 mg/L, and 1.02–4.94, 0.04–0.32 mg/L during pre-monsoon and post-monsoon, respectively. The various concentration of heavy metal in tricity indicates characteristics of intermediate leachate (Renou et al. 2008).
3.2 Physiochemical characteristic of groundwater
Groundwater sample was collected in pre-monsoon (June) and post-monsoon (October) to quantify the contamination of leachate. Variation in the concentration of pH, EC, TDS, total alkalinity, total hardness, calcium, magnesium, COD and BOD shown in Fig. 1. pH in pre-monsoon varies 6.8–7.7 and 7.1–7.4 in post-monsoon. All the samples were within the permissible limit 6.5–8.5 (BIS 2012) except S2, S22 which was found to be slightly acidic. Acidic nature of water represents the dissolution of carbon dioxide and organic acid (fulvic and humic acid) obtained from decaying and leaching of plant material and organic material from landfill (Khound et al. 2012).
EC of water samples during pre-monsoon ranges from 220 to 1550 µmhos/cm and during post-monsoon 136–1039 µmhos/cm reflecting fresh to moderately saline. High EC in sample S1 in close vicinity of Chandigarh landfill site and Sample 23 in close vicinity of Mohali landfill site may be due to percolation of leachate in groundwater. Elevated value of EC 2300–1400 µmhos/cm in groundwater sample collected from vicinity of Kandy landfill site was reported by Nirmla and Jagath (2013).
TDS in pre-monsoon varies from 180 to 679 mg/L and in post-monsoon 161–664 mg/L. Near about 50% sample of water have concentration above desirable limit, i.e., 500 mg/L (according to BIS 2012). Abd El-Salam and Abu-Zuid (2014) reported high value of TDS in groundwater ranges from 2855–16,276 mg/L due to leachate contamination. Groundwater possesses inorganic and organic compounds in small amount.
Total alkalinity of water ranges 28–372 mg/L in pre-monsoon, 24–148 mg/L during post-monsoon period. All the samples were below the permissible limit, i.e., 600 mg/L. Likewise total hardness in tricity groundwater varies 36–464 mg/L during pre-monsoon and 64–456 mg/L during post-monsoon period. Total hardness of groundwater higher then alkalinity indicates non-carbonate hardness in groundwater (Bikundia and Mohan 2014). Calcium and magnesium range from 14,120 mg/L; 8–77 mg/L in pre-monsoon 16–83 mg/L; 10–64 mg/L in post-monsoon. Nearly about 40% of sample are above the desirable limits 200 mg/L BIS (2012). Comparison of physicochemical, microbiological, and heavy metal with BIS (2012) and WHO (2005) shown in supplementary Table S2.
COD in nearby groundwater sample of tricity landfill varies ND (non-detected) − 128 mg/L and ND-80 mg/L during pre- and post-monsoon sampling. Highest COD value was found in S1 (64 mg/L), S6 (32 mg/L), S 40 (128 mg/L), S 38 (96 mg/L), and S 22 (80 mg/L) and are located in close vicinity of the landfill sites. Sample S 40, S 38 is in the Ambedkar colony which is adjacent to the wetland and Mohali landfill sites. Wetland receives leachate from the Mohali landfill site and domestic effluent from the Ambedkar colony. This attributes to high value of COD in both samples. As wetland plays a very crucial role in the groundwater recharge, if the wetland is contaminated due to anthropological activities, it is the possible chances to contaminate the groundwater also. BOD of groundwater ranges ND— 24 mg/L in pre-monsoon and ND—12 mg/L in post-monsoon. All samples were safe from BOD contamination except S1, S15, S22, and S39. An S39 sample is in Ambedkar colony, whereas S22 is within the landfill site. The presence of BOD contamination in these two samples may be due to presence of contaminate in a leachate and domestic effluent.
