Abstract
Anticoagulant rodenticides (ARs) are increasingly recognized as a threat to non-target species including native wildlife. Fishers (Pekania pennanti) are generally considered deep forest inhabitants that are not expected to have high exposure to ARs. To evaluate the distribution and levels of ARs in fishers, we analyzed liver samples from fisher carcasses (N = 45) opportunistically trapped across Vermont and New Hampshire between 2018 and 2019. Liquid chromatography-mass spectrometry was used to detect and quantify 11 different ARs in the liver tissue of each fisher at the time of trapping. All but one sample analyzed were positive for exposure to ARs, and 84% were positive for more than one type of AR. The most prevalent ARs detected were diphacinone (96%) and brodifacoum (80%). No samples had detectable levels of coumachlor, coumafuryl, difenacoum, pindone, or warfarin. These results are mostly consistent with findings for fishers in California as well as with a variety of rodent specializing avifauna throughout the Northeast USA but, show a higher prevalence of exposure and a different distribution of AR types than other studies. These results help establish current baseline exposure to ARs in fishers in the Northeast USA and suggest that ARs could pose a threat to wild mesocarnivore species in this region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anticoagulant rodenticides (ARs) are widely used to control rodent populations and reduce their damage in both urban and agricultural areas. In general, ARs are vitamin K1 antagonists that limit the synthesis of blood clotting factors (Hellemans et al., 1963) and herein will be categorized into first-, intermediate-, and second-generation ARs as outlined by Rattner and Harvey (2020): first-generation anticoagulant rodenticides (FGARs) include dicoumarol, coumachlor, coumafuryl, and warfarin; intermediate generation anticoagulant rodenticides (IGARs) include chlorophacinone, diphacinone, and pindone; second-generation anticoagulant rodenticides (SGARs) include brodifacoum, bromadiolone, difenacoum, and difethialone. First-generation ARs are less potent and have a shorter half-life, requiring rodents to repeatedly consume bait over several feedings in order to receive a lethal dose. In contrast, SGARs have a longer half-life and are more potent, usually making a single feeding lethal. Intermediate generation ARs fall in between but are more like FGARs. Even if a target rodent has consumed a lethal dose of an AR compound, it will not succumb to the effects until a latent period of four to nine days later (Meehan, 1985). This allows the rodent to repeatedly consume ARs beyond the lethal dose and become even more toxic to potential predators and scavengers. During this latent period, poisoned rodents uncharacteristically spend more time in open areas in a lethargic state—making them more susceptible to predation and enhancing the potential for non-target predator and scavenger exposure (Cox & Smith, 1992). This secondary toxicity and potential biomagnification could have damaging effects in ecosystems. Predators that exclusively or predominantly feed on rodents have proven to be at risk of secondary toxicity. Examples are well documented in avian species in the Northeast USA including four different raptor species in Massachusetts (Murray, 2011, 2017; Murray & Tseng, 2008) and in seven different species across New York, New Jersey, and Connecticut (Stansley et al., 2014; Stone et al., 1999).
While ARs are detectable in a variety of biological samples, the liver is the optimal tissue for AR detection (Imran et al., 2015). Anticoagulant rodenticides will persist in the liver long after detectable levels in tissues like blood have dropped. Attempts to monitor for the presence of ARs in livers of wildlife are normally limited to post-mortem sample analysis, hindering the ability to understand toxicant level changes over time and only providing a snapshot at the time of death.
Currently, ARs are regulated under the Federal Insecticide, Fungicide, and Rodenticide Act by the Environmental Protection Agency (EPA). The act states that ARs need to be strictly kept within 50 feet of a building for consumer use and 50–100 feet from man-made structures for professional and agricultural use, specifically to reduce the potential risk to non-target wildlife (Bradbury, 2008). While these regulations should help protect more rural wildlife that do not regularly approach buildings, AR toxicities in such mammals via secondary exposure have rarely been studied. This could be because wildlife that avoid human populated areas are presumed to have minimal risk to AR exposure.
