Abstract
Results of a two-dimensional gas chromatograph time of flight mass spectroscopy (GCXGC-TOFMS) determination of polychlorinated dibenzo-p-dioxin (PCDDs) and polychlorinated dibenzofurans (PCDF) in sediments and catfish samples collected from the Msimbazi River are presented here. Samples were extracted using USEPA Method 1613. PCDD/Fs congeners in sediments ranged from 2.0 to 393.0 and 0.7 to 654.8 pg/g in the dry and wet seasons, respectively. 1,2,3,4,7,8,9-HepCDF was detected at the highest concentration, but all were lower than the USA action level of 1000 pg/g. Toxicity for each of the sampling points ranged from 19.7 to 36.5, with a mean concentration of 27.0 pg WHO 2005-TEQ g−1 in the dry season and 2.0 to 38.7 with a mean concentration of 20.7 pg WHO 2005-TEQ g−1 in the wet season. Analysis of variance (ANOVA) showed that there was no significant difference between PCDD/Fs TEQ during the dry and wet seasons (p = 0.08; α = 0.05). The highest TEQ value was estimated at Jangwani in the wet season. Toxicity of PCDD/Fs in catfish collected from the Msimbazi River ranged from 9.3 to 145.2, with a mean of 61.2 pg WHO2005-TEQg−1. Tetrachlorodibenzo dioxin (2, 3, 7, and 8-TCDD) was detected in all fish samples and ranged from 3.5 to 12.7 with a mean of 8.1 pg/g. The concentration of TCDD in fish exceeded the Agency for Toxic Substance and Diseases Registry MRL, thus posing a probable high risk to people whose dietary requirements depend on fish from the Msimbazi River.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated dibenzo-p-dioxin (PCDDs) and polychlorinated dibenzofurans (PCDF) are among persistent organic pollutants (POPs) that are ubiquitous in the environment, primarily as unintentional byproducts of combustion. They persist, bioaccumulate through the food chain, and cause adverse effects on human beings and the ecosystem. Initiatives have been taken globally to reduce the production and release of intentionally produced POPs, such as pesticides and industrial chemicals to the environment. Although PCDD/Fs can be formed naturally in forest fires and volcanic eruptions, their main sources are anthropogenic, such as the open burning and incineration of wastes, as well as emissions from industrial processes (Jones & De Voogt, 1999; Pan et al., 2010; Rovira et al., 2010; UNEP, 2001). Monitoring of POPs is of great concern because of the adverse effects that they inflict on human beings and the environment. PCDD/Fs have acute and chronic effects on the immune, nervous, endocrine, and reproductive systems, including their carcinogenic potential (Bi et al., 2020; Mikolajczyk et al., 2021; USEPA, 1985).
PCDD/Fs enter aquatic ecosystems, persist for long periods of time, and bioaccumulate and biomagnify through the food chain. Studies have reported their presence in sediments and in aquatic species such as fish (Lee et al., 2007; Mikolajczyk et al., 2021; Shin et al., 2020). Exposure of aquatic organisms to PCDD/Fs can also affect them in different ways, such as mortality, decreased growth rate, and weight loss. There are several factors that affect the accumulation of PCDD/Fs in marine organisms. Common factors are body weight, lipid content, and trophic levels. Fish is relatively higher in the food chain; hence, they can bioaccumulate PCDD/Fs in their fat tissues in muscles, 100,000 times the surrounding environment (Castro et al., 2013). Catfish live at the bottom of the river and feed on sediments; hence, they accumulate PCDD/Fs adsorbed on the sediments. This underscores the need to assess PCDD/Fs contaminations in sediments and fish in rivers.
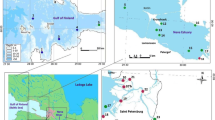
Msimbazi River flows across Dar es Salaam City from the higher areas of Kisarawe in the coastal region of Tanzania. It passes along the industrial and residential areas and discharges into the Indian Ocean. The river is about 35 km long, with three tributaries: Sinza, Ubungo, and Luhanga Rivers (Kondoro, 1992). The river is important to dwellers who depend on vegetable cultivation besides the river. Msimbazi River catchment area is surrounded by industrial effluents, sewer effluents, abattoir effluents, hospital waste incinerators, power stations, high-traffic roads, and massive informal settlements. All these are speculated to be potential sources of PCDD/Fs (Kassenga & Mbuligwe, 2009; Mbuligwe & Kaseva, 2005; Wirgin & Chambers, 2021). Furthermore, the river is misused by people around, in which during dry season massive wastes are dumped and burned. As a result of these activities, there is a need to establish the levels of PCDD/Fs in the Msimbazi River and also to predict their sources and exposure to humans through catfish consumption in order to safeguard human health and the ecosystem.
There has been a historical trend of pollution in the Msimbazi River dating back to 1986. Several studies have been conducted in this river in order to assess heavy metals and organic matter pollution (Ak'habuhaya & Lodenius, 1988; Chanzi, 2017; Mbuligwe & Kaseva, 2005; Mihale, 2021; Miraji et al., 2020; Sawe et al., 2021). Literature showed that no results had been reported on the concentrations of PCDD/Fs despite there being a myriad of potential sources of these pollutants. The aim of this study thus was to establish levels, distribution patterns, and sources of PCDD/Fs in the Msimbazi River and establish toxin exposure patterns of humans through fish consumption.
