Abstract
Increasing reports of cyanobacteria or cyanotoxins around the world expose a major threat for the environment, animal, and human health. Current water treatment processes are ineffective at eliminating cyanotoxins; hence, risk management relies mostly on early detection and on the development of specific regulatory frameworks. In developed countries, well-documented monitoring activities offer a good assessment of the cyanobacterial and/or cyanotoxin status and are used to prevent intoxications. In developing countries such as Peru, despite their potential threat to the environment and public health, cyanobacteria and cyanotoxins are still poorly studied. We found that the regulatory measures regarding cyanobacteria and/or cyanotoxin are almost non-existent. We also present and discuss some examples of recent monitoring efforts underwent by isolated local authorities and scientific reports that, whereas limited, may provide some important insights to be considered nationally. A revision of the available information of planktonic cyanobacteria or cyanotoxins in Peruvian freshwater lentic water bodies revealed a total of 50 documented reports of 15 different genera across 19 water bodies, including the reported highly toxic Dolichospermum and Microcystis. A unique case of microcystin-LR has been documented. We propose some recommendations to be implemented to improve potential toxic cyanobacteria risk management that include incorporating a widespread monitoring of cyanobacterial communities in lakes and reservoirs used for human consumption via specific guidelines. Aligning Peruvian regulations on cyanobacteria and cyanotoxins to international standards may also support law enforcement and ensure compliance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria (known colloquially as “blue-green algae”) are a ubiquitous and diverse group of photosynthetic gram-negative bacteria inhabiting terrestrial, freshwater, and marine ecosystems throughout the planet. They are considered responsible for the oxygenation of early earth’s atmosphere starting more than three billion years ago and were identified as the precursors to chloroplast organelles in eukaryotic algae and higher plants (Miller et al., 2017). Cyanobacteria nowadays play major roles in the biogeochemical cycles. Like algae and terrestrial plants, cyanobacteria contain chlorophyll and other pigments and thus perform photosynthesis. Some cyanobacteria are also capable of fixing nitrogen via specialized cells (Herrero et al., 2001). Cyanobacteria’s abundance is often (but not always) related to eutrophication, which is the general term used by aquatic scientists to describe the suite of symptoms that a lake exhibits in response to overloading of nutrients (mainly nitrogen and phosphorus). One of the most alarming symptoms of eutrophication is the appearance of floating algal blooms, causing high water turbidity and generating anoxia in the deeper parts of lakes from the decay of dead algal material. The lack of oxygen can in turn cause the death of several aquatic organisms such as invertebrates, fish, and amphibians (Schindler et al., 2008). Cyanobacterial blooms can also affect the odor and taste characteristics of water, putting at risk water provisioning.
Some cyanobacteria may produce toxins (called cyanotoxins) that can represent risks of illness and mortality to humans and animals (Carmichael et al., 2001; Svircév et al., 2019). Current water treatment processes are ineffective at eliminating toxins. Thus, risk management relies mostly on the detection of potentially toxic cyanobacteria or cyanotoxins in water and the development of specific regulatory frameworks such as thresholds for water quality standards. The global economic impacts of harmful algal blooms (called HABs) have not been fully calculated, but the cost of eutrophication on fisheries, drinking water treatment, and the health of humans and livestock is likely to be billions of dollars per year (Anderson et al., 2000). Unfortunately, the magnitude and frequency of harmful cyanobacterial blooms and cyanobacteria related poisoning cases has increased in many areas of the planet over the last decades (Svircév et al., 2019). In South America, toxic cyanobacteria and cyanotoxins have mostly been documented in Brazil, followed by some cases in Argentina, Chile, Uruguay (Svircév et al., 2019), and more recently also in Colombia and Peru (Muñoz et al., 2021). A notable example of human health hazard caused by cyanobacteria in South America occurred in Caruaru, Brazil, in February 1996, where an outbreak of acute liver failure occurred at a dialysis center and 116 of 131 patients experienced visual disturbances, nausea, and vomiting after routine hemodialysis treatment and 76 died. The examination of phytoplankton from the dialysis clinic’s water source led to the identification of two groups of cyanobacterial toxins named microcystin and cylindrospermopsin (Carmichael et al., 2001).
