Abstract
Increasing urbanisation and industrialisation of the Visakhapatnam region have brought domestic sewage and industrial wastewater discharge into the coastal ocean. This study examines the indicator and pathogenic bacteria’s quantitative abundance and antibiotic susceptibility. This study collected surface and subsurface water samples from ten different regions (147 stations; 294 samples), including 12 industrial discharge points, surrounding stations and two harbours from the coast of Pydibheemavaram to Tuni. Physicochemical parameters like salinity, temperature, fluorescence, pH, total suspended matter, nutrients, chlorophyll-a and dissolved oxygen showed a difference between regions. We noticed the presence of indicator (Escherichia coli and Enterococcus faecalis) and pathogenic (Aeromonas hydrophila, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella and Shigella, Vibrio cholera and Vibrio parahaemolyticus) bacteria among the samples. Waters from the near harbour and Visakhapatnam steel plant showed lower bacterial load with no direct input from industries to the coastal water. Samples collected during the industrial discharge period had a higher bacterial load, including E. coli. Enteric bacteria were found in higher numbers at most stations. Some isolates were resistant to multiple antibiotics with higher antibiotic resistance and multiple antibiotic resistance indexes compared with the other coastal water habitats in the Bay of Bengal. The occurrence of these bacteria above the standard limits and with multiple antibiotic resistance in the study region may pose a potential threat to the local inhabitants. It can create an alarming situation in the coastal waters in the study region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is considered one of the major carriers of various diseases. More than 5 million people have mortality from water-related diseases annually (Gleick, 2002). Among the water-borne diseases, cholera alone is responsible for 21,000 to 143,000 deaths worldwide caused by a pathogenic bacterium, Vibrio cholerae (Ali et al., 2015). Pathogenic bacteria-mediated diseases are widespread and often fatal after viruses. Antibiotic resistance in pathogenic bacteria makes them multidrug-resistant, contributing to the spread and difficulty in treating the diseases caused by them. Studies from coastal water, mariculture sites (Chen et al., 2017; Zou et al., 2011) and wastewater treatment, pharmaceutical plants (Obayiuwana et al., 2018) have shown the presence of both antibiotic-resistant bacteria and antibiotic residues in them. Furthermore, 40% of the world’s population lives within 100 km of the coast (Farmasi & Dan, 2017). Therefore, these people are always at immediate risk of contracting water-borne diseases from consuming seafood and other water-related activities, including fishing and recreation (Fowsul Ameer, 2017; Majra & Gur, 2009; Ralston et al., 2011).
Developing countries like India, Bangladesh and Myanmar surround the Bay of Bengal region from three sides. The population living in this region is one-third of the world. Among these, India has a vast coastline of 7516.6 km. According to a census by the Center for International Earth Science Information Network (CIESIN, 2007) (http://www.ciesin.org), the lower elevation zones of the East coast of India are densely populated. In addition to the population pressure, the presence of industries, thermal power plants and activities of harbours add to the increasing pollution and diseases along coastal regions. Moreover, Indian water receives a considerable amount of discharge from pharmaceutical companies in terms of active pharmaceutical ingredients (Larsson, 2008). A report by Reddy and Kostenzer (2011) indicates that the East coastal population of India is more vulnerable and at higher risk of contracting diseases.
Visakhapatnam, one of the major cities on the East coast of India, also suffers from industrial-derived pollution. This city’s industrial region falls under India’s eight major industrial regions, with many industries related to maritime, petroleum chemicals, pharmaceuticals, etc. The presence of two ports, one primary and a minor, adds to the industrial activities of this region. The pollution levels and the resulting contaminants in the coastal water of the city have been studied in the past, indicating its rise in recent times (Dileep & Prameela, 2021; Sarma et al., 1996). Microbiological studies have reported that treated/untreated sewage and urban run-off carry disease-causing bacteria that impair the coastal waters the most compared to other contaminants (Naidoo & Olaniran, 2014; Pandey et al., 2014).
Studies by Clark et al. (2003) and Lakshmi et al. (2019) showed coastal waters of Visakhapatnam city have a high pollution index in terms of different indicator organisms such as total coliforms, faecal coliforms and protozoan cysts. The city’s fishing harbour and shipyard are primarily targeted for microbiological pollution studies, which showed that the abundance of selected pathogenic bacteria is higher than that on other city beaches (Babu et al., 2014; Myla et al., 2015; Sailaja et al., 2013). Heterotrophic bacterial counts, total coliforms, Enterococcus faecalis and Pseudomonas aeruginosa-like organisms in off-Visakhapatnam coastal waters were shaped by physicochemical conditions (Sudha Rani et al., 2018). Salinity, temperature and suspended particulate matter among these parameters influenced the presence and abundance of a pathogenic bacterium V. cholerae (Khandeparker et al., 2020).
The city across its coast has many industrial outfalls into the ocean. There are no studies on bacterial abundance and pollution near these industrial discharge regions. However, a study on plankton community composition from this region suggests no impact from the discharge sites (Shaik et al., 2017). The current study targets the coastal waters of the Visakhapatnam district, where major industrial discharge points are spread across a 150-km coastal area. The study aims to determine whether the heterotrophic and pathogenic bacterial abundance varies across the coast, and whether physicochemical parameters of the region have any role to play with the bacterial load. It tries to determine the effect of industrial discharge on the selected pathogenic bacterial groups in the coastal waters. Also, it attempts to detect the antibiotic resistance potential in the bacteria isolated from different discharge points. As part of this study, various physical, chemical and microbiological parameters are measured and analysed from these coastal waters. The parameters are then compared in groups to understand their variability, dependence and underlying cause with support from statistical analyses. The current study gives a primary understanding of various pathogenic bacteria present in these waters and their resistance to the different antibiotics from this region. This kind of study from the industrial coastal belt of Visakhapatnam is crucial and first from this region.
Materials and methods
Study area
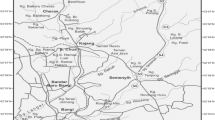
In this study, the coastline from Pydibhimabaram to Tuni in the district of Visakhapatnam was chosen. This region covers ten central sampling locations, including treated effluent discharge points from industries and harbours (Fig. 1). The details of the study sites, industries, type of discharge and the number of stations sampled from each station are listed in Table 1. Except for 12 major discharge points, nearby stations varying in numbers from 9 to 24 surrounding the discharge points are also sampled. A total of 147 stations were sampled as part of this study. All these stations are between 0.5 and 6.6 km from the coast. For easier understanding, the stations are broadly divided into three groups, i.e. north stations (NS), middle stations (MS) and south stations (SS), based on their geographic location and time of collection. The NS included PBM and TPM; the MS included CHP, VSH, GPH and VSP; and the SS included TVM, PDM, NKP and TUN.