Materials and methods
Chemicals and reagents
For extraction, HPLC-grade toluene, hexane, and dichloromethane were purchased from Riedel-de Haën (Germany), sulfuric acid (ACS reagent), copper powder, basic alumina, and high purity silica gel (100–200 mesh) were obtained from Sigma-Aldrich, Poole, Dorset, and Dublin (Republic of Ireland). Anhydrous sodium sulfate was purchased from Rochelle Chemicals, Merck, Central Carolina (USA). Cellulose extraction thimbles were purchased from Whatman International Ltd (England). Internal standards (EDF 4145) isotopically labeled and unlabeled PCDD/Fs standards (EDF 4999) were purchased from Cambridge Isotope Laboratories, CIL, Andover, MA (USA).
Sediment sampling
Purposive sampling of sediments was conducted at the Msimbazi River during the dry and wet seasons. Sampling points were chosen based on identified industrial or biomass-burning activities that were speculated to be point sources of PCDD/Fs, as seen in Fig. 1. Samples were collected from the main Msimbazi River and its main tributaries, such as Luhanga, Ubungo, and Sinza (Fig. 1). One sample, i.e., M0, was collected upstream and used as a control to assess the contamination of PCDD/Fs exclusive of the speculated point sources.
The sediments were coded M0–M7 and were sampled at depths of 10–20 cm using a hand corer that was 30 cm long and 6 cm internal diameter (id). Three (3) subsamples were obtained from each sampling point to form a composite sample and mixed on-site. After mixing and homogenizing, about 300 g of sample were wrapped in aluminum foil and zipped into labeled sample bags. Coordinates as seen in Table 1, for each sampling point were recorded using a Geographic Positioning System (GPS).
Sampling in the dry season was done from January to February 2017 and in the wet season from May to June 2017. All the samples were transported to the University of Dar es Salaam, Department of Chemistry, and stored at 4 °C until they were shipped by air on cold chains (USEPA, 1994) to the University of Botswana for extraction and analysis.
Fish sampling
Random fish sampling from the Msimbazi River was done by collecting fish of different sizes. Samples were wrapped in aluminum foil, placed in sample bags, and then in an ice-cooled box. They were frozen until they were shipped by air on cold chains to the University of Botswana for extraction and analysis (USEPA, 1994).
Sediment extraction and clean up before PCDD/F analysis
Sediment samples were air-dried on aluminum foil, ground, and sieved using a 2-mm mesh sieve before extraction. USEPA method 1613 was used for sediments extraction in which forty grams (40 g) of each sample were spiked with 13C-labeled standard containing all the 17 congeners of PCDD/Fs then mixed with an equal amount of anhydrous sodium sulfate (Na2SO4), and then Soxhlet extracted for 16 h with toluene. The extracts were concentrated to 1 mL using a rotary evaporator. The first clean-up was performed by eluting the extracts through a multilayer silica gel column. The extracts were then concentrated to 1 mL and further clean-up was performed through an alumina column from which PCDD/Fs were eluted using 50% dichloromethane in hexane (USEPA, 1994).
Fish extraction before PCDD/Fs analysis
The US EPA Method 1613 was used for extraction. Composite samples were made from fish samples collected from the same sampling point with approximately the same length. All fish were deboned, and muscles were blended using a food processor to form homogenous samples. Then, 30 g of homogenized fish samples were placed in 500-mL bottles with screwed lids and spiked with 13C-labeled standard containing all 17 congeners of PCDD/Fs. Two hundred (200) mL of 6 M HCl were added to the sample, followed by 200 mL of a mixture of methylene chloride and hexane (1:1). The same amount was added to the quality control aliquots. The mixture was shaken for 10 min and vented in the hood to release pressure, then placed on a shaker, which ensured the mixture was in constant motion for 12 h. The mixture was then removed from the shaker and allowed to settle so that the aqueous layer could be separated. The solvent was then decanted with glass fiber and filtered using a column containing 10 g of granular anhydrous Na2SO4. After extraction, acidic silica was used to remove oil, which was then cleaned up and ready for analysis (USEPA, 1994, 2008).
GCXGC-TOFMS instrumentation
GCxGC-TOFMS instrument housed at the University of Witwatersrand in South Africa was used for analysis. The system is a Pegasus 4D, Leco Corp., St. Joseph (USA), equipped with a multi-purpose autosampler (Gerstel, Mülheim, Germany). Liquid nitrogen was used for cold jets and air from an in-house system during modulation. Instrument control and data processing were carried out using ChromaTOF software, version 4.5, Leco Corp., St. Joseph, MI (USA). In this study, one pair of columns, i.e., the Rxi-5 Sil MS/Rtx-200 for GCxGC from the Restek Corporation, Bellefonte, PA (USA) were optimized for primary and secondary separations. The full set of instrumental parameters used were as follow: First dimension column Rxi-5SilMS (30 m, 0.25 mm id, and 0.25 µm df) and second dimension column Rtx-200 (1.1 m × 0.25 mm id × 0.25 µm df). Carrier gas was helium at a flow rate of 1 mL/min in constant flow mode. In total, 2 µL of sample extracts were injected using the splitless mode. The injector temperature was 250 °C. The oven temperatures were programmed as follows: 140 °C for 1 min, then ramped to 180 °C at 30 °C/min and then to 250 °C at 2 °C/min to 300 °C and held there for 20 min. The 2D column temperature was the same as primary, offset by 20 °C with a transfer line temperature of 280 °C and modulator temperature offset of 30 °C for 3 s. The mass range was 100–500 amu at an acquisition rate of 50 spe/s. The detector voltage was 1900 V. Electron ionization was done with electron energy cantered at 70 eV.