3.3 Cation chemistry
Concentrations of ammoniacal nitrogen in pre-monsoon ranges 0.4–9.8 mg/L and 0.09–6.8 mg/L in post-monsoon. Around 50% of water sample near Chandigarh landfill site has ammoniacal nitrogen above the permissible limit, i.e., 0.5 mg/L BIS (2012). Highest value of ammoniacal nitrogen, i.e., 11 mg/L was in S22 which was found near to Mohali landfill site. Same result with ammoniacal nitrogen, i.e., 11.61 mg/L in vicinity area of Malaysia landfill site has been reported by Abd El-Salam and Abu-Zuid (2014). It clearly indicates that landfill was potential source of water contamination. Ammoniacal nitrogen is a stable ion and act as a trace element of landfill leachate (Mor et al. 2006). Sodium and potassium are the important minerals occurring in nature. High value of both elements in groundwater acts as indicator of landfill pollution, as sodium and potassium are not affected by the microbiological activity within the waste heap of landfill site and enter into groundwater (Hanipha and Zahir Hussain 2013). Sodium and potassium in groundwater varies 39–74 mg/L: 17–40 mg/L in pre-monsoon and 19–53 mg/L: 12–43 mg/L post-monsoon.
3.4 Anion chemistry
Sulfate concentration in pre-monsoon ranges 3.4–57 mg/L and in post-monsoon range 1.88–40.4 m g/L. Sulfate concentration of groundwater was below the permissible limit, i.e., 250 mg/L BIS (2012). This attributes to the presence of sulfate-reducing bacteria in soil or groundwater that competes with methane-producing microbes for the available organic carbon, resulting in the formation of hydrogen sulfide by reduction of sulfates into sulfides (Maiti et al. 2016). In present study, chloride varies 15–115 mg/L in pre-monsoon and 9–67 mg/L in post-monsoon, indicates that the groundwater quality was enormously affected by the migrated leachate from the landfill site. Haarstad and Mæhlum (2007) suggested chloride ion is not debilitated by biochemical as well as geological process and forms a strong contaminated plume which contaminates water sources. High chloride concentration was found in groundwater sample within the 75 m of Bhalaswa landfill site (Jhamnani and Singh 2009). Fluoride has permissible limit 1.5 mg/L (BIS 2012) and all the groundwater samples were found within the limit. Fluoride ranges ND—1.38 mg/L during pre-monsoon and ND—0.07 mg/L during post-monsoon. Phosphate and nitrate can act as nutrients for the microorganism but excess of these in drinking water affect the water quality and make unfit for its user (Wang et al. 2016; Wang et al. 2018). However, both were within the limit and not detected. Phosphate concentration in pre-monsoon ranges from 0.01 to 0.29 mg/L and during post-monsoon 0.001–0.21 mg/L, while nitrate in water during pre-monsoon varies from ND—3.14 mg/L and ND—3.01 mg/L in post-monsoon. Excess of nitrogen in blood leads to methemoglobinemia like condition in cell by reducing the oxygen-binding capacity of hemoglobin (Sadeq et al. 2008). Concentration of ammoniacal nitrogen, sodium, potassium, chloride, sulfate, phosphate, fluoride, and nitrate are shown in Fig. 2.
3.5 Heavy metals
Macroelements such as lead, zinc, copper in pre-monsoon range ND-0.007 mg/L; ND-4.037 mg/L; ND-0.27 mg/L, respectively, and ND-0.005 mg/L; ND − 0.4 mg/L; ND-0.07 mg/L in post-monsoon. Metal percolation in groundwater depends on soil adsorption ability, rate of reaction, and groundwater movement (Behbahaninia et al. 2010). Metals concentration in groundwater was below the detection limit, i.e., copper 1.5 mg/L, lead 0.01 mg/L and zinc 15 mg/L (Fig. 3). Lower value of heavy metals detected in the water source was mainly due to adsorption of metal on soil strata or by the organic matter in the soil (Nagarajan et al. 2012).