Fishers (Pekania pennanti) are believed to avoid open and fragmented landscapes and preferentially reside in dense coniferous forests, a habitat that usually keeps them away from densely human-populated areas (Powell, 1993). Habitat loss, human encroachment, and possibly predator release have allowed fisher populations to expand in recent years especially in the Northeast USA where they may be increasingly found in peri-urban areas (LaPoint et al., 2015). Their diet is composed of mice, voles, shrews, rabbits, and a variety of other mostly small to medium-sized mammals, reptiles, birds, insects, berries (Powell, 1993), and even carrion (Zielinski et al., 1999). On the west coast, where some fisher populations are federally endangered, AR toxicity research has been ongoing and has shown fishers to be at high risk (Gabriel et al., 2012, 2015). In the Northeast where fishers are not threatened, AR exposure has not been well studied.
The land use in Vermont (VT) and New Hampshire (NH) is very similar with both states having a mosaic of farmland, human-developed land, and forested land interspersed across the state. Both VT and NH are highly forested 74% and 79%, respectively (Snyder & Sinclair, 2017, USDA Forest Service, 2021), allowing remote habitat for fishers to be secluded from humans and our introduced toxicants. The objective of this study was to determine if opportunistically sampled fishers in VT and NH are being exposed to ARs.
Methods
Sample collection
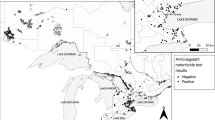
Carcasses were obtained by the Vermont Fish and Wildlife Department (VFWD) from animals legally trapped during the December 1st through December 31st 2018 season. Fishers from the New Hampshire Fish and Game (NHFG) legal trappings were between November 25th and December 31st, 2019. Carcasses are reported to the town area where trapped, not precisely geolocated (Supplemental Tables 1 and 2). The 30 fisher samples from VT were selected from those trapped in 23 different towns across the state with an effort to get a broad geographic distribution and to limit repeated samples (no more than two) from the same town. The 15 samples from NH were from 10 towns and representative of the distribution of harvested animals during the trapping season. The samples analyzed were from 17 of the 24 counties in NH and VT. Fisher age was determined using lower premolar 4 teeth for cementum annulus analysis at a specialized laboratory (Matson’s Laboratory, Manhattan, MT; Arthur et al., 1992, Poole et al., 1994).
After collection, the VT carcasses were stored at − 20 °C until March 2019 when they were allowed to thaw for processing. Livers were dissected from thawed carcasses and placed into polyethylene storage bags (Whirl–pak, Nasco, Fort Atkinson, WI). These samples were re-frozen and shipped to the New Hampshire Veterinary Diagnostics Laboratory (NHVDL) and then transferred to Cummings School of Veterinary Medicine at Tufts where they remained frozen until being subsampled or directly submitted for analysis in May 2019. The NH carcasses were submitted to the NHVDL after harvest, and livers were dissected, placed in Whirl-pak bags, and refrozen. All samples were sent for screening and quantification analysis of eleven ARs at the Pennsylvania Animal Diagnostic Laboratory System (PADLS) Toxicology Laboratory according to standard methods (Vudathala et al., 2010).
Quantitative AR results were based on the calibration curve prepared and run on the day of analysis. If the level of a rodenticide was below the established cutoff, but a peak was seen on the chromatogram at the correct retention time and with an appropriate ion ratio, it was reported as a “trace” amount detected. Screening and quantitative analysis was done for the following compounds at the specified detection limits: brodifacoum (0.010 ppm), bromadiolone (0.025 ppm), chlorophacinone (0.050 ppm), coumachlor (0.100 ppm), coumafuryl (0.100 ppm), dicoumarol (0.100 ppm), difenacoum (0.010 ppm), difethialone (0.050 ppm), diphacinone (0.050 ppm), pindone (0.100 ppm), and warfarin (0.100 ppm).
Prevalence results within our study and with other studies were compared using 2 × 2 contingency tables with two-tailed Fisher’s exact test.
