Abstract
Presently, microplastic pollution has emerged as a growing environmental risk around the world. Nevertheless, knowledge of the occurrence and characteristics of microplastics in tropical agricultural soil is limited. This study investigated the pollution of surface soil microplastics in two agricultural farms located at Klang Valley, Malaysia. An extraction method based on density separation by using saturated extraction solution (sodium sulfate, ρ = 2 g cm−3 and sucrose, ρ = 1.59 g cm−3 with a ratio 1:1, v/v) was carried out. The study revealed the mean particle size of soil microplastics with 3260.76 ± 880.38 μm in farm A and 2822.31 ± 408.48 μm in farm B. The dominant types of soil microplastics were fragments and films with major colors of white (59%) and transparent (28%) in farm A, while black (52%) and white (37.6%) in farm B. Representatives of soil microplastics detected polymers of polyvinyl chloride (PVC), high density polyethylene (HDPE), polycarbonate (PC), and polystyrene (PS). The sources of plastic products were black and white plastic pipes, black plastic films for vegetation, fertilizer bottles, plastic water containers and polystyrene storage boxes, and the breakdown processes, contributed to the microplastic pollution in these farms. The outcomes of this study will establish a better understanding of microplastic pollution in tropical agricultural soil in the Southeast Asian region. The findings would be beneficial as supportive reference for the endeavor to reduce microplastic pollution in agricultural soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
World production of plastics has tremendously increased and is predicted to be four times by 2050, with 8.4% yearly growth rate (Geyer et al., 2017; Plastics Europe, 2021; Ritchie & Roser, 2020; Suaria et al., 2016). Currently, plastic contamination is a significant factor contributing to the universal decrease of biodiversity and poses a huge threat to the viability of the global system and human health (Cárdenas-Alcaide et al., 2022; Fan et al., 2022; Kumar et al., 2022). Although plastics have a low degradation rate due to their high durability, in due course, plastics progressively break down into smaller particles and are classified based on size as macroplastics (> 2 cm), mesoplastics (5 mm–2 cm), microplastics (< 5 mm), and nanoplastics (< 1 mm) (Afrin et al., 2020; Allen et al., 2022; Neves et al., 2022). Research has reported that soil serves as a precursory medium of microplastics, which, over time, is degraded further or is repeatedly remobilized (Huo et al., 2022; Liwarska-Bizukojc, 2021; Zhang, Jin, et al., 2022). The small size and toxicity of plasticizers and additives in plastic materials raise caution on potential negative impacts on the terrestrial ecosystem (Ainali et al., 2022; Tong et al., 2022; Wu et al., 2021). The risks of microplastic pollution in terrestrial ecosystems have initiated research on the repercussions of microplastic contamination on land, including agricultural crops (Li et al., 2020). Typically, after crops are harvested, plastic materials, such as films are abandoned on the farms, leading to degradation into microplastics which induces a change in physicochemical attributes of the soil (Lian et al., 2020; Qi et al., 2020).
To date, studies on microplastics in agricultural soil have mostly been carried out in temperate and subtropical regions, such as south China and southeast South America (Pérez-Reverón et al., 2022; Truong et al., 2021). A recent study by Sa’adu and Farsang (2023), reported that although 60% of studies on microplastics contamination in agricultural soils have been conducted in Asia; however, most studies were conducted in China and Japan. Additionally, current studies in China have discovered microplastics in agricultural soil and vegetable farmlands with an abundance of 50–12,560 items/kg (Chen et al., 2020a; Wang et al., 2022; Xu et al., 2022; Zhang, Zhang, et al., 2022). Meanwhile, in Malaysia, till date, only a study by Praveena et al. (2023), has investigated the occurrence of microplastics in agricultural soil in Malaysia, indicating a wide gap in research on terrestrial microplastics, hence, requiring more focus on agricultural microplastics in Malaysian soil. On the other hand, previous studies have mainly emphasized on the abundance and composition of microplastic types in marine and freshwater environments in Malaysia (Amelia et al., 2020; Ibrahim et al., 2022; Karami et al., 2016, 2017; Karbalaei et al., 2019; Khalik et al., 2018; Md Amin et al., 2020; Pariatamby et al., 2020; Sarijan et al., 2018; Yang Hwi et al., 2020). On the other hand, with the use of agricultural plastics in the Southeast Asian region at 4.1 million tons in 2018 and is expected to increase to 7 million tons by 2030, plastic films is the largest contributor to agricultural plastics; and the abundance of it is expected to rise due to increasing quantities of greenhouses, greater reliance on mulching activities, and wider usage of plastic products (FAO, 2021; Sintim & Flury, 2017). However, studies on especially microplastic occurrence pertaining to agricultural soil in the tropical Southeast Asian region, including Malaysia, are still insufficient and remain ambiguous, leading to the scarcity of information, not only on the impacts of plastics usage but also on the potential sources of microplastics (Babel et al., 2022; Henseler et al., 2019).
The sources of microplastics in agricultural soil are wide and varied and consistently cause accumulation of microplastics in the soil (Chen et al., 2020a; Zhang, Lykaki, et al., 2022). These sources possibly originate from plastic products, such as fertilizer bags and plastic films, shade nets, vinyl tunnels, and sludge or compost utilization (Afrin et al., 2020; Bläsing & Amelung, 2018; Hurley & Nizzetto, 2018; Liu, Li, et al., 2022). The major plastic source that contributes to the highest quantity of agricultural microplastics is plastic films, comprising greenhouse films (3.5 million tons), mulch films (2.5 million tons), and silage films (1.4 million tons) (FAO, 2021). Plastic films, which are used in agricultural soil to control soil temperature and moisture, reduce soil nutrient loss, prevent soil erosion, and improve water use efficiency, will eventually degrade into diverse fragments (Gao et al., 2019; Kader et al., 2019). In contrast, the source of fiber particles includes synthetic fabrics, such as nylon and polyester, nylon nets, string, ropes, cables, and fiber residue in irrigation waters (Geyer et al., 2022; Priyanka & Govindarajulu, 2023; Zhang, Wang, et al., 2022). Qi et al. (2020) reported that among the abundance of soil microplastics, particles of polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET), and polyvinyl chloride (PVC) were predominant. This is due to the slow breakdown of the polymers, owing to the addition of plasticizers to soften the plastics, usage of antioxidants, and flame retardants in plastic products to prevent the degradation process in an outdoor environment (Rawtani et al., 2023). However, the tropical region has environmental factors, such as daily high temperature of 33 °C and ultraviolet radiation of an index of 14, which increase the possibility of speeding up the breakdown of the polymers. Therefore, plastic product is likely degraded, which contributes to the abundance of tropical agricultural soil.
This study aims to elucidate the occurrence, characteristics (particle size, color, and shape), and polymer identification of microplastics in vegetable farmlands; to provide an insight into possible sources of microplastic pollution in vegetable farmlands; and to compare the outcomes of this study with other related studies. The findings of this study will broaden the present knowledge and understanding of microplastic pollution in vegetable farmlands, particularly in the tropical terrestrial environments in the Southeast Asian region, specifically in Malaysia. The research data would be an additional reference for the cutback and mitigation of sources and occurrence of microplastics in agricultural soil. The study of microplastics in agricultural soil includes monitoring the occurrence and types of microplastics in soil, which in due time, enables understanding of the effectiveness of contamination control and waste management methods. Apart from that, conveying the findings to the community such as the public, farmers and shareholders increases awareness on the effects of microplastics in agricultural soil.