In many countries, especially in developing ones, a first general picture of the extent of cyanobacterial and cyanotoxin occurrence is emerging through different isolated activities such as phytoplankton and/or cyanobacterial monitoring programs, cyanotoxin exploration activities, surveys, and also from programs for reducing eutrophication in order to improve the quality of aquatic habitats. Often, these efforts are funded partially by public authorities or by water utilities interested in having a first overview of targeted water bodies or as a spin-off from other programs (e.g., long-standing phytoplankton monitoring of drinking water reservoirs). Based on the findings from such still rare initiatives, measures to protect public health from cyanotoxins such as interdiction of swimming or water consumption have been implemented even if formal regulations are not yet in place.
In Peru, toxic cyanobacteria and cyanotoxins are still poorly explored. However, the potential threat to the environment and public health due to cyanotoxins is getting increasing attention from the media, the scientific community, and water-related authorities (Muñoz et al., 2021). In this document, we start presenting the current regulatory measures related to toxic cyanobacteria in Peru. We also present and discuss some examples of recent monitoring efforts underwent by Peruvian authorities, local governments, and scientific reports that despite their limitations may provide some important insights to be considered by water management authorities. Next, after conducting a literature revision and collating the information available, we provide a general overview about the reports of planktonic cyanobacteria or cyanotoxins in Peruvian freshwater lentic water bodies. We finally propose some improvements for toxic cyanobacteria risk management to be implemented by Peruvian authorities.
Regulatory framework regarding cyanobacteria and cyanotoxins in Peru
Peru has five core regulations regarding the evaluation of water quality:
-
(i)
National strategy to improve the quality of water resources (chief resolution, RJ-N ° 042–2016-ANA).
-
(ii)
National protocol for the monitoring of the quality of water resources (chief resolution, RJ-N °010–2016-ANA).
-
(iii)
Classification of surface continental water bodies (chief resolution, RJ-N ° 056–2018-ANA).
-
(iv)
Environmental quality standards for water, established by Supreme Decree (No. 004–2017-MINAM). MINAM is the Ministry of the Environment.
-
(v)
Regulation of water quality for human consumption (supreme decree, DS No. 031–2010-SA).
In these regulations and according to the categories established globally in the National Environmental Quality Standard for Water (ECA-Water), freshwater bodies are classified into the following four categories:
-
Category 1: population and recreational
-
Category 2: coastal and inland marine extraction and farming activities
-
Category 3: irrigation of vegetables and animal drinking
-
Category 4: conservation of the aquatic environment
According to the current Peruvian legislation, the concentration of cyanotoxins (specifically microcystin-LR) cannot exceed a concentration of 1.0 µg/L for category 1, which is in accordance with the World Health Organization (WHO) safety guidelines for microcystin-LR in source water for human consumption purposes (Chorus & Bartram, 1999). A microbiological parameter is also defined for category 1 water bodies. This is, the concentration of free-living organisms (OVL) (algae, protozoa, copepods, rotifers, nematodes, in all their living stages) should not exceed 5 × 106 organisms per liter. However, OVL does not establish a limit concentration for cyanobacteria alone, which is the metric being used by the WHO in the evaluation of cyanobacteria around the world. For water bodies in categories 2 and 3, no mentions to either cyanobacteria or cyanotoxins exist. For category 4 water bodies, it is only recommended that the chlorophyll-a concentration should not exceed 0.008 mg/L (8.0 µg/L).
The WHO also recommends that guidelines about cyanobacteria thresholds respond to the situation of each country in terms of its geographical location, hydrographic network, land use, and natural levels of phytoplankton productivity. This requires a thorough knowledge of the local situation regarding the magnitude of the distribution of cyanobacteria and cyanotoxins and the type of ecosystem that they affect most frequently (Chorus & Welker, 2021). In Peru, some spontaneous local water authorities such as Autoridad Autónoma de Majes (AUTODEMA) are using a framework that has been developed largely from the perspective of the drinking water supply operators and that is also important for the manager of the raw water supply (Chorus & Bartram, 1999). This framework includes three levels of awareness:
-
(a)
Vigilance level (200 cells/mL cyanobacteria)
-
(b)
Alert level I (2000 cells/mL cyanobacteria)
-
(c)
Alert level II (100,000 cells/mL cyanobacteria)
More recently, Chorus and Welker (2021) published another awareness framework based on cyanobacterial biovolume:
-
(a)
Vigilance level (10 colonies or 50 filaments/mL cyanobacteria)
-
(b)
Alert level I (≥ 0.3 mm.3/L cyanobacteria)
-
(c)
Alert level II ((≥ 4.0 mm.3/L cyanobacteria)
These guidelines are used provisionally in raw water evaluations until the authorities decide a more precise local guideline for cyanobacteria.