Results
The 45 fishers tested comprised 24 males, 20 females, and 1 unidentified individual and had an age class distribution of 28 juveniles, 9 subadults (~ 1 year old), 7 adults (≥ 2 years old), and 1 that was unidentified. All but 1 fisher (98%) tested positive (trace or higher) for at least 1 of the 11 ARs (Table 1). Most fishers (84%) were positive for more than 1 type of AR, and more than half of fishers (60%) were exposed to 3 or more different AR compounds. Additionally, 1 individual from each state, both male, was exposed to 5 ARs. The most frequently detected AR compound was diphacinone, which is considered intermediate generation and was detected in 43 of the 45 samples (96%) at a range of 0.05 to 0.96 ppm. Brodifacoum was the most frequently detected SGAR with 36 samples (80%) testing positive at levels ranging from 0.01 to 0.47 ppm. The other ARs were detected with the following prevalence: bromadiolone (49%), chlorophacinone (20%), difethialone (20%), and dicoumarol (2%). Aside from 1 sample that had trace levels of dicoumarol, no samples had detectable levels of any FGARS (coumachlor, coumafuryl, difenacoum, or warfarin) nor the IGAR, pindone. Fishers from NH and VT had similar patterns for prevalence and detected levels of the different ARs. The high prevalence of exposure meant fishers positive for at least one AR were found in 32 of 33 towns sampled, which deterred further geographic analysis. With the exception of chlorophacinone and bromadiolone, juvenile fishers had lower prevalence of detected ARs than other age classes, but the only statistically significant difference was their lower prevalence of bromadiolone exposure compared to all older classes (36% vs. 75%, P = 0.027, Fisher’s exact test).
Discussion
The results of this study demonstrate that fishers in the Northeast USA have a high prevalence of exposure to a variety of ARs. Non-target wildlife exposure to ARs in the Northeast is well documented in birds of prey. In Massachusetts (MA), 95–99% of liver samples from birds of prey have been found positive for brodifacoum (Murray, 2011, 2017; Table 2). The high prevalence of brodifacoum exposure in our study (80%) is consistent with these regional findings as well as with a survey of fishers in CA where 92–97% of samples were positive for brodifacoum (Gabriel et al., 2012; Table 2). Our results are also consistent with CA fisher and MA raptor surveys in finding little or no exposure to FGARs, or the SGAR difenacoum, and low to intermediate (3–49%) prevalence of exposure to bromadiolone and difethialone. However, our study found a higher prevalence of IGAR exposure with diphacinone prevalence at 96% being significantly higher than even the most exposed (15%) population of southern Sierra CA fishers (P < 0.0001, Fisher’s exact test). While the exact amount and use of ARs by region are difficult to obtain, a 2017 survey of pest management companies in MA revealed that of the companies surveyed, the compounds predominantly used were bromadiolone, difethialone, brodifacoum, and diphacinone after 2011 (Memmott et al., 2017).
The specific levels of individual AR concentrations are difficult to interpret because these values are labile with expected decline after initial exposure and deposition in the liver. Moreover, the half-life of these compounds in fishers, and indeed any carnivores, is largely unknown (Kopanke et al., 2018). Likewise, lethal doses have not been determined for fishers for any of these compounds and studies of other mustelids have found large disparities even for the most common compound, brodifacoum (Alterio, 1996; Alterio et al., 1997; Fournier-Chambrillon et al., 2004). Clinical disease and presumed death in fishers has been observed in individual fishers in CA with AR concentrations in the liver of 0.38–0.66 ppm brodifacoum, 0.11–0.17 ppm bromadiolone, and trace amounts of chlorophacinone or diphacinone. While we did not detect brodifacoum levels that high in any of the VT fishers, we did find 2 NH fishers within that range (0.39 and 0.47 ppm). Our study also found individuals, which were notably live trapped, with much higher levels of bromadiolone (up to 0.29 ppm) and diphacinone (up to 0.96 ppm).
Although the cause of death for the fishers in our study was exclusively from being trapped for their pelts, sub-lethal AR levels may still be a risk for ineffective blood clotting. Fishers often suffer lacerations from prey while hunting, or through intraspecific trauma while defending territory. Minor wounds that would have otherwise healed could prove much more severe as has been seen in raptors (Erickson & Urban, 2004). Persistent, sub-lethal AR levels may also suppress normal immune functions making predators more susceptible to diseases. Furthermore, neonatal transfer of ARs from a lactating female fisher to altricial kits has been documented (Gabriel et al., 2012) and may affect species fitness.
While this study starts to fill an information gap, there were limitations. Sample collection was opportunistic from available trapped fishers and location data was limited to the town where fishers were trapped. More intentional sampling and specific geolocations from a larger dataset would further our understanding of AR exposure risk in New England fishers and allow proper assessment of risk factors including proximity to human activity and infrastructure. The carcass storage conditions between trapping and initial liver collection presumably varied widely within the maximum of 84 h by which carcasses needed to be tagged by state authorities. The December time frame would predictably ensure a level of natural cold storage though some tissue degradation might occur. However, this should not be a significant factor as AR compounds persist in tissue samples and would only be expected to be higher in fresh tissues; the liver values found in this study are therefore a minimum.