Materials and methods
Site description
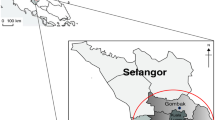
In this study, two vegetable farmlands were identified in Semenyih (Farm A) and Puchong (Farm B), both located in Klang Valley, Selangor (Fig. 1). Selangor is a state in central Malaysia with a tropical climate with an average daily high temperature of 33 °C and annual ultraviolet radiation index of 14. The state is responsible for around 75% of the total vegetable production in Malaysia. Besides, both farms plant various types of vegetation for the local market and export to other countries.
Farm A which was established since 2017, is located on high grounds in Semenyih with limited land area and structured rectangular vegetable plots. Therefore, as the farm is based in highlands, there is limited exposure to tropical high temperatures and ultraviolet radiation. Furthermore, there is limited movement of people and no vehicle movement. Soil preparation in vegetable plots, planting seedlings and harvesting is the main operational activities on farm A. Plastics on farm A (Fig. S1) were large white pipes used to produce nitrogen-infused water for vegetation, while smaller black pipes provided water to the vegetable plots. Moreover, some vegetation plots have white plastic frameworks supporting, black plastic netting, and transparent covers to protect the vegetation from high temperatures and direct sunlight. Plastic drums and trays were used for storage purposes. After the harvest of its vegetation, the young farm cleans up the utilized soil by removing unwanted plant stems, roots, and weed as well as foreign materials such as plastic particles. Although there is initiative to restore the soil health, however, the farm still practices minimal usage of plastic materials to avoid contamination. Furthermore, wastes such as the plastic materials used in operational activities are managed and disposed in an appropriate manner. Additionally, it is assumed that the breakdown of plastic materials present in the farm to microplastics takes a longer duration as the farm is still newly established with minimal exposure to weathering and atmospheric deposition due to limited movement of people and vehicles.
On the other hand, farm B was established and operational for the last 35 years. It is situated on flat low grounds in Puchong and is more of a typical traditional farming on a large farm area. The farm is well organized with defined rows of vegetation. However, the farm is vastly open and is greatly exposed to high temperatures and ultraviolet radiation. Farm B has an active movement of people who work there. Additionally, some vehicle movement is present due to roads located nearby the farm. Agricultural activities, such as soil plowing, seeding, cultivation, weeding, and harvesting are constantly carried out on the farm. Major sources of plastic products on the farm (Fig. S2) are black plastic films for cultivating vegetation, fertilizer bottles and plastic water containers, polystyrene storage boxes, plastic bags, and plastic wastes. In order to prevent soil erosion due to rain and other operational activities, the black plastic bags and films are vastly used for seedlings and sprouting vegetation. Apart from that, wastes especially plastic materials from operational activities are typically disposed in a nearby area on the farm itself. Due to heavy weathering, vast movement of humans and vehicles, including the long-term environmental effects of the age-old established farm, there is possibility of small microplastics particles being present on the farm.
Summary of methodology
The flowchart of the methodology for detection and identification of microplastics in soil is presented in Fig. 2. It includes sample preparation, determination of soil chemical properties, extraction of surface soil
Sampling
The surface agricultural soil samples were obtained from 40 different locations, with 20 locations from each farm, respectively. One surface soil sample was collected from each location, providing a total of 20 surface soil samples from each farm. The surface soil samples were collected at a depth of 20 cm in an area of 20 × 20 cm2 using a stainless-steel shovel. About 1 kg of soil samples were collected and packed individually in a box lined with aluminum foil for each sampling site. Large visible foreign particles were removed from the soil and air-dried for 7 days. These surface soil samples were homogenized with a mortar and pestle and sieved with a 5-mm stainless steel sieve.
Determination of soil chemical properties
The pH levels of the agricultural surface soil samples were determined by mixing 10 g of soil sample with 20 ml of deionized water, sealed with aluminum foil and left to stand. After 30 min, the pH of the soil was measured by using a calibrated pH meter (Thermo Scientific Eutech pH 450, USA). Meanwhile, the content of soil organic matter was determined through oxidation by adding 20 g of surface soil sample to 100 ml of Fenton’s reagent (50 ml of Ferrous catalyst solution and 50 ml of 30% hydrogen peroxide solution adjusted to pH 3) in a fume hood for 12 h at room temperature. A 100-mm filter paper (Whatman No.1) was weighed prior to the filtration process. The digested surface soil was then filtered with the same filter paper and glass funnel. The filtered surface soil was left overnight to air-dry. After 24 h, the dried filter paper containing the filtered surface soil was weighed. The content of surface soil organic matter was determined through digestion efficiency with following calculations (Mbachu et al., 2021; Prihandari et al., 2021).
Wi is the initial weight of soil
Wa is the weight of dry filter paper after filtration
Wb is the weight of dry filter paper before filtration
Extraction of soil microplastics through density separation
At room temperature, 50 g of sieved surface soil sample was treated with 100 mL of Fenton’s reagent for 12 h to digest organic matter. After filtration, the soil sample was placed in a glass beaker with 100 mL of saturated extraction solution (sodium sulfate, ρ = 2 g cm−3 and sucrose, ρ = 1.59 g cm−3 with a ratio 1:1 v/v). The suspension was stirred with a magnetic stirrer (Heidolph, Germany) for 30 min and then shaken for 2 h at 150 rpm with an orbital shaker (Boeco, Germany). The glass beaker with soil sample was sealed with aluminum foil and left to stand at room temperature for 24 h. After density separation, the floated microplastics were extracted through filtration by using 24-cm filter paper. The extracted microplastics were rinsed with distilled water to remove excess residue, placed on clear paper in a glass petri dish, and air-dried in a contained environment. Each surface soil sample was run in triplicates, and the density separation process was repeated three times to extract the microplastics from each surface soil sample thoroughly.
Microplastics identification and quantification
Filter paper of the extracted particles was observed under a compound microscope (CHK2-F-GS, Olympus, Japan) with a magnification of 40× and photographed. Identification of plastics and non-plastic particles in these photos was distinguished using a key for the classification of microplastic particles by (Lusher et al., 2020). The photos were used to identify the color and shape and recorded. While for particle size, the same photos and Image J 1.53 (http://imagej.nih.gov), an open-source particle analysis software was utilized. Lastly, the same filter paper was analyzed for the composition of the plastic polymers by using attenuated total reflectance—Fourier transform infrared (ATR-FTIR) spectroscopy (Perkin Elmer Frontier, USA). The FTIR spectrum range was from 650 to 4000 cm−1, and the spectra were then compared to a standard library. Only nine particles were selected as representatives and analyzed for this purpose.
Quality assurance and quality control
During sampling, all surface soil samples were collected in clean containers lined with aluminum foil and any usage of plastic tools was avoided. Triplicate tests for microplastics extraction were carried out for each surface soil sample. A total of ten positive controls were used for the field soil samples to ensure optimum quality control. Sterilized deionized water was used for chemical preparations and carried out in a fume hood. During all processes of preparation, digestion, and density separation, aluminum foil was used to cover the glass wares and containers to reduce exposure to external environment. Cotton lab coats and latex gloves were used throughout the laboratory work. Cabinets and work benches were cleaned and sterilized constantly to maintain cleanliness and reduce contamination.
Statistical analysis
Descriptive analysis was used to analyze mean, minimum, maximum, and standard deviation of particle size. All data, graphs, and statistical analysis were performed by using Excel M365 (version A1), while the Kruskal Wallis test and correlation analysis were performed using SPSS version 27. The Kruskal Wallis test was performed to compare the means of variables (microplastic particle size, organic matter, pH) between Farm A and Farm B, to give comprehension into whether there are significant differences of findings between the two farms. The confidence level was set at 95%.