3.6 Microbiological analysis
Apart from physicochemical parameters pathological bacteria like E.coli, Pseudomonas aeruginosa, Enterococci, Salmonella are also predominate in the leachate and can percolate in nearby water source (Kalčíková et al. 2014). 122 groundwater samples were analyzed and compared with Mac Cardy’s. Ideally, total coliform should not be present in the groundwater sample, as it is the indicator of organic pollutant. Total coliforms were calculated from MacCardy’s table, and bacteriological results of water sample are shown in Table 2. Bacteriological result of groundwater sample represents high pathological contamination with varying percentage indicates human and animal waste intrusion. Result obtained shows that 40.6% samples in pre-monsoon were unsatisfactory and unfit for human consumption. High percentage (52.2%) of groundwater samples was found unsatisfactory in post-monsoon due to discharge of animal feces and domestic waste and rain water. Elevated value of fecal coliform in post-monsoon is as a result of rise in water table which leads to aerobic conditions and thus increasing the survival rate of the bacteria as reported Pujari et al. (2011). Groundwater samples S39 and S38 collected from the close vicinity of the Mohali landfill and wetland (marshy) shows high organic contamination. This was mainly due to open defecation around the wet land, open drain as well as the landfill leachate causing the infiltration of the pathologic contamination through subsoil. Most of the samples that were located in the close vicinity of the Panchkula landfill show no organic contamination because of deep aquifer, which some extent act as barrier for groundwater contamination.
3.7 Statistical analysis
3.7.1 Hydrochemical facies of groundwater
Piper (1953) classified the alkali and alkaline earth metal of ground with reference to hydro chemical processes with the help of piper diagram. Piper diagram of pre- and post-monsoon is shown in Fig. 4. Piper plot include 2 triangle, one represents cation other anion. The combination of anion and cation shows a single diamond shape (Tank and Chandel 2010). Diamond facies of pre-monsoon sample represents strong acid (So2−4 + Cl−) and weak acid (CO32− + HCO32−) and cation triangle represents (Ca2+ + Mg2+) exceed then sodium potassium. No significant change in the pre-monsoon and post-monsoon piper diagram was observed. Results of present study are in agreement with the result obtained by the Sadashivaiah et al. (2008) who reported dominance of sulfate and chloride and calcium and magnesium hardness in drinking water in Karnataka.
3.7.2 Correlation
Correlation measures the association between the two variables. Correlation shows positive and negative as well as strong association among the variables. In pre-monsoon pH has shown strong correlation with NH4+ and Cu2+ and negative correlation with total hardness, cations, anions, total alkalinity, heavy metal, whereas EC shows strong correlation with TDS, TH, Mg2+ and Ca2+. Further TH, Mg2+, Ca2+, TA, Cl−, SO42− show good correlation with each other. Correlation matrix of pre-monsoon and post-monsoon is shown in supplementary Tables 3 and 4. EC depends on TDS, because it mainly constitutes of TDS in water which is ionic in nature. Cl− shows good relation with Na+ which represents anthropological source of contamination. EC shows controlled correlation with Cu2+ which represents that these ions to some extent associate with the ion–exchange mechanism in aquifers (Srivastava and Ramanathan 2008). EC–TDS, TH-Ca2+, Mg2+–Ca2+, Na+–SO42−, TA-Cl− show significant correlated pairs which represent that all of them have same origin (Bikundia and Mohan 2014). In post-monsoon major cations, calcium, magnesium, sodium, and potassium were positively correlated with total alkalinity, pH, chloride, total dissolved solid, and ammoniacal nitrogen (Aboyeji and Eigbokhan 2016).
