Abstract
Mining generates large quantities of mineral processing wastes that are typically stored in mine tailings (MT) ponds. Long-term exposure of the surrounding areas to the material from the tailings ponds has been reported to have adverse effects on both human health and the environment. The purpose of this study was to evaluate the ability of Atriplex atacamensis Phil. to phytostabilize metals (Cu, Fe, Mn, and Zn) and sulfur (S) when grown directly on mine tailings with and without compost (C) and humic substance (HS). The stress status of A. atacamensis Phil. was also evaluated through the 13C isotopic composition of bulk leaves. A 120-day greenhouse experiment was conducted and three treatments were evaluated: (i) MT without any amendments (control), (ii) MT + C (dose: 89 ton ha−1), and (iii) MT + HS (0.72 ton ha−1). Mine tailings material exhibited low salinity, alkaline pH, high extractable S–SO4 concentrations, and low fertility; total Fe, Mn, and Zn concentrations were within the reference range for mine tailings, but total Cu concentrations were high at 1860 ± 236 mg kg−1. The HS had higher pH, EC, CEC, and available concentrations of N, P, and K than compost, while S-SO4 concentrations were similar in both amendments. 13C NMR analysis showed that the HS contained more alkyl, aromatic, and phenolic groups, while the compost was dominated by O-alkyl and carboxyl groups. At the end of the experiment, the MT + C treatment achieved a significant decrease in Cu, Fe, and Mn concentrations in the roots and aboveground parts of A. atacamensis Phil. and an increase in Zn values in both tissues. Both amendments increased the sulfur content in the aboveground parts, while metal concentrations under the HS treatment proved similar to control. Furthermore, the δ13CV-PDB values obtained in this study indicate that the organic amendments did not cause additional physiological stress to the plants compared to the MT treatment. Overall, A. atacamensis Phil. was shown to have the ability to phytostabilize metals and sulfur, making it a potential candidate species for in situ evaluation of the phytostabilization process on mine tailings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mining industry generates large quantities of mineral processing wastes, which are typically stored in tailings ponds. The wastes may consist of a mixture of water, fine solids, sand, and multiple chemical elements in high concentrations, such as metals, metalloids, and sulfur (Santibañez et al., 2011). It is estimated that the global production of mining wastes exceeds 10 billion tons per year (Adiansyah et al., 2015). Chile, the world’s largest copper producer and the second-largest molybdenum producer, currently has 24 billion tons of tailings deposited in the country’s 746 tailings storage facilities (102 operating, 471 idle, and 173 abandoned), with an average of 530 million tons of mining wastes added annually (SERNAGEOMIN, 2018). Prior to 2011, the country did not have laws regulating mining operation closure and rehabilitation practices, which led to the emergence of many unregulated tailings storage sites. Law 20.551, passed in 2011, authorized such regulation to protect the life, health, and safety of people, ensure the chemical and physical integrity of tailings facilities and reduce their impact on water, soil, and air (DOE, 2011).
Areas subjected to long-term exposure to mine tailings have been widely reported to pose a risk to human health and the environment due to leaching losses of toxic metal(loid)s and aeolian transport and dispersion of waste materials (Sanchez-Lopez et al., 2015; Wang et al., 2017; Xie & van Zyl, 2020). The magnitude of these effects depends on the physicochemical properties of the tailings, including metal(loid) mobility, pH, concentration, bioavailability, composition, and organic content (Alloway, 2010). In general, pH is the crucial factor related to metal(loid) mobility since it is associated with adsorption, desorption, and soluble complexes formation of them (Hooda, 2010). For instance, soils and mine tailings under acidic conditions, low content of cation exchange capacity (CEC), and organic matter favor increased trace elements mobility (Adriano, 2001; Alloway, 2010; Hooda, 2010). In contrast, alkaline pH and high concentrations of those properties contribute to decreasing trace element’s mobility in those environments. Several studies over the last decade have recommended phytoremediation as a form of bioremediation strategy that consists in putting a vegetative cover over contaminated soil or tailings sites containing high concentrations of potentially toxic substances (Acuña et al., 2021; Parraga-Aguado et al., 2014; Pérez et al., 2021; Tapia et al., 2020). This technique uses select plant species to stabilize and reduce the mobility of chemical elements by accumulating them in plant organs, immobilizing them in the rhizosphere, reducing leaching, and depleting substrate moisture through transpiration (Wang et al., 2017). Phytoremediation can be separated into two types: phytostabilization and phytoextraction. Phytostabilization is associated with plants that preferentially accumulate the elements in roots. On the other hand, in phytoextraction, the elements are accumulated in the aboveground parts and then cut to remove the pollutant. In arid and semiarid zones, the phytostabilization technique is more recommended for mine tailings or soils since a permanent vegetation cover can prevent erosion in the zone (Méndez & Maier, 2008). However, due to the low biological activity and nutrient concentrations of tailings substrates under arid and semi-arid conditions, the use of organic amendments was found to be crucial to improve substrate quality, fertility, and plant growth rates (Menares et al., 2017). Furthermore, organic amendments are known to affect the adsorption and complexation of metal(loid)s by reducing their mobility and availability in soil and mine waste substrates, in part due to the presence of alkyl, phenol, carboxyl, and aromatic functional groups that are involved in and control the metal complexation mechanism (Senesi, 2018; Teixeira et al., 2011). Therefore, the physicochemical properties and the dosage of the amendments should be carefully analyzed to ensure they are compatible with the chosen phytostabilization strategy and do not lead to increased mobility of the elements or a decrease in pH. In this regard, commercial humic substances and amendments made from organic waste, such as compost, have been recommended as suitable substrate improvers for mine tailings’ treatment (Duarte et al., 2019; Manzano et al., 2016; Tapia et al., 2017). However, regardless of the type of organic amendments used, their effectiveness will always depend on the plant species selected for the phytostabilization process.