Results and discussion
Composition of the surface soil samples of Farm A and B
The findings of surface soil pH, organic matter, and microplastics particle size distribution of farm A and farm B are presented in Table 1. The mean average of surface soil pH level for farm A was 5.99 ± 0.22 with the minimum pH of 5.56 and the maximum pH of 6.36. The mean average surface soil pH level for farm B was 6.45 ± 0.28 with the minimum pH of 6.03 and the maximum pH of 6.90. The range of pH levels for farm A was between 5.5 and 6.5 and farm B was between 6.0 and 7.0. In this study, findings demonstrated that surface soil samples from both, Farm A and Farm B were of slightly acidic soils. However, surface soil from farm A was more acidic compared to farm B due to a lower average of soil pH at 5.99. A factor that lowers soil pH levels in surface soil is the abundance of organic matter (Hong et al., 2019; Wu et al., 2022). The coefficient of variation (CV) was calculated based on the study by Aronhime et al. (2014), to indicate the reliability and reproducibility of the findings in this study. The coefficient of variation (CV) of pH levels for farm A was 3.67%, while for farm B, it was 4.34%. The low CV indicates that the data points are closely clustered around the mean, suggesting consistent and reliable results. Thus, the CV percentages obtained show a high level of reproducibility in pH measurements for both, farm A and farm B. The findings demonstrate the reliability of the analytical methods used in this study.
Farm A presented a mean average of soil organic matter at 33.24 ± 5.86%, with the minimum at 19.86% and the maximum at 42.82%. Farm B presented a mean average of soil organic matter at 22.17 ± 5.61%, with the minimum at 15.67% and the maximum at 37.65%. Comparatively, the soil organic matter content of farm A was significantly higher than farm B with an 11.07% difference in mean average. This could be due to influence of pH levels affecting the organic matter content. Several possible factors such as climate, soil texture, soil hydrology, land use, and vegetation affect the outcomes of agricultural soil organic matter content (Du et al., 2021). The type of vegetation plays a part in organic matter content as it increases the buildup of soil organic matter by decreasing wind erosion on soil surface (Du et al., 2021; Wang et al., 2021). Therefore, farm A which consists of contained vegetable plots has high possibility of retaining plant residues in soil, hence, increasing organic matter in soil whereas farm B which has an open and large area has potential of losing plant residues over time. Loss of organic matter in soil is also possibly because of erosion of surface soil which could be due to heavy rainfall (Bot et al., 2005). The coefficient of variation (CV) for organic matter content for farm A was 17.63%, while for farm B, it was 25.30%, indicating the variability in organic matter content within both farms. Although the CV of 17.63% for farm A suggests a relatively lower degree of variation, while indicating relatively consistent results, the CV of 25.30% for farm B indicates a slightly higher degree of variability but is still within an acceptable range. Regardless of the differences in CV percentages between farm A and farm B, it can be concluded that the organic matter measurements on both farms are reasonably reproducible.
The average particle size of microplastics in the surface soil in farm A was 3260.76 ± 880.38 μm with a minimum particle size of 2920.84 μm and a maximum of 3540.96 μm. Meanwhile, farm B presented smaller particle size of soil microplastics of 2822.31 ± 408.48 μm. The minimum particle size was 2288.58 μm and maximum was 3299.29 μm. Degradation of plastics to microplastics was achieved through physicochemical effects, such as exposure to high temperature and ultraviolet radiation as well as mechanical wear. Therefore, generating the aggregation of plastics of small particle size (Andrady et al., 2022; Song et al., 2017; Yu et al., 2022). Furthermore, in vegetable farmlands, soil microplastics undergo intense physical and mechanical action through agricultural activities, such as cultivation, plowing, seed sowing, harvesting, and frequent tilling of the soil, thus, inducing further deterioration of microplastics into smaller particles (Chen & Yan, 2020; Zhang, Zhao, et al., 2022).
Occurrence of microplastics particles in agricultural farms
The descriptive statistics of microplastics particle size distribution from farm A and farm B are represented in Table 2. Farm A presented findings of microplastic particle size in surface soil with 3260.76 ± 880.38 μm, much larger size than in farm B. This could be due to the fact that the plastic sources on the farm underwent a slow breakdown process as the farm was not exposed to high temperatures as it is located on high grounds. Similarly, exposure to ultraviolet radiation was also limited as there was black plastic netting to protect the vegetation and parts of the farm. Additionally, the black netting limits the exposure to the tropical high temperature and ultraviolet radiation on the white plastic framework and smaller black pipes in the vegetation plots. Furthermore, due to the large size of white pipes, blue-colored storage drums, and plastic trays, it eventually took a longer duration to become microplastic particles of much smaller size. Plastics remain long in the environment and take time to degrade depending on factors, such as temperature, type, and size of plastics (Manzoor et al., 2022). Although farm A carried out agricultural activities, such as soil preparation, seeding, and cultivation, the large microplastic particle size showed that there was little mechanical abrasion on the plastic sources.
In contrast, farm B had agricultural soil microplastic particle size of 2822.31 ± 408.48 μm which was smaller than microplastic particle size in farm A. A reasonable cause could be that the vast and open area in farm B enabled full exposure to the high temperature of the tropics. Another possibility is that some ultraviolet rays may radiate through the farm, hence, causing soil microplastics to be further broken down into smaller-sized particles. Plastic bottles and polystyrene storage boxes were exposed to these harsh environmental factors. Similarly, black plastic films that were used for initial cultivation were exposed to the same environmental effects. Besides, there were many activities on the farm that caused mechanical action on the plastic sources and broke down into small-sized soil microplastics. Constant and intense soil tilling, cultivation, and harvesting, including weeding, are major agricultural activities on large farm which lead to microplastic abrasion, thus, producing smaller microplastic particles. Furthermore, people and vehicle movement in the farm area enhance the microplastics breakdown.
The coefficient of variation (CV) for soil microplastic particle size detected on farm A was 27.00%, while on farm B, it was 14.47%. The CV percentages indicate the variability in soil microplastic particle sizes within both the farms. Although the CV of 27.00% for farm A suggests a relatively higher degree of variation, however, the variability falls within an acceptable range. Meanwhile, the CV of 14.47% for farm B indicates a lower degree of variability, thus indicating relatively consistent findings. Hence, it can be concluded that the soil microplastic particle sizes on both farms are reproducible.
Characterization of microplastics particles in farm A and farm B
The shape distribution of soil microplastics in farm A and farm B is demonstrated in Fig. 3. The dominant shape of microplastics in agricultural soil in both farms was primarily fragments. Farm A obtained a total of 69.9% of fragments and 30.1% of films. Farm B acquired a total of 93.8% of fragments and 6.2% of films. Fig. S3 shows the shapes of fragments and films of agricultural soil microplastics from farm A and farm B.