Organic matter determination
Samples were air dried and sieved using a 2-mm sieve. Two grams was weighed and placed in crucibles that had been preheated at 400 °C for 1 h. The weight in grams (g) of an empty crucible (W1) was recorded together with the weight of both the crucible and sample after heating in an oven for 24 h at 105 °C (W105). Finally, samples were heated in a furnace for 16 h at 375 °C and then weighed (W375) after cooling. Percent organic matter contents were then calculated using Eq. 1:
where OMC is the organic matter content, W105 is the weight in grams (g) of the sample after heating in an oven at 105 °C for 24 h, and W375 is the weight in grams (g) of the sample after heated in a furnace at 375 °C for 16 h.
Quality control, standards, and method optimization using isotope dilution
The EDF 7999 standard, containing all 17 congeners of PCDD/Fs at different concentrations, was used for method optimization. The concentration of the original standard stock solution was 400 pg/µL for tetrachlorodibenzo dioxin/furans, 4000 pg/µL for octachlorodibenzo dioxins/furans, and 2000 pg/µL for the rest of the congeners. Dilution was done 100- to 200-fold to prepare calibration standards. Isotope dilution was used for the calibration of the native compounds (PCDD/Fs) with labeled analogs according to USEPA method 1613. Since the native and the labeled compounds respond in a similar way at a certain concentration, the relative response (RR) of each native, i.e., relative to its labeled analog, was determined by using the area response of the exact m/z ratios for each calibration standard. The same amounts of labeled compounds were added to every sample prior to analysis. RR values were used with the initial calibration data to determine the concentrations of PCDD/Fs in the extracts. The quality control parameters, i.e., the linearity, the percent recoveries, accuracy, and precision, have been reported by this research group (Pius et al., 2020).
Data analysis
Statistical analysis of data was done using Microsoft Excel 365 and PLS_Toolbox version 9.0 for MATLAB Release 23,592, 2021 copyright (C) 1995–2021 Eigenvector Research, Inc. (PLS Toolbox 9.0, 2021). MATLAB R2021b for academic use was used in this study.
Results and discussion
Dry and wet season organic matter content (OMC) in sediments from the Msimbazi River
The significance of organic matter in relation to PCDD/Fs concentration has been described previously (Pius et al., 2020). Dry and wet seasons % OMC varied from 0.05 to 4.55, with a mean of 0.93, and from 0.01 to 9.39, with a mean of 1.63, respectively. The slightly higher % OMC during the wet season could have been due to heavy rains, which lead to erosion and deposition of organic matter from human habitats to the river (Table 2). A similar study by Carreira et al. (2015) reported the influence of the Paraíba do Sul River in Brazil as a source and distributor of organic matter in sediments. They found high organic matter content in river sediments during the wet season. Although the OMC in this study was slightly higher during the wet season, ANOVA showed that there was no significant difference in the average OMC content in the dry and wet seasons (p = 0.58, α = 0.05). Since the analysis was performed at two different depths, i.e., the upper and lower, it became imperative to correlate the two sampling levels. In the dry season, the % OMC ranged from 0.05 to 2.15 with a mean of 0.84 for the upper-level sediments, while it ranged from 0.05 to 4.55 with a mean of 1.03 for lower-level sediments. In the wet season, the % OMC ranged from 0.03 to 8.16, with a mean of 1.50 for upper-level sediments, and ranged from 0.01 to 9.39, with a mean of 1.75 for lower-level sediments. Although there was a slight difference between the (A) % OMC in the upper and lower-level sediments, ANOVA showed that there was no significant difference in both the dry and wet seasons (p = 0.77 and 0.88, α = 0.05). This confirmed that both the upper and lower organic matter originated from the same source and justified performing PCDD/Fs analysis for a homogenous mixture of the upper and lower sediments.
Downstream closer to the Indian Ocean, in samples M6 and M7, the % OMCs were higher compared to upstream samples in both the dry and the wet seasons. This could be due to the deposition and accumulation of organic matter from all tributaries. The same results were reported in the Paraíba do Sul River in Brazil study (Carreira et al., 2015). Presence of organic matter in sediments of the Msimbazi River was indicative of the presence of other organic pollutants, which justified the analysis of PCDD/Fs in the same samples.
Dry and wet season PCDD/Fs concentration in sediments
The concentrations of 17 congeners of PCDD/Fs in sediments during the dry and wet seasons ranged from 2.0 to 393.0 and 0.7 to 654.8 pg/g dry weight, respectively, as seen in Figs. 2 and 3. Since this was the first PCDD/Fs concentration study in Tanzanian rivers, comparisons were made with results from studies elsewhere. The results were compared with the USA action level set at 1000 pg/g for PCDD/Fs, and all were lower.
From Figs. 2 and 3, congener concentrations varied between the sampling sites, which indicated that the PCDD/Fs in the samples originated from different sources. In the sample M0 collected upstream before discharge from suspected point sources, several congeners of PCDD/Fs were detected, with PCDFs being predominant over PCDDs. This meant that the ratio of PCDDF to PCDD was greater than one (1) and implied that their sources were mainly from open burning. This observation has been observed and reported by Everaert and Baeyens (2002).