Monitoring efforts and inventories regarding cyanobacteria in Peru
Given the importance of protecting and recovering the quality of water resources and identifying potential sources of impact on its quality, the Peruvian National Water Authority (ANA) started surveillance activities since 2009. However, biological parameters such as phytoplankton and cyanobacteria are still not considered in most of these monitoring activities due to the lack of resources, qualified personnel, regulations, and well-defined guidelines. Also, most of limnological research in Peru has been focused in diatoms (Tapia, 2008), and cyanobacteria are still scarcely studied despite that cyanobacteria harmful algae blooms (CyanoHABs) are recognized as the most prevalent and problematic HABs in freshwaters worldwide over more than a decade (Lopez et al., 2008). An interesting case of recent monitoring efforts on cyanobacteria takes place in the Arequipa region. A monitoring carried out since January 2016 by AUTODEMA in the reservoirs belonging to the Chili Regulado and Colca-Siguas system (known as SCHRYCS) includes a monthly water monitoring program that intermittently incorporates cyanobacteria identification and quantification based on microscopy (https://www.autodema.gob.pe/resultados-laboratorio-de-calidad-de-agua/). This water quality surveillance program was established as a result of an event in the city of Arequipa in September 2014, where the population reported alterations in the odor and taste characteristics of the drinking water distributed in the city. Lakes and reservoirs included in the monitoring data are Aguada Blanca, Condorama, Dique de los Españoles, and El Pañe. Unfortunately, these monitoring activities have not been consistent. Not all lakes have been monitored regularly every month, and cyanobacteria identification is often not done. For instance, the monitoring program was almost completely suspended in 2020 due to COVID-19 restrictions. Also, in 2021, sampling sites in several lakes were changed. Biological data, including reports of cyanobacteria using microscopy, started only in January 2017. In any case, the monitoring strategy for the SCHRYCS regarding cyanobacteria was designed as a long-term plan that included activities such as:
-
(i)
Implementation of monthly hydrobiological assays
-
(ii)
Implementation of methodologies for the quantification of abundance and composition of planktonic communities focused on cyanobacteria
-
(iii)
Implementation of an early warning alert on cyanobacteria’s blooms based on estimations of their biomass concentrations
-
(iv)
Implementation of thresholds for cyanobacteria abundance alert levels in drinking water
-
(v)
Identification of potential drivers of contamination that would trigger cyanobacteria bloom formation
This monitoring program (SCHRYCS) allowed, for instance, determining the presence of Dolichospermum cf. circinale, a potentially toxic species in the pelagic zone of the El Pañe reservoir since March 2017 and in Dique los Españoles in April 2022. Hence, the Chilca-Colca system in the Arequipa region sets a precedent in Peru on the inclusion of biological parameters (including cyanobacteria identification and counting) in raw water quality evaluations in the country’s reservoirs, recognizing it as a preventive tool against this type of event.
Reports of planktonic cyanobacteria and cyanotoxins in lakes and reservoirs in Peru
Most freshwater systems used for human consumption in Peru are located in the Andes mountains at high altitude (ca. 3500 to 4200 m.a.s.l.), which makes them hard to sample and to be monitored. As mentioned above, Peruvian lentic freshwater systems have been or are sampled only occasionally or seasonally in search for cyanobacteria or cyanotoxins. In order to gather all the available information regarding previous observations of cyanobacteria and/or cyanotoxins in Peruvian lakes and reservoirs, a detailed search was performed on the Internet for any type of publication (i.e., article, scientific report, technical report, thesis, newspaper, blog, webpage) using the following keywords: cyano* OR cyanobacteria OR cyanotoxins AND Peru OR Peruvian in Spanish or any other language. The search for information on cyanobacteria from Peru also included specialized scientific browsers such as Web of Knowledge, Research Gate, and SciVal. Documents or webpages that included samples from freshwater lakes and reservoirs were retained for further analysis, whereas marine, coastal, or riverine studies were discarded. Finally, only references that presented detailed enough information on the date and location of the sampling procedure in addition to a proper identification of the cyanobacteria or the cyanotoxins present in each water body are reported in this study. Given the scarcity of quantitative information in basic parameters such as abundance or concentration and the heterogeneity on the sampling methodologies, a qualitative (i.e., presence) analysis on the prevalence of cyanobacteria/cyanotoxins was conducted.