Soil microplastic particle in fragment shape was widely found in farm A and farm B. These fragment microplastics can be derived from various plastic products. Plastic sources, as observed in farm A, included plastic net covers and plastic frameworks for vegetation plots that were used to protect some of the vegetation from direct sunlight. Additionally, large white and small black pipes were used for water supply to the vegetation plots. Meanwhile, large plastic drums and trays were used as storage containers on the farm. All these agricultural plastic products that are widely used on the farm generally decompose into fragment-shaped microplastics. In addition, transparent shade plastics on the farm may have decomposed into microplastic films. Farm B presented fragments from plastic storage boxes, polystyrene foam boxes, and various types of plastic bottles. Due to the heat in the widely exposed and largely open farm area, these plastic sources tend to degrade into fragment-shaped microplastics. Film-shaped microplastics that were found on farm B were presumably from the black plastic covers or films that were used to protect vegetation during initial cultivation. These plastic films used to preserve soil moisture and prevent weed growth eventually degraded into thin films (Conventional and Biodegradable Plastics in Agriculture, 2021; Mansoor et al., 2022). Furthermore, the black plastic covers were not efficiently disposed and were left on the farm as plastic waste which could have led to film degradation. Apart from that, agricultural activities on farm B, comprising physical and mechanical action, such as plowing to open shallow furrows in the ground for cultivation, irrigation, weeding, and harvesting the crops, tend to cause abrasions, thus, breaking down the plastics into smaller fragments and films.
The soil microplastics in farm A and farm B had highly assorted colors and were classified into eight categories: black, white, transparent, blue, yellow, red, brown, and green. As presented in Fig. 4, the color with the highest average percentage for farm A was white (59%), followed by transparent (28%), blue (7%), yellow (2%), and black (2%); red and brown were the least at 1% each, respectively. Farm B provided findings with the highest average percentage of black (52%), white (37.6%), transparent (5%), blue (2.5%), red (1.6%), and brown (1%); and the lowest was green (0.%). White microplastic particles were the most dominant color from farm A, while black color was dominant in microplastic particles from farm B.
Wide usage of white plastic pipes as frameworks for placing nets over the vegetation may have resulted in the largest proportion of white microplastics in farm A, and the transparent plastic covers contributed to the proportion of transparent microplastics (Fig. S1). Comparatively, farm A had obtained only 2% of black-colored microplastics due to black netting usage for vegetation and seedling soil bags. Apart from the significant white and transparent colors, blue colored microplastics were notably present in farm A. The presence of large, blue-colored plastic drums and trays in farm A were the possible sources of blue microplastics. Sources of the black-colored microplastics in farm B were from plastic covers that were vastly used on the agricultural soil surrounding the newly sprouting vegetation (Fig. S2). Plastic products, such as fertilizer bottles and polystyrene boxes were contributors to white microplastics, followed by transparent microplastics from vegetation covers. There were black and blue plastic bags spotted in farm B which appeared to be wastes and not properly disposed.
The Kruskal Wallis analysis showed a significant difference, with p value in organic matter (p < 0.05) and pH levels (p < 0.05) between both the farms. Contrastingly, for microplastic particle size, the Kruskal Wallis test demonstrated a non-significant difference with p > 0.05, between both farms. Thus, it was concluded that there may not be any significant difference in microplastic particle size between the farms. Although there are variations in the surroundings and backgrounds of both the farms, if the environmental atmosphere such as climate, temperature, humidity, and precipitation are similar, it can lead to comparable microplastic particle size distributions.
Apart from that, a correlation analysis was performed to study the relationship between the particle size of the extracted soil microplastics and environmental factors such as soil organic matter and soil pH. The data set was not normally distributed, hence, a Spearman’s rho test for non-parametric correlation was carried out. Table 3 shows the correlation between the microplastics particle size and environmental factors of farm A and farm B. The microplastics particle size in farm A had a significant and strong positive correlation with the soil organic matter. Zhang, Jin, et al. (2022) documented that the morphology of soil microplastics is affected by the interaction between microplastics and organic matter. Furthermore, the presence of organic matter decreases the repulsion force in soil, hence, promoting aggregation which affects microplastics particle size (Zhang, Jin, et al., 2022). Meanwhile, there is no relationship between microplastics particle size and soil pH in farm A as the correlation is 0.02. Similarly, soil organic matter and soil pH presented a correlation of 0.04, hence, determining a no relationship between both environmental factors in farm A. Farm B presented no relationship between the microplastics particle size and both environmental factors, thus, determining that microplastics particle size are not affected by the tested environmental factors. However, there are possibilities of the microplastics particle size being affected by the existence of other types of environmental factors such as temperature and ultraviolet radiation, agricultural activities, and presence of soil organisms. Microplastics particle size in agricultural soils can vary due to degradation through chemical changes of plastics, that are caused by temperature fluctuations and ultraviolet radiations (Chen et al., 2020b; Qi et al., 2020; Y. Zhou et al., 2020). Additionally, Chen et al. (2020a) reported that anthropogenic activities such as plowing, cultivation, weeding, and harvesting can possibly affect microplastics particle size due to mechanical abrasions. A recent study by Feng et al. (2023) found that diverse insects and microorganisms have a vital role in plastic biodegradation. Hence, various types of environmental factors have a hand in shaping the particle size of soil microplastics.
Plastics polymer identification using ATR-FTIR analysis
To further understand the composition of the soil microplastic particles, ten selected microplastics particles (five from each farm) were selected as representatives for the analysis by using attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR). Among the ten microplastic particles, only nine was analyzed as one sample was deemed insufficient, and the results were compared against a spectrum library for polymer type. The FTIR spectrum range was from 650 to 4000 cm−1. Fig. S4 represents the spectrum of the nine microplastic samples. The identified polymer compositions were polyvinyl chloride (PVC), high-density polyethylene (HDPE), polycarbonate (PC), and polystyrene (PS).
The analysis of the microplastics presented characteristic peaks that were consistent with the polymer spectral library. Sample FA_1 showed peaks at 2918 cm−1, 1457 cm−1, 912 cm−1, and 688 cm−1 followed by FA_2 with 2950 cm−1, 1451 cm−1, 912 cm−1, and 690 cm−1. FA_3 showed peaks at 2917 cm−1, 1451 cm−1, 912 cm−1, and 694 cm−1; FB_1 peaked at 2968 cm−1, 1425 cm−1, 912 cm−1, and 690 cm−1, and FA_4 had characteristic peaks at 2949 cm−1, 1451 cm−1, 913 cm−1, and 690 cm−1. The polymer spectral library revealed characteristic peaks of polyvinylchloride (PVC) plastics near 2913 cm−1, 1426 cm−1, 961 cm−1, and 687 cm−1. Peaks near 2910 cm−1 and 2972 cm−1 represented CH2 asymmetrical stretching, 1451 cm−1 with C–H stretching, and near 691 cm−1 represented trans C–H wagging. Therefore, the characteristic peaks of samples FA_1, FA_2, FA_3, FB_1, and FA_4 were consistent with the characteristic peaks of PVC.
Two samples, FB_2 and FB_3 represented high-density polyethylene (HDPE) with characteristic peaks at 2915 cm−1, 2848 cm−1, 716 cm−1 and 2915 cm−1, 2848 cm−1, and 717 cm−1, respectively. The samples matched the peaks of HDPE spectrum at 2916 cm−1, 2848 cm−1, and 719 cm−1. The peak of 2915 cm−1 had CH2 asymmetric C–H stretch; 2848 cm−1 had CH2 symmetric C–H stretch, and 716 cm−1 and 717 cm−1 had CH2 rock. The polystyrene (PS) polymer was identified through sample FB_4 with peaks at 3593 cm−1, 1452 cm−1, 751 cm−1, and 692 cm−1. The PS spectrum revealed peaks at 3299 cm−1, 1492 cm−1, 753 cm−1, and 697 cm−1. The peaks above 3000 cm−1 had C–H stretches from aromatic rings, 1452 cm−1 had an aromatic ring, and 751 cm−1 with C−H group. Additionally, the polycarbonate (PC) spectrum showed peaks at 1771 cm−1 and 1503 cm−1. Sample FA_5 had peaks at 1768 cm−1 and 1506 cm−1 which were consistent with polycarbonate. Peaks 1768 cm−1 represented carbonyl peaks C=O and 1506 cm−1 with phenol ring stretching. As a result, it can be determined that there were significant particles of PVC, HDPE, PC, and PS in the agricultural soils of farm A and farm B.