The selection of appropriate plant species for a phytostabilization project must ensure that plants can grow in highly saline or alkaline conditions, tolerate high concentrations of metals, adapt to dry periods in a semi-arid climate, and grow relatively quickly (Marques et al., 2009; Wu et al., 2013). The genus Atriplex, widely distributed in arid and semi-arid regions of the world, are halophyte plants that have been studied and reported as a promising candidate species for the phytostabilization of soils and mine tailings with variable metal(loid) content and salinity (Acosta et al., 2018; Fernandez et al., 2016; Tapia et al., 2013, 2020). Hydroponic experiments with Atriplex atacamensis Phil. (A. atacamensis), a Chilean endemic from the world’s driest Atacama desert, have shown that it can stabilize metalloids such as arsenic (Orrego et al., 2020) and thrive under high concentrations of copper and salinity in an arid environment (Fernandez et al., 2016; Tapia et al., 2013). However, no studies have investigated the phytostabilizing potential of A. atacamensis grown directly on mine tailings.
Carbon isotopic composition (δ13C) has been used to provide insights related to metabolic, chemical, and physical processes associated with carbon transformation processes in plants (Robinson et al., 2000). In that sense, several studies have utilized this analysis as an integral measure to evaluate environmental stress on plants (Merchant et al., 2012), responses to salt stress on physiological mechanisms (Araus et al., 2021; Borzouei et al., 2020), and drought effect (Gouveia et al., 2019; Mariotte et al., 2013; Mårtensson et al., 2017). However, there are also no studies that have evaluated the stress level of plants grown on mine tailings using the 13C isotope as a physiological indicator, considering that mine tailings can have chemical and physical conditions adverse to plant growth. Therefore, the objectives of this study were (i) to evaluate the phytostabilizing ability of A. atacamensis for copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), and sulfur (S) when grown on mine tailings with two different organic amendments, and ii) to determine whether the 13C isotopic composition can be used as a potential stress indicator for the species under study.
Materials and methods
Sampling and characterization of mine tailings and organic amendments
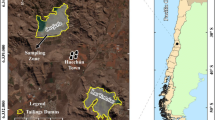
The tailings samples were obtained from the wall of the Ovejería tailings pond of the Codelco Andina Division (33°2′23,25″S 70°47′29,02″W) in Til Til, Santiago Metropolitan Region, Chile. The climate of the tailings pond area is semi-arid and rainy in winter, classified as BSk according to Köppen-Geiger (Peel et al., 2007). The mean annual precipitation is 219 mm and the mean annual minimum and maximum temperatures are 6.7 °C and 27.6 °C, respectively (Santibañez, 2017). Compost and a commercial humic substance (HS) (Pow Humus® WSG 85, Humintech GmbH, Germany) were used as amendments. The humic substance was a mixture of water-soluble humates obtained by alkaline extraction from German leonhardite; Tapia et al. (2017) characterized the German product and reported total extractable carbon (TEC), humic acid carbon (HAC), and fulvic acid carbon (FAC) concentrations (g kg−1) of 131.2 ± 27.2, 116.8 ± 4.3 and 14.4 ± 0.5, respectively. The compost used for the experiment consisted of vegetable residue and branch trimmings composted at the Department of Environmental Management, La Pintana District, Santiago de Chile.
Electrical conductivity (EC) and pH of the compost were measured in a 1:5 w/v compost:water suspension using a Hanna HI 3222 m (Woonsocket, Rhode Island, USA). For mine tailings, pH was evaluated in a 1:2.5 w/v suspension and EC was measured in a saturated extract. Total organic carbon (TOC) in all three types of samples was determined according to Walkley and Black (1946) by oxidation with K2Cr2O7-H2SO4 and determination using FeSO4·7H2O as titrant. Organic matter (OM) was calculated using the following equation: OM (%) = TOC × 1.724. Samples were digested with HF-HClO4-HCl-HNO3 (Dold & Fontboté, 2001), and total Cu, Fe, Mn, and Zn concentrations were subsequently determined using atomic absorption spectrophotometry (AAS) (PerkinElmer 3110). DTPA solution (0.005 M DTPA, 0.01 M CaCl2, and 0.1 M triethanolamine adjusted to pH 7.3) was used to measure available Cu, Fe, Mn, and Zn, and concentrations were determined by AAS (Baker & Amacher, 1982). Available sulfur (S-SO4) was extracted with Ca(H2PO4)2 and measured by turbidimetry using a UV–Vis spectrophotometer (Hach DR5000, USA) (Sing et al., 1995). Available nitrogen (N) was obtained by extraction with KCl followed by distillation and NH4 titration (Mulvaney, 1996). The Olsen method was used to extract available phosphorus (P) from samples using sodium hydrogen carbonate (0.5 M and pH 8.5) (Kuo, 1996). In addition, available potassium (K) was extracted using ammonium acetate (1 M and pH 7.0) with the concentration determined by the AAS method.
To determine the TEC of the compost, 0.5 g compost was mixed with 25 mL of 0.5 M NaOH and shaken for 48 h. The solution was centrifuged and the supernatant was separated from the precipitate. Concentrated HCl was added to the supernatant containing TEC until pH ~ 1.0 to precipitate the humic acid fraction (HAF) from the solution. The fulvic acid fraction (FAF) was separated from the HAF by centrifuge (Schnitzer, 1982) and the concentrations of TEC and C in FAF were determined by oxidation with 1.0 N K2Cr2O7 and phenanthroline as indicator followed by titration with 1.0 N FeSO4·7H2O (Nelson & Sommers, 1982). HAC concentration was calculated using the following equation: HAC = TEC – FAC (Romero et al., 2007; Senesi, 1989; Tapia et al., 2017).