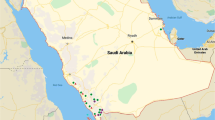
Although scarce, some publications provide taxonomic and qualitative records of potentially toxic cyanobacteria presence at Peruvian lakes or dams (for some examples, see Richerson et al., 1986; Dejoux & Iltis, 1992; Montoya & Golubic, 1991; Montoya, 2004; Komárek & Komárková-Legnerová, 2007; Johnson et al., 2008; Salbatier & Osorio, 2012; Montoya et al., 2014; Mendoza-Carbajal, 2016; Komárková et al., 2016; Montoya et al., 2017; see Muñoz et al., 2021 for a revision). The genus Anabaena was reported as part of phytoplanktonic composition of the Andean lake Paucarani (Tacna region; Franco et al., 1996). Later on, in 2004, the species Anabaena sphaerica, A. spiroides, A. flos-aquae, Microcystis aeruginosa, and Aphanizomenon flos-aquae were registered as forming films at Puerto Viejo lake (Lima region; Montoya, 2004). In 2014, a Microcystis population was also described in the Puno bay in Lake Titicaca (Montoya et al., 2014), and it was mentioned that suppression of the cyanobacterial developments was achieved after the restriction of waste disposal into the lake and by the strict regulation of human activities. The microalgae in the Laguna Los Patos (Sullana province in Piura), between March 2013 and February 2014, were analyzed and qualitatively determined that Anabaena constricta and Oscillatoria tenuis were present throughout the year (Sabalú, 2014). In 2016, for the first time, the cyanobacteria Sphaerocavum brasiliense and Microcystis wesenbergii were reported in an algal bloom in the Huacachina lagoon (Ica region, Mendoza-Carbajal, 2016). At Piuray lake, located in the District of Chinchero (Cusco region), Anabaenopsis was described as one of the main genera in the water column of this water system (Rosas & Miranda, 2015). At Puerto Viejo Lake, in the Cañete province (Lima region), microcystin-LR was registered at a concentration of 327 µg/L (Salbatier & Osorio, 2012). This could represent, to our knowledge, the only public report about this cyanotoxin in Peru to date. Overall, potentially toxic cyanobacteria have been reported in nineteen lentic water bodies across Peru (Table 1, Fig. 1).
In these 19 water bodies, a total of 15 genera (Table 2) of potentially toxic cyanobacteria have been identified, belonging to 4 orders: Chroococcales (2 genera), Nostocales (7 genera), Oscillatoriales (4 genera), and Synechococcales (2 genera). The more widespread genera are Dolichospermum and Oscillatoria, reported in 12 and 10 lakes or reservoirs, respectively. Lakes Huacachina and Lagunillas presented the highest genus richness with seven different taxa of cyanobacteria being reported.
Recommendations
Experts agree that the incidence of cyanobacteria has been increasing worldwide, and with warming climates and continued nutrient loading from human activities, it is very possible that the number and severity of toxic blooms may continue to increase. The evidence presented in this document indicates that, despite the major threats to water security, the current knowledge about the presence of cyanobacteria or cyanotoxins in Peru is very limited. Some interesting initiatives such as the one implemented in the SCHRYCS in the Arequipa region are emerging but remain scarce and inconsistent due to their costs and logistics. It is necessary to specifically include cyanobacteria guidelines and analyses in regular monitoring of lentic systems (lakes and reservoirs) in Peru, especially those used for human consumption. Faced with the health risks exposed in this study (odor/taste alterations and potential toxicity), this biological parameter (cyanobacteria) should be specifically considered in the national water quality standards, so that sampling for quantitative analysis (cells or ml per liter) is carried out regularly. Attention should be drawn to the “reporting unit”; as proposed by the WHO, as cyanobacteria toxicity is produced by “cyanobacteria cells,” however, the Peruvian metric still uses “organisms,” which is not precise enough to carry out adequate evaluations of cyanobacterial risk. Although microcystin-LR are included in water quality standards, cyanobacterial guidelines need to be included in monitoring activities to get a more comprehensive evaluation of the potential risks associated with their presence. Cyanobacteria monitoring could provide an adequate assessment of freshwater algal blooms, along with understanding the causes and consequences of these events (Lopez et al., 2008). Not all cyanobacteria produce toxins, but it has been hypothesized that the aquatic environment with increasing water temperatures will favor the bloom of toxin-producing harmful cyanobacteria (Cheung et al., 2013). Some genus of cyanobacteria described in Peru such as Dolichospermum and Microcystis is highly toxic. Therefore, potential risk of toxic cyanobacterial blooms is a reality for some freshwater systems evaluated in Peru. Complicating the problem is the fact that most lakes for human use in Peru are not currently being targeted for nutrient reductions, because of the absence of blooms so far. However, eutrophication could trigger the dominance by cyanobacteria and the onset of harmful algal blooms in a warmer future. In this context, one preventive tool would be the establishment of monthly monitoring plans for the total phytoplankton community, including cyanobacteria, to evaluate their cellular concentrations in raw water bodies aimed for human consumption. Of the 743 dams inventoried by the National Water Authority (ANA), only a small percentage report hydrobiological analyses of their pelagic zones (emphasis on phytoplankton and cyanobacteria communities). As instances of cyanobacterial blooms increase around the world, there is a need to obtain quantitative data on these biological communities on a regular basis to mitigate events that could compromise the quality of the raw water from which cities are supplied.