3.8 Effect of depth, age and distance on groundwater contamination
Groundwater contamination due to migration of the leachate from the unlined dumping site is affected by various parameters such as chemical composition of leachate, depth, distance as well as the age of the water source. Further, concentration of Cl−, NH4+, COD, Na+ and K+ was considered to estimate the extent of contamination in terms of age, radial distance, and depth. Table 3 evidently shows the trends of each parameter at various depth, age and distance. These trends indicate the vulnerability of shallow aquifer for the ionic as well as organic leachate pollutant. As the depth of the water sample increases, the value of Cl−, NH4+, and COD found to be decreases. In addition, variation of different parameter with age and distance was also estimated to assess the groundwater quality. Result inferred that groundwater located in between 0 and 1 km from the landfill exhibits high ionic composition of Cl−, NH4+, and COD as compared to the sample located within 1–2 and 2–3 km, and confirmed the contamination produced by the occurrence of leachate. In contrast to this, Na+ and K+ show high concentration within the distance of 2–3 km and age of 10–20 years. This may be linked to the percolation of fertilizer residues from the near by agriculture field. In agreement with present study, Reyes-lópez et al. (2008) reported value of the Na+ and K+ reach 3500 mg/L in the groundwater sample located within 20 m from the Guadalupe Victoria landfill.
The study found that groundwater sample close to the Mohali and Chandigarh landfill site shows an elevated value of tracer element at a different depth, age, and radial distance as compared to the water sample collected near Panchkula landfill site. This may be due to presence of the shallow water table and fracture rock aquifer in Mohali and Dadumajra that allow the leachate plume to move more easily through these rocks and help to enter into unconfined aquifer (Shivendra and Ramaraju 2015). The risk of pollution highly depends on the nature of the geological strata and thickness of the unsaturated zone. Mohali and Chandigarh mainly dominated by alluvial soil and aquifer in this soil are generally shallow (Sidhu et al. 2009) with thin unsaturated zone as compared to the Panchkula landfill site.
3.9 Human health risk assessment
As mentioned above, the concentration of Zn2+ and Pb2+ in the groundwater sample was lower than the drinking water standards, whereas Cu2+ and NH4+ were above the acceptable limit. The human health risk assessment of Cu2+, Zn2+, and Pb2+ showed HQ values less than 1 during pre-monsoon and post-monsoon (Table 4). It indicates non-carcinogenic health risk. The HQ value of Zn2+ in Chandigarh was found high (0.46 mg/kg/day) in pre-monsoon as compared to Mohali (0.24 mg/kg/day) and Panchkula (0.00005 mg/kg/day) and 0.36 mg/kg/day, 0.24 mg/kg/day and 0.0005 mg/kg/day in post-monsoon. Likewise, the same pattern was observed for Cu2+ where HQ varies 0.01 mg/kg/day, 0.006 mg/kg/day in Mohali and 0.01, 0.001 mg/kg/day in Panchkula and 0.03 mg/kg/day, 0.025 mg/kg/day in Chandigarh during pre-monsoon and post-monsoon. In addition, the human health risk of Pb2+ at Mohali (0.28 mg/kg day) was higher than the two sites Chandigarh (0.11 mg/kg/day) and Panchkula (ND) in pre-monsoon. HQ value of the Cu2+, Zn2+ and Pb2+ less than one was also reported by Adamu et al. (2015) and Pokkate et al. (2013). Similarly, HI values of Cu2+, Zn2+ and Pb2+ in tricity were found to be 0.61 mg/kg/day, 0.53 mg/kg/day and 0.01 mg/kg/day in pre-monsoon and 0.38 mg/kg/day, 0.24 mg/kg/day and 0.01 mg/kg/day in post-monsoon were less than one and represent non-carcinogen health risk. A HI value of Panchkula was found 0.36 mg/kg/day in pre-monsoon which represents that Panchkula landfill site has least effect on the health of population which reside in vicinity area of landfill. Whereas HI value of Chandigarh landfill site (0.61 mg/kg/day) indicated high risk to the population followed by Mohali and Panchkula.