The most evident representative of the polymers was of polyvinyl chloride (PVC). Fig. S1 shows large white PVC pipes that were used in farm A for water nitrification process in farming. Additionally, these pipes had smaller outlets that support a small sum of miniature plant growth. Black-colored PVC pipes were also found across the vegetation plots to provide sufficient water to the vegetation. Apart from that, white pipes made of PVC, with a smaller diameter stand over some of the vegetation plots as support for plastic netting to protect the vegetation. High-density polyethylene (HDPE) films were used on farm B for initial land cultivation and crop growth as well as to minimize soil erosion (Fig. S2). However, these HDPE black films were not disposed in a proper manner after use, causing plastic contamination. Polycarbonate that was also detected in surface soil samples was possibly from fertilizer and water bottles. Meanwhile, polystyrene (PS) was from sources of boxes that were probably used as storage boxes. All these possible sources of polymer contributed to microplastic contamination in agricultural soil.
Comparison between current and previous studies on microplastics particles in agricultural soil
A comparison of the current and previous studies on microplastics characteristics in agricultural soil is presented in Table 4. The current study revealed that both farm A and farm B had a distribution of microplastics with much larger particle sizes as compared to the findings of other related studies.
Although the different methodologies adopted in these studies have made the comparison across the studies difficult, differences in size distribution were due to agricultural soil sampling from various depths and exposure to plastic breakdown as opposed to surface soil sampling in the current study. Even so, other studies have findings of soil microplastic particle size which are smaller than 500 μm (Cao et al., 2021; Chen & Yan, 2020). Previous studies had also established findings of microplastic particles that were < 1000 μm in size, with 75.5% from farmlands in Yunnan (Zhang, Wang, et al., 2022) and 48.8% from agricultural soils in Shanghai (Liu, Wang, et al., 2022), and Yu et al. (2021) disclosed similar results with 84.4% of farmland soil microplastics. Yang et al. (2021) stated that plastic debris is broken down to smaller sized particles through several physical and chemical effects, such as temperature, ultraviolet radiation, and mechanical wear. In previous studies, the range of microplastic particle size was particularly < 1000 μm. Although this current study sampled surface agricultural soil, the microplastic particle size was mostly > 2000 μm. This outcome allows the possibilities that in previous studies, there has been exposures to ultraviolet rays and high temperatures for a long duration on the plastics as compared to farm A and farm B. Furthermore, mechanical abrasion on the plastics, due to physical and agricultural activities in both farms (current study), could have been less vigorous than in previous studies. A recent study by Ren and Ni (2022) investigated the combined effects of mechanical abrasion driven by wind, and ultraviolet ray exposure on microplastics break down on agricultural plastic films namely polyethylene (PE) and polyvinyl chloride (PVC). Apart from the mechanical abrasion from the friction of plastics with soil particles due to agricultural activities, wind speed also plays a part in the breakdown of microplastics to smaller particle sizes. However, the limitation is that the study was applied only to transparent agricultural plastic films, and further research is required for non-transparent agricultural plastic films.
The current study presented microplastic shapes of fragments and films on the surface agricultural soil. Similarly, findings in previous studies suggested that an average of 51.7% of agricultural soil microplastics in the suburbs of Wuhan, China were fragments (Chen & Yan, 2020; Xu et al., 2022; Zhang, Zhao, et al., 2022). Some possible sources of microplastic particles in fragment shape were polyethylene nets, plastic storage containers, foam boxes, and plastic bottles (Chen & Yan, 2020). Films that were identified in the current study were mainly from plastic covers used for the initial cultivation of vegetation. Unlike this current study, previous studies have also identified microplastics, such as foam, pellets, fibers, and microbeads (Chen & Yan, 2020; Yu et al., 2021; Yu et al., 2022; Zhang, Jin, et al., 2022). Kim et al. (2021) stated that through weathering and breakdown process, a higher quantity of plastic products which are not effectively recycled and are inadequately disposed would become primary sources of agricultural soil microplastics with various shapes.
Microplastics that were extracted in the current study were in various colors with black, white, and transparent as the major colors. Similarly, previous studies also had a fair distribution of microplastic colors. The variation in the distribution of color of the agricultural soil microplastics indicates the presence of divergent sources of microplastics (Zhang, Zhang, et al., 2022). Black and transparent microplastics may originate from plastic covers for protecting the vegetation. Meanwhile, other colors may come from plastic bottles and bags, drums and trays, and waste products in the agricultural soils. Zhang, Lykaki, et al. (2022) stated that green microplastics derived from green plastic ropes and woven bags in the study area of agricultural soil in Yunnan, China. The colors of microplastics are highly depending on the plastic products used on agricultural farms.
The comparison study determined that all previous studies as well as the current study had outcomes of prominent polymers indicating the impacts of long-term use of plastic products in agricultural soil. The current study identified four types of polymers, such as polyvinyl chloride (PVC), high-density polyethylene (HDPE), polystyrene (PS), and polycarbonate (PC). Chen and Yan (2020) and Zhang, Wang, et al. (2022) similarly detected PVC in agricultural soils in Wuhan and Sichuan, respectively. Additionally, all comparison studies had identified polyethylene in agricultural soil samples. Polymers like polyamide, polyolefin, polyester, and acrylic that were detected in previous studies were associated with plastic products, such as weathered package bag, plastic basket, plastic tape, agrochemical package bags, synthetic fiber, and woven bags (Cao et al., 2021; Chen & Yan, 2020; Yu et al., 2021; Zhang, Zhao, et al., 2022; Zhou et al., 2021). Zhang, Zhao, et al. (2022) reported that the presence of polystyrene was due to the degradation of styrofoam plastics that were normally used in packaging and thermal insulation for the transport of vegetation in agricultural soil.
Conclusion
This study investigated the presence of surface soil microplastics in two tropical agricultural farms. Surface soil microplastics were identified in both farms with microplastics mean particle size of 3260.76 ± 880.38 μm in farm A and 2822.31 ± 408.48 μm in farm B. The microplastic particle size in the current study was larger, as compared to the sizes of microplastics from other related studies. The dominant and primary shapes of microplastics in both farms were fragments and films. Apart from that, the major colors of microplastics identified in farm A were white and transparent, followed by farm B with black and white. The representatives of soil microplastics were identified as polyvinyl chloride (PVC), polycarbonate (PC), high-density polyethylene (HDPE) and polystyrene (PS). The sources contributing to agricultural soil microplastics were plastic products, such as plastic and foam boxes, pesticide and insecticide bottles, packaging plastic bags, and vegetation covers. This study enables a clearer and better understanding of microplastic pollution in tropical agricultural soil and illustrates as a guide for further research on the threat of microplastics in agriculture.
Data availability
All the data and materials have been reported in this manuscript.