Sample collection and analyses
Samples were collected during two time periods. During the 1st phase of sampling (8 to 11 Dec 2018), Water samples from all the stations from CHP, VSH, GPH and VSP were collected. In the 2nd phase of sampling (24 Dec 2018 to 1 Jan 2019), water samples from the rest of the stations were collected. The second sampling phase was conducted to collect water samples from the stations that included industrial discharge points during their respective discharge period. A Niskin bottle (10 l) was used for a sample collection from 147 stations along the study area’s coast. Both surface (below 0.5 m from the surface) and bottom (above 1 m from the bottom) water samples were collected from each station and fixed/preserved accordingly for downstream analyses.
Physicochemical parameters
Vertical profiles of pressure, temperature (°C) and salinity (ppt) were measured using a portable conductivity, temperature and depth profiler (SBE 19 plus; Sea-Bird Electronics, USA). First, bubble-free water is collected for dissolved oxygen (DO) and immediately fixed with Winkler’s reagents, which were analysed later by the Winkler titration method following Carritt and Carpenter (1966). Water samples were collected in cleaned plastic bottles for nutrient analysis and fixed by adding mercury chloride (for ammonia, nitrate, nitrite and phosphate). The concentrations of nutrients were measured following standard spectrophotometric procedures (Grasshoff et al., 1983). A 2-l water sample was filtered through a GF/F filter (0.5-μm pore size, Whatman). Chlorophyll-a on the filter was extracted with 90% acetone at 4 °C in the dark for 12 h and then spectrofluorometrically analysed. Samples collected in acid-washed glass bottles were analysed for pH by potentiometric method (Dickson et al., 2007) using 835 Titrando (Metrohm, Switzerland). For total suspended matter (TSM), 1–2 l of water samples were filtered through 0.22-µm filter paper (MCE, 47 mm, Millipore), and they were dried, weighed and calculated in grammes per litre. The average values, along with standard deviations of the physicochemical parameters measured, are presented in Table 2.
Bacteriological parameters
About 100 ml of sample was taken into a pre-sterilised screw-capped bottle for bacterial analysis from the surface and bottom water from the Niskin water sampler’s nozzle. All samples were collected after rinsing the sterile collected bottle with precautions required for microbiological analysis while wearing gloves. Samples were kept in an ice box for transportation to the lab, where it is analysed soon after or kept at 4 °C whenever necessary to stunt bacterial growth (APHA et al., 2012; Ramaiah et al., 2004). The bacteriological examinations were carried out following Ramaiah et al. (2004) and Prasad et al. (2015) to enumerate heterotrophic, indicator and few pathogenic bacteria. A 100-µl sample was taken, spread on various plates and incubated at 25 °C for 24–48 h. Sample dilution (10 times) was performed whenever the plate became overcrowded and re-plated. All the samples were plated in replicates. The number of colonies was then counted using a colony counter. The specific colonies (unique to the organism of interest) on the respective agar media (HIMEDIA) were quantified, and the results were expressed in colony-forming units (CFU ml−1) after averaging out from the replicate plates according to Nagvenkar and Ramaiah (2009). Different selective, isolation and differential media were used for the specific bacterial groups. Heterotrophic bacterial counts were determined on R2A agar. Enumeration of coliforms on MacConkey agar, Aeromonas hydrophila on Aeromonas Isolation agar, Enterococcus faecalis, Escherichia coli, Klebsiella pneumonia, Proteus mirabilis, Staphylococcus aureus on Universal differential medium, Pseudomonas sp. like Pseudomonas aeruginosa on Cetrimide agar, Salmonella spp. and Shigella spp. on xylose-lysine-deoxycholate agar, and Vibrio spp. like Vibrio cholerae, Vibrio fluvialis, Vibrio parahaemolyticus and Vibrio vulnificus on thiosulphate citrate bile salts sucrose agar were determined.
Antibiogram
We isolated presumptive pathogenic bacterial colonies from respective agar plates using the streak plate method. Forty-six isolates from respective agar plates were transferred to 1 ml of Mueller Hinton broth and left to grow overnight. The concentrations of the cultures were adjusted to 0.5 McFarland (OD of 0.1 at 600 nm) before plating. Mueller Hinton agar plates were prepared, and new cultures from the tubes were spread evenly using sterilised cotton swabs covering the whole surface. A total of 21 antibiotic discs used for testing the sensitivity/resistance of Enterobacteriaceae isolates as per Clinical and Laboratory Standards Institute (CLSI) guidelines are cefuroxime, cefoxitin, ceftazidime, ceftriaxone, cefoperazone, cefotaxime, cefepime, piperacillin, piperacillin/tazobactam, ampicillin/sulbactam, nalidixic acid, norfloxacin, ciprofloxacin, gentamicin, amikacin, imipenem, meropenem, azithromycin, tetracycline, aztreonam and chloramphenicol (C). These antibiotic discs were placed on MH agar plates and left overnight to grow. The next day, the diameter of the inhibition zones was measured using a scale specifically for this purpose. Among them, 11 antibiotics are considered for Pseudomonas aeruginosa isolates as per CLSI guidelines in M100 (CLSI, 2017).
The resulting inhibition zones were compared with the inhibition zone diameter range given in the current CLSI guidelines that determine the inhibition zone diameter breakpoint where the bacterial strain is resistant, intermediate or susceptible to the given antibiotic, which follows the Kirby-Bauer disc diffusion method (Bauer et al., 1966). For the Enterobacteriaceae family, 21, and Pseudomonas, 11 antibiotics were taken for testing. The antibacterial resistance (AR) index for these samples based on the isolates was determined.
where y is the number of resistant microbes in the sample, n is the population size and x is the total number of antibiotics used.
For isolates resistant to 3 or more antibiotics, multiple antibiotic resistance (MAR) index was calculated.
where a is the no. of antibiotics, the isolate is resistant to; b is the no. of antibiotics, the isolate is exposed to/tested.
Statistical analyses
To compare the means of the parameters and whether they vary significantly spatially, pair-wise statistical analyses were performed between NS, MS and SS, combining both values in surface and sub-surface water samples. A parametric Tukey’s honestly significant difference (HSD) test was performed for physicochemical parameters (Table 3). A non-parametric Dunn’s post hoc test is carried out for bacteriological parameters (Table 4). Principal component analysis (PCA) was performed to infer the effect of physical (temperature, salinity, PAR and fluorescence) and chemical parameters (dissolved oxygen, chlorophyll-a, total suspended matter, pH, ammonium, nitrate, nitrite and phosphate) on bacteriological parameters (heterotrophic bacterial count, total coliforms, Escherichia coli, Enterococcus faecalis, Klebsiella pneumonia, Aeromonas hydrophila, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella, Shigella, Vibrio cholera and Vibrio parahaemolyticus) for surface samples and bottom samples separately. All the statistical analyses were performed using PAST 4.03 software.