In both the dry and wet seasons, regardless of the sampling point, the dominant congeners were 1,2,3,4,6,7,8-HepCDD, 1,2,3,4,6,7,8-HepCDF, 1,2,4,7,8-PeCDD, OctaCDD, and TCDF. High concentrations of highly chlorinated-substituted PCDD/Fs such as octa and hepta signify atmospheric deposition as the major source of PCDD/F. Several studies have also reported the release of PCDD/Fs, especially highly chlorinated substituted congeners, from engines propelled by diesel (Chang et al., 2014; Laroo et al., 2011). It is also possible that the detected PCDD/Fs were deposited in the Msimbazi River from diesel engine vehicles from garages, car wash stations, and high-traffic roads such as Morogoro, Kawawa, SamNujoma, and Mandela roads near or along the river or its tributaries at different points.
During the wet season, in addition to wM0, two more sites (wM6 and wM7) had their PCDDF concentrations higher than PCDDs. This suggests atmospheric deposition as a major source of PCDD/Fs. The concentration of PCDD/Fs at the sampling site close to the Indian Ocean (wM7) in the wet season was higher than during dry season (M7). Since this was the lowest point of the river, this could have been due to sediment-bound PCDD/Fs that had eroded from the upper to the lower steam of the river. Movement of sediment-bound pollutants from upper to lower steams have been reported in the literature (Dinç et al., 2021; Verta et al., 2009; Weber et al., 2008). Concentration of PCDD/Fs in samples collected at Jangwani (M5) was relatively higher than those from other sampling points. This could be in addition to accumulation of sediment-bound PCDD/Fs from other streams such as Ubungo and Luhanga, heavy traffic along Morogoro road, car wash stations, parking slots, and garages.
Toxicity potential of PCDD/Fs at different sampling points along the Msimbazi River
TEQs indicate the magnitude of toxicity of PCDD/Fs congeners relative to 2, 3, 7, and 8-TCDD, which is the most toxic congener assigned a toxic equivalent factor (TEF) of 1. TEQs were calculated by summation of the products of the concentration of individual congeners at every sampling point to toxic equivalent factor (TEF) established by the World Health Organization (WHO). The total PCDD/Fs concentrations varied widely from one point to another along the river and its tributaries. In the dry season, the TEQ varied from 19.7 to 36.5, mean of 27 pg TEQ WHO2005/g, and in the wet season, it varied from 2.0 to 38.72, with a mean of 20.70 pgTEQ WHO2005 g−1, as seen in Fig. 4a and b.
During the dry season, the lowest TEQ value was found upstream of the river (M0). This was the point before the Msimbazi River passed through the suspected point source of contamination. Furthermore, 86% of the TEQ in this site was contributed by 1,2,3,7,8-PeCDD. It was speculated that the PeCDD could have precipitated due to fair deposition since this was the point that was assumed not to be affected by point sources of PCDD/Fs. This observation corroborated with other studies that reported the existence of PeCDD in sediments because of air depositions (Sundqvist et al., 2009; Verta et al., 2007). The highest TEQ was found in Luhanga River (M2), followed by M1, which is the main Msimbazi River at Vingunguti. All these points had their TEQs mainly contributed by low chlorinated PCDD/Fs such as PeCDD and TCDF. The elevated TEQ values in Luhanga River could be attributed to Nida textile factory, which is located near the river. This also corroborates well with other studies that have reported the release of PCDD/Fs to the environment from textile industries (Huang et al., 2021; Klasmeier & McLachlan, 1998; Križanec & Majcen Le Marechal, 2006).
During the wet season, the lowest TEQ was estimated at Sinza River sampling site wM6, followed by sampling site M7, i.e., the last point before the river poured into the Indian Ocean. At this point, there was a mixing of water from the ocean and the river, especially during the wet season. The low TEQ of PCDD/Fs was attributed to the washout of sediment-bound PCDD/Fs to the ocean. The highest TEQ was estimated at Jangwani, sampling site wM5, a point where Ubungo and Luhanga merge with the main Msimbazi River downstream. The elevated TEQ at this point could be due to the movement of sediment-bound PCDD/Fs from upstream and from other tributaries. At this point, there were high traffic and parking slots for the rapid bus transport.
Seasonal variation of TEQ of PCDD/Fs in sediments from the Msimbazi River
Concentrations of PCDD/Fs were lower during the dry season compared to where they were detected during the wet season, as seen in Fig. 5.
During the dry season, the water in the river had receded, and it was speculated that the sediment-bound PCDD/Fs were deposited at the riverbed. During the wet season, there was a big variation of PCDD/Fs from one sampling point to another. This could be attributed to the movement of sediment-bound PCDD/Fs due to the rainwater flow responsible for washing out sediment-bound PCDDFs from one point to another.
However, ANOVA showed that there was no significant difference between PCDD/Fs TEQ values during the dry and wet seasons (p = 0.08; α = 0.05). Notable was the detection of PCDD/Fs upstream of the Msimbazi River (M0), before the suspected PCDD/Fs point sources. This suggested that PCDD/Fs in the Msimbazi River were not only from point sources such as industrial effluent but also atmospheric deposition because of the open burning of wastes and biomass that are common practices in many places in Dar es Salaam.