Conclusion
There are fast increasing cases of cyanobacterial blooms around the world, and Peru freshwater lentic water bodies seem to be no exception. This is supported by the available, although scarce, reports summarized in this document. Considering the various risks to human health and environmental services that cyanobacteria’s presence implies, further evaluation of their community composition and population dynamics for Peru lentic water systems is urgently needed. Furthermore, to avoid contingencies that can negatively impact the quality of raw water supplies for urban populations, routine monitoring of cyanobacterial communities will be necessary. Moreover, aligning Peruvian regulations on cyanobacteria and cyanotoxins to international standards will be required to allow enforcement and ensure compliance.
Data availability
The datasets generated and analyzed during the current study are presented in the main manuscript.
References
Anderson, D. M., Hoagland, P., Kaoru, Y., & White, A. W. (2000). Estimated annual economic impacts from harmful algal blooms (HABs) in the United States, Technical Report WHOI-2000–11, Woods Hole Oceanographic Institution.
Carmichael, W. W., Azevedo, S. M., An, J. S., Molica, R. J., Jochimsen, E. M., Lau, S., Rinehart, K. L., Shaw, G. R., & Eaglesham, G. K. (2001). Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environmental Health Perspectives, 109(7), 663–668.
Cheung, M. Y., Liang, S., & Lee, J. (2013). Toxin-producing cyanobacteria in freshwater: A review of the problems, effects on drinking water safety, and efforts for protecting public health. Journal of Microbiology, 51, 1–10.
Chorus, I., & Bartram, J. (1999). Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management. E & FN Spon.
Chorus, I., & Welker, M. (2021). Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management (2nd ed.). CRC Press.
Dejoux, C., & Iltis, A. (1992). Lake Titicaca – A synthesis of limnological knowledge. Kluwer Academic Publishers.
Franco, J., Delgado, V., & Sulca, L. (1996). https://revistas.unjbg.edu.pe/index.php/cyd/article/view/101/94. Accessed 13 Dec 2022.
Herrero, A., Muro-Pastor, A. M., & Flores, E. (2001). Nitrogen control in cyanobacteria. Journal of Bacteriology, 183, 411–425.
Johnson, H. E., King, S. R., Banack, S. A., Webster, C., Callanaupa, W. J., & Cox, P. A. (2008). Cyanobacteria (Nostoc commune) used as a dietary item in the Peruvian highlands produce the neurotoxic amino acid BMAA. Journal of Ethnopharmacology, 118, 159–165.
Komárek, J., & Komárková-Legnerová, J. (2007). Several rare freshwater planktic Cyanobacteria (cyanoprokaryotes) from reservoirs in South America. Hoehnea, 34(1), 49–58.
Komárková, J., Montoya, H., & Komárek, J. (2016). Cyanobacterial water bloom of Limnoraphis robusta in the Lago Mayor of Lake Titicaca. Can it develop? Hydrobiologia, 764, 249–258.
Lopez, C. B., Jewett, E. B., Dortch, Q., Walton, B. T., & Hudnell, H. K. (2008). Scientific assessment of freshwater harmful algal blooms. Interagency Working Group on Harmful Algal Blooms, Hypoxia, and Human Health of the Joint Subcommittee on Ocean Science and Technology. Washington, D.C., USA.