13C-NMR solid-state
Solid-state 13C NMR spectroscopy was performed on a Bruker 400 MHz AVANCE III HD spectrometer equipped with a 4 mm triple resonance probe. A cross-polarization spinning method was used with a magic angle at a spinning speed of 14 kHz and a contact time of 1 ms. The pulse delay was 500 ms, which was proven to be long enough to avoid saturation. The 13C chemical shifts were referenced to external tetramethylsilane (= 0 ppm) and adjusted with glycine (= 176.04 ppm) as an external standard. The spectra were subdivided into the following chemical shift regions: alkyl C (0–45 ppm), N-alkyl C (45–60 ppm), O-alkyl C (60–93 ppm), Di-O-alkyl C (93–110), aromatic C (110–140 ppm), carboxyl C (160–190 ppm), and amide-ketonic C (190–220 ppm) (Stevenson, 1994). The relative intensity distribution of 13C was determined by integrating the signal intensities in the various chemical shift regions using an integration routine supplied with the instrument software. Corrections were made to the intensity distribution of the spinning sidebands (Knicker & Lüdemann, 1995).
Experimental assay under controlled conditions
The pot experiment was conducted under greenhouse conditions at the Faculty of Agricultural Science of the University of Chile (33°34′11,20″S 70°37′54,50″W). from February to May 2018 (summer and fall seasons) The mean temperature in the greenhouse during the study period was 21.7 °C, which was similar to the mean temperature (18.1 °C) in the tailings pond area reported by the weather station of the mining company. The plants of the Chilean endemic species A. atacamensis (20 – 25 cm height) were planted directly into the Ovejería tailings pond using polyethylene pots with a 1 L capacity. Prior to transplanting, A. atacamensis plants had been grown in pearlite and irrigated with tap water. The treatments were: mine tailings with no added amendment (MT); mine tailings with added HS at a dose of 0.72 ton ha−1 (1.30 g pot−1) (MT + HS); and mine tailings with added compost at 89 ton ha−1 (158 g pot−1 or 5% w/w) (MT + C). Each treatment had four replicates. The HS dose was calculated based on the results of Tapia et al. (2019) (360 kg ha−1) and the product cost of 12 USD kg−1.
Total Cu, Fe, Mn, Zn, S (mg kg−1), and TOC (g kg−1) concentrations, dry weight (g), and δ13CV-PDB values of the plants before transplanting are given in Table 1. The amendments were applied in solid form, and each treatment studied was repeated four times. The trial was conducted in a randomized block design. In the first month of the experiment, plants were watered with a nutrient solution consisting of NH4H2PO4 (0.12 g L−1), KNO3 (0.5 g L−1), Ca(NO3)2 (0.49 g L−1), and MgSO47H2O (0.49 g L−1), three times per week, 200 mL per pot, to ensure proper plant establishment in the first days of the experiment. From March to May, treatments were irrigated with tap water (pH: 7.92; EC: 1.51 dS m−1; Cu: 0.03 mg L−1; Fe: 0.39 mg L−1; Mn: 0.01 mg L−1 and Zn: 0.02 mg L−1) twice a week with a dose of 200 mL per pot.
Analysis of plants
At 120 days into the trial, four plants per treatment were harvested and washed with distilled water. The aboveground parts (leaves + stems) and roots were separated and dried in an oven at 70° ± 5 °C for 24 h to determine dry weight. Roots and aboveground parts were ground and 0.5 g of each replicate were autoclaved with 6 mL 65% HNO3, 4 mL H2O2, and 6 mL distilled water at 125 °C and 1.5 kPa for 40 min (Moreno-Jiménez et al., 2012). The concentrations of Cu, Fe, Mn, and Zn were determined by AAS. For sulfur content, 0.5 g of each sample was calcined in Mg(NO3)2, dissolved in 2 M HCl, and BaSO4 turbidity was measured using a UV–Vis spectrophotometer (Lachica et al., 1973). Results were expressed in mg kg−1 dry matter. All reagents used were of analytical grade quality (Merck, Darmstadt, Germany), and deionized water (18.2 MΩ.cm at 25 °C) obtained using a Milli-Q system (Merck, Darmstadt, Germany) was used for the preparation of reagents and standards. The detection limit of the AAS (PerkinElmer 3110) for Cu, Fe, Mn, and Zn is 0.05 mg L−1. The Soil and Water Chemistry laboratory from the Faculty of Agricultural Science, University of Chile, is accredited by the Servicio Agricola y Ganadero (SAG), an official government organization associated with the Agriculture Ministry of Chile (RE 7849, 2020). Internal samples reference from the Soil and Water Chemistry Laboratory were used to verify the accuracy and precision of the samples measured. In general, for metal-enriched samples (tailings, soils, plants, and waters) the recovery rate is between 98 and 102%.
Total carbon concentration and δ13CV-PDB determination in plants
Representative leaf samples of the upper, middle, and lower parts of A. atacamensis were collected at each treatment iteration and placed in pre-labeled envelopes. The samples were oven-dried (Memmert, model UF 160, Germany) at 65 °C to constant weight and ground to a fine powder in a mill (IKA, model MF10 basic, Germany) (Cook et al., 2018). Approximately 1.0 mg of each sample was weighed on an analytical balance (Precisa, model 125A, Switzerland) and sealed in a tin capsule. Capsules were placed in a 96-well microtiter plate and used for isotopic analysis. Leaf carbon isotopic composition and total carbon were measured using an isotope ratio mass spectrometer (Sercon, model Integra2, UK). Results were expressed as ‰ for δ13C and g kg−1 dry matter for leaf total carbon. The accuracy of the instrument was 0.2‰ for δ13C.
Phytoremediation indexes and metals uptake
The translocation index (TI) was used to evaluate the migration of elements from soils or mine tailings to plant tissues (Acuña et al., 2021; Dede & Ozdemir, 2016; Tapia et al., 2019). The TI of Cu, Fe, Mn, Zn, and S in plant samples was calculated as follows (Eq. 1):
where CAP is the concentration of the element in the aboveground parts (mg kg−1) and CR is the concentration of the element in the roots (mg kg−1). The values obtained were interpreted as follows: TI > 1 indicates phytoextraction of the element by A. atacamensis, and TI < 1 indicates the species’ phytostabilization ability.
