Abstract
The increasing proximity of the Dudumbia dumpsite, an open dumpsite in Navrongo, Ghana, to human settlements necessitates an investigation of the soil quality to safeguard the environment from heavy metal toxicity. This study examined the impact of waste dumping activities on the physicochemical properties of the soil, as well as the level of heavy metal (Pb, Cd, Ni, Cr, As, Hg, Cu, Mn, and Zn) contamination and associated risks. Various contamination and risk assessment tools were used, including the geoaccumulation index (Igeo), pollution load index (PLI), potential ecological risk (Er), and potential ecological risk index (PERI). The study found significant improvements in notable soil attributes such as phosphorus (P), organic carbon (C), total nitrogen (N), calcium (Ca), magnesium (Mg), potassium (K), sodium (Na), and effective cation exchange capacity, with percentage increases ranging from 50.8 to 2078.3%. Igeo values ranged from 2.07 to 6.20, indicating contamination levels from moderate to extreme. The PLI and PERI values were 16.241 and 1810, respectively. The Er values for the heavy metals ranged from 36 to 607, indicating ecological risk levels from low to very high, with Cd and Hg posing very high risks. These results suggest that while the dumpsite soil shows improvements in some characteristics favourable for plant cultivation, waste dumping significantly contributes to heavy metal contamination. The soil at the dumpsite is deteriorated and poses significant health risks, particularly due to Cd and Hg. Therefore, remediation efforts should prioritise mitigating the risks posed by Cd and Hg.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities generate vast quantities of waste originating from various sources, including residential, commercial, industrial, agricultural, and institutional activities (Buenrostro et al., 2001; Kumar et al., 2024). Many different kinds of waste are generated, including organic waste (e.g. food scraps, yard waste, animal waste), inorganic waste (e.g. plastics, metals, glass), hazardous waste (e.g. chemicals, medical waste), municipal solid wastes (e.g. food wastes, packaged goods, disposable goods, used electronics), and commercial and industrial wastes (e.g. demolition debris, incineration residues, refinery sludges) (EPA, 2023). The increase in global population, urbanisation, and economic activities has led to a corresponding rise in waste production, posing significant challenges for waste management systems worldwide (Amasuomo & Baird, 2016).
Effective waste management practices are essential to mitigate the adverse environmental and health impacts of waste. These practices include waste reduction, reuse, recycling, energy recovery, and disposal (EPA, 2023). However, in many African countries, as well as other underdeveloped and developing regions, sustainable waste management practices such as reuse, recycling, and energy recovery face numerous challenges, including limited infrastructure, inadequate funding, and lack of regulatory enforcement (Dissanayake et al., 2022). Consequently, open dumpsites are a common waste management practice in these areas due to their low cost (Aluko et al., 2022). These sites are often characterised by the uncontrolled disposal of diverse categories of untreated wastes (Kumar & Agrawal, 2020). Pollutants, especially heavy metals, leach from these waste materials into the surrounding environment, accumulating in the soil and potentially entering the food chain (Sharma & Kumar, 2021). This contamination can have long-lasting adverse effects on both the environment and human health (Briffa et al., 2020; Shah et al., 2018).
The proximity of polluted sites to human settlements heightens the risks associated with environmental contamination. Residents living near polluted sites are exposed to higher levels of pollutants, which can lead to various health issues, including respiratory problems, skin disorders, and other chronic conditions (Briffa et al., 2020; Capelo et al., 2022). The presence of heavy metals in the environment is particularly concerning due to their toxicity and persistence (Ali et al., 2019).
The Dudumbia dumpsite in Navrongo, Ghana, exemplifies an unsustainable waste management practice. Moreover, the expansion of settlements in Navrongo is prominent, with development encroaching closer to the Dudumbia dumpsite. This increasing proximity heightens the risk of exposure to environmental pollutants, including heavy metals, for the local population. Addressing the potential contamination at the Dudumbia dumpsite is critical to protect public health and ensure sustainable living conditions for the community.
This study aims to identify a suitable control site with similar characteristics to the Dudumbia dumpsite and use it to examine changes in physicochemical properties, as well as the level and risk of heavy metal (Pb, Cd, Ni, Cr, As, Hg, Cu, Mn, and Zn) contamination resulting from open waste dumping activities. These heavy metals are common environmental contaminants with associated human health risks linked to anthropogenic activities. The outcomes of this research provide insights into which heavy metals should be the focus of environmental regulations and monitoring plans at the site. Furthermore, understanding the heavy metal pollution status of the dumpsite soil will be valuable for planning potential remediation efforts to safeguard the biota in the environment.