Additionally, data was subjected to partial least squares discriminant analysis (PLS-DA) using PLS_Toolbox version 9.0 for MATLAB Release 23,592, 2021 copyright (C) 1995–2021 Eigenvector Research, Inc. (PLS Toolbox 9.0, 2021). MATLAB R2021b for academic use was used in this instance. This was done to see if the two classes, i.e., dry and wet seasons, could be distinguished. The PLS-DA model was built using training data and exhibited a receiver operating characteristic (ROC) curve of sensitivity in the wet season concentrations of PCDD/Fs above a threshold of 0.8 and the area under the curve (AUC) of 0.9688, while in the dry season, the values were above 0.8 and 0.9531, respectively. These model parameters were used to predict test data, from which the confusion matrix showed an overall classification error of 12.5%, which was the same as 12.5% for the prediction results. The variance was significant only up to approximately 3 latent variables, as deduced from the X variance captured (%) versus the latent variable. The score plot of latent variable (LV) 1 with a variance of 22.9% versus latent variable (LV) 3 with a variance of 11.96% is shown in Fig. 7.
As seen in Fig. 6, two classes were clearly distinguished for the sediment PCDD/Fs concentrations in the dry and wet seasons. Furthermore, a biplot, i.e., scores plot, with latent variable 1 (LV1) carrying the same variance at 22.9% and LV3 carrying 11.96% superimposed on the loadings plot is shown in Fig. 7. From Fig. 7, the wet season showed higher concentrations of PCDD/Fs, which were closely correlated. The observation underscores the fact that there was movement of sediment from the sampling points where PCDD/Fs were detected in high concentrations during the dry season to the sampling points where they were detected in higher concentrations during the wet season.
The variable importance in projection (VIP) scores versus latent variables showed the following as the most important variables: (i) 1,2,3,7,8-PeCDF, (ii) 1,2,3,4,7,8-HexCDF, (iii) 2,3,4,7,8-PeCDF, (iv) 1,2,4,7,8-PeCDD, and (v) 1,2,3,6,7,8-HexCDD, 1,2,3,4,6,7,8-HepCDD, OctaCDD, and 1,2,3,7,8,9-HexCDF. These PCDD/Fs contributed to the classification observed in the scores and loadings plot and qualified the observation made in Figs. 2 and 3, where the dominant analytes were a subset of these latent variables.
In this study, all the sediments collected along the Msimbazi River exceeded the Interim Sediment Quality Guideline (ISQG) in Canada and USA for dioxins quoted 2,3,7,7-TCDD toxicity equivalents, which are 0.85 and 2.5 ng/kg (dw), respectively. There is a possibility that the sediments could cause detrimental effects on sensitive organisms at all trophic levels (Canadian Council of Ministers of the Environment, 2002; Cook, 1993). Fifty percent (50%) of dry and wet season sediments presented in this study exceeded the American guideline specified as a high risk to sensitive species 25 ng TEQ/kg dw for mammals and wildlife. Except for M0, M1, M4, M6, and M7, all other stations for both wet and dry seasons had their TEQs exceeding the upper limit of 20 pg WHO-dw set by the European Commission for Sediments.
PCDD/Fs reported in this study were lower than the levels reported in River Nile in Egypt. PCDD/Fs in the river Nile were suggested to be high due to commercial formulation of DDT in which PCDD/Fs are found as impurities (El-Kady et al., 2007). This could be the reason why their TEQs were very high. PCDD/Fs reported in this study were relatively higher than the TEQs reported in South Africa, but their sources were closely related to open burning of wood and domestic wastes with small contribution from industrial activities (Nieuwoudt et al., 2009). The river Elbe, in Central Europe was reported to have higher PCDD/Fs concentrations compared to the levels in the Msimbazi River reported in this study (Götz et al., 2017).
PCDD/Fs in catfish collected from the Msimbazi River
Concentrations of PCDD/Fs congeners detected in fish samples are presented as log10concetrations for clarity in Fig. 8. Out of 17 congeners, four, namely, 1,2,4,7,8-PeCDD, 1,2,3,4,6,7,8-HepCDD, and 1,2,3,4,7,8,9-HepCDF were most dominant. Concentrations of these congeners were also high in sediments analyzed from the Msimbazi River, indicating a common source of PCDD/Fs in sediments and in fish.
PCDD/Fs in fish collected from the Msimbazi River presented in WH02005 fresh weight ranged from 9.3 to 145.2, with a mean of 61.2 pg WHO2005-TEQg−1 as seen in Fig. 9.
The TEQs estimated in fish were very high compared to values reported in sediments. This could be due to the long-time accumulation of PCDD/Fs in fish fat tissue compared to accumulation in sediments. The mean concentration of PCDD/Fs was higher than the European Commission regulation value (3.5 pg WHO-TEQ pg/g) fw for muscle meat of fish and fishery products (European Commission, 2011), suggesting a health risk for consumers of fish from the Msimbazi River. 2,3,7,8-TCDD was detected in all fish samples, ranging from 3.5 to 12.7 with a mean of 8.1 pg/g. The minimum risk level for hazardous substances established by the Agency for Toxic Substance and Diseases Registry prescribes a minimum residue level (MRL) of 0.0002 ugkg−1 day−1 of 2,3,7,8-TCDD to be eaten on average by a person of 70 kg (Chou et al., 1998). With a mean value of 8.1 pg/g TCDD obtained in this study, even less than 1 g of fish per person per day would still exceed the MRL. This surfaces a probable high risk of consuming catfish from the Msimbazi River. About 50% of fish samples had their PCDFs concentrations higher than PCDDs, so the ratio of PCDF to PCDD was higher than 1. This suggested that the PCDD/Fs, which accumulated in fish, could have been from open burning of waste and biomass.