The bioconcentration factor (BCF) was calculated to evaluate the trace elements’ uptake of A. atacamensis and its potential hyperaccumulation efficiency in the roots (Lam et al., 2017; Taeprayoon et al., 2022). The BCF of Cu, Fe, Mn, and Zn in roots was calculated according to Eq. 2.
where CR is the concentration in roots (mg kg−1) and CMT is the trace element total concentration in the mine tailings (mg kg−1). According to Kamari et al. (2014) and Lam et al. (2017), BCF values < 1.0 indicate plants are excluder, between 1.0 and 10, are accumulator, and BCF > 10 indicate a potential trace elements hyperaccumulation by A. atacamensis.
Additionally, the metals and S uptake contents were calculated in roots and the aboveground parts by A. atacamensis (Eq. 3). The metals and S uptake contents indicate the concentration in plant tissues that were taken by the root system based on plant biomass; this analysis evaluates the potential phytostabilization capacity of plants studied for bioremediation in soils and tailings (Meeinkuirt et al., 2016; Taeprayoon et al., 2022; Tapia et al., 2020).
where CAP/R is the concentration in roots or aboveground parts (mg kg−1) and BAP/R is the roots or aboveground parts dry biomass (kg). The metals and S uptake contents were expressed in mg tissues−1.
Statistical analyses
Concentrations (mg kg−1) and uptake (mg) of Cu, Fe, Mn, and Zn, percent S, dry matter content, total carbon (g kg−1), δ13CV-PDB, TI, and BCF at 120 days were compared across treatments by one-way analysis of variance (ANOVA). Tukey’s test (p ≤ 0.05) was used to evaluate statistically significant differences among treatments. Statistical analyses were performed using InfoStat v.2017 software.
Results and discussion
Chemical characterization of mine tailings and organic amendments
The results of the chemical characterization (Table 2) showed that mine tailings had low salinity, alkaline pH, and low fertility, consistent with the detected N, P, K, CEC, and OM levels (Tapia et al., 2017). As expected, high concentrations of extractable S-SO4 were detected, which is in agreement with the findings of Dold and Fontboté (2001), who showed that pyrite oxidation reactions result in the release of metal(loid)s and sulfates into solution. Total Fe, Mn, and Zn concentrations were within the global reference range for tailings reported by Hossner and Shahandeh (2005) (Fe: 0.4–57%; Mn: 10–4000 mg kg−1 and Zn: 1.0–5000 mg kg−1); however, total Cu concentration was more than twice the maximum content reported by these authors (1–750 mg kg−1). In Chile, soils and mine tailings typically exceed background Cu concentrations reported by other authors, such as 2–250 mg kg−1 according to Sparks (2003), and 14–109 mg kg−1 according to Kabata-Pendias (2011). The reason is that some geographic areas of the country are naturally enriched in Cu (Oyarzún et al., 2016). Values ranging from 436 to 2564 mg kg−1 Cu in mine tailings in central Chile (Dold & Fontboté, 2001), 4393 mg kg−1 in the north-central zone (Santibañez et al, 2011), and 1.6% Cu in northern Chile (Lam et al., 2018) have been reported.
For regulatory purposes, the use of a risk assessment code (RAC) has been recommended to assess the level of risk associated with the available concentrations of metal(loid)s in sediments, soils, and mine tailings that may potentially migrate from the exchangeable and carbonate fractions (Mileusnić et al., 2014; Singh & Lee, 2015; Tapia et al., 2017). According to the five levels of RAC, available concentrations < 1% pose no risk of migration; concentrations of 1 to 10% are low risk; 11 to 30% are medium risk; 31 to 50% are high risk; and above 75% are very high risk (Sun & Chen, 2018). According to the RAC matrix, the available Cu, Fe, and Zn concentrations found in this study, 1.86%, 1.23%, and 6.07%, respectively, indicate a low risk of migration, while the Mn concentration of 0.85% indicates no risk.
Turning to organic amendments, we found that the HS had higher pH, EC, CEC, and available N, P, and K concentrations than the compost, while both amendments had similar TOC percentages and S-SO4 concentrations. The observed chemical parameters of the amendments were favorable for improving the fertility of the tailings substrate to ensure adequate growth and development of A. atacamensis. In addition, the alkaline pH of the compost and the HS reduced the mobility of the metals studied, while the salinity provided by both amendments stimulated plant growth (Pardo et al., 2017; Tapia et al., 2020). For metallic elements, we found that both total and available concentrations of Cu, Fe, Mn, and Zn were higher in the compost than in the HS; however, none of the metallic elements in either amendment exceeded their respective concentrations in the tailings. The main difference between the two amendments was the higher concentration of HA-C and FA-C in the HS compared to the compost, which was due to the higher moisture content in the HS. This difference indicates the higher reactivity of the HS to cationic elements, which may reduce the solubility and mobility of metallic elements through adsorption or the formation of less mobile complexes (Boruvka & Drábek, 2004; Clemente et al., 2006).
The 13C NMR spectra shown in Fig. 1 indicate that the chemical structures of the amendments are dissimilar, presumably due to the different source materials of the compost and HS (plant matter vs. leonardite). As for the main spectral peaks, the relative areas of the C-groups are shown in Table 3. The analysis showed that the alkyl-C group is the dominant spectral region for both amendments and is linked to humic acids with aliphatic structures, recalcitrant short-chain components such as cutin and suberin, and volatile fatty acids (Lin et al., 2018; Tambone et al., 2019). Meanwhile, the content of O-alkyl groups in the compost was higher than that of the HS fraction, indicating the presence of labile organic compounds such as carbohydrates, cellulose, and hemicellulose, which are usually abundant in plant-based amendments and soils with plant residues (Bonanomi et al., 2018; Lin et al., 2018; Paetsch et al., 2016).