Conclusions
The widespread and often improper use of ARs may be a contributing factor to the bioaccumulation of ARs in rodent populations and could contribute to biomagnification of ARs within non-target species through secondary toxicity. This has important implications for the regulation of ARs and how they might negatively impact broader ecosystems. Larger predators including some common in the Northeast (e.g., coyote, bobcat, and domestic dog) have been known to kill or scavenge fishers (Gabriel et al., 2015), which could compound their own secondary AR exposure from rodents. As past research has shown, the loss of a major predator or predators could severely impact the balance of a sensitive or well-established ecosystem (Sergio et al., 2008; Zielinski, 2014). In addition to these effects in the trophic cascade, ARs may also persist in our ecosystem posing more environmental concerns (Hernández et al., 2013; Liu et al., 2015).
This study shows trapped fishers are highly exposed to a wide spectrum of ARs across Vermont and New Hampshire. Whether this tends to be true of fishers across the Northeast and whether this exposure poses a significant health risk are open but important questions. Further understanding the source of ARs and their influence up the food chain from rodents to fishers is equally important and could help identify possible interventions including policies that may minimize fisher and similar mesocarnivore exposure to ARs. For example, it would be helpful to know whether fishers are directly consuming rat poison, rodents poisoned by legal human AR use, or are consuming forest rodents that might be poisoned from illegal and mislabeled use of ARs.
Importantly, regardless of the source and whether the AR use is legal or not, the near universal exposure of the fishers sampled suggests that AR exposure is widespread and represents an underestimated health risk to wild fishers. It follows that the same risk exists for other mammalian rodent predators such as bobcats, foxes, and marten, all species essential to their ecosystems in the Northeast. Wider use of AR alternatives, policy intervention, more public outreach and education about the propensity for secondary toxicity, and wider surveillance for wildlife health effects are warranted.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Alterio, N. (1996). Secondary poisoning of stoats (Mustela erminea), feral ferrets (Mustela furo), and feral house cats (Felis catus) by the anticoagulant poison, brodifacoum. New Zealand Journal of Zoology, 23(4), 331–338. https://doi.org/10.1080/03014223.1996.9518092
Alterio, N., Brown, K., & Moller, H. (1997). Secondary poisoning of mustelids in a New Zealand Nothofagus forest. Journal of Zoology (1987), 243(4), 863–869. https://doi.org/10.1111/j.1469-7998.1997.tb01986.x
Arthur, S. M., Cross, R. A., Paragi, T. F., & William, B. K. (1992). Precision and utility of cementum annuli for estimating ages of fishers. Wildlife Society Bulletin, 20(4), 402–405.
Bradbury, S. (2008). Risk mitigation decision for ten rodenticides. United States Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substance, Washington DC.
Cox, P., & Smith, R. H. (1992). Rodenticide ecotoxicology: pre-lethal effects of anticoagulants on rat behaviour. Proceedings of the Vertebrate Pest Conference, 15. Retrieved from https://escholarship.org/uc/item/5883j4pn Accessed 16 Mar 2021
Erickson, W. A., & Urban, D. J. (2004). Potential risks of nine rodenticides to birds and nontarget mammals: a comparative approach. US Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Washington, DC, pp. 225. http://pesticideresearch.com/site/docs/bulletins/EPAComparisonRodenticideRisks.pdf Accessed 28 Apr 2021
Fournier-Chambrillon, C., Berny, P. J., Coiffier, O., Barbedienne, P., Dassé, B., Delas, G., Galineau, H., Mazet, A., Pouzenc, P., Rosoux, R., & Fournier, P. (2004). Evidence of secondary poisoning of free-ranging riparian mustelids by anticoagulant rodenticides in France: implications for conservation of European mink (Mustela lutreola). Journal of Wildlife Diseases, 40(4), 688–695. https://doi.org/10.7589/0090-3558-40.4.688
Gabriel, M. W., Woods, L. W., Poppenga, R., Sweitzer, R. A., Thompson, C., Matthews, S. M., Higley, J. M., Keller, S. M., Purcell, K., Barrett, R. H., Wengert, G. M., Sacks, B. N., & Clifford, D. L. (2012). Anticoagulant rodenticides on our public and community lands: spatial distribution of exposure and poisoning of a rare forest carnivore. PLoS ONE. https://doi.org/10.1371/journal.pone.0040163
Gabriel, M. W., Woods, L. W., Wengert, G. M., Stephenson, N., Higley, J. M., Thompson, C., Matthews, S. M., Sweitzer, R. A., Purcell, K., Barrett, R. H., Keller, S. M., Gaffney, P., Jones, M., Poppenga, R., Foley, J. E., Brown, R. N., Clifford, D. L., & Sacks, B. N. (2015). Patterns of natural and human-caused mortality factors of a rare forest carnivore, the fisher (Pekania pennanti) in California. PLoS ONE. https://doi.org/10.1371/journal.pone.0140640
Hellemans, J., Vorlat, M., & Verstraete, M. (1963). Survival time of prothrombin and factors VII, IX and X after completely synthesis blocking doses of coumarin derivatives (Vol. 9).