Materials and methods
Sampling sites
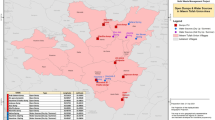
Dudumbia dumpsite lies along Navrongo-Paga road, in the Kassena Nankana Municipal in the Upper East Region of Ghana (Fig. 1). It is on the left side of the N10 road and on the left side when moving from Navrongo towards Paga.
Waste dumping activities at the site commenced in the 1980s when the area was characterised by a dense forest. It is now a cleared land serving as a central waste site shared by both Navrongo and a neighbouring town, Paga. As years go by, the forests around the dumpsite are being cleared and houses are being built near the dumpsite.
Various types of waste were identified at the dumpsite, including domestic, agricultural, and industrial refuse. This assortment comprises hospital wastes such as used syringes, needles, and test kits, as well as expired beverages and canned products like tomatoes, mackerel, and canned milk. Additionally, it includes discarded toilets, damaged car tyres, car parts, spoiled fruits, vegetables, deceased animals, wood, papers, and plastics, among others. Moreover, the activities of waste scavengers were observed at the site, as they picked what they considered valuable disposables. Some were seen burning tyres to extract the metallic components.
The control site selected is about 250 m away from the dumpsite. Situated across the primary N10 road along the Navrongo-Paga route, it stands in direct opposition to the dumpsite (Fig. 2).
Furthermore, in the aerial view illustrated (Fig. 2), the prominent red road extends past the control site and serves as a designated landing area for helicopters visiting both Navrongo and Paga. The control site is an undeveloped area with a clean history, devoid of any waste dumping activities or other actions that could directly raise the concentration of heavy metals on the site.
Soil sampling
The soil samples were collected in May 2023, from both the study site (Dudumbia dumpsite) and the control site. The dumpsite was demarcated into four zones, and ten grab samples were collected from each zone at regular intervals, ensuring comprehensive coverage. Stainless steel shovel, spade, and hand trowel were used to clear the refuse over the topsoil, loosen the topsoil particles, and collect grab samples within a consistent depth of 0–20 cm, respectively. Approximately 200 g of soil was collected at each sampling spot. The same approach was applied at the control site. This resulted in a total of eighty samples, each of which was kept in a sampling bag for subsequent treatments.
Sample preparation
Sampling units collected from both the dumpsite and the control site soils were mixed thoroughly to form separate aggregate soil samples. This process ensured the creation of distinct aggregate samples representing the dumpsite soil and the control site soil. Both aggregate samples were air-dried in a well-ventilated dust-free area at ambient conditions, spread out in thin layers to facilitate the natural evaporation of moisture. During air-drying, manual techniques were employed for gentle disaggregation, ensuring the removal of any clumps or compacted material. After air-drying and disaggregation, they were each sieved through 2-mm mesh and each mixed thoroughly. Subsequently, 500 g each of both the study site soil sample and the control site soil sample were weighed and taken for physicochemical characterisation. Finally, 50 g was taken from each of the remaining portions of the study site soil sample and the control site soil sample. They were dried at 110 °C to constant weight using a drying oven, allowed to cool down outside the oven, and kept in a desiccator until needed for digestion. The soil sample treatments for the measurement of the concentrations of volatile heavy metals Hg and As did not involve drying at 110 °C.
Physicochemical characterisation
Soil pH was determined using a glass calomel electrode (in soil/water, 1:2.5) (FAO, 2021a) and readings taken from the H1 9017 microprocessor pH meter. The determination of total nitrogen and available phosphorus was performed through Kjeldahl digestion and distillation procedures (Bremner, 1996) and the Bray and Kurtz P-1 method (Sims, 2009), respectively. Soil organic carbon content was determined using the Walkley–Black procedure (Nelson & Sommers, 1983). The concentrations of exchangeable cations (potassium, sodium, calcium, and magnesium) were determined by employing the ammonium acetate method (Thomas, 1983). Soil’s texture experiment was determined by the hydrometer method (Huluka & Miller, 2014), and the type of soil was determined using the United States Department of Agriculture (USDA) Natural Resources Conservation Service’s Soil Textural triangle (Groenendyk et al., 2015). Soil electrical conductivity was measured by a unified standard (soil/water, 1:5) (FAO, 2021b).