The proportion of aromatic C in the HS in this study was 1.95 times higher than in the compost, due to the longer degradation time of leonardite OM and the tendency of O-alkyl groups to decompose into aromatic, phenolic, and carboxyl groups (Wang et al., 2015). Our observations were consistent with that trend, as the O-alkyl region of the HS was three times smaller than that of the compost, but had more aromatic and phenolic groups. The abundance of aromatic and phenolic compounds in the HS may also indicate the predominance of structures derived from recalcitrant or organic compounds such as lignin and polyphenols (Lin et al., 2018) involved in the reactions between metals and humic substances. Taking into account additional factors, such as the degree of alkylation and maturity, this factor may be considered responsible for the metal adsorption behavior (Li et al., 2015). The relative region of the carboxyl group in this study was fairly similar in both amendments but slightly larger in the compost. This group plays an important role in the binding process of metal ions due to its high affinity to metallic elements (Senesi, 2018), facilitating the formation of heavy metal complexes and the stabilization of metallic elements in mine tailings or contaminated soils. These findings are consistent with those of Mayans et al. (2019), who conducted a chemical analysis of commercial HS derived from leonardite and found a greater relative area of alkyl-C, aromatic-C, and phenolic-C groups compared to composts derived from plant matter or animal manure. These authors also found that HS had a greater ability to complex with Cu due to its phenolic and carboxylic groups and was more thermostable due to its high recalcitrant OM.
Metallic elements and plant sulfur
Overall, the addition of the HS did not significantly increase Cu, Fe, Mn, or Zn concentrations in the aboveground parts or roots of A. atacamensis compared to the MT treatment (Table 4). However, the slight increase in root Cu concentrations in the MT + HS treatment suggests that the alkalinity of both the HS and the tailings material and the predominance of aromatic and alkyl fractions in the HS, as indicated by 13C NMR results, reduced the mobility of metal complexes and facilitated their adsorption on A. atacamensis root tissues. A similar trend for Cu was reported by Tapia et al. (2020), who evaluated Atriplex nummularia Lindl. (A. nummularia) in the same tailings, reporting root concentrations of 155 ± 4.40 mg kg−1 without amendments and 179 ± 21.1 mg kg−1 with 5% w/w commercial humic substance. Our results for root Cu concentrations were higher compared to other studies in mine tailings using species of the genus Atriplex. Thus, in Atriplex halimus L. (A. halimus) grown in mine tailings (total Cu: 1,080 to 2,201 mg kg−1), Midhat et al. (2019) reported maximum root Cu values of 51.5 ± 0.72 mg kg−1, and Tapia et al. (2019) in tailings with humic substances (at a dose of 120 kg ha−1) reported maximum Cu concentrations of 185 ± 37.8 mg kg−1. Furthermore, Acosta et al. (2018) found root Cu concentrations of less than 20 mg kg−1 in A. halimus grown in unamended tailings (pH: 7.4 ± 0.2; DTPA-Cu: 2.3 ± 0.3 mg kg−1). Meanwhile, these results may have one drawback, which has to do with the high solubility of HS, making it vulnerable to leaching losses, especially if only one low dose is applied. An alternative that could improve HS efficiency would be to use higher doses with piecemeal application over time; however, the high cost (12 USD kg−1) of commercial humic substances makes them unsuitable for continuous use, given those areas covered by phytoremediation are usually extensive.
Interestingly, the MT + C treatment resulted in significantly lower concentrations of Cu, Fe, and Mn in both aboveground parts and roots compared to the MT and MT + HS treatments. These results suggest that compost application likely immobilized Cu, Fe, and Mn by forming stable organometallic compounds adsorbed on the OM, thereby reducing the phytoavailability of these metals. The higher amount of compost applied (158 g pot−1) compared to the HS (1.30 g pot−1) clearly played a role because the MT + C treatment contained proportionally more aromatic, phenolic, and carboxyl groups that have higher metal complexing capacity than other functional groups (Mayans et al., 2019). Usually, metallic elements are firmly incorporated into the OM via metal-chelate compounds and are believed to persist as long as the properties of the OM do not change significantly over time (Hooda, 2010). Depending on the element, the number of atoms bound to the metal ion, the number of rings formed, pH, and metal concentration, the stability of metal-chelate complexes may vary to a greater or lesser extent (Cu+2 > Ni+2 > Co+2 > Zn+2 > Fe+2 > Mn+2) (Stevenson, 1994). Duarte et al. (2019) evaluated the effect of progressively rising composted manure doses (from 1 to 12% w/w) on chemical fractionation and metal retention in mine tailings and reported a 28.5% decrease in EDTA-extractable Cu and a decrease in interchangeable metal fractions (bioavailable to plants) at the highest doses. A similar trend was observed by Khan and Jones (2009), who reported that a 10% dose of green manure compost in Cu mine tailings (Cu total: 1905 mg kg−1) decreased Cu and Fe extractability over time.
The opposite situation was observed with the Zn content in A. atacamensis, however, as the MT + C treatment significantly increased the Zn concentration in the aboveground parts compared to the MT and MT + SH treatments. The increase was probably due to the formation of soluble complexes that increased Zn availability to plants. Manzano et al. (2016), who evaluated metal mobility in mine tailings supplemented with compost (4% w/w), reported an increase in exchangeable Zn due to the formation of soluble complexes between Zn and dissolved organic carbon supplied with the amendment. In addition, the compost used may itself be a significant source of metals such as calcium and magnesium, which can affect Zn mobility due to ionic competition (Branzini & Zubillaga, 2012). Furthermore, bioavailability tests showed weaker Zn binding to humic acids due to lower affinity (Kang et al., 2011).