Hernández, A. M., Bernal, J., Bernal, J. L., Martín, M. T., Caminero, C., & Nozal, M. J. (2013). Simultaneous determination of nine anticoagulant rodenticides in soil and water by LC-ESI-MS. Journal of Separation Science, 36(16), 2593–2601. https://doi.org/10.1002/jssc.201300310
Imran, M., Shafi, H., Wattoo, S. A., Chaudhary, M. T., & Usman, H. F. (2015). Analytical methods for determination of anticoagulant rodenticides in biological samples. Forensic Science International, 253, 94–102. https://doi.org/10.1016/j.forsciint.2015.06.008
Kopanke, J. H., Horak, K. E., Musselman, E., Miller, C. A., Bennett, K., Olver, C. S., Volker, S. F., VandeWoude, S., & Bevins, S. N. (2018). Effects of low-level brodifacoum exposure on the feline immune response. Scientific Reports, 8(1), 8168. https://doi.org/10.1038/s41598-018-26558-3
LaPoint, S. D., Belant, J. L., & Kays, R. W. (2015). Mesopredator release facilitates range expansion in fisher: mesopredator release and range expansion in fisher. Animal Conservation, 18(1), 50–61. https://doi.org/10.1111/acv.12138
Liu, J., Xiong, K., Ye, X., Zhang, J., Yang, Y., & Ji, L. (2015). Toxicity and bioaccumulation of bromadiolone to earthworm Eisenia fetida. Chemosphere (oxford), 135, 250–256. https://doi.org/10.1016/j.chemosphere.2015.04.058
Meehan, A. P. (1985). Rats and mice: their biology and control. The Rentokil Library. The Quarterly Review of Biology, 60(4), 523–524. https://doi.org/10.1086/414646
Memmott, K., Murray, M., & Rutberg, A. (2017). Use of anticoagulant rodenticides by pest management professionals in Massachusetts, USA. Ecotoxicology, 26(1), 90–96. https://doi.org/10.1007/s10646-016-1744-5
Murray, M. (2011). Anticoagulant rodenticide exposure and toxicosis in four species of birds of prey presented to a wildlife clinic in Massachusetts, 2006–2010. Journal of Zoo and Wildlife Medicine, 42(1), 88–97. https://doi.org/10.1638/2010-0188.1. PMID: 22946375.
Murray, M. (2017). Anticoagulant rodenticide exposure and toxicosis in four species of birds of prey in Massachusetts, USA, 2012–2016, in relation to use of rodenticides by pest management professionals. Ecotoxicology, 26(8), 1041–1050. https://doi.org/10.1007/s10646-017-1832-1
Murray, M., & Tseng, F. (2008). Diagnosis and treatment of secondary anticoagulant rodenticide toxicosis in a red-tailed hawk (Buteo jamaicensis). Journal of Avian Medicine and Surgery, 22(1), 41–46. https://doi.org/10.1647/2007-012R.1
Poole, K. G., Matson, G. M., Strickland, M. A., Magoun, A. J., Graf, R. P., & Dix, L. M. (1994). Age and sex determination for American martens and fishers. In S. W. Buskirk, A. S. Harestad, M. G. Raphael, & R. A. Powell (Eds.), Martens, sables, and fishers: biology and conservation.
Powell, R. A. (1993). The fisher: life history, ecology, and behavior (2nd ed.). University of Minnesota Press.