References
Afrin, S., Uddin, M. K., & Rahman, M. M. (2020). Microplastics contamination in the soil from Urban Landfill site, Dhaka, Bangladesh. Heliyon, 6(11). https://doi.org/10.1016/j.heliyon.2020.e05572
Ainali, N. M., Kalaronis, D., Evgenidou, E., Kyzas, G. Z., Bobori, D. C., Kaloyianni, M., Yang, X., Bikiaris, D. N., & Lambropoulou, D. A. (2022). Do poly(lactic acid) microplastics instigate a threat? A perception for their dynamic towards environmental pollution and toxicity. In Science of the Total Environment (Vol. 832). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2022.155014
Allen, S., Allen, D., Karbalaei, S., Maselli, V., & Walker, T. R. (2022). Micro(nano)plastics sources, fate, and effects: What we know after ten years of research. Journal of Hazardous Materials Advances, 6, 100057. https://doi.org/10.1016/j.hazadv.2022.100057
Amelia, T. S. M., Sukri, S. N. F., Jaapar, A. N., Md Amin, R., & Bhubalan, K. (2020). Uptake and egestion of polyhydroxyalkanoate microbeads in the marine copepod Nitokra lacustris pacifica. Journal of Sustainability Science and Management, 15(4), 45–53. https://doi.org/10.46754/jssm.2020.06.005
Andrady, A. L., Barnes, P. W., Bornman, J. F., Gouin, T., Madronich, S., White, C. C., Zepp, R. G., & Jansen, M. A. K. (2022). Oxidation and fragmentation of plastics in a changing environment; from UV-radiation to biological degradation. In Science of the Total Environment (Vol. 851). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2022.158022
Aronhime, S., Calcagno, C., Jajamovich, G. H., Dyvorne, H. A., Robson, P., Dieterich, D., Isabel Fiel, M., Martel-Laferriere, V., Chatterji, M., Rusinek, H., & Taouli, B. (2014). DCE-MRI of the liver: Effect of linear and nonlinear conversions on hepatic perfusion quantification and reproducibility. Journal of Magnetic Resonance Imaging, 40(1), 90–98. https://doi.org/10.1002/jmri.24341
Babel, S., Ta, A. T., Nguyen, T. P. L., Sembiring, E., Setiadi, T., & Sharp, A. (2022). Microplastics pollution in selected rivers from Southeast Asia. APN Science Bulletin, 2022(1), 5–17. https://doi.org/10.30852/sb.2022.1741
Bläsing, M., & Amelung, W. (2018). Plastics in soil: Analytical methods and possible sources. In Science of the Total Environment (Vol. 612, pp. 422–435). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2017.08.086
Bot, A., Benites, J., & Food and Agriculture Organization of the United Nations. (2005). The importance of soil organic matter : Key to drought-resistant soil and sustained food production. Food and Agriculture Organization of the United Nations.
Cao, L., Wu, D., Liu, P., Hu, W., Xu, L., Sun, Y., Wu, Q., Tian, K., Huang, B., Yoon, S. J., Kwon, B. O., & Khim, J. S. (2021). Occurrence, distribution and affecting factors of microplastics in agricultural soils along the lower reaches of Yangtze River, China. Science of the Total Environment, 794. https://doi.org/10.1016/j.scitotenv.2021.148694
Cárdenas-Alcaide, M. F., Godínez-Alemán, J. A., González-González, R. B., Iqbal, H. M. N., & Parra-Saldívar, R. (2022). Environmental impact and mitigation of micro(nano)plastics pollution using green catalytic tools and green analytical methods. Green Analytical Chemistry, 3, 100031. https://doi.org/10.1016/j.greeac.2022.100031
Chen, X., & Yan, N. (2020). A brief overview of renewable plastics. Materials Today Sustainability, 7–8. https://doi.org/10.1016/j.mtsust.2019.100031
Chen, Y., Leng, Y., Liu, X., & Wang, J. (2020a). Microplastic pollution in vegetable farmlands of suburb Wuhan, central China. Environmental Pollution, 257. https://doi.org/10.1016/j.envpol.2019.113449
Chen, Y., Leng, Y., Liu, X., & Wang, J. (2020b). Microplastic pollution in vegetable farmlands of suburb Wuhan,Central China. Environmental Pollution, 257. https://doi.org/10.1016/j.envpol.2019.113449
Conventional and biodegradable plastics in agriculture. (2021). http://www.europa.eu. Accessed 7 Jul 2023.
Du, Z., Gao, B., Ou, C., Du, Z., Yang, J., Batsaikhan, B., Dorjgotov, B., Yun, W., & Zhu, D. (2021). A quantitative analysis of factors influencing organic matter concentration in the topsoil of black soil in northeast China based on spatial heterogeneous patterns. ISPRS International Journal of Geo-Information, 10(5). https://doi.org/10.3390/ijgi10050348
Fan, P., Yu, H., Xi, B., & Tan, W. (2022). A review on the occurrence and influence of biodegradable microplastics in soil ecosystems: Are biodegradable plastics substitute or threat? In Environment International (Vol. 163). Elsevier Ltd. https://doi.org/10.1016/j.envint.2022.107244
FAO. (2021). Assessment of agricultural plastics and their sustainability: A call for action. In Assessment of agricultural plastics and their sustainability: A call for action. FAO. https://doi.org/10.4060/cb7856en
Feng, S., Wang, H., Wang, Y., & Cheng, Q. (2023). A review of the occurrence and degradation of biodegradable microplastics in soil environments. In Science of the Total Environment (Vol. 904). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2023.166855
Gao, H., Yan, C., Liu, Q., Ding, W., Chen, B., & Li, Z. (2019). Effects of plastic mulching and plastic residue on agricultural production: A meta-analysis. In Science of the Total Environment (Vol. 651, pp. 484–492). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2018.09.105
Geyer, R., Gavigan, J., Jackson, A. M., Saccomanno, V. R., Suh, S., & Gleason, M. G. (2022). Quantity and fate of synthetic microfiber emissions from apparel washing in California and strategies for their reduction. Environmental Pollution, 298. https://doi.org/10.1016/j.envpol.2022.118835
Geyer, R., Jambeck, J. R., & Law, K. L. (2017). Production, use, and fate of all plastics ever made. http://advances.sciencemag.org/. Accessed 24 Jul 2023.
Henseler, M., Brandes, E., & Kreins, P. (2019). Microplastics in agricultural soils: A new challenge not only for agro-environmental policy?