The aboveground and root content of Zn in this study (Table 4) was lower compared to other studies of the genus Atriplex on mine tailings. For example, Acosta et al. (2018) reported leaf Zn range of 300 to 400 mg kg−1 and root Zn content of 250 to 300 mg kg−1 in A. halimus grown on mine tailings (pH 7.5 and available Zn: 402 mg kg−1) supplemented with pig manure (dose 3 L m−2). Similar results were published about Atriplex lentiformis (Torr.) by Gil-Loaiza et al. (2016), who supplemented tailings with compost in doses of 15% and 20% w/w (pH 2.5 and extractable Zn: 1800 mg kg−1), reporting shoot Zn content in the range of 506 ± 253 to 936 ± 305 mg kg −1. The lower concentrations observed in the Chilean species may be related to the amount of available Zn in the tailings in our study (15.0 ± 1.17 mg kg−1), considering that another species, Carpobrotus aequilaterus (Haw.), grown in the same tailings with the addition of HS (30 kg ha−1), exhibited the same concentrations in the aboveground parts as in our experiment (38.2 ± 7.6 mg kg−1) (Tapia et al., 2017).
As for S concentrations (Table 4), A. atacamensis had between 0.21 ± 0.01 and 0.35 ± 0.04% in the aboveground parts and between 0.38 ± 0.04 to 0.42 ± 0.06% in the roots, which is consistent with typical S concentrations in plants (0.20–0.50%), according to Havlin et al. (2014). In addition, the sulfur and metal concentrations obtained in this study were below the limit for livestock feed plants (NRC, 2005), which was set to prevent contaminants from moving up the food chain through livestock grazing on live or dead plants at the senescence stage (Pérez-de-Mora et al., 2011). In fact, the sulfur concentrations in our experiment were similar to the results reported by other authors who studied the genus Atriplex as livestock feed. Koning et al. (2019) evaluated A. nummularia and Atriplex amnicola Lindl. for free-range poultry feed and reported leaf sulfur contents of 0.60 and 0.20%, respectively. Similarly, Norman et al. (2004) investigated the nutritional value of two species of the genus Atriplex used in commercial grazing systems and found S concentrations in the range of 0.38 ± 0.07% and 0.48 ± 0.12. In addition, Mahipala et al. (2009) reported concentrations ranging from 0.09 ± 0.01% to 0.47 ± 0.04% in Atriplex species grown in the Mediterranean climate and investigated as potential ruminant feed.
Regarding S phytoremediation, the use of higher plants has been proposed as an effective strategy in gypsum soils with toxic concentrations of sulfate, considering the diversity of metabolism, accumulation, and role of S as a major nutrient in plants (Ruiz et al., 2003). In this regard, several species common in semi-arid and arid habitats and capable of accumulating leaf S concentrations of 2.5 to 8.2% dry matter have been classified as thiophore plants and recommended as candidates for use in remediation strategies (Ernst, 1998). The global environmental issue related to S and mine tailings is the acid mine drainage phenomenon, which is promoted by bacterial oxidation of sulfide minerals such as pyrite (FeS2). This process is characterized by increasing the acidity of tailings and the available concentration of metals and sulfate, which are more susceptible to leaching (Lottermoser, 2010). Therefore, adequate species are needed to remove metals and sulfate from mine tailings. In general, few studies have evaluated the sulfur content of plants grown directly on mine tailings. Parraga-Aguado et al. (2014) reported shoot/leaf S concentrations of 0.61 ± 0.10% in A. halimus, grown naturally on mine tailings in semi-arid climates in Spain, and concluded that this halophytic plant could be used for desalination of mine tailings. In Chile, Tapia et al. (2017) used Carpobrotus aequilaterus (Haw.) with the HS at a rate of 60 kg ha−1 and obtained aboveground S content of 0.44 ± 0.03% and root S of 0.65 ± 0.30% at 120 days, which is higher than the concentrations reported in this study. However, A. nummularia grown on the same mine tailings with and without amendments (compost 5% w/w and humic substance 5% w/w) by Tapia et al. (2020) exhibited concentrations in aboveground parts as high as 2%. The authors concluded that this species is a potential thiophore plant and that the addition of commercial humic substances improves its ability to phytostabilize sulfur.
In our experiment, the addition of organic amendments significantly increased the aboveground S concentrations in A. atacamensis compared to the MT treatment (Table 4). However, despite using the same genus as Tapia et al. (2020), our results were insufficient to recommend the Chilean endemic A. atacamensis as a potential sulfur accumulator. The significant increase in S content in the MT + C and MT + HS treatments can be attributed to the contribution of S–SO4 from the organic amendments incorporated in the mine tailings (87% contribution in the MT + C treatment and 81% in the MT + HS treatment). Furthermore, the average temperature of 21.7 °C during the study period was within the optimal range (20–40 °C) for increased S mineralization due to OM influx from the amendments and probably contributed to S-SO4 availability in solution (Havlin et al., 2014).
Phytostabilization indexes, metals uptake, and δ13CV−PDB in leaves
TI values (Fig. 2) for all elements and treatments evaluated were below unity, indicating that this species tends to concentrate Cu, Fe, Mn, Zn, and S in the roots rather than in the aboveground parts, with or without organic supplementation of the tailings’ substrate. Furthermore, the values of Cu and Fe were less than 0.1, indicating that A. atacamensis has a greater ability to phytostabilize these elements compared to the other metals studied. Several studies (Acuña et al., 2021; Eissa & Almaroai, 2019; Lam et al., 2017; Rabier et al., 2014) reported TI values lower than 1.0 for the genus Atriplex for various metal(loid)s in tailings and contaminated soils and concluded that this genus has remarkable tolerance to high metal(loid) concentrations while accumulating metals primarily in its roots rather than in the shoots and stems. Regarding the BCF (Fig. 3), the values for all treatments and elements were different, indicating that the metal accumulation capacity in roots by A. atacamensis varies between elements. In addition, the BCF results showed values below unity, classifying this specie as an excluder plant for Cu, Fe, Mn, and Zn; however, these results are explained by the significant difference between the metal concentrations in the mine tailings and the root contents. The BCF results for Cu, Fe, and Mn in the MT + C treatment followed a trend to have significantly lower values than MT and MT + HS, which are also reflected in the concentration of those elements (Table 4). These results are attributed to the compost addition and its effect on metals, decreasing the bioavailability by forming organometallic compounds adsorbed on the OM. The BCF values < 1.0 are frequently found for Cu, Fe, Mn, and Zn in the genus Atriplex; for instance, Lam et al. (2017) studied A. nummularia in mine tailings and found similar BCF values for Cu (0.12–0.17), Fe (0.03–0.10), Mn (0.11–0.33), and Zn (0.11–0.27), concluding that A. nummularia can be considered as an excluder specie for those elements. However, although BFC classifies A. atacamensis as an excluder species, our TI results (Fig. 2) show that A. atacamensis was mainly an accumulator for Cu and Fe, and can have the potential for phytostabilization in its roots which agree with TI results reported by Tapia et al. (2020) for Cu (0.15 – 0.20) in A. nummularia cultivated in mine tailings, considered a phytostabilization species for Cu after 120 days of trial.