Rattner, B. A., & Harvey, J. J. (2020). Challenges in the interpretation of anticoagulant rodenticide residues and toxicity in predatory and scavenging birds. Pest Management Science. https://doi.org/10.1002/ps.6137
Sergio, F., Caro, T., Brown, D., Clucas, B., Hunter, J., Ketchum, J., Mchugh, K., & Hiraldo, F. (2008). Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Annual Review of Ecology, Evolution, and Systematics, 39(1), 1–19. https://doi.org/10.1146/annurev.ecolsys.39.110707.173545
Snyder, M. C., & Sinclair, S. J. (2017). 2017 Vermont forest action plan. Department of Forests, Parks and Recreation. https://fpr.vermont.gov/sites/fpr/files/Forest_and_Forestry/Vermont_Forests/Library/2017_VT_ForestActionPlan.pdf. Accessed 29 Dec 2022
Stansley, W., Cummings, M., Vudathala, D., & Murphy, L. A. (2014). Anticoagulant rodenticides in red-tailed hawks, Buteo jamaicensis, and great horned owls, Bubo virginianus, from New Jersey, USA, 2008–2010. Bulletin of Environmental Contamination and Toxicology, 92(1), 6–9. https://doi.org/10.1007/s00128-013-1135-z
Stone, W. B., Okoniewski, J. C., & Stedelin, J. R. (1999). Poisoning of wildlife with anticoagulant rodenticides in New York. Journal of Wildlife Diseases, 35(2).
USDA Forest Service. (2021). Forests of New Hampshire, 2020. Resource Update FS-335. Madison, WI: U.S. Department of Agriculture, Forest Service. 2p. https://doi.org/10.2737/FS-RU-335
Vudathala, D., Cummings, M., & Murphy, L. (2010). Analysis of multiple anticoagulant rodenticides in animal blood and liver tissue using principles of QuEChERS method. Journal of Analytical Toxicology, 34(5), 273–279. https://doi.org/10.1093/jat/34.5.273
Zielinski, W. J. (2014). The forest carnivores: marten and fisher. In: J. W. Long, L. Quinn-Davidson, & C. N. Skinner (Eds.), Science synthesis to support socioecological resilience in the Sierra Nevada and southern Cascade Range. Gen. Tech. Rep. PSW-GTR-247. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: 393–435. Chap. 7.1.
Zielinski, W. J., Duncan, N. P., Farmer, E. C., Truex, R. L., Clevenger, A. P., & Barrett, R. H. (1999). Diet of fishers (Martes pennanti) at the southernmost extent of their range. Journal of Mammalogy, 80(3), 961–971. https://doi.org/10.2307/1383266
Acknowledgements
The authors would like to acknowledge the contributions of the anonymous reviewers as well as the following people and organizations: the Vermont and New Hampshire Trappers Association, volunteers and staff at the Vermont Fish and Wildlife Department and New Hampshire Fish and Game Department, Fanwood foundation, Wildlife Heritage Foundation of New Hampshire, USFWS Sport Fish Wildlife Restoration Program, and Tufts Cummings School of Veterinary Medicine particularly, the Center for Conservation Medicine.
Funding
Partial financial support was provided for analysis from the Fanwood Foundation, Wildlife Heritage Foundation of New Hampshire, and USFWS Sport Fish Wildlife Restoration Program.
Author information
Authors and Affiliations
Contributions
Jacqueline Y Buckley and Christopher Whittier were the main drivers for the concept, design, data analysis, interpretation, writing, and prepared tables for the project. David Needle, Walter Cottrell, Patrick Tate, and Kimberly Royar performed sample acquisition and assisted with data interpretation and writing. David Needle additionally coordinated between state agencies and diagnostic laboratories and performed post-mortem sample collections. All Authors provided critical revisions and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Ethics approval
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted. This study made opportunistic use of carcasses from fishers legally harvested and in the possession of the Vermont Fish and Wildlife and New Hampshire Fish and Game Departments and was therefore exempt from the Tufts University and University of New Hampshire Animal Care and Use Committees (IACUC) protocol approval.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buckley, J.Y., Needle, D., Royar, K. et al. High prevalence of anticoagulant rodenticide exposure in New England Fishers (Pekania pennanti). Environ Monit Assess 195, 1348 (2023). https://doi.org/10.1007/s10661-023-11919-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11919-x