4 Conclusions
Leachate sample analyzed for the physiochemical parameters, anions, and cations were found above the permissible limit. The elevated levels of ammoniacal nitrogen and chloride were observed in most of the samples, which falls in the vicinity of Chandigarh and Mohali landfill site. This indicates that landfill leachate has contaminated the groundwater to some extent. Microbiological analysis of water sample reveals 40.6% samples, were unsatisfactory during pre-monsoon sampling and 22.1% samples were suspicious and 4.7% satisfactory, whereas during post-monsoon 52.2% were unsatisfactory, 13.6% suspicious and 3.1% satisfactory. Hazard index of Chandigarh groundwater was 0.61 mg/kg/day, 0.38 mg/kg/day during pre-monsoon and post-monsoon sampling, respectively. Likewise the Hazard Index value of Mohali and Panchkula was 0.53 mg/kg/day, 0.01 mg/kg/day in pre-monsoon and 0.24 mg/kg/day and 0.01 mg/kg/day during the post-monsoon season. It suggested acceptable level of non-carcinogenic health risk, but high value of Hazard Index in Chandigarh indicate higher risk of the population living in the vicinity of the Chandigarh landfill site as compared to Panchkula and Mohali landfills.
References
Abd El-Salam, M. M., & Abu-Zuid, G. I. (2014). Impact of landfill leachate on the groundwater quality: A case study in Egypt. Journal of Advanced Research. https://doi.org/10.1016/j.jare.2014.02.003.
Abdul, S., Al, H., Sulaiman, H., Suliman, F. E., & Abdallah, O. (2014). Assessment of heavy metals in leachate of an unlined landfill in the sultanate of Oman. International Journal of Environmental Science and Development,5(1), 10–13. https://doi.org/10.7763/IJESD.2014.V5.451.
Aboyeji, O. S., & Eigbokhan, S. F. (2016). Evaluations of groundwater contamination by leachates around Olusosun open dumpsite in Lagos metropolis, southwest Nigeria. Journal of Environmental Management,183, 333–341. https://doi.org/10.1016/j.jenvman.2016.09.002.
Adamu, C. I., Nganje, T. N., & Edet, A. (2015). Environmental nanotechnology, monitoring & management heavy metal contamination and health risk assessment associated with abandoned barite mines in Cross River State, southeastern Nigeria. Environmental Nanotechnology, Monitoring & Management,3, 10–21. https://doi.org/10.1016/j.enmm.2014.11.001.
Akinbile, C. O., Yusoff, M. S., & Area, A. S. (2011). Environmental impact of leachate pollution on groundwater supplies in Akure, Nigeria. International Journal of Environment Science and Development, 2(1).
Alexandre Bogas, J., & Gomes, A. (2015). Non-steady-state accelerated chloride penetration resistance of structural lightweight aggregate concrete. Cement & Concrete Composites,60, 111–122. https://doi.org/10.1016/j.cemconcomp.2015.04.001.
Al-Tarazi, E., Abu Rajab, J., Al-Naqa, A., & El-Waheidi, M. (2008). Detecting leachate plumes and groundwater pollution at Ruseifa municipal landfill utilizing VLF-EM method. Journal of Applied Geophysics,65(3–4), 121–131. https://doi.org/10.1016/j.jappgeo.2008.06.005.
Aluko, O. O., Sridhar, M. K. C., & Oluwande, P. A. (2003). Characterization of leachates from a municipal solid waste landfill site in Ibadan, Nigeria. Journal of Environment Health Research,2(1), 32–37.
Behbahaninia, A., Mirbagheri, S. A., & Nouri, J. (2010). Effects of sludge from wastewater treatment plants on heavy metals transport to soils and groundwater. Iranian Journal of Environmental Health Science & Engineering,7(5), 401–406.
Bhatt, A. H., Altouqi, S., Karanjekar, R. V., Sahadat Hossain, M. D., Chen, V. P., & Sattler, M. S. (2016). Preliminary regression models for estimating first-order rate constants for removal of BOD and COD from landfill leachate. Environmental Technology & Innovation,5(February), 188–198. https://doi.org/10.1016/j.eti.2016.02.002.
Bhupander, K., & Mukherjee, D. P. (2011). Assessment of human health risk for arsenic, copper, nickel, mercury and zinc in fish collected from tropical wetlands in. Advanced Life Science and Technologyife Science and Technology,2, 13–25.
Bikundia, D. S., & Mohan, D. (2014). Major ion chemistry of the ground water at the Khoda Village, Ghaziabad, India. Sustainability of Water Quality and Ecology,3, 133–150. https://doi.org/10.1016/j.swaqe.2014.12.001.