Results obtained from this study were higher than values obtained in lake whitefish from the Canadian Great Lakes (22–54 TEQ g−1) (Bhavsar et al., 2008) and those reported in fish from Lake Victoria in East Africa, i.e., 0.001 to 0.09 WHO-TEQ pg/g (Ssebugere et al., 2013).
Conclusions
Results demonstrate that sediments and fish were contaminated by PCDD/Fs at levels higher than international standards, such as the USA action levels for PCDD/Fs, which can cause detrimental effects to human and aquatic life. ANOVA and PLS-DA statistical evaluations showed that elevated concentrations of PCDD/Fs in some segments of the river arose due to movement of sediment-bound PCDD/Fs from the upper to the lower river stream. There was washout of sediment-bound PCDD/Fs at points close to the Indian Ocean, suggesting a large environmental impact of PCDD/Fs from the Msimbazi River. The amount of organic matter positively correlated with PCDD/Fs concentration, suggesting common sources with PCDD/Fs. Classification of congeners suggested different sources of PCDD/Fs. PCDD/Fs were also detected up-stream of the Msimbazi River and attributed to atmospheric deposition from open burning of wastes and biomass, which is a common practice in Dar es Salaam, Tanzania. The concentration of TCDD in fish exceeded the Agency for Toxic Substance and Diseases Registry MRL, thus posing a probable high risk to people whose dietary requirements depend on fish from the Msimbazi River.
Data availability
All data generated or analyzed during this study are included in this published article. If more data are needed for specific purposes, they are available from the corresponding author upon request.
References
Ak’habuhaya, J., & Lodenius, M. (1988). Metal pollution of river Msimbazi, Tanzania. Environment International, 14(6), 511–514. https://doi.org/10.1016/0160-4120(88)90412-6
Bhavsar, S. P., Awad, E., Fletcher, R., Hayton, A., Somers, K. M., Kolic, T., & Reiner, E. J. (2008). Temporal trends and spatial distribution of dioxins and furans in lake trout or lake whitefish from the Canadian Great Lakes. Chemosphere, 73(1), S158–S165. https://doi.org/10.1016/j.chemosphere.2007.05.100
Bi, C., Chen, Y., Zhao, Z., Li, Q., Zhou, Q., Ye, Z., & Ge, X. (2020). Characteristics, sources and health risks of toxic species (PCDD/Fs, PAHs and heavy metals) in PM2.5 during fall and winter in an industrial area. Chemosphere, 238, 124620. https://doi.org/10.1016/j.chemosphere.2019.124620
Canadian Council of Ministers of the Environment. (2002). Canadian environmental quality guidelines (Vol. 2): Canadian Council of Ministers of the Environment.
Carreira, R. S., Cordeiro, L. G., Oliveira, D. R., Baeta, A., & Wagener, A. L. (2015). Source and distribution of organic matter in sediments in the SE Brazilian continental shelf influenced by river discharges: An approach using stable isotopes and molecular markers. Journal of Marine Systems, 141, 80–89. https://doi.org/10.1016/j.jmarsys.2014.05.017
Castro, J., Rotllant, G., Ábalos, M., Parera, J., Dachs, J., & Joan, B. (2013). Accumulation of dioxins in deep-sea crustaceans, fish and sediments from a submarine canyon (NW Mediterranean). Progress in Oceanography, 118, 260–272. https://doi.org/10.1016/j.pocean.2013.07.017
Chang, Y.-C., Lee, W.-J., Wang, L.-C., Yang, H.-H., Cheng, M.-T., Lu, J.-H., & Young, L.-H. (2014). Effects of waste cooking oil-based biodiesel on the toxic organic pollutant emissions from a diesel engine. Applied Energy, 113, 631–638. https://doi.org/10.1016/j.apenergy.2013.08.005
Chanzi, G. (2017). Heavy metal pollution assessment along Msimbazi River, Tanzania. Journal of Scientometric Research, 17(5), 1–8. https://doi.org/10.9734/JSRR/2017/38526
Chou, C.-H.S.J., Holler, J., & De Rosa, C. T. (1998). Minimal risk levels (MRLs) for hazardous substances. Journal of Clean Technology in Environmental Toxicolology and Occupational Medicine, 7(1), 1–24.
Cook, P. (1993). Interim report on data and methods for assessment of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin risks to aquatic life and associated wildlife. Retrieved February 27, 2023, from https://archive.epa.gov/raf/web/pdf/dioxin_risks_aquatic_life_and_wildlife_1993.pdf
Dinç, B., Çelebi, A., Avaz, G., Canlı, O., Güzel, B., Eren, B., & Yetis, U. (2021). Spatial distribution and source identification of persistent organic pollutants in the sediments of the Yeşilırmak River and coastal area in the Black Sea. Marine Pollution Bulletin, 172, 112884. https://doi.org/10.1016/j.marpolbul.2021.112884
El-Kady, A. A., Abdel-Wahhab, M. A., Henkelmann, B., Belal, M. H., Morsi, M. K. S., Galal, S. M., & Schramm, K.-W. (2007). Polychlorinated biphenyl, polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofuran residues in sediments and fish of the River Nile in the Cairo region. Chemosphere, 68(9), 1660–1668. https://doi.org/10.1016/j.chemosphere.2007.03.066
European Commission. (2011). Commission Regulation (EU) no 1259/2011 of 2 December 2011 amending regulation (EC) No 1881/2006 as regards maximum levels for dioxins, dioxin-like PCBs and non dioxin-like PCBs in foodstuffs. Official Journal of the European Union, 320, 18–23.