Soil sample digestion
Soil digestion was performed using the aqua regia method (Adesewa & Morenikeji, 2017). A 1 g of prepared soil sample was weighed into a 250-mL beaker, and 15 mL of freshly prepared aqua regia was added. The beaker containing the mixture was covered with a watch glass and heated gently on a hot plate in a fume hood at approximately 60–70 °C until brown fumes disappeared, and the volume of the mixture was reduced to approximately 5–3 mL. The mixture was removed from the hotplate, allowed to cool, and diluted with 20 mL of distilled deionised water (DDW). The resulting mixture was filtered through Whatman 42 filter paper into a 100-mL standard volumetric flask. The filtrate was diluted to a final volume of 100 mL using DDW. The resulting solution was transferred into a plastic bottle and kept for the analysis of heavy metals. The digestion was performed in multiple replicates for the dumpsite soil and the control site soil.
Heavy metal contamination and risk assessment
The heavy metal contamination and risk assessment of the Dudumbia dumpsite soil were conducted through a multi-step approach. This involved determining the concentration of the heavy metals, statistically comparing the measured concentrations with those of the control site, evaluating the geoaccumulation index (Igeo) of each heavy metal, comparing the concentrations of the heavy metals with permissible limits in soils established by international guidelines, assessing the potential ecological risk of individual heavy metals and their overall potential ecological risk (PERI), and evaluating the pollution load index (PLI).
Heavy metal analysis by atomic absorption spectrophotometry
Solutions obtained from the digestion procedure were sequentially introduced into an Agilent 240 AA atomic absorption spectrophotometer (AAS), manufactured by Agilent Technologies, USA. The instrument was calibrated beforehand for measuring heavy metal concentrations in aqueous solutions. The formula used for the calculation of the mg/kg concentrations of the heavy metals in the soils is as follows:
where B represents the concentration (in mg/L) of the heavy metal present in the solution prepared for metal analysis as measured from AAS, V is the volume (in L) of the solution prepared for AAS analysis, and M (in kg) is the mass of the soil sample subjected to acid digestion.
Correction for moisture content was made to obtain the concentration of the volatile elements Hg and As in a dried (to constant weight) sample of the soil. The correction was calculated using the following formula:
Statistical analysis
The acquired data on metal concentrations in the dumpsite soil and the control site soil obtained from replicate measurements were summarised using means and standard deviations.
Contamination level and risk assessment
The geoaccumulation index (Igeo) (Asamoah et al., 2021; Muller, 1969) for each metal was determined to assess the extent of soil contamination resulting from waste dumping activities at the Dudumbia dumpsite. The higher the Igeo, the more contaminated the site is with respect to a particular heavy metal. The formula used for the calculation of Igeo is as follows:
where Cn is the concentration of the heavy metal in the sample (mg/kg), and Bn is the background concentration of the heavy metal (mg/kg). The concentrations of the heavy metals in the control site soil were used as the background concentrations.
The Igeo values of the heavy metals in the dumpsite soil were interpreted using the Igeo classification. According to the classification, Igeo < 0 indicates that the study site is uncontaminated with the heavy metal; 0 < Igeo < 1 indicates that the study site is uncontaminated/moderately contaminated with the heavy metal; 1 < Igeo < 2 indicates that the study site is moderately contaminated with the heavy metal; 2 < Igeo < 3 indicates that the study site is moderately/strongly contaminated with the heavy metal; 3 < Igeo < 4 indicates that the study site is strongly contaminated with the heavy metal; 4 < Igeo < 5 indicates that the study site is strongly/extremely contaminated with the heavy metal, and 5 < Igeo indicates that the study site is extremely contaminated with the heavy metal (Muller, 1969).
The pollution load index (PLI) (Rahmanian & Safari, 2022) of the heavy metals in the dumpsite soil was determined to examine the overall contamination status. Heavy metal PLI of the dumpsite soil was determined using the equation:
where \({C}_{n}^{zth}\) is the measured concentration of the zth heavy metal in the dumpsite soil, and \({B}_{n}^{zth}\) is the background concentration of zth heavy metal.
The value obtained was interpreted using the PLI classification. According to the classification, PLI < 1 indicates perfection, PLI = 1 suggests that only baseline levels of the pollutants are present, and PLI > 1 suggests deterioration of the site quality.
Potential ecological risk (Er) (Hakanson, 1980; Karimian et al., 2021) for each heavy metal under study was evaluated to determine the ecological risk associated with its contamination in the dumpsite soil. Er for a heavy metal in the dumpsite soil was determined using the equation:
where Ti is the toxic response factor of heavy metal i. The values for Pb, Cd, Ni, Cr, As, Hg, Cu, Mn, and Zn are 5, 30, 5, 2, 10, 40, 5, 1, and 1, respectively. Cn is the measured concentration of the heavy metal in the sample (mg/kg); Bn is the background concentration of the metal (mg/kg).