Results
Physicochemical parameters
The range of physicochemical parameters in both surface and bottom waters is summarised in Table 2 and Supplementary Figs. 2 and 3. The VSH and GPH stations were deeper compared to all other stations. They are also far from the coast (Supplementary Fig. 3). The mean temperature for the NS and SS groups varied between 24 and 25 °C. In contrast, the temperature of the MS group was 1 °C higher in both surface and subsurface waters (Table 2, Supplementary Figs. 2 and 3). The temperature in SS was found to be a little lower as compared to NS. In MS, the subsurface water temperature was more than the surface compared to NS and SS, where the temperature in the water column is more or less constant. Salinity showed an opposite trend as opposed to temperature. The salinity of MS was 1 ppt lower than SS and NS. The subsurface salinity of MS was 1 to 3 ppt higher than the surface water. Lower pH was found in MS compared to SS and NS (Table 2, Supplementary Figs. 2 and 3). Higher pH values were observed in TVM, PDM and TUN (Table 2, Supplementary Figs. 2 and 3). Stations of TVM showed a little higher chlorophyll-a than others (Table 2, Supplementary Figs. 2 and 3). TSM was higher in MS than SS and NS (Table 2, Figs. 2 and 3) and higher in surface and bottom waters. DO concentration was slightly higher on the surface than in the subsurface water. DO is less in both surface and bottom waters in the MS, especially in VSH (Table 2, Supplementary Figs. 2 and 3). Subsurface waters showed higher nitrate, nitrite and phosphate concentrations than surface waters, and ammonia concentration did not differ much. Nitrite concentration showed a higher trend towards SS (Table 2, Supplementary Figs. 2 and 3). Ammonia concentration was higher in TUN in surface and bottom waters (Table 2, Supplementary Figs. 2 and 3).
Heterotrophic, indicator and pathogenic bacteria
The range of all bacterial counts (CFU/ml) in both surface and bottom waters are summarised in Supplementary Table 1, and the trend is shown in Figs. 2 and 3. Surface water HBC was found to be higher in CHP stations, followed by VSP, NKP, GPH and TVM stations, lowest was found in VSH stations (Fig. 2). Similarly, CHP stations had the highest HBC in subsurface water (Fig. 3). HBC of subsurface waters was more compared to surface in most of the stations. Surface water total coliform (TC) counts were highest in PDM stations and lowest in the MS group except for CHP. Subsurface TC was highest in TVM stations (Fig. 3). Escherichia coli (EC) showed a different pattern; surface EC numbers were found to be higher in the NS group and 2 stations of the SS group (PDM, NKP). Similarly, PBM, TPM and PDM stations had higher subsurface EC counts and CHP stations (Fig. 3). VSH, GPH and VSP stations had the lowest EC numbers. Enterococcus faecalis (EF) was present only in PBM, TPM and CHP stations in surface and subsurface waters. Klebsiella pneumoniae (KP) was in relatively higher numbers in PBM and TUN stations (Figs. 2 and 3). KP counts in other stations are found to be low. Proteus mirabilis (PM) was higher in TUN stations, followed by VSH, GPH and VSP (Fig. 2) in surface waters. In subsurface waters, they were high in PBM (Fig. 3). Though the counts of Staphylococcus aureus were less across all the stations, some signatures were found in stations of PBM, TPM, CHP, VSP and TVM (Figs. 2 and 3). Aeromonas hydrophila (AH) was found in surface and subsurface waters NS and SS group and was less in MS (Figs. 2 and 3). Vibrio cholera (VC) and Vibrio parahaemolyticus (VP) were primarily found in the subsurface and surface waters of NS group stations (Figs. 2 and 3). VP was additionally found in CHP and some stations of NKP (Figs. 2 and 3). Pseudomonas aeruginosa (PA) was found in most stations in higher numbers than other pathogenic bacteria (Figs. 2 and 3). Their numbers were highest on the surface waters of TPM and GPH, followed by CHP and PBM stations. In the subsurface water, PA counts were highest in TVM, followed by CHP, TPM and TVM (Figs. 2 and 3).
Antibiotic susceptibility test
Among the 28 isolates belonging to the Enterobacteriaceae family, only 3 were sensitive to all antibiotics, and among the 18 Pseudomonas isolates, 5 were sensitive to all antibiotics. Out of all the 46 isolates, 38 were resistant to at least 1 antibiotic used in the study (Tables 5 and 6). Isolates from the Enterobacteriaceae family showed resistance to more antibiotics than Pseudomonas (Tables 5 and 6). Many Enterobacteriaceae isolates were resistant antibiotics such as aztreonam (24), cefuroxime (12), cefotaxime (12), ceftazidime (14), cefepime (12) and azithromycin (11). No Enterobacteriaceae isolates were resistant to chloramphenicol, norfloxacin and tetracycline. Among the Enterobacteriaceae isolates, 85% and among Pseudomonas, 66% were resistant to aztreonam, followed by ceftazidime (50%, 27%) and cefepime (42%, 22%) for Enterobacteriaceae and Pseudomonas respectively. In the case of Enterobacteriaceae isolates, 42.8%, 42.8% and 39.2% were resistant to cefuroxime, cefotaxime and azithromycin, respectively. Similar inference can be drawn in terms of AR indexes for each antibiotic, with aztreonam having an AR index of 0.04, followed by ceftazidime (0.023), cefuroxime (0.02), cefotaxime (0.02) and cefepime (0.02) (Table 5). Isolates belonging to the genera Proteus and Escherichia showed the most resistance to the antibiotics tested (Table 5). Aztreonam had the highest AR index of 0.06 among the Pseudomonas isolates, followed by 0.025 for ceftazidime and 0.02 for cefepime (Table 6). Among 28 isolates of the Enterobacteriaceae family, 13 had a MAR index more than or equal to 0.2, and among Pseudomonas, the number was 4 among the 18 isolates (Table 7).
Statistical analyses
Temperature, fluorescence, pH and DO concentrations showed significant variation in their means among the 3 stations when compared pair-wise (p-value < 0.05) at a 95% confidence interval (Tukey’s HSD test) (Table 3). The salinity of the SS group showed a significant difference compared to NS and MS groups. Nutrients did not show significant variation among the 3 groups except for phosphate. The MS group was found to be different from the other two groups in terms of values of different physicochemical parameters. Dunn’s post hoc test for the non-parametric distribution of bacteriological parameters suggested a significant difference between the 3 groups (Table 4). Aeromonas hydrophila, Salmonella Shigella-like organisms and Klebsiella pneumoniae numbers showed a significant difference in all three groups, as inferred by the pair-wise test at a 95% significance level. The test suggested NS group differs from other groups regarding various bacterial counts.
The surface sample PCA (Fig. 4) has two significant clusters. The first cluster included stations of the MS group along the direction of higher temperature and TSM load and opposite to salinity. The other cluster contained mostly the SS group (TVM, PDM, NKP) and one from the NS group (TPM) in the direction of higher salinity, pH, fluorescence, chlorophyll-a and TC. PBM was separate from others and showed positive variation with dissolved oxygen concentration, EC, EF, AH, KP, VC and VP. The first two components explained the 22.82% variation in the data (PC1: 13.61%; PC2: 9.21%) in the PCA.