Mendoza-Carbajal, L. H. (2016). The genus Sphaerocavum and the dominance of S. brasiliense and Microcystis wesenbergii (Microcystaceae, Cyanophyceae) in the algae bloom of Huacachina lagoon, Peru. Revista Peruana De Biología, 23(1), 053–060.
Miller, T. R., Beversdorf, L. J., Weirich, C. A., & Bartlett, S. L. (2017). Cyanobacterial toxins of the Laurentian Great lakes, their toxicological effects, and numerical limits in drinking water. Marine Drugs, 15(6), 160.
Montoya, H. (2004). Flora y ecología algal del ecosistema lagunar de Puerto Viejo, departamento de Lima. https://sisbib.unmsm.edu.pe/bibvirtualdata/publicaciones/magistri/n1_2006/a01.pdf. Accessed 13 Dec 2022.
Montoya, H., & Golubic, S. (1991). Morphological variability in natural populations of mat-forming cyanobacteria in the Salinas of Huacho, Lima, Peru. Algological Studies, 64, 423–441.
Montoya, H., Gómez, J., Mariano, M., Jara, E., Mayta, E., & Benavente, M. (2017). Phenotypic diversity of the cyanobacterium Pseudophormidium tenue (Oscillatoriales, Microcoleaceae), new record for Peru. Arnaldoa, 24(1), 369–382.
Montoya, H., Komárková, J., & Komárek, J. (2014). Cyanobacterial species, potentially forming water-blooms in the Lake Titicaca (Peru). Arnaldoa, 21(2), 381–390.
Muñoz, M., Cirés, S., de Pedro, Z. M., et al. (2021). Overview of toxic cyanobacteria and cyanotoxins in Ibero-American freshwaters: Challenges for risk management and opportunities for removal by advanced technologies. Science of the Total Environment, 761, 143197.
Richerson, P. J., Neale, P. J., Wurtsbaugh, W., Alfaro, T. R., & Warwick, V. (1986). Patterns of temporal variation in Lake Titicaca. A high-altitude tropical lake. I. Background, physical and chemical processes, and primary production. Hydrobiologia, 138, 205–220.
Rosas, J., & Miranda, G. J. (2015). Estructura temporal y espacial de las comunidades planctónicas de la laguna de Piuray – Chinchero – Cusco. Dissertation, Universidad nacional de San Antonio Abad del Cusco. http://repositorio.unsaac.edu.pe/bitstream/handle/20.500.12918/145/253t20150005.pdf?sequence=1&isAllowed=y. Accessed 13 Dec 2022.
Sabalú, C. (2014). Microalgas en la laguna Los Patos, la Horca – Querecotillo, Sullana. Dissertation, Universidad Nacional de Piura. https://repositorio.unp.edu.pe/bitstream/handle/UNP/293/BIS-SAB-MED-14.pdf?sequence=1&isAllowed=y. Accessed 13 Dec 2022.
Salbatier, M., & Osorio, S. (2012). Blooms of toxic cyanobacteria in Peru: first report of microcystin hepatotoxin in recreational lagoons. The Biologist (Lima), vol. 10, Suplemento Especial. https://sisbib.unmsm.edu.pe/bvrevistas/biologist/v10_sup/pdf/a04v10.pdf. Accessed 13 Dec 2022.
Schindler, D. W., Hecky, R. E., Findlay, D. L., Stainton, M. P., Parker, B. R., Paterson, M. J., Beaty, K. G., Lyng, M., & Kasian, S. E. M. (2008). Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. PNAS, 105(32), 11254–11258.
Svircév, Z., Lalić, D., Bojadžija Savić, G., Tokodi, N., Drobac Backović, D., Chen, L., Meriluoto, J., & Codd, G. A. (2019). Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Archives of Toxicology, 93, 2429–2481.
Tapia, P. M. (2008). Diatoms as bioindicators of pollution in the Mantaro River, Central Andes, Peru. International Journal of Environment and Health, 2(1).
Funding
This work was funded by Prociencia Peru, grant # 002–2020, awarded to AS, DR, MSM, and PV.
Author information
Authors and Affiliations
Contributions
AS and PV collected the information presented and wrote the main manuscript. DR, AR, and MSM provided comments and edits to the document.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salazar-Torres, A., Robles, D., Reyes, A. et al. Evaluation of planktonic cyanobacteria in Peruvian freshwater lentic water bodies: prevalence and regulatory framework to aid policy making. Environ Monit Assess 195, 852 (2023). https://doi.org/10.1007/s10661-023-11487-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11487-0