The values obtained were interpreted using the Er ecological risk classification. Er < 40 indicates low risk; 40–80 indicates moderate risk; 80–160 indicates considerable risk; 160–320 indicates high risk, and Er > 320 indicates very high risk.
PERI (Hakanson, 1980; Karimian et al., 2021; Wang et al., 2022) was calculated to evaluate the overall ecological risks associated with heavy metal contamination in the dumpsite soil. It was calculated using the equation:
The value obtained was interpreted using the PERI ecological risk classification. According to the classification, PERI < 150 indicates low ecological risk, 150–300 indicates moderate ecological risk, 300–600 indicates strong ecological risk, and PERI > 600 indicates quite strong ecological risk.
The concentrations of the heavy metals in the dumpsite soil were also examined for compliance with WHO permissible limits in soil. This is crucial for indicating pollution (Adagunodo et al., 2018). A breach often necessitates remediation to reduce contamination and mitigate health risks.
Analytical quality control
To ensure the reliability of our results, rigorous quality control measures were implemented during both sampling and analysis stages. Analytical-grade reagents were used throughout the study. Multiple samples were collected from various parts of the dumpsite and the control site, ensuring wide coverage and representativeness. Distinct sets of sample collection and treatment tools were dedicated to each site to prevent cross-contamination of the studied pollutants. Calibration standard reagents for each heavy metal were used to prepare five standard solutions covering a concentration range specific to each heavy metal (0–15 mg/L for lead, 0–1.5 mg/L for cadmium, 0–10 mg/L for nickel, 0–6 mg/L for chromium, 0–1.5 mg/L for arsenic, 0–10 mg/L for mercury, 0–5 mg/L for copper, 0–3 mg/L for manganese, and 0–10 mg/L for zinc). These solutions were crucial for establishing a strong correlation (with r2 = 0.99) between absorbance readings from AAS and the corresponding heavy metal concentrations. The linear regression equations derived from these calibration plots (Table 6) were characterised by intercepts at the origin and were utilised to assess unknown concentrations of heavy metals in solutions using the same instrument. Additionally, a blank sample analysis was conducted to monitor and correct any potential reagent errors during the determination of heavy metal concentrations in the solution of the heavy metals obtained after the digestion of the soil sample. The blank sample was prepared by carrying out the digestion process without the soil sample, following the same procedure as for the soil sample, and diluting the resulting solution for AAS analysis. The concentrations of the heavy metals in the blank solution were measured and found to be 0 ppm for each metal.
Results and discussion
Physicochemical characteristics
The control site exhibits close similarity in soil texture and porosity with the dumpsite soil (Table 1; Fig. 4). These similarities, along with its location within the same geographical area and its uncontaminated history, make the control site a good reference point for accurately assessing the impact of waste dumping activities on the dumpsite soil. The observed differences can be attributed to the activities at the dumpsite.
The pH of the dumpsite soil is slightly higher than that of the control site (Table 2). This suggests the presence of a higher concentration of basic cations in the dumpsite soil (Neina, 2019). Similarly, the elevated electrical conductivity in the dumpsite soil compared to the control site soil indicates an increase in the concentration of dissolved ions, which could include both essential and heavy metal ions (Abira, 2008). These observations align with higher concentrations of calcium (Ca), magnesium (Mg), potassium (K), and sodium (Na) in the dumpsite soil compared to the control site soil. The rise in the concentrations of these elements may be attributed to the decomposition of organic waste materials at the dumpsite, leading to the release of soil nutrients such as cations (Asare & Száková, 2023). This explanation corresponds to the results obtained for nitrogen, organic matter, organic carbon, and phosphorus, which also show higher concentrations in the dumpsite soil compared to the control site soil.
Moreover, the dumpsite soil demonstrates a notably greater water retention capacity (Table 1). This observation aligns with the findings regarding organic matter. It is plausible that the higher water retention capacity of the dumpsite soil is attributed to a higher organic matter content, considering that organic carbon is known to enhance soil’s water-holding abilities (Ankenbauer & Loheide, 2017; Rawls et al., 2003). Thus, it is reasonable to assume that the increased presence of organic matter in the dumpsite soil is the underlying factor contributing to its enhanced water retention capacity.
The dumpsite soil not only boasts greater nutrient abundance but also exhibits a significantly heightened capacity for retaining and exchanging crucial cations, including Ca2⁺, Mg2⁺, K⁺, and Na⁺. This is evident in the measured effective cation exchange capacity (eCEC), with the dumpsite soil displaying a markedly higher eCEC value in contrast to the control site soil (Table 2). The characteristics developed by the dumpsite soil hold substantial potential for promoting nutrient availability, thereby offering favourable conditions for plant growth (Headlee et al., 2014).