Translocation index of Cu, Fe, Mn, Zn, and S for Atriplex atacamensis Phil. cultivated in mine tailing (MT), mine tailings + humic substance (MT + HS), and mine tailing + compost (MT + C). Values below the dotted line indicate the phytostabilization of elements. Values are mean ± standard deviation (n = 4). Different letters between treatments show statistically significant differences according to the Tukey test (p ≤ 0.05)
Bioconcentration factor of Cu, Fe, Mn, and Zn for Atriplex atacamensis Phil. cultivated in mine tailing (MT), mine tailings + humic substance (MT + HS), and mine tailing + compost (MT + C). Values are mean ± standard deviation (n = 4). Different letters between treatments show statistically significant differences according to the Tukey test (p ≤ 0.05)
The metals and S uptake values are shown in Fig. 4. The results indicate that A. atacamensis tends to accumulate more Cu and Fe in its roots than the aboveground parts, which agrees with the TI values (TI < 0.1) discussed above (Fig. 2). The treatments MT and MT + HS had Cu and Fe contents statistically higher than MT + C due to the compost influence on Cu and Fe stabilization in MT + C, which decreases the bioavailability for plants. Those results followed a similar pattern to the Cu and Fe concentrations (Table 4), which showed lower concentrations in MT + C than in MT and MT + HS. Adding compost reduces metal mobility as a result of precipitation and sorption processes as metal-chelate compounds formed are firmly incorporated into the OM of the substrate (Hooda, 2010). Mainly for Cu and Fe, the alkaline pH, high CEC, and the carboxyl groups associated with the high compost dose added could increase adsorption and precipitation processes in the mine tailings. Thus, adequate amendment doses are crucial to improving fertility, metal stabilization, growth rate, and elements’ uptake in plants established in mine tailings under arid and semi-arid conditions. In our study, A. atacamensis showed a higher Cu uptake capacity for roots and aboveground parts than the Atriplex nummularia studied by Tapia et al. (2020) in the same mine tailings with compost and humic substance addition (5% w/w dose); indicating that A. atacamensis could be more suitable for Cu phytostabilization than A. nummularia. However, for S phytoremediation purposes, the species studied by Tapia et al. (2020) would be more suitable as significantly could accumulate higher S content in roots and aboveground parts than A. atacamensis.
Uptake content (mg) of Cu, Fe, Mn, Zn, and S for Atriplex atacamensis Phil. cultivated in mine tailing (MT), mine tailings + humic substance (MT + HS), and mine tailing + compost (MT + C). Values are mean ± standard deviation (n = 4). Different letters between treatments and tissues show statistically significant differences according to the Tukey test (p ≤ 0.05)
Dry weight analysis (Fig. 5) showed that both organic amendments significantly reduced the dry weight of aboveground parts, while compost additionally reduced the dry weight of roots compared to the MT treatment. The reduction was attributed to metal immobilization by compost, which reduced Fe concentrations in A. atacamensis Phil. to deficiency levels (< 150 mg kg−1 leaves) inducing a series of physiological responses in plants, including reduced photosynthetic activity, inhibited root elongation, increased apical root diameter, and abundant root hair formation (Marschner, 2012). This conclusion is supported by the lower leaf TOC content in the MT + C treatment compared to the MT and MT + HS treatments (Table 5).
Dry weight of Atriplex atacamensis Phil. cultivated in mine tailing (MT), mine tailings + humic substance (MT + HS), and mine tailing + compost (MT + C). Values are mean ± standard deviation (n = 4). Different letters between treatments indicate statistically significant differences according to the Tukey test (p ≤ 0.05)
Measurements of carbon isotopic composition in leaf dry matter in C4 plants can be used to assess changes in the ratio of intercellular and ambient CO2 concentrations (Ci/Ca-ratio), photosynthetic and post-photosynthetic fractionations (Ellsworth & Cousins, 2016), as well as drought, salt, and nitrogen stresses (Walker and Sinclair, 1992; Fravolini et al., 2002; Monneveux et al., 2007). Our results (Tables 1 and 5) showed that A. atacamensis leaf δ13CV-PDB values at 120 days increased by 4.63%, 5.89%, and 3.09% in the MT, MT + HS, and MT + C treatments, respectively, compared to the values in plants established in pearlite. This was likely due to the salinity of the tailings’ material used. Walker and Sinclair (1992) reported a trend toward decreased discrimination in the leaf 13C composition in the Atriplex species against 13C in CO2 in the air with increasing soil conductivity. In addition, Meinzer and Zhu (1999) observed that the decrease in discrimination against 13C and other physiological parameters analyzed affected the efficiency of C4 photosynthesis in Atriplex lentiformis under salinity stress. The addition of organic amendments made no significant difference in the 13C composition at the end of the experiment (Table 5), indicating that the amendments applied and their doses did not cause any additional salt stress beyond that exerted by the mine tailings material. The evaluation of this parameter may serve as a first approximation for the study of other physiological parameters of plants grown in tailings, such as stomatal conductance, photosynthesis, Ci/Ca ratio, leaf temperature, and leaf water potential, in order to elucidate the effect of organic amendments in mine tailings on stress in plants grown and evaluated under in situ conditions.