EPA. (2005). Provisional peer reviewed toxicity values for ammonia.
EPA. (2014). Framework for human health risk assessment to inform decision making framework for human health risk assessment to inform decision making.
Haarstad, K., & Mæhlum, T. (2007). Electrical conductivity and chloride reduction in leachate treatment systems. Journal of Environmental Engineering,133(6), 659–664. https://doi.org/10.1061/(ASCE)0733-9372(2007).
Hanipha, M., & Zahir Hussain, A. (2013). Study of groundwater quality at Dindigul Town, Tamilnadu, India. International Research Journal of Environment Science,2(1), 68–73.
Jhamnani, B., & Singh, S. (2009). Groundwater contamination due to Bhalaswa landfill site in New Delhi. International Journal of Environment Science and Engineering,1(3), 121–125. Retrieved from http://idc-online.com/technical_references/pdfs/civil_engineering/GroundwaterContamination.pdf.
Kalčíková, G., Babič, J., Pavko, A., & Gotvajn, A. Ž. (2014). Fungal and enzymatic treatment of mature municipal landfill leachate. Waste Management (New York, N.Y.),34(4), 798–803. https://doi.org/10.1016/j.wasman.2013.12.017.
Kaur, K., Ravindra, K., & Mor, S. (2015). A Glance at the World-Waste management policies in India: Can we address the implementation challenges. Waste Management, 37, I-II.
Khai, N. M. (2012). Chemical precipitation of ammonia and phosphate from Nam Son Landfill Leachate, Hanoi. Iranica Journal of Energy & Environment,3, 32–36. https://doi.org/10.5829/idosi.ijee.2012.03.05.06.
Khound, N. J., Phukon, P., & Bhattacharyya, K. G. (2012). A comparative study of ground water and surface water quality in the Jia—Bharali river basin, Assam, India with reference to physico-chemical characteristics. International Journal of Applied Science and Engineering Research,1(3), 512–521. https://doi.org/10.6088/ijaser.0020101052.
Magombeyi, M., & Nyengera, R. (2012). The impact of municipal landfill on surface and ground water quality in Bulawayo, Zimbabwe. Wudpeckerresearchjournals.Org, 1 (November), 251–258. Retrieved from http://www.wudpeckerresearchjournals.org/JESWR/Pdf/2012/November/MagombeyiandNyengera.pdf.
Maiti, S. K., Hazra, T., & Dutta, A. (2016). Characterization of leachate and its impact on surface and groundwater quality of a closed dumpsite—A case study at Dhapa, Kolkata, India. Procedia Environmental Sciences,35, 391–399. https://doi.org/10.1016/j.proenv.2016.07.019.
Mangimbulude, J. C., Van Breukelen, B. M., Krave, A. S., Van Straalen, N. M., & Röling, W. F. M. (2009). Seasonal dynamics in leachate hydrochemistry and natural attenuation in surface run-off water from a tropical landfill. Waste Management,29(2), 829–838. https://doi.org/10.1016/j.wasman.2008.06.020.
Mor, S., Ravindra, K., Dahiya, R. P., & Chandra, A. (2006a). Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environmental Monitoring and Assessment, 118(1–3), 435–456. https://doi.org/10.1007/s10661-006-1505-7.
Mor, S., Ravindra, K., De Visscher, A., Dahiya, R. P., & Chandra, A. (2006b). Municipal solid waste characterization and its assessment for potential methane generation: A case study. Science of the Total Environment, 371(1–3), 1–10.
Mor, S., Kaur, K., & Khaiwal, R. (2016). SWOT analysis of waste management practices in Chandigarh, India and prospects for sustainable cities. Journal of Environmental Biology, 37(3), 327.
Mor, S., Negi, P., & Kaiwal, R. (2018). Assessment of groundwater pollution by landfills in India using leachate pollution index and estimation of error. Environmental Nanotechnology, Monitoring & Management. https://doi.org/10.1016/j.enmm.2018.09.002.