The results obtained align with those of a prior study conducted by Simeon and Ambah (Simeon & Ambah, 2013), which similarly demonstrated the enhancement of soil physicochemical properties conducive to soil nutrient nourishment due to the influence of municipal solid waste.
Heavy metal contamination and risk assessment
Following the analysis of multiple replicates for heavy metal concentrations in both dumpsite and control site soils, it is noteworthy that the mean concentrations of all the heavy metals in the dumpsite soil consistently exceed those in the control site soil (Table 3; Figs. 5, 6, 7, 8, 9, 10, 11, 12, and 13).
These results unequivocally demonstrate that the waste disposal operations at the Dudumbia dumpsite have notably and consistently elevated the concentrations of Pb, Cd, Ni, Cr, As, Hg, Cu, Mn, and Zn in the dumpsite soil. This elevation in heavy metal concentrations can be attributed to the types of waste disposed of at the site.
Considering the sampling site description, the co-occurrence of elevated Cd and other metals and the presence of specific types of waste, such as canned products (tomatoes, mackerel, and canned milk) (Al Zabadi et al., 2018; Maduabuchi et al., 2006), discarded car parts (Elinder, 2019), dumped tyres (Adeyi & Oladoye, 2020; Shakya et al., 2006), plastics (Turner & Filella, 2021), and burning activities at the site (Adeyi & Oladoye, 2020; Soubra et al., 2021), suggest a possible correlation. The contribution of Pb could result from the deposition of Pb-containing materials such as vehicle battery remnants and metallic or alloy waste (Twumasi et al., 2016). The contribution of Hg could be from waste from electrical and electronic equipment such as batteries and fluorescent lamps and medical equipment wastes like thermometers and sphygmomanometers (Chalkidis et al., 2020; Cheng & Hu, 2012).
The contribution of waste dumping activities to the heavy metals in the dumpsite soil is further supported by the Igeo values of the heavy metals (Fig. 3), which evaluates the enrichment measure of each heavy metal in the dumpsite soil. The Igeo values indicate contamination levels ranging from moderate (Igeo value of 2.07) to extreme (Igeo value of 6.20).
Geoaccumulation index calculated for the heavy metals. Igeo < 0 indicates dumpsite soil is uncontaminated with the metal; 0 < Igeo < 1 indicates uncontaminated/moderately contaminated; 1 < Igeo < 2 indicates moderate contamination; 2 < Igeo < 3 indicates moderately/strongly contaminated; 3 < Igeo < 4 indicates strongly contaminated; 4 < Igeo < 5 indicates strongly/extremely contaminated, and 5 < Igeo indicates extremely contaminated
Furthermore, the findings reveal a range of contamination intensities among the heavy metals. As exhibits the lowest contamination level, followed by Pb, Cu, Mn, Ni, Hg, Cd, and Cr, with Zn presenting the highest degree of contamination.
Values 16.241 and 1810 obtained for PLI and PERI, respectively, for the dumpsite soil suggest significant deterioration in the soil quality due to heavy metal contamination, posing a very high risk to the biota in the environment.
Comparison of the concentration of heavy metals in the dumpsite soil with the WHO permissible limits (Table 4) reveals that, while heavy metals such as Pb, Ni, Cr, As, Hg, Cu, Mn, and Zn have their concentrations below the permissible limits, Cd has its concentrations exceeding its permissible limits.
Complementing this comparison are the results obtained for the potential ecological risk (Er) for each of the heavy metals (Table 5).
These results align with previous findings: Mn, Cu, and Pb show low ecological risk; Ni, As, and Cr show moderate ecological risks; Zn shows considerable ecological risk; and Cd and Hg show very high ecological risks.
Implications at Dudumbia dumpsite
The Dudumbia dumpsite, initially a thick forest, has undergone extensive changes over the years. It has evolved into a central waste site shared by Navrongo and the neighbouring town, Paga. This transformation has involved significant deforestation, the construction of houses nearby, and ongoing construction activities. Consequently, pollution of the soil at this site is expected to have detrimental effects on the local environment, particularly affecting nearby residents and those who come into direct contact with the dumpsite.
The Igeo values of all the heavy metals indicate that waste dumping activities have significantly contaminated the dumpsite soil. This implies that all the studied heavy metals contribute to the contamination and should not be excluded from the environmental regulation and monitoring plans at the dumpsite. Prioritisation for monitoring can be based on the magnitude of the Igeo values, which follow the following order: Zn > Cr > Cd > Hg > Ni > Mn > Cu > Pb > As.