Principal component analysis of surface samples (Temp, temperature; Sal, salinity; PAR; DO, dissolved oxygen; Chl-a, chlorophyll-a; TSM, total suspended matter; Frsn, fluorescence; pH; NH4, ammonium; NO3, nitrate; NO2, nitrite; PO4, phosphate; HBC, heterotrophic bacterial count; TC, total coliforms; EC, Escherichia coli; EF, Enterococcus faecalis; KP, Klebsiella pneumonia; AH, Aeromonas hydrophila; PM, Proteus mirabilis; PA, Pseudomonas aeruginosa; SS, Salmonella- and Shigella-like organisms; VC, Vibrio cholera; and VP; Vibrio parahaemolyticus)
In the bottom samples, a similar grouping is seen (Fig. 5) but it is not that prominent compared to the former arrangement of the surface samples. Again, MS group stations are clustered with CHP far from the main group. The grouping was in the direction of temperature, TSM and salinity. The second cluster was found to be present in the middle with the SS group (PDM, TUN, NKP) except TVM. TVM was present in the direction of TC, PA and SS counts. Station PDM fell in the direction of EC < EF, VC and PM, separated from other stations. The first two components in this PCA explained a 23.99% variation in the data (PC1: 14.63%; PC2: 9.36%).
Principal component analysis of bottom samples (Temp, temperature; Sal, salinity; PAR; DO, dissolved oxygen; Chl-a, chlorophyll-a; TSM, total suspended matter; Frsn, fluorescence; pH; NH4, ammonium; NO3, nitrate; NO2, nitrite; PO4, phosphate; HBC, heterotrophic bacterial count; TC, total coliforms; EC, Escherichia coli; EF, Enterococcus faecalis; KP, Klebsiella pneumonia; AH, Aeromonas hydrophila; PM, Proteus mirabilis; PA, Pseudomonas aeruginosa; SS, Salmonella- and Shigella-like organisms; VC, Vibrio cholera and VP, Vibrio parahaemolyticus)
Discussion
Effect of rainfall on physicochemical parameters
A temperature difference of 2 °C in the MS group station surface waters compared to other groups can be attributed to the time of collection. Water samples from these places were collected during the 1st phase of sampling, whereas other samples were collected during the 2nd phase. The water temperature corresponds to the air temperature recorded during these times, which was 23 to 30 °C during early December and decreased to 17 to 27 °C during late December. The lower salinity observed in the MS group is attributed to rainfall near these places before the period of sampling (Supplementary Fig. 1). Except for these variations, the usual trend of salinity towards the southern station is increasing because the northern Bay of Bengal is directly affected by freshwater influx from rivers that drain into the ocean. During December, East India Coastal Current is southwards (Shankar et al., 1996), which brings a gradual change in salinity which increases downwards to the South. The stations which have a 3–4-ppt increase in salinity from surface to subsurface waters of VSH, GPH and VSP have a depth of 25 m or more, whereas other stations where salinity has a maximum 1 ppt difference between the surface and subsurface waters have depths under 15 m. A little lower pH of these stations can also be attributed to the rainfall, as rainwater has a lower pH. These particular stations also had 2 to 2.5 times higher TSM than other stations, corresponding to lower chlorophyll-a contents, as higher TSM can reduce the availability of sunlight, reducing phytoplankton abundance (Sarma et al., 2010; Sudha Rani et al., 2018). This result is supported by the PCA (Figs. 4 and 5), where total suspended matter is grouped with stations VSH, GPH and VSP. Among these stations, the highest TSM load of GPH stations may depend on the port activity and dredging caused by the port authority, fishing nets and trawling. Nutrients like ammonium, nitrate, nitrite and phosphate did not differ greatly. Principal component analysis of surface and bottom waters shows the significant effect of rainfall on the stations where two different groups are formed, one including the MS group with higher temperature and low salinity, and another with other stations with lower temperature and higher salinity conditions (Figs. 4 and 5).
Spatial distribution of bacterial numbers
Sample properties of all the stations showed variation in two groups based on the time of collection, affecting the water’s physical and chemical properties. During this sampling, changes in physical parameters are more prominent than chemical. The waters of MS group stations were warmer, less saline and less alkaline compared to other stations from which water samples were collected 13 days later. Heterotrophic bacteria use organic matter for energy production; their abundance in most samples indicates their presence is not affected considerably by the physical property change of the water column. While heterotrophic bacterial counts and total coliform counts are helpful in the prediction of the microbial processes that are going on in the water column, specific bacteria have been used as indicators of heavy metal pollution, faecal contamination, wastewater pollution and oil contamination (Sumampouw & Risjani, 2014). As enteric and faecal-origin bacteria like Escherichia coli and Enterococcus faecalis have a narrow host range of humans and other warm-blooded animals, they help determine the source of contamination and the extent of anthropogenic pollution (Brandt et al., 2017; Wheeler et al., 2002). Indicator bacteria like E. coli and E. feacalis counts showed a particular pattern in this study. Both were highest in PBM. E. faecalis was found in higher numbers in NS regions (TPM and CHP) and was mostly absent after that, except in a few stations. E. coli numbers were high in similar stations as E. faecalis, including PDM. These bacteria were present in fewer numbers in other stations and were very few in VSH and GPH stations. All these stations (PBM, TPM, CHP, PDM and TVM) are located near the coast where small villages, including fishing villages, are present. The sampling location PBM is close to the biggest village, among others. PCA of both surface and bottom water also indicates PBM stations have a higher abundance of E. coli, E. faecalis, Aeromonas hydrophila, Klebsiella pneumoniae, Vibrio cholerae and Vibrio parahaemolyticus as compared to others (Figs. 4 and 5). The presence of villages can affect the health of coastal water negatively. Land run-off into the ocean from these places can be affected by human and animal faeces via domestic sewage, open defecation and water from animal sheds. This water can harbour various enteric and pathogenic bacteria. Less commonly used indicator bacteria like K. pneumoniae were found in VSH and GPH along with northern stations. Another pathogenic bacterium, Proteus mirabilis, showed a reverse pattern in its presence. They were found in most of the stations of the MS group (VSH, GPH and VSP) and the bottom waters of a few other stations. Though these waters have few E. coli and E. faecalis and are present offshore, enteric bacteria like P. mirabilis show potential faecal contamination from the port region. The presence of opportunistic pathogenic bacteria like Pseudomonas aeruginosa in all of the stations in high abundance shows their adaptability to spatial and temporal variations of different parameters, as indicated by their genome study (Silby et al., 2011). Fish pathogenic bacteria like A. hydrophila counts were higher in bottom waters and did not show considerable variation spatially. Vibrio groups that included V. cholerae and V. parahaemolyticus were present primarily in the northern stations, including PBM, TPM and CHP. A study by Prasanthan et al. (2011) from the Kerala coast has shown that V. cholerae and V. parahaemolyticus have a positive correlation with each other similar to our study where these two are co-present in the stations. Increased counts of E. coli, A. hydrophila and P. mirabilis in the bottom waters of most of the stations indicate mixing activity near-bottom sediment and their mode of respiration (as these bacteria are facultative anaerobes). The difference between 24 days during samplings and rainfall during earlier sampling days effectively controlled these bacterial numbers. Additionally, bacterial abundance data directly from the discharge waters of PBM, CHP, TVM and NKP (from the industry before discharge into the sea) showed consistency with our data from the corresponding discharge points in the sea (Supplementary Table 2).