Hong, S., Gan, P., & Chen, A. (2019). Environmental controls on soil pH in planted forest and its response to nitrogen deposition. Environmental Research, 172, 159–165. https://doi.org/10.1016/j.envres.2019.02.020
Huo, Y., Dijkstra, F. A., Possell, M., & Singh, B. (2022). Ecotoxicological effects of plastics on plants, soil fauna and microorganisms: A meta-analysis. In Environmental Pollution (Vol. 310). Elsevier Ltd. https://doi.org/10.1016/j.envpol.2022.119892
Hurley, R. R., & Nizzetto, L. (2018). Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. In Current Opinion in Environmental Science and Health (Vol. 1, pp. 6–11). Elsevier B.V. https://doi.org/10.1016/j.coesh.2017.10.006
Hwi, T. Y., Ibrahim, Y. S., & Khalik, W. M. (2020). Microplastic abundance, distribution, and composition in Sungai Dungun, Terengganu, Malaysia. Sains Malaysiana, 49(7), 1479–1490. https://doi.org/10.17576/jsm-2020-4907-01
Ibrahim, N. A., Anuar, M. H. H. K., Mazlan, M. A., Nahar, A. M. A., Alian, S. N. N. S., Zakaria, N. A., Hashim, M. A., & Kamil, K. A. (2022). Abundance, uptake, presence and biodegradation of microplastics in Malaysian context: A systematic literature review. Journal of Sustainability Science and Management, 17(2), 221–239. https://doi.org/10.46754/jssm.2022.02.016
Kader, M. A., Singha, A., Begum, M. A., Jewel, A., Khan, F. H., & Khan, N. I. (2019). Mulching as water-saving technique in dryland agriculture: Review article. Bulletin of the National Research Centre, 43(1). https://doi.org/10.1186/s42269-019-0186-7
Karami, A., Golieskardi, A., Ho, Y. B., Larat, V., & Salamatinia, B. (2017). Microplastics in eviscerated flesh and excised organs of dried fish. Scientific Reports, 7(1). https://doi.org/10.1038/s41598-017-05828-6
Karami, A., Romano, N., Galloway, T., & Hamzah, H. (2016). Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environmental Research, 151, 58–70. https://doi.org/10.1016/j.envres.2016.07.024
Karbalaei, S., Golieskardi, A., Hamzah, H. B., Abdulwahid, S., Hanachi, P., Walker, T. R., & Karami, A. (2019). Abundance and characteristics of microplastics in commercial marine fish from Malaysia. Marine Pollution Bulletin, 148, 5–15. https://doi.org/10.1016/j.marpolbul.2019.07.072
Khalik, W. M. A. W. M., Ibrahim, Y. S., Tuan Anuar, S., Govindasamy, S., & Baharuddin, N. F. (2018). Microplastics analysis in Malaysian marine waters: A field study of Kuala Nerus and Kuantan. Marine Pollution Bulletin, 135, 451–457. https://doi.org/10.1016/j.marpolbul.2018.07.052
Kim, S. K., Kim, J. S., Lee, H., & Lee, H. J. (2021). Abundance and characteristics of microplastics in soils with different agricultural practices: Importance of sources with internal origin and environmental fate. Journal of Hazardous Materials, 403. https://doi.org/10.1016/j.jhazmat.2020.123997
Kumar, R., Manna, C., Padha, S., Verma, A., Sharma, P., Dhar, A., Ghosh, A., & Bhattacharya, P. (2022). Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere, 298. https://doi.org/10.1016/j.chemosphere.2022.134267
Li, J., Song, Y., & Cai, Y. (2020). Focus topics on microplastics in soil: Analytical methods, occurrence, transport, and ecological risks. In Environmental Pollution (Vol. 257). Elsevier Ltd. https://doi.org/10.1016/j.envpol.2019.113570
Lian, J., Wu, J., Xiong, H., Zeb, A., Yang, T., Su, X., Su, L., & Liu, W. (2020). Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). Journal of Hazardous Materials, 385. https://doi.org/10.1016/j.jhazmat.2019.121620
Liu, B., Li, W., Pan, X., & Zhang, D. (2022). The persistently breaking trade-offs of three-decade plastic film mulching: Microplastic pollution, soil degradation and reduced cotton yield. Journal of Hazardous Materials, 439. https://doi.org/10.1016/j.jhazmat.2022.129586
Liu, H., Wang, X., Shi, Q., Liu, Y., Lei, H., & Chen, Y. (2022). Microplastics in arid soils: Impact of different cropping systems (Altay, Xinjiang). Environmental Pollution, 303. https://doi.org/10.1016/j.envpol.2022.119162
Liwarska-Bizukojc, E. (2021). Effect of (bio)plastics on soil environment: A review. In Science of the Total Environment (Vol. 795). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2021.148889
Lusher, A. L., Bråte, I. L. N., Munno, K., Hurley, R. R., & Welden, N. A. (2020). Is it or isn’t it: The importance of visual classification in microplastic characterization. Applied Spectroscopy, 74(9), 1139–1153. https://doi.org/10.1177/0003702820930733
Mansoor, Z., Tchuenbou-Magaia, F., Kowalczuk, M., Adamus, G., Manning, G., Parati, M., Radecka, I., & Khan, H. (2022). Polymers use as mulch films in agriculture—A review of history, problems and current trends. Polymers, 14(23), 5062. https://doi.org/10.3390/polym14235062
Manzoor, S., Naqash, N., Rashid, G., & Singh, R. (2022). Plastic material degradation and formation of microplastic in the environment: A review. Materials Today: Proceedings, 56, 3254–3260. https://doi.org/10.1016/j.matpr.2021.09.379
Mbachu, O., Jenkins, G., Pratt, C., & Kaparaju, P. (2021). Enzymatic purification of microplastics in soil. MethodsX, 8. https://doi.org/10.1016/j.mex.2021.101254
Md Amin, R., Sohaimi, E. S., Anuar, S. T., & Bachok, Z. (2020). Microplastic ingestion by zooplankton in Terengganu coastal waters, southern South China Sea. Marine Pollution Bulletin, 150. https://doi.org/10.1016/j.marpolbul.2019.110616
Neves, C. V., Gaylarde, C. C., Neto, J. A., Vieira, K. S., Pierri, B., Waite, C. C., Scott, D. C., & da Fonseca, E. M. (2022). The transfer and resulting negative effects of nano- and micro-plastics through the aquatic trophic web—A discreet threat to human health. Water Biology and Security, 100080. https://doi.org/10.1016/j.watbs.2022.100080
Pariatamby, A., Hamid, F. S., Bhatti, M. S., Anuar, N., & Anuar, N. (2020). Status of microplastic pollution in aquatic ecosystem with a case study on cherating river, Malaysia. Journal of Engineering and Technological Sciences, 52(2), 222–241. https://doi.org/10.5614/j.eng.technol.sci.2020.52.2.7
Pérez-Reverón, R., Álvarez-Méndez, S. J., Kropp, R. M., Perdomo-González, A., Hernández-Borges, J., & Díaz-Peña, F. J. (2022). Microplastics in agricultural systems: Analytical methodologies and effects on soil quality and crop yield. Agriculture, 12(8), 1162. https://doi.org/10.3390/agriculture12081162
PlascticsEurope. (2021). Plastics-the Facts 2021 An analysis of European plastics production, demand and waste data. Plastics Europe. https://www.plasticseurope.org
Praveena, S. M., Hisham M. A. F. I., & Nafisyah A. L. (2023). Microplastics pollution in agricultural farms soils; preliminary findings from tropical environment (Klang Valley, Malaysia). Environmental Monitoring and Assessment, 195, 650. https://doi.org/10.1007/s10661-023-11250
Prihandari, R., Karnpanit, W., Kittibunchakul, S., & Kemsawasd, V. (2021). Development of optimal digesting conditions for microplastic analysis in dried seaweed gracilaria fisheri. Foods, 10(9). https://doi.org/10.3390/foods10092118
Priyanka, R., & Govindarajulu, B. (2023). Transportation of microplastics from the rural area into the urban area of the Kosasthalaiyar River in the metropolitan city of Chennai. Regional Studies in Marine Science, 66. https://doi.org/10.1016/j.rsma.2023.103151
Qi, R., Jones, D. L., Li, Z., Liu, Q., & Yan, C. (2020). Behavior of microplastics and plastic film residues in the soil environment: A critical review. In Science of the Total Environment (Vol. 703). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2019.134722
Rawtani, D., Satish, S., & Rao, P. (2023). Flame retardancy of nanocomposites with emphasis on Halloysite nanotubes. Journal of Industrial and Engineering Chemistry, 125, 1–13. https://doi.org/10.1016/j.jiec.2023.04.036
Ren, S. Y., & Ni, H. G. (2022). A method for measuring the emissions of in situ agricultural plastic film microplastics by ultraviolet and mechanical abrasion. Science of the Total Environment, 819. https://doi.org/10.1016/j.scitotenv.2021.152041
Ritchie, H., & Roser, M. (2020). Plastic Pollution-Our World in Data. https://ourworldindata.org/plastic-pollution. Accessed 11 Jul 2023.