Beyond contamination, the site exhibits deterioration and poses a very high risk as suggested by PLI, PERI, and the individual Er for each heavy metal. The very high ecological risks associated with Cd and Hg imply that individuals who have direct contact with the dumpsite or reside nearby are at significant risk of suffering from the adverse effects of environmental pollution by these heavy metals. In light of this, considering measures to address Cd and Hg contamination, such as soil remediation or targeted interventions, is considerable.
Conclusions
Waste dumping activities have led to improvements in some soil properties, such as phosphorus (P), organic carbon (C), total nitrogen (N), calcium (Ca), magnesium (Mg), potassium (K), sodium (Na), and effective cation exchange capacity. These improvements can enhance conditions for plant cultivation. However, our findings reveal that the activities at the dumpsite contribute significantly to contamination by all the heavy metals investigated: Zn, Cr, Cd, Hg, Ni, Mn, Cu, Pb, and As. Moreover, the dumpsite soil shows signs of significant deterioration, with Cd and Hg presenting very high ecological risks.
Given the dumpsite’s proximity to human settlements, environmental regulation and monitoring plans at the site must focus on all these heavy metals to protect public health. To ensure the environment is safe, especially if future land reuse is anticipated, remediation efforts must prioritise mitigating the risks posed by Cd and Hg.
Data availability
The datasets generated and/or analysed during this study are available upon request.
References
Abira, M. A. (2008). A pilot constructed treatment wetland for pulp and paper mill wastewater: Performance, processes and implications for the Nzoia River, Kenya, UNESCO-IHE PhD. CRC Press. https://books.google.com.gh/books?id=-P4xSv7j9S0C. Accessed 27 Oct 2023.
Adagunodo, T. A., Sunmonu, L. A., & Emetere, M. E. (2018). Heavy metals’ data in soils for agricultural activities. Data in Brief, 18, 1847–1855. https://doi.org/10.1016/j.dib.2018.04.115
Adesewa, A., & Morenikeji, O. (2017). Helminths and heavy metals in soils from a dumpsite in Ibadan city, Nigeria. Journal of Preventive Medicine and Hygiene, 58(4), E328.
Adeyi, A. A., & Oladoye, P. O. (2020). Assessment of heavy metals in simulated leachates and the ashes of end-of-life tyres. International Journal of Environment and Waste Management, 25(4), 474–486.
Al Zabadi, H., Sayeh, G., & Jodeh, S. (2018). Environmental exposure assessment of cadmium, lead, copper and zinc in different Palestinian canned foods. Agriculture & Food Security, 7(1), 50. https://doi.org/10.1186/s40066-018-0205-1
Ali, H., Khan, E., & Ilahi, I. (2019). Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. Journal of Chemistry, 2019. https://doi.org/10.1155/2019/6730305
Aluko, O. O., Obafemi, T. H., Obiajunwa, P. O., Obiajunwa, C. J., Obisanya, O. A., Odanye, O. H., & Odeleye, A. O. (2022). Solid waste management and health hazards associated with residence around open dumpsites in heterogeneous urban settlements in Southwest Nigeria. International Journal of Environmental Health Research, 32(6), 1313–1328.
Amasuomo, E., & Baird, J. (2016). The concept of waste and waste management. J. Mgmt. & Sustainability, 6, 88.
Ankenbauer, K. J., & Loheide, S. P. (2017). The effects of soil organic matter on soil water retention and plant water use in a meadow of the Sierra Nevada, CA. Hydrological Processes, 31(4), 891–901.
Asamoah, B. D., Asare, A., Okpati, S. W., & Aidoo, P. (2021). Heavy metal levels and their ecological risks in surface soils at Sunyani magazine in the Bono Region of Ghana. Scientific African, 13, e00937. https://doi.org/10.1016/j.sciaf.2021.e00937
Asare, M. O., & Száková, J. (2023). Are anthropogenic soils from dumpsites suitable for arable fields? Evaluation of soil fertility and transfer of potentially toxic elements to plants. Plant and Soil, 486(1), 307–322. https://doi.org/10.1007/s11104-023-05870-6
Bremner, J. M. (1996). Nitrogen-total. Methods of soil analysis: Part 3. Chemical Methods, 5, 1085–1121.