A study from a marine coastal zone in Italy found that the counts of E. coli, Aeromonas spp. and Vibrio are in the maximum range of 102 CFU/100 ml of sample (Maugeri et al., 2004). This bacterial range is lower than the present study’s data. A study by Mudryk et al. (2014) from the coastal Baltic Sea showed the counts of Aeromonas to be higher compared to Pseudomonas and E. coli. In contrast, the highest numbers were seen for Pseudomonas counts in the present study. When studies from the Indian coast are concerned regarding indicator and pathogenic bacteria, the East Coast (off Chennai) showed a higher abundance of total viable counts and total coliforms as compared to the West Coast of India (off Kerala) (Sudhanandh et al., 2012; Vignesh et al., 2012). A study from Chennai coastal water showed TVC/heterotrophic bacterial numbers to be 10 times higher than this study from the coast of Andhra Pradesh (Vignesh et al., 2012). There have been many similar studies from Visakhapatnam coastal waters (primarily beaches and Visakhapatnam harbour) which show the water of this region with high pollution index concerning indicator bacteria (Clark et al., 2003; Khandeparker et al., 2020; Kumar et al., 2017). The current study compares how these bacteria are distributed along the coast from industrial discharge sites and harbours. Moreover, the abundance of indicator bacteria is higher than the standard limit in the study region (Enterococcus faecalis more than 35 CFU/100 ml set in recreational water). The coliform counts also far exceeded the faecal coliform limit set by the central pollution control board, India, i.e., 100 CFU/100 ml.
Occurrence of antibiotic-resistant bacterial isolates
Several Enterobacteriaceae isolates were resistant to antibiotics azithromycin, aztreonam, cefuroxime, cefotaxime, ceftazidime and cefepime. Except for azithromycin, other antibiotics mentioned have β-lactam rings in their structure. Many bacteria target these β-lactam rings and hydrolyse these antibiotics as part of their antibiotic resistance mechanism by producing β-lactamase (Paterson & Bonomo, 2005). Bacteria with extended-spectrum β-lactamase activity from Enterobacteriaceae and Pseudomonas can confer resistance against most β-lactam antibiotics such as penicillin, cephalosporins and aztreonam (Pang et al., 2019; Paterson & Bonomo, 2005). Resistance of many isolates to cefepime, a 4th generation of cephalosporin, is concerning. Azithromycin, which belongs to the macrolide class of antibiotics, was shown resistant by 39% of isolates belonging to Enterobacteriaceae. Surprisingly, only one Pseudomonas isolate showed resistance to an old broad-spectrum antibiotic like tetracycline. The same result was seen in the case of another antibiotic, chloramphenicol. Few isolates showed resistance to ampicillin/sulbactam, gentamicin and ciprofloxacin. Few isolates showed resistance to the carbapenem class of antibiotics used in the study (imipenem and meropenem). Carbapenems are generally used as last resort antibiotics, which are not frequently administered to help prevent antibiotic resistance against them. In our study, antibiotics belonging to cephalosporin family were shown most resistance from the bacterial isolates. As discovered by Farooqui et al. (2019) from medical audit data, β-lactam cephalosporins had the highest prescription rate, followed by β-lactam penicillin in India. This could be a reason for the earlier result as antibiotic resistance in bacteria is dependent on uses of antibiotics in those areas (Alipour et al., 2014). The current study deduced 23 out of 46 isolates (50%) to have a MAR index of more than 0.2. A MAR index greater than 0.2 suggests contamination from a source with higher use of antibiotics (Ayandele et al., 2020). There are various sources from which antibiotic-resistant bacteria can be originated. These bacteria can enter the natural environment by various means, from human faeces, livestock manure slurry, agricultural land run-off, sewage water, wild bird and animals, and pharmaceutical industry discharge during manufacturing process of antibiotics (Wellington et al., 2013).
A study by Obayiuwana et al. (2018) pointed out that pharmaceutical wastewater discharge can be a hot spot for genetic determinants for antibiotic resistance in bacteria. In the current study, antibiotic-resistant isolates can be attributed to the widespread open-defecation practices near the coastal villages. As there are several pharmaceutical companies’ discharge points along the study area, they can act as a determinant too. Compared to the data obtained from isolates from different port regions along the east coast of India, these antibiotic susceptibility data in terms of AR and MAR indexes showed a considerable variation (Unpublished data). Data from the abovementioned port regions were collected during monsoon seasons (August 2019) and pre-monsoon (June 2020). When the percentage of bacterial isolates with a MAR index of more than 0.2 were considered, the current study has a higher (0.46) percentage value than 0.36% and 0.26% of the sampling mentioned above (Supplementary Table 3). The higher values of the indexes in the present study can be attributed to the closeness of the sampling sites to human settlements and the sampling time which is December, when dilution by fresh water is at the lowest. However, antibiotic susceptibility alone cannot correctly provide a whole understanding of the antibiotic resistance phenomenon. Chemical analysis of the water concerning the measurement of antibiotic residues is a better way to lay the foundation for determining how much quantity of these antibiotics are discharged into the coastal water in the first place. Furthermore, assessing the isolates for the presence of specific antibiotic resistance genes will be beneficial in the future.
A comparative analysis of antibiotic susceptibility studies from various coastal sites is presented in Table 8. In the present study, bacterial isolates showed more resistance to the antibiotics such as aztreonam, ceftazidime, cefepime and cefuroxime. This pattern is very different from other studies. The case of antibiotics that have less resistance from isolates is similar to other studies. Antibiotic susceptibility test from the Enterobacteriaceae family from Veraval, West Coast of India, showed 80% resistance to ampicillin (Maloo et al., 2014). The present study shows less than 10% for the same antibiotic. The same study also reported no resistance to antibiotics of the cephalosporin family, whereas in the present study, 10–50% resistance is seen for antibiotics of the same family. In the case of MAR, the percentage of isolates from other regions is 23.46 to 100%. The isolates’ percentage in current study is in between these values. A study from Veraval, on the West coast of India, found 100% resistance in case of Enterobacter, which is high compared to the current study from the East coast of India.
Antibiotic resistance of Pseudomonas spp. in the present study is low compared to the Enterobacteriaceae family. However, there are no similar studies where the comparison can be performed for the same. The source of antibiotic resistance in the region can be anything, which can come from pharmaceutical companies or faecal matter from nearby coastal villages. Some studies suggest discharge from antibiotic manufacturing/pharmaceutical sites leads to the development and dissemination of multidrug-resistant bacteria (González-Plaza et al., 2019; Hubeny et al., 2021). Sewage contamination also affects marine life, including fish, as Al-Bahry et al. (2009) studied when antibiotic tests were performed on bacteria isolated from fish. The multi-drug-resistant bacteria in this study show this region’s risk factors.