Everaert, K., & Baeyens, J. (2002). The formation and emission of dioxins in large scale thermal processes. Chemosphere, 46(3), 439–448. https://doi.org/10.1016/S0045-6535(01)00143-6
Götz, R., Bergemann, M., Stachel, B., & Umlauf, G. (2017). Dioxin in the river Elbe. Chemosphere, 183, 229–241. https://doi.org/10.1016/j.chemosphere.2017.05.090
Huang, J., Opoku, P. A., Guang, L., Ke, L., & Norgbey, E. (2021). A multi-emission analysis of organic and inorganic pollutants during the combustion of sludge with high and low calorific value coals. Environmental Science and Pollution Research, 28, 65399–65409. https://doi.org/10.1007/s11356-021-15301-7
Jones, K., & De Voogt, P. (1999). Persistent organic pollutants (POPs): State of the science. Environmental Pollution, 100(1), 209–221. https://doi.org/10.1016/S0269-7491(99)00098-6
Kassenga, G., & Mbuligwe, S. E. (2009). Impacts of a solid waste disposal site on soil, surface water and groundwater quality in Dar es Salaam City, Tanzania. Journal of Sustainable Development in Africa, 10. Retrieved February 27, 2023 from https://jsd-africa.com/Jsda/V10N4_Spring2009/PDF/ImpactSolidDisposalSite.pdf
Klasmeier, J., & McLachlan, M. S. (1998). PCDD/Fs in textiles—part 1: A screening method for detection of octachlorodibenzo-p-dioxin and octachlorodibenzofuran. Chemosphere, 36(7), 1627–1635. https://doi.org/10.1016/S0045-6535(97)10093-5
Kondoro, J. (1992). Dispension of heavy metals along Msimbazi basin in Dar es Salaam, Tanzania. Tanzanian Journal of Science, 23, 1–10.
Križanec, B., & Majcen Le Marechal, A. (2006). Dioxins and dioxin-like persistent organic pollutants in textiles and chemicals in the textile sector. Croatica Chemica Acta, 79(2), 177–186. Retrieved December 27, 2022, from https://hrcak.srce.hr/4120
Laroo, C. A., Schenk, C. R., Sanchez, L. J., & McDonald, J. (2011). Emissions of PCDD/Fs, PCBs, and PAHs from a modern diesel engine equipped with catalyzed emission control systems. Environmental Science Technology, 45(15), 6420–6428. https://doi.org/10.1021/es104220f
Lee, K. T., Lee, J. H., Lee, J. S., Park, K. H., Kim, S. K., Shim, W. J., & Oh, J. R. (2007). Human exposure to dioxin-like compounds in fish and shellfish consumed in South Korea. Human and Ecolological Risk Assessment, 13(1), 223–235. https://doi.org/10.1080/10807030601105365
Mbuligwe, S. E., & Kaseva, M. E. (2005). Pollution and self-cleansing of an urban river in a developing country: A case study in Dar es Salaam, Tanzania. Environmental Management, 36(2), 328–342. https://doi.org/10.1007/s00267-003-0068-4
Mihale, M. J. (2021). Metal contamination in sediments of coastal rivers around Dar es Salaam, Tanzania. Huria Journal, 27(2), 72–92. Retrieved February 27, 2023, from https://journals.out.ac.tz/index.php/huria/article/view/894/720
Mikolajczyk, S., Warenik-Bany, M., & Pajurek, M. (2021). PCDD/Fs and PCBs in Baltic fish–recent data, risk for consumers. Marine Pollution Bulletin, 171, 112763. https://doi.org/10.1016/j.marpolbul.2021.112763
Miraji, H., Mureithi, E., Othman, O. C., & Ngassapa, F. (2020). 1-D transport behaviour of emerging contaminants in the unidirectional flowing surface waters of the Msimbazi River. Tanzanian Journal of Science, 46(2), 371–382.