Briffa, J., Sinagra, E., & Blundell, R. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon, 6(9). https://doi.org/10.1016/j.heliyon.2020.e04691
Buenrostro, O., Bocco, G., & Cram, S. (2001). Classification of sources of municipal solid wastes in developing countries. Resources, Conservation and Recycling, 32(1), 29–41. https://doi.org/10.1016/S0921-3449(00)00094-X
Capelo, R., Rohlman, D. S., Jara, R., García, T., Viñas, J., Lorca, J. A., Contreras Llanes, M., & Alguacil, J. (2022). Residence in an area with environmental exposure to heavy metals and neurobehavioral performance in children 9–11 years old: An explorative study. International Journal of Environmental Research and Public Health, 19(8), 4732.
Chalkidis, A., Jampaiah, D., Aryana, A., Wood, C. D., Hartley, P. G., Sabri, Y. M., & Bhargava, S. K. (2020). Mercury-bearing wastes: Sources, policies and treatment technologies for mercury recovery and safe disposal. Journal of Environmental Management, 270, 110945. https://doi.org/10.1016/j.jenvman.2020.110945
Cheng, H., & Hu, Y. (2012). Mercury in municipal solid waste in China and its control: A review. Environmental Science & Technology, 46(2), 593–605. https://doi.org/10.1021/es2026517
Dissanayake, P., Dissanayaka, D., Rankoth, L. M., & Abeysinghe, G. (2022). Waste management challenges in developing countries. In Waste technology for emerging economies (1st ed., pp. 5–28). CRC Press.
Elinder, C. G. (2019). Cadmium: Uses, occurrence, and intake. In Cadmium and health (1st ed., pp. 23–64). CRC Press.
EPA. (2023). Wastes. Retrieved 25 May from https://www.epa.gov/report-environment/wastes. Accessed 17 May 2024.
FAO. (2021a). Standard operating procedure for soil pH determination. FAO.
FAO. (2021b). Standard operating procedure for soil electrical conductivity, soil/water, 1:5. FAO.
Groenendyk, D. G., Ferre, T. P., Thorp, K. R., & Rice, A. K. (2015). Hydrologic-process-based soil texture classifications for improved visualization of landscape function. PLoS ONE, 10(6), e0131299.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14(8), 975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Headlee, W. L., Brewer, C. E., & Hall, R. B. (2014). Biochar as a substitute for vermiculite in potting mix for hybrid poplar. BioEnergy Research, 7(1), 120–131. https://doi.org/10.1007/s12155-013-9355-y
Huluka, G., & Miller, R. (2014). Particle size determination by hydrometer method. Southern Cooperative Series Bulletin, 419, 180–184.
Karimian, S., Shekoohiyan, S., & Moussavi, G. (2021). Health and ecological risk assessment and simulation of heavy metal-contaminated soil of Tehran landfill. RSC Advances, 11(14), 8080–8095.
Kumar, A., & Agrawal, A. (2020). Recent trends in solid waste management status, challenges, and potential for the future Indian cities – a review. Current Research in Environmental Sustainability, 2, 100011. https://doi.org/10.1016/j.crsust.2020.100011
Kumar, R., Kaur, A., Sharma, S., Bharti, H., & Kumar, R. (2024). Advancements and challenges in agriculture waste management: A comprehensive. Educational Administration: Theory and Practice, 30(5), 7253–7273.
Maduabuchi, J. M. U., Nzegwu, C. N., Adigba, E. O., Aloke, R. U., Ezomike, C. N., Okocha, C. E., Obi, E., & Orisakwe, O. E. (2006). Lead and cadmium exposures from canned and non-canned beverages in Nigeria: A public health concern. Science of The Total Environment, 366(2), 621–626. https://doi.org/10.1016/j.scitotenv.2005.12.015
Muller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal, 2, 108–118.
Neina, D. (2019). The role of soil pH in plant nutrition and soil remediation. Applied and Environmental Soil Science, 2019, 1–9.
Nelson, D. A., & Sommers, L. E. (1983). Total carbon, organic carbon, and organic matter. Methods of soil analysis: Part 2 chemical and microbiological properties, 9, 539–579.
Ogunlana, R., Korode, A., & Ajibade, Z. (2020). Assessing the level of heavy metals concentration in soil around transformer at Akoko community of OndoState, Nigeria. Journal of Applied Sciences and Environmental Management, 24(12), 2183–2189.
Ojiabo, K. T., Umeojiakor, C. T., Nwanwe, C. C., & Ekpemandu, J. C. (2020). Investigation of the concentration of heavy metals in soil samples from Nekede- Owerri mechanic village. In National Conference on Engineering and Technology (vol 1, issue 1). NACET Journal.