Conclusion
Indicator (Escherichia coli and Enterococcus faecalis) and pathogenic (Aeromonas hydrophila, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella and Shigella, Vibrio cholera and Vibrio parahaemolyticus) bacteria are found to be present in high numbers in the study region. Heterotrophic bacterial abundance was comparable in all the stations except a few. However, enteric and pathogenic bacteria showed spatial variation in the studied region, highest in the northern stations, followed by south stations and least in the middle stations. Counts of E. faecalis were found to be 103 to 104 times higher in Pydibhimavaram and Tammayyapalem stations than the standard for marine recreational water. It is found that the rainfall affects the physiochemical characteristics of the middle stations, which in turn impact the bacterial load. The variation in the bacterial abundance in the study sites can be attributed to on the presence of coastal villages and industrial discharge points. Antibiotic susceptibility tests of the isolates also revealed multidrug-resistant bacteria in these waters, which is of great concern. Continuous monitoring of these bacteria, their resistance potential and presence of antibiotic residues, their dissemination, persistence in natural waters and their source determination can lead to a clearer understanding of this grave situation.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author upon reasonable request.
References
Adeniji, O. O., Sibanda, T., & Okoh, A. I. (2020). Molecular detection of antibiotic resistance and virulence gene determinants of Enterococcus species isolated from coastal water in the Eastern Cape Province, South Africa. International Journal of Environmental Studies, 78(2), 1–20. https://doi.org/10.1080/00207233.2020.1785759
Al-Bahry, S. N., Mahmoud, I. Y., Al-Belushi, K. I. A., Elshafie, A. E., Al-Harthy, A., & Bakheit, C. K. (2009). Coastal sewage discharge and its impact on fish with reference to antibiotic resistant enteric bacteria and enteric pathogens as bio-indicators of pollution. Chemosphere, 77(11), 1534–1539. https://doi.org/10.1016/j.chemosphere.2009.09.052
Ali, M., Nelson, A. R., Lopez, A. L., & Sack, D. A. (2015). Updated global burden of Cholera in endemic countries. PLoS Neglected Tropical Diseases, 9(6), e0003832. https://doi.org/10.1371/journal.pntd.0003832
Alipour, M., Hajiesmaili, R., Talebjannat, M., & Yahyapour, Y. (2014). Identification and antimicrobial resistance of Enterococcus Spp. isolated from the river and coastal waters in Northern Iran. Scientific World Journal. https://doi.org/10.1155/2014/287458
APHA, AWWA, & WEF. (2012). Standard Methods for examination of water and wastewater. 22nd ed. Washington: American Public Health Association, 1360, ISBN 978–087553–013–0. Retrieved July 8, 2022, from http://www.standardmethods.org/
Ayandele, A. A., Oladipo, E. K., Oyebisi, O., & Kaka, M. O. (2020). Prevalence of multi-antibiotic resistant Escherichia coli and Klebsiella species obtained from a tertiary medical institution in Oyo State, Nigeria. Qatar Medical Journal, 1, 9. https://doi.org/10.5339/qmj.2020.9
Babu, K. R., Hima S. V., Reddy, K. V. S., Anand, K. G., Pratap, G. V., & Raju, M. R. (2014). Studies on microbial status and characteristic features from polluted coastal habits at Visakhapatnam, India. International Journal of Multidisciplinary and Current Research, 2, 113–117. Retrieved November 11, 2019, from http://ijmcr.com/wp-content/uploads/2014/01/Paper19113-117.pdf
Bauer, A. W., Kirby, W. M. M., Serris, J. C., & Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disc method. American Journal of Clinical Pathology, 45(4), 493–496.
Baya, A. M., Brayton, P. R., Brown, V. L., Grimes, D. J., Russek-Cohen, E., & Colwell, R. R. (1986). Coincident plasmids and antimicrobial resistance in marine bacteria isolated from polluted and unpolluted Atlantic Ocean samples. Applied and Environmental Microbiology, 51(6), 1285–1292. https://doi.org/10.1128/aem.51.6.1285-1292.1986
Belding, C., & Boopathy, R. (2018). Presence of antibiotic-resistant bacteria and antibiotic resistance genes in coastal recreational waters of southeast Louisiana, USA. Journal of Water Supply: Research and Technology - AQUA, 67(8), 800–809. https://doi.org/10.2166/aqua.2018.076
Brandth, M. J., Johnson, K. M., Elphinston, A. J., & Ratnayaka, D. D. (2017). Twort’s water supply (Seventh edition). Microbiology and Biology of Water. https://doi.org/10.1016/B978-0-08-100025-0.00007-7
Carritt, D. E., & Carpenter, J. H. (1966). Comparison and evaluation of currently employed modifications of Winkler method for determining dissolved oxygen in seawater–a Nasco report. Journal of Marine Research, 24(3), 286–318.
Chen, C. Q., Zheng, L., Zhou, J. L., & Zhao, H. (2017). Persistence and risk of antibiotic residues and antibiotic resistance genes in major mariculture sites in Southeast China. Science of the Total Environment, 580, 1175–1184. https://doi.org/10.1016/j.scitotenv.2016.12.075
CIESIN (Center for International Earth Science Information Network), Columbia University. (2007). Population Density within and outside of a 10m Low Elevation Coastal Zone. Retrieved November 10, 2019, from http://www.ciesin.org
Clark, A., Turner, T., Dorothy, K. P., Goutham, J., Kalavati, C., & Rajanna, B. (2003). Health hazards due to pollution of waters along the coast of Visakhapatnam, east coast of India. Ecotoxicology and Environmental Safety, 56(3), 390–397. https://doi.org/10.1016/S0147-6513(03)00098-8
CLSI. (2017) Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. CLSI guideline M100 Wayne, PA: Clinical and Laboratory Standards Institute.
De Souza, M. J., Nair, S., Loka Bharathi, P. A., & Chandramohan, D. (2006). Metal and antibiotic-resistance in psychrotrophic bacteria from Antarctic Marine waters. Ecotoxicology, 15(4), 379–384. https://doi.org/10.1007/s10646-006-0068-2
Dickson, A. G., Sabine, C. L., & Christian, J. R. (2007). Guide to best practices for ocean CO2 measurements. PICES Special Publication, 3.
Dileep, G., & Prameela, M. (2021). Waste water pollution in coastal city: A study in the city of Visakhapatnam, India. Ecology, Environment and Conservation, 25(3), 1309–1314.