Nieuwoudt, C., Quinn, L. P., Pieters, R., Jordaan, I., Visser, M., Kylin, H., & Bouwman, H. (2009). Dioxin-like chemicals in soil and sediment from residential and industrial areas in central South Africa. Chemosphere, 76(6), 774–783. https://doi.org/10.1016/j.chemosphere.2009.04.064
Pan, J., Yang, Y., Geng, C., Yeung, L., Cao, X., & Dai, T. (2010). Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and dibenzofurans in marine and lacustrine sediments from the Shandong Peninsula, China. Journal of Hazardous Materials, 176(1), 274–279. https://doi.org/10.1016/j.jhazmat.2009.11.024
Pius, C., Koosaletse-Mswela, P., Sichilongo, K., & Dikinya, O. (2020). Mapping polychlorinated dibenzo-p-dioxins/dibenzofurans in soils around Pugu municipal dump site in Dar es Salaam, Tanzania: implications on dermal and soil ingestion exposure for people in the peripheral. Environmental Pollution, 258, 113665. https://doi.org/10.1007/s11356-018-3629-z
Rovira, J., Mari, M., Nadal, M., Schuhmacher, M., & Domingo, J. L. (2010). Environmental monitoring of metals, PCDD/Fs and PCBs as a complementary tool of biological surveillance to assess human health risks. Chemosphere, 80(10), 1183–1189. https://doi.org/10.1016/j.chemosphere.2010.06.016
Sawe, S. F., Shilla, D. A., & Machiwa, J. F. (2021). Lead (Pb) contamination trends in Msimbazi estuary reconstructed from 210Pb-dated sediment cores (Msimbazi River Tanzania). Environmental Forensics. https://doi.org/10.1080/15275922.2020.1805823
Shin, E.-S., Jeong, Y., Barghi, M., Seo, S.-H., Kwon, S. Y., & Chang, Y.-S. (2020). Internal distribution and fate of persistent organic contaminants (PCDD/Fs, DL-PCBs, HBCDs, TBBPA, and PFASs) in a Bos Taurus. Environmental Pollution, 267, 115306. https://doi.org/10.1016/j.envpol.2020.115306
Ssebugere, P., Kiremire, B. T., Henkelmann, B., Bernhöft, S., Kasozi, G. N., Wasswa, J., & Schramm, K.-W. (2013). PCDD/Fs and dioxin-like PCBs in fish species from Lake Victoria, East Africa. Chemosphere, 92(3), 317–321. https://doi.org/10.1016/j.chemosphere.2013.03.033
Sundqvist, K. L., Tysklind, M., Geladi, P., Cato, I., & Wiberg, K. (2009). Congener fingerprints of tetra-through octa-chlorinated dibenzo-p-dioxins and dibenzofurans in Baltic surface sediments and their relations to potential sources. Chemosphere, 77(5), 612–620. https://doi.org/10.1016/j.chemosphere.2009.08.057
UNEP. (2001). Stockholm convention. Retrieved March 20, 2018, from UNEP http://chm.pops.int/Countries/StatusofRatifications/PartiesandSignatoires/tabid/4500/Default.aspx
USEPA. (1985). Health assessment document for polychlorinated dibenzo -p-dioxins. Washington DC. Retrieved February 27, 2023, from https://www.nal.usda.gov/exhibits/speccoll/items/show/6491
USEPA. (1994). Tetra-through octa-chlorinated dioxins and furans by isotope dilution HRGC/HRMS revision B, 821/B-94–005 Method 1613: office of water engineering and analysis division (4303). Retrieved February 27, 2023, from https://www.epa.gov/sites/default/files/2015-08/documents/method_1613b_1994.pdf
USEPA. (2008). Method 1668B chlorinated biphenyl congeners in water, soil, sediment, biosolids, and tissue by HRGC/HRMS. Retrieved February 27, 2023, from https://f.hubspotusercontent00.net/hubfs/6549100/EPA%20Method%201668B.pdf
Verta, M., Kiviranta, H., Salo, S., Malve, O., Korhonen, M., Verkasalo, P. K., & Päätalo, K. (2009). A decision framework for possible remediation of contaminated sediments in the River Kymijoki, Finland. Environmental Science and Pollution Research, 16(1), 95–105. https://doi.org/10.1007/s11356-008-0061-9
Verta, M., Salo, S., Korhonen, M., Assmuth, T., Kiviranta, H., Koistinen, J., & Tysklind, M. (2007). Dioxin concentrations in sediments of the Baltic Sea - a survey of existing data. Chemosphere, 67(9), 1762–1775. https://doi.org/10.1016/j.chemosphere.2006.05.125
Weber, R., Gaus, C., Tysklind, M., Johnston, P., Forter, M., Hollert, H., & Masunaga, S. (2008). Dioxin-and POP-contaminated sites - contemporary and future relevance and challenges. Environmental Science and Pollution Research, 15(5), 363. https://doi.org/10.1007/s11356-008-0024-1
Wirgin, I., & Chambers, R. C. (2021). Hepatic Burdens of PCB and PCDD/F Congeners in federally endangered shortnose sturgeon and Atlantic sturgeon from the Hudson River, New York, USA: burden patterns and potential consequences in offspring. Retrieved February 27, 2023, from https://doi.org/10.21203/rs.3.rs-1086966/v1
Acknowledgements
The authors would like to thank the University of Botswana for material support. C. Pius thanks Transdisciplinary Training for Resource Efficiency and Climate Change Adaptation in Africa (TRECCAfrica) for financial support.
Author information
Authors and Affiliations
Contributions
Sample collection, preparation, and analysis were performed by Christina Pius. The first draft of the manuscript was written by Christina Pius. Authors Pulane Koosaletse-Mswela, Kwenga Sichilongo, and Oagile Dikinya commented on earlier versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All authors have read, understood, and complied as applicable, with the statement on “Ethical responsibilities of authors” as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.”
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pius, C., Koosaletse-Mswela, P., Dikinya, O. et al. Polychlorinated dibenzo-p-dioxin/dibenzofurans (PCDD/Fs) contamination in sediments and fish from Msimbazi River in Dar es Salam, Tanzania: patterns, sources, and their exposure to humans. Environ Monit Assess 195, 631 (2023). https://doi.org/10.1007/s10661-023-11241-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11241-6