Rahmanian, M., & Safari, Y. (2022). Contamination factor and pollution load index to estimate source apportionment of selected heavy metals in soils around a cement factory, SW Iran. Archives of Agronomy and Soil Science, 68(7), 903–913. https://doi.org/10.1080/03650340.2020.1861252
Rawls, W. J., Pachepsky, Y. A., Ritchie, J. C., Sobecki, T. M., & Bloodworth, H. (2003). Effect of soil organic carbon on soil water retention. Geoderma, 116(1), 61–76. https://doi.org/10.1016/S0016-7061(03)00094-6
Shah, B. A., Oluyinka, O. A., Patel, A. V., & Bagia, M. I. (2018). Impacts of industrial and agricultural chemical wastes on freshwater resources. JSM Chem, 6(1), 1052.
Shakya, P. R., Shrestha, P., Tamrakar, C. S., & Bhattarai, P. K. (2006). Studies and determination of heavy metals in waste tyres and their impacts on the environment. Pakistan Journal of Analytical & Environmental Chemistry, 7(2), 7.
Sharma, P., & Kumar, S. (2021). Characterization and phytotoxicity assessment of organic pollutants in old and fresh municipal solid wastes at open dump site: A case study. Environmental Technology and Innovation, 24, 101938. https://doi.org/10.1016/j.eti.2021.101938
Simeon, P. O., & Ambah, B. (2013). Effect of municipal solid waste on the growth of maize (Zea mays L.). International Letters of Natural Sciences, 2. https://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-64b022f0-3da0-4cca-a1a5-3e0502718654. Accessed 27 Oct 2023.
Sims, J. T. (2009). Soil test phosphorus: Bray and Kurtz P-1. In Methods of phosphorus analysis for soils, sediments, residuals, and waters (2nd ed., pp. 13). SERA-IEG 17.
Soubra, G., Massoud, M. A., Alameddine, I., Al Hindi, M., & Sukhn, C. (2021). Assessing the environmental risk and pollution status of soil and water resources in the vicinity of municipal solid waste dumpsites. Environmental Monitoring and Assessment, 193(12), 857. https://doi.org/10.1007/s10661-021-09640-8
Thomas, G. W. (1983). Exchangeable cations. Methods of soil analysis: Part 2 chemical and microbiological properties, 9, 159–165.
Turner, A., & Filella, M. (2021). Hazardous metal additives in plastics and their environmental impacts. Environment International, 156, 106622. https://doi.org/10.1016/j.envint.2021.106622
Twumasi, P., Tandoh, M. A., Borbi, M. A., Ajoke, A. R., Owusu-Tenkorang, E., Okoro, R., & Dumevi, R. M. (2016). Assessment of the levels of cadmium and lead in soil and vegetable samples from selected dumpsites in the Kumasi Metropolis of Ghana. African Journal of Agricultural Research, 11(18), 1608–1616.
Wang, Q., Duan, W., Yao, X., Guo, X., Liu, D., Gao, W., & Zhang, J. (2022). Distribution and ecological risk assessment of heavy metals in the sediments of Changli ecological monitoring area, northwest of Bohai Bay, China. Environmental Pollutants and Bioavailability, 34(1), 180–189. https://doi.org/10.1080/26395940.2022.2069599
Acknowledgements
The authors appreciate CSIR-Soil Research Institute, Kumasi, Ghana, for generously providing access to their valuable research facilities. We also express our sincere appreciation to Mr. Charles Asante of CSIR-Soil Research Institute, Kumasi, for his invaluable assistance in performing the crucial analysis. Their support and expertise were instrumental in the successful execution of this work.
Author information
Authors and Affiliations
Contributions
Olutayo Abiodun Oluyinka: conceptualisation, methodology, formal analysis, data curation, writing—original draft, writing—review and editing, visualisation, and validation. Mary-Magdalene Pedavoah: writing—original draft, writing—review and editing. James Abugri: visualisation, writing—review and editing. Emmanuel Olajide Oyelude: writing—review and editing. Richard Mosobil: writing—review and editing. Kpono Amos: field work, laboratory work, and visualisation. Donatus Akasokeba Asamannaba: field work and laboratory work. Abdul-Waris Issahaku: field work and laboratory work. Abdul-Karim Kabah Isshak: field work and laboratory work. Nsoh Ali Aberinga: field work and laboratory work.
Corresponding author
Ethics declarations
Ethics approval
All authors have read, comprehended, and, as applicable, adhered to the guidelines outlined in the “Ethical Responsibilities of Authors” as stated in the Instructions for Authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Table 6
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oluyinka, O.A., Pedavoah, MM., Abugri, J. et al. Soil quality and heavy metal contamination in an open dumpsite in Navrongo, Ghana. Environ Monit Assess 196, 781 (2024). https://doi.org/10.1007/s10661-024-12930-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-12930-6