Divyashree, M., Vijaya Kumar, D., Ballamoole, K. K., Shetty, A., & V., Chakraborty, A., & Karunasagar, I. (2020). Occurrence of antibiotic resistance among Gram negative bacteria isolated from effluents of fish processing plants in and around Mangalore. International Journal of Environmental Health Research, 30(6), 653–660. https://doi.org/10.1080/09603123.2019.1618799
Farmasi, J., & Dan, S. (2017). Factsheet: People and oceans. The Ocean Conference, United Nations, 14(1), 55–64. Retrieved March 17, 2022, from https://www.un.org/sustainabledevelopment/wp-content/uploads/2017/05/Ocean-fact-sheet-package.pdf
Farooqui, H. H., Mehta, A., & Selvaraj, S. (2019). Outpatient antibiotic prescription rate and pattern in the private sector in India: Evidence from medical audit data. PLoS One, 14(11), e0224848. https://doi.org/10.1371/journal.pone.0224848
Fowsul Ameer, M. L. (2017). Water-Borne Diseases and the Their Challenges in the Coastal of Ampara District in Sri Lanka. Wnofns, 9, 7–18. Retrieved February 5, 2023, from www.worldnewsnaturalsciences.com
Gambino, D., Savoca, D., Sucato, A., Gargano, V., Gentile, A., Pantano, L., Vicari, D., & Alduina, R. (2022). Occurrence of antibiotic resistance in the Mediterranean Sea. Antibiotics, 11(3), 1–9. https://doi.org/10.3390/antibiotics11030332
Gleick, P. H. (2002). Dirty Water: Estimated Deaths from Water-Related Diseases 2000–2020. Pacific Institute Research Report, 1–12.
González-Plaza, J. J., Blau, K., Milaković, M., Jurina, T., Smalla, K., & Udiković-Kolić, N. (2019). Antibiotic-manufacturing sites are hot-spots for the release and spread of antibiotic resistance genes and mobile genetic elements in receiving aquatic environments. Environment International, 130, 104735. https://doi.org/10.1016/j.envint.2019.04.007
Grasshoff, K., Ehrhardt, M., & Kremling, K. (1983). Methods of Seawater Analysis (pp. 89–224). Weinheim: Chemie.
Hubeny, J., Harnisz, M., Korzeniewska, E., Buta, M., Zieliński, W., Rolbiecki, D., Giebułtowicz, J., Nałęcz-Jawecki, G., & Płaza, G. (2021). Industrialization as a source of heavy metals and antibiotics which can enhance the antibiotic resistance in wastewater, sewage sludge and river water. PLoS ONE, 16(6), 1–24. https://doi.org/10.1371/journal.pone.0252691
Khandeparker, L., Desai, D. V., Sawant, S. S., Krishnamurthy, V., & Anil, A. C. (2020). Spatio-temporal variations in bacterial abundance with an emphasis on fecal indicator bacteria and Vibrio spp. In and around Visakhapatnam Port, East Coast of India. ASEAN Journal on Science and Technology for Development, 37(3), 91–99. https://doi.org/10.29037/AJSTD.619
Kumar, V. M., Janakiram, P., Sivaprasad, B., & Jayasree, L. (2017). Physico-chemical parameters and bacterial abundance in coastal water of Visakhapatnam, Bay of Bengal, India, with special reference to Pseudomonas sp. and Vibrio sp. Indian Journal of Geo-Marine Sciences, 46(8), 1588–1595.
Lakshmi, P., Kumar, P., & Babu, R. (2019). Studies on cultural, biochemical and seasonal variations of bacterial load from coastal waters of Bheemili, Visakhapatnam. International Journal of Recent Innovations in Academic Research, 3(4), 259–265.
Larsson, D. G. J. (2008). Drug production facilities – An overlooked discharge source for pharmaceuticals to the environment. Pharmaceuticals in the Environment, 37–42. https://doi.org/10.1007/978-3-540-74664-5_3
Majra, J. P., & Gur, A. (2009). Climate change and health: Why should India be concerned? Indian Journal of Occupational and Environmental Medicine, 13(1), 11–6. https://doi.org/10.4103/0019-5278.50717. PMID: 20165606; PMCID: PMC2822161.
Maloo, A., Borade, S., Dhawde, R., Gajbhiye, S. N., & Dastager, S. G. (2014). Occurrence and distribution of multiple antibiotic-resistant bacteria of Enterobacteriaceae family in waters of Veraval coast, India. Environmental and Experimental Biology, 12, 43–50.
Matyar, F. (2012). Antibiotic and heavy metal resistance in bacteria isolated from the Eastern Mediterranean Sea coast. Bulletin of Environmental Contamination and Toxicology, 89(3), 551–556. https://doi.org/10.1007/s00128-012-0726-4
Maugeri, T. L., Carbone, M., Fera, M. T., Irrera, G. P., & Gugliandolo, C. (2004). Distribution of potentially pathogenic bacteria as free living and plankton associated in a marine coastal zone. Journal of Applied Microbiology, 97(2), 354–361. https://doi.org/10.1111/j.1365-2672.2004.02303.x
Moore, D. F., Guzman, J. A., & McGee, C. (2008). Species distribution and antimicrobial resistance of enterococci isolated from surface and ocean water. Journal of Applied Microbiology, 105(4), 1017–1025. https://doi.org/10.1111/j.1365-2672.2008.03828.x
Mudryk, Z. J., Gackowska, J., Skórczewski, P., Perliński, P., & Zdanowicz, M. (2014). Occurrence of potentially human pathogenic bacteria in the seawater and in the sand of the recreational coastal beach in the southern Baltic Sea. Oceanological and Hydrobiological Studies, 43(4), 366–373. https://doi.org/10.2478/s13545-014-0154-7
Myla, S. C., Ganesh, P. R. C., Amaranth, D., SanthiSudha, B., & Samantha, M. H. (2015). Bacteria of the recreational beach waters of Visakhapatnam, India. International Journal of Recent Scientific Research, 6(2006), 7929–7932.
Naidoo, S., & Olaniran, A. O. (2014). Treated wastewater effluent as a source of microbial pollution of surface water resources. International Journal of Environmental Research and Public Health, 11(1), 249–270. https://doi.org/10.3390/ijerph110100249
Nagvenkar, G. S., & Ramaiah, N. (2009). Abundance of sewage-pollution indicator and human pathogenic bacteria in a tropical estuarine complex. Environmental Monitoring and Assessment, 155(1–4), 245–256. https://doi.org/10.1007/s10661-008-0432-1
Obayiuwana, A., Ogunjobi, A., Yang, M., & Ibekwe, M. (2018). Characterization of bacterial communities and their antibiotic resistance profiles in wastewaters obtained from pharmaceutical facilities in Lagos and Ogun states, Nigeria. International Journal of Environmental Research and Public Health, 15(7), 1365. https://doi.org/10.3390/ijerph15071365
Pandey, P. K., Kass, P. H., Soupir, M. L., Biswas, S., & Singh, V. P. (2014). Contamination of water resources by pathogenic bacteria. AMB Express, 4, 1–16. https://doi.org/10.1186/s13568-014-0051-x
Pang, Z., Raudonis, R., Glick, B. R., Lin, T.-J., & Cheng, Z. (2019). Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnology Advances, 37(1), 177–192.