Sa’adu, I., & Farsang, A. (2023). Plastic contamination in agricultural soils: a review. In Environmental Sciences Europe (Vol. 35). Springer. https://doi.org/10.1186/s12302-023-00720-9
Sarijan, S., Azman, S., Said, M. I. M., Andu, Y., & Zon, N. F. (2018). Microplastics in sediment from Skudai and Tebrau river, Malaysia: A preliminary study. In MATEC Web of Conferences (Vol. 250). EDP Sciences. https://doi.org/10.1051/matecconf/201825006012
Sintim, H. Y., & Flury, M. (2017). Is biodegradable plastic mulch the solution to agriculture’s plastic problem? In Environmental Science and Technology (Vol. 51, Issue 3, pp. 1068–1069). American Chemical Society. https://doi.org/10.1021/acs.est.6b06042
Song, Y. K., Hong, S. H., Jang, M., Han, G. M., Jung, S. W., & Shim, W. J. (2017). Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environmental Science and Technology, 51(8), 4368–4376. https://doi.org/10.1021/acs.est.6b06155
Suaria, G., Avio, C. G., Mineo, A., Lattin, G. L., Magaldi, M. G., Belmonte, G., Moore, C. J., Regoli, F., & Aliani, S. (2016). The Mediterranean plastic soup: Synthetic polymers in Mediterranean surface waters. Scientific Reports, 6. https://doi.org/10.1038/srep37551
Tong, H., Zhong, X., Duan, Z., Yi, X., Cheng, F., Xu, W., & Yang, X. (2022). Micro- and nanoplastics released from biodegradable and conventional plastics during degradation: Formation, aging factors, and toxicity. Science of the Total Environment, 833. https://doi.org/10.1016/j.scitotenv.2022.155275
Truong, T.-N.-S., Strady, E., Kieu-Le, T.-C., Tran, Q.-V., Le, T.-M.-T., & Thuong, Q.-T. (2021). Microplastic in atmospheric fallouts of a developing Southeast Asian megacity under tropical climate. https://doi.org/10.1016/j.chemosphere.2021.129874ï
Wang, K., Chen, W., Tian, J., Niu, F., Xing, Y., Wu, Y., Zhang, R., Zheng, J., & Xu, L. (2022). Accumulation of microplastics in greenhouse soil after long-term plastic film mulching in Beijing, China. Science of the Total Environment, 828. https://doi.org/10.1016/j.scitotenv.2022.154544
Wang, Y., Zhang, Z., & Chen, X. (2021). Quantifying influences of natural and anthropogenic factors on vegetation changes based on geodetector: A case study in the Poyang lake basin, China. Remote Sensing, 13(24). https://doi.org/10.3390/rs13245081
Wu, X., Lu, J., Du, M., Xu, X., Beiyuan, J., Sarkar, B., Bolan, N., Xu, W., Xu, S., Chen, X., Wu, F., & Wang, H. (2021). Particulate plastics-plant interaction in soil and its implications: A review. In Science of the Total Environment (Vol. 792). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2021.148337
Wu, Z., Sun, X., Sun, Y., Yan, J., Zhao, Y., & Chen, J. (2022). Soil acidification and factors controlling topsoil pH shift of cropland in central China from 2008 to 2018. Geoderma, 408. https://doi.org/10.1016/j.geoderma.2021.115586
Xu, G., Yang, L., Xu, L., & Yang, J. (2022). Soil microplastic pollution under different land uses in tropics, southwestern China. Chemosphere, 289. https://doi.org/10.1016/j.chemosphere.2021.133176
Yang, L., Zhang, Y., Kang, S., Wang, Z., & Wu, C. (2021). Microplastics in soil: A review on methods, occurrence, sources, and potential risk. In Science of the Total Environment (Vol. 780). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2021.146546
Yu, H., Zhang, Y., Tan, W., & Zhang, Z. (2022). Microplastics as an emerging environmental pollutant in agricultural soils: Effects on ecosystems and human health. In Frontiers in Environmental Science (Vol. 10). Frontiers Media S.A. https://doi.org/10.3389/fenvs.2022.855292
Yu, L., Zhang, J., Liu, Y., Chen, L., Tao, S., & Liu, W. (2021). Distribution characteristics of microplastics in agricultural soils from the largest vegetable production base in China. Science of the Total Environment, 756. https://doi.org/10.1016/j.scitotenv.2020.143860
Zhang, H., Jin, T., Geng, M., Cui, K., Peng, J., Luo, G., Delgado, A. N., Zhou, Y., Liu, J., & Fei, J. (2022). Occurrence of microplastics from plastic fragments in cultivated soil of Sichuan Province: The key controls. Water (Switzerland), 14(9). https://doi.org/10.3390/w14091417
Zhang, J., Zhang, Q., Maa, J. P. Y., Shen, X., Liang, J., Yu, L., Ge, L., & Wang, G. (2022). Effects of organic matter on interaction forces between polystyrene microplastics: An experimental study. Science of the Total Environment, 844. https://doi.org/10.1016/j.scitotenv.2022.157186
Zhang, Y., Wang, K., Chen, W., Ba, Y., Khan, K., Chen, W., Tu, C., Chen, C., & Xu, L. (2022). Effects of land use and landscape on the occurrence and distribution of microplastics in soil, China. Science of the Total Environment, 847. https://doi.org/10.1016/j.scitotenv.2022.157598
Zhang, Y. Q., Lykaki, M., Markiewicz, M., Alrajoula, M. T., Kraas, C., & Stolte, S. (2022). Environmental contamination by microplastics originating from textiles: Emission, transport, fate and toxicity. Journal of Hazardous Materials, 430. https://doi.org/10.1016/j.jhazmat.2022.128453
Zhang, Z., Zhao, S., Chen, L., Duan, C., Zhang, X., & Fang, L. (2022). A review of microplastics in soil: Occurrence, analytical methods, combined contamination and risks. Environmental Pollution, 306. https://doi.org/10.1016/j.envpol.2022.119374
Zhou, J., Wen, Y., Marshall, M. R., Zhao, J., Gui, H., Yang, Y., Zeng, Z., Jones, D. L., & Zang, H. (2021). Microplastics as an emerging threat to plant and soil health in agroecosystems. In Science of the Total Environment (Vol. 787). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2021.147444
Zhou, Y., Wang, J., Zou, M., Jia, Z., Zhou, S., & Li, Y. (2020). Microplastics in soils: A review of methods, occurrence, fate, transport, ecological and environmental risks. Science of the Total Environment, 748. https://doi.org/10.1016/j.scitotenv.2020.141368
Funding
The first author’s postgraduate study is funded by Graduate Research Assistance scheme (GRA), Universiti Putra Malaysia. This work is supported by Fundamental Research Grant Scheme, Ministry of Higher Education (Malaysia) with reference code: FRGS/1/2019/WAB05/UPM/02/2.
Author information
Authors and Affiliations
Contributions
Main idea and conceptualization: Sarva Mangala Praveena; investigation, analysis of data, writing—original draft, editing: Shyamala Devi Shanmugam; review and supervision: Sarva Mangala Praveena, Samsuri Abdul Wahid, Josephine Ying Chyi Liew; funding acquisition: Sarva Mangala Praveena
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Consent to participate
All authors give consent to participate in the publication of this manuscript.
Consent for publication
All authors give consent to publish this manuscript in this respective journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2306 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shanmugam, S.D., Praveena, S.M., Wahid, S.A. et al. Occurrence and characteristics of microplastics pollution in tropical agricultural soils in Klang Valley, Malaysia. Environ Monit Assess 196, 144 (2024). https://doi.org/10.1007/s10661-024-12330-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-12330-w