Nagarajan, R., Thirumalaisamy, S., & Lakshumanan, E. (2012). Impact of leachate on groundwater pollution due to non-engineered municipal solid waste landfill sites of Erode City, Tamil Nadu, India. Iranian Journal of Environmental Health Science & Engineering,9(1), 35. https://doi.org/10.1186/1735-2746-9-35.
Naveen, B., Mahapatra Madhab, D., Sitharam, T., Sivapullaiah, P., & Ramachandra, T. (2017). Physico-chemical and biological characterization of urban municipal landfill leachate. Environmental Pollution,220, 1–12. https://doi.org/10.1016/j.envpol.2016.09.002.
Nirmla, D., & Jagath, G. (2013). Leachate characterization and surface groundwater pollution at municipal solid waste landfill of Gohagoda, Sri Lanka. International Journal of Scientific Research,3(11), 1–7.
Piper, A. M. (1953). A graphic procedure in the geological interpretation of water analysis. Groundwater note no. 12, US Geology Survey Department, Washington, DC.
Pokkate, W., Serilert, C., Siriwong, W., & Mark, R. (2013). Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agriculture area in Ubon Ratchthani province, Thailand. Envitonment Geochemical Health. http://doi.org/10.1007/s10653-013-9537-8.
Pujari, P. R., Labhasetwar, P. K., Mahore, P., & Ganguly, A. K. (2011). Assessment of the impact of on-site sanitation systems on groundwater pollution in two diverse geological settings—A case study from India. http://doi.org/10.1007/s10661-011-1965-2.
Rajasekhar, B., Nambi, I. M., & Kumar, S. (2018). Human health risk assessment of ground water contaminated with petroleum PAHs using Monte Carlo simulations: A case study of an Indian Metropolitan City. Journal of Environmental Management,205, 183–191. https://doi.org/10.1016/j.jenvman.2017.09.078.
Raju, M. V. S. (2012). Contamination of ground water due to landfill leachate. International Journal of Engineering Research,53(1), 48–53.
Rao, J., Hari Babu, B., Swami, A. V. V. S., & Sumithra, S. (2013). Chemical characteristics of ground water of Vuyyuru. Part of East Coast of India Abstract: Journal of Environment Research and Technology,3(2), 225–232.
Ravindra, K., Kaur, K., & Mor, S. (2015). System analysis of municipal solid waste management in Chandigarh and minimization practices for cleaner emissions. Journal of Cleaner production, 89, 251–256.
Ravindra, K., Kaur, K., & Mor, S. (2016). Occupational exposure to the municipal solid waste workers in Chandigarh, India. Waste Management & Research, 34(11), 1192–1195.
Renou, S., Givaudan, J. G., Poulain, S., Dirassouyan, F., & Moulin, P. (2008). Landfill leachate treatment: Review and opportunity. Journal of Hazardous Materials,150(3), 468–493. https://doi.org/10.1016/j.jhazmat.2007.09.077.
Reyes-lópez, J. A., Ramírez-hernández, J., & Lázaro-mancilla, O. (2008). Assessment of groundwater contamination by landfill leachate: A case in México,28, 33–39. https://doi.org/10.1016/j.wasman.2008.03.024.
Sadashivaiah, C., Ramakrishnaiah, C. R., & Ranganna, G. (2008). Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk, Karnataka state, India. International Journal of Environmental Research and Public Health, 5(3), 158–164. https://doi.org/10.3390/ijerph5030158.
Sadeq, M., Moe, C. L., Attarassi, B., Cherkaoui, I., ElAouad, R., & Idrissi, L. (2008). Drinking water nitrate and prevalence of methemoglobinemia among infants and children aged 1–7 years in Moroccan areas. International Journal of Hygiene and Environmental Health,211(5–6), 546–554. https://doi.org/10.1016/j.ijheh.2007.09.009.