Paterson, D. L., & Bonomo, R. A. (2005). Extended-spectrum beta-Lactamases: A clinical update. Clinical Microbiology Reviews, 18(4), 657–686. https://doi.org/10.1128/CMR.18.4.657-686.2005
Prasad, V. R., Srinivas, T. N. R., & Sarma, V. V. S. S. (2015). Influence of river discharge on abundance and dissemination of heterotrophic, indicator and pathogenic bacteria along the east coast of India. Marine Pollution Bulletin, 95(1), 115–125. https://doi.org/10.1016/j.marpolbul.2015.04.032
Prasanthan, V., Udayakumar, P., Sarathkumar.,& Ouseph, P. P. (2011). Influence of abiotic environmental factors on the abundance and distribution of Vibrio species in coastal waters of Kerala, India. Indian Journal of Geo-Marine Sciences, 40(4), 587–592.https://doi.org/10.2166/wh.2011.157
Ralston, E. P., Kite-Powell, H., & Beet, A. (2011). An estimate of the cost of acute health effects from food- and water-borne marine pathogens and toxins in the USA. Journal of Water and Health, 9(4), 680–694. https://doi.org/10.2166/wh.2011.157
Ramaiah, N., Kolhe, V., & Sadhasivan, A. (2004). Abundance of pollution indicator and pathogenic bacteria in Mumbai waters. Current Science, 87(4), 435–439.
Reddy, R. K., & Kostenzer, J. (2011). Health of the East Coast population of India, ACCESS Health International Inc., Centre for Market Solutions, Indian School of Bussiness. Retrieved November 18, 2019, from www.accessh.org
Sabry, S. A., Ghozlan, H. A., & Abou-Zeid, D. M. (1997). Metal tolerance and antibiotic resistance patterns of a bacterial population isolated from sea water. Journal of Applied Microbiology, 82(2), 245–252. https://doi.org/10.1111/j.1365-2672.1997.tb02858.x
Sailaja, V. H., Archana, A., & Babu, K. R. (2013). Assessment of fecal indicator bacteria in the coastal waters of Visakhapatnam, India. Pelagia Research Library, 4(4), 432–435.
Sarma, V. V., Vara Prasad, S. J. D., Gupta, G. V. M., & Sudhakar, U. (1996). Petroleum hydrocarbons and trace metals in Visakhapatnam harbour and Kakinada Bay, east coast of India. Indian Journal of Marine Sciences, 25, 148–150.
Sarma, V. V. S. S., Prasad, V. R., Kumar, B. S. K., Rajeev, K., Devi, B. M. M., Reddy, N. P. C., Sarma, V. V., & Kumar, M. D. (2010). Intra-annual variability in nutrients in the Godavari estuary, India. Continental Shelf Research, 30(19), 2005–2014. https://doi.org/10.1016/j.csr.2010.10.001
Shaik, A. U. R., Biswas, H., Surendra Babu, N., Reddy, N. P. C., & Ansari, Z. A. (2017). Investigating the impacts of treated effluent discharge on coastal water health (Visakhapatnam, SW coast of Bay of Bengal, India). Environmental Monitoring and Assessment, 189(12), 1–15. https://doi.org/10.1007/s10661-017-6344-1
Shankar, D., McCreary, J. P., Han, W., & Shetye, S. R. (1996). Dynamics of the East India Coastal Current 1. Analytic solutions forced by interior Ekman pumping and local alongshore winds. Journal of Geophysical Research C: Oceans, 101(C6), 13975–13991. https://doi.org/10.1029/96JC00559
Silby, M. W., Winstanley, C., Godfrey, S. A. C., Levy, S. B., & Jackson, R. W. (2011). Pseudomonas genomes: Diverse and adaptable. FEMS Microbiology Reviews, 35(4), 652–680. https://doi.org/10.1111/j.1574-6976.2011.00269.x
Sudhanandh, V. S., Udayakumar, P., Faisal, A. K., Potty, V. P., Ouseph, P. P., Prasanthan, V., & Narendra Babu, K. (2012). Distribution of potentially pathogenic enteric bacteria in coastal sea waters along the Southern Kerala coast. India. Journal of Environmental Biology, 33(1), 61–66.
Sudha Rani, P., Sampath Kumar, G., Mukherjee, J., Srinivas, T. N. R., & Sarma, V. V. S. S. (2018). Perennial occurrence of heterotrophic, indicator and pathogenic bacteria in the coastal Bay of Bengal (off Visakhapatnam) - Impact of physical and atmospheric processes. Marine Pollution Bulletin, 127(2), 412–423. https://doi.org/10.1016/j.marpolbul.2017.12.023
Sumampouw, O. J., & Risjani, Y. (2014). Bacteria as indicators of environmental pollution: Review. International Journal of Ecosystem, 4(6), 251–258. https://doi.org/10.5923/j.ije.20140406.03
Vignesh, S., Muthukumar, K., & Arthur James, R. (2012). Antibiotic resistant pathogens versus human impacts: A study from three eco-regions of the Chennai coast, southern India. Marine Pollution Bulletin, 64(4), 790–800. https://doi.org/10.1016/j.marpolbul.2012.01.015
Wellington, E. M. H., Boxall, A. B. A., Cross, P., Feil, E. J., Gaze, W. H., Hawkey, P. M., Johnson-Rollings, A. S., Jones, D. L., Lee, N. M., Otten, W., Thomas, C. M., & Williams, A. P. (2013). The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. The Lancet Infectious Diseases, 13(2), 155–165. https://doi.org/10.1016/S1473-3099(12)70317-1
Wheeler, A. L., Hartel, P. G., Godfrey, D. G., Hill, J. L., & Segars, W. I. (2002). Potential of Enterococcus faecalis as a Human Fecal Indicator for Microbial Source Tracking. Journal of Environmental Quality, 31(4), 1286–1293. https://doi.org/10.2134/jeq2002.1286
You, K. G., Bong, C. W., & Lee, C. W. (2012). Antimicrobial resistance in bacteria isolated from tropical coastal waters of Peninsular Malaysia. Malaysian Journal of Science, 31(2), 111–120. https://doi.org/10.22452/mjs.vol31no2.9
Zou, S., Xu, W., Zhang, R., Tang, J., Chen, Y., & Zhang, G. (2011). Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities. Environmental Pollution, 159(10), 2913–2920. https://doi.org/10.1016/j.envpol.2011.04.037
Acknowledgements
We thank the Director, CSIR-National Institute of Oceanography, Goa, Scientist-In-Charge, NIO, RC, Visakhapatnam, for the encouragement and support. We are thankful for the help of the boat crew and their assistance during sampling. It is the NIO contribution number.
Funding
This work was supported by the AP Pollution Control Board, Visakhapatnam (SSP 3180) and OLP 1710.
Author information
Authors and Affiliations
Contributions
Srinivas T. N. R.: conceptualisation, project administration, methodology, writing—review and editing. Swarnaprava Behera: methodology; writing—original draft, review and editing, visualisation; software; Sri Rama Krishna Moturi: writing—review and editing; Geethika Gudapati, Sravani, M., Satyanarayana Reddy, T.: formal analysis, investigation, data curation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Behera, S., Tanuku, N.R.S., Moturi, S.R.K. et al. Anthropogenic impact and antibiotic resistance among the indicator and pathogenic bacteria from several industrial and sewage discharge points along the coast from Pydibhimavaram to Tuni, East Coast of India. Environ Monit Assess 195, 546 (2023). https://doi.org/10.1007/s10661-023-11083-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11083-2