Sarvajayakesavalu, S., Lakshminarayanan, D., George, J., Magesh, S. B., Anilkumar, K. M., Brammanandhan, G. M., et al. (2018). Groundwater for sustainable development geographic information system mapping of gross alpha/beta activity concentrations in ground water samples from Karnataka, India: A preliminary study. Groundwater for Sustainable Development,6(November 2016), 164–168. https://doi.org/10.1016/j.gsd.2017.12.003.
Shivaprasad, H., Nagarajappa, D. P., & Sham Sundar, K. M. (2014). A study on physico-chemical characteristics of borewell water in sugar town, Mandya City, Karnataka State, India. Internation Journal of Engineering and Application,4(7), 112–123.
Shivendra, B. T., & Ramaraju, H. K. (2015). Impact of onsite sanitation system on groundwater in different geological settings of Peri Urban areas. Aquatic Procedia,4(Icwrcoe), 1162–1172. https://doi.org/10.1016/j.aqpro.2015.02.148.
Shukla, H. C., Gupta, P., Mehta, H. C., & Hebert, J. (2002). Descriptive epidemiology of body mass index of an urban adult population in western India. Journal of Epidemiology Community Health,56, 876–880.
Sidhu, S. P., & Sharma, B. D. (2009). Characteristics and classification of arid zone soils of Punjab, India. Arid Soil Research and Rehabilation, 4(4), 223–232.
Siraj, K., & Kitte, S. A. (2013). Analysis of copper, zinc and lead using atomic absorption spectrophotometer in ground water of Jimma town of Southwestern Ethiopia. International Journal of Chemical and Analytical Science,4(4), 201–204. https://doi.org/10.1016/j.ijcas.2013.07.006.
Srivastava, S. K., & Ramanathan, A. L. (2008). Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environmental Geology,53(7), 1509–1528. https://doi.org/10.1007/s00254-007-0762-2.
Sun, C., Zhao, W., Zhang, Q., Yu, X., Zheng, X., & Zhao, J. (2016). Spatial distribution, sources apportionment and health risk of metals in topsoil in Beijing, China. Environment Research and Public Health. https://doi.org/10.3390/ijerph13070727.
Tank, D. K., & Chandel, C. P. S. (2010). Analysis of the major ion constituents in groundwater of Jaipur City. Nature and Science,8(10), 1–7.
Wang, J., He, J., & Chen, H. (2012). Science of the total environment assessment of groundwater contamination risk using hazard quantification, a modified DRASTIC model and groundwater value, Beijing Plain, China. Science of the Total Environment,432, 216–226. https://doi.org/10.1016/j.scitotenv.2012.06.005.
Wang, Z., Jiang, Y., Kumar, M., Wang, J., Yang, X., & Amjad, A. (2018). Nitrate removal by combined heterotrophic and autotrophic denitrification processes: Impact of coexistent ions. Bioresource Technology,250(December 2017), 838–845. https://doi.org/10.1016/j.biortech.2017.12.009.
Wang, W., Ma, C., Zhang, Y., Yang, S., Shao, Y., & Wang, X. (2016). Phosphate adsorption performance of a novel filter substrate made from drinking water treatment residuals. Journal of Environmental Sciences. https://doi.org/10.1016/j.jes.2016.01.010.
Woldeyohans, A. M., Worku, T., Kloos, H., & Mulat, W. (2014). Treatment of leachate by recirculating through dumped solid waste in a sanitary landfill in Addis Ababa, Ethiopia. Ecological Engineering,73, 254–259. https://doi.org/10.1016/j.ecoleng.2014.09.052.
Yaqout-Al, A., & Hamoda, M. F. (2003). Evaluation of landfill leachate in arid climate—A case study. Environment International,29(5969), 593–600. https://doi.org/10.1016/S0160-4120(03)00018-7.
Funding
Funding was provided by University Grants Commission (IN) (Grant No. MANF-2013-14-BUD-28213).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Negi, P., Mor, S. & Ravindra, K. Impact of landfill leachate on the groundwater quality in three cities of North India and health risk assessment. Environ Dev Sustain 22, 1455–1474 (2020). https://doi.org/10.1007/s10668-018-0257-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-018-0257-1