Conclusions
The study showed that Atriplex atacamensis Phil. grown in a tailings pond successfully fulfills the phytostabilizing function with or without the addition of organic amendments, resulting in higher concentrations of Cu, Fe, Mn, Zn, and S in the roots, but not in the aboveground parts. The addition of the humic substance did not make a significant difference compared to the MT treatment, but the compost was able to reduce the concentrations of Cu, Fe, and Mn in both roots and aboveground parts by immobilizing these metals, which also was demonstrated with the uptake values obtained. The opposite situation was observed in both plant tissues with respect to Zn content. These results are attributable, firstly, to the differences in the chemical structure of the amendments determined by 13C NMR analysis and, secondly, to the dose of compost used, which provided a greater number of functional groups with a higher complexation capacity to metals. Our δ13CV-PDB values for the compost and HS treatments showed that Atriplex atacamensis Phil. experienced no greater salt stress at 120 days compared to the treatment without organic amendments. Overall, Atriplex atacamensis Phil. demonstrated the ability to phytostabilize metals and sulfur and may thus be considered a suitable candidate species for in situ evaluation of the phytostabilization process of mine tailings.
Data availability
All data included in this paper can be made available upon request to the corresponding author.
References
Acosta, J. A., Abbaspour, A., Martínez, G. R., Martínez-Martínez, S., Zornoza, R., Gabarrón, M., & Faz, A. (2018). Phytoremediation of mine tailings with Atriplex halimus and organic/inorganic amendments: A five-year field case study. Chemosphere, 2014, 71–78. https://doi.org/10.1016/j.chemosphere.2018.04.027
Acuña, E., Castillo, B., Queupuan, M., Casanova, M., & Tapia, Y. (2021). Assisted phytoremediation of lead contaminated soil using Atriplex halimus and its effect on some soil physical properties. International Journal of Environmental Science and Technology, 18(7), 1925–1938. https://doi.org/10.1007/s13762-020-02978-5
Adiansyah, J. S., Rosano, M., Vink, S., & Keir, G. (2015). A framework for a sustainable approach to mine tailings management: Disposal strategies. Journal of Cleaner Production, 108, 1050–1062. https://doi.org/10.1016/j.jclepro.2015.07.139
Adriano, D. C. (2001). Trace Elements in Terrestrial Environments (2nd ed.). Springer.
Alloway, B. J. (2010). Heavy metals in soils. In Trace metals and metalloids in soil and their bioavailability (3rd ed.). N.Y., USA: Springer.
Araus, J. L., Rezzouk, F. Z., Thushar, S., Shahid, M., Elouafi, I. A., Bort, J., & Serret, M. D. (2021). Effect of irrigation salinity and ecotype on the growth, physiological indicators and seed yield and quality of Salicornia europaea. Plant Science., 304, 110819. https://doi.org/10.1016/j.plantsci.2021.110819
Baker, D., & Amacher, M. (1982). In A. L. Page (Ed.), Nickel, copper, zinc and cadmium (pp. 331–332). American Society of Agronomic Inc, Soil Science Society of American Inc, Madison, Winconsin.
Bonanomi, G., Ippolito, F., Cesarano, G., Vinale, F., Lombardi, N., Castro, A., Woo, S., & Scala, F. (2018). Biochar chemistry defined by 13C-CPMAS NMR explains opposite effects on soilborne microbes and crop plants. Applied Soil Ecology, 124, 351–361. https://doi.org/10.1016/j.apsoil.2017.11.027
Boruvka, L., & Drábek, O. (2004). Heavy metal distribution between fractions of humic substances in heavily polluted soils. Plant Soil and Environment, 50(8), 339–345. https://doi.org/10.17221/4041-PSE
Borzouei, A., Mousavi Shalmani, M. A., & Eskandari, A. (2020). Effects of salt and nitrogen on physiological indices and carbon isotope discrimination of wheat cultivars in the northeast of Iran. J. of Integr. Agr., 19(3), 656–667. https://doi.org/10.1016/S2095-3119(19)62629-8
Branzini, A., & Zubillaga, M. S. (2012). Comparative use of soil organic and inorganic amendments in heavy metals stabilization. Applied and Environmental Soil Science, 2012, 1–7. https://doi.org/10.1155/2012/721032
Clemente, R., Escobar, A., & Bernal, M. P. (2006). Heavy metals fractionation and organic matter mineralization in contaminated calcareous soil amended with organic materials. Bioresource Technology, 97, 1894–1901. https://doi.org/10.1016/j.biortech.2005.08.018
Cook, C., Erkila, B., Chakraborty, S., Tipple, B., Cerling, T., & Ehleringer, J. (2018). Stable isotope biogeochemistry and ecology: Laboratory manual (p. 173p). Middletown.
Dede, G., & Ozdemir, S. (2016). Effects of elemental sulphur on heavy metal uptake by plants growing on municipal sewage sludge. Journal of Environmental Management, 166, 103–108. https://doi.org/10.1016/j.jenvman.2015.10.015
DOE. (2011). Diario Oficial de la República de Chile. Ministerio de Minería, pp. 56 (N°40107. 11/11/2011).
Dold, B., & Fontboté, L. (2001). Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. Journal of Geochemical Exploration, 74, 3–55. https://doi.org/10.1016/S0375-6742(01)00174-1
Duarte, V. M., Carrillo-González, R., Lozano, M. L., & Carrasco, V. (2019). Fractionation of heavy metals in mine tailings amended with composted manure. Soil and Sediment Contamination, 28, 148–161. https://doi.org/10.1080/15320383.2018.1553931
Eissa, M. A., & Almaroai, Y. A. (2019). Phytoremediation capacity of some forage plants grown on a metals-contaminated soil. Soil Sediment Contamination, 28(6), 569–581. https://doi.org/10.1080/15320383.2019.1634674
Ellsworth, P. Z., & Cousins, A. B. (2016). Carbon isotopes and water use efficiency in C4 plants. Current Opinion in Plant Biology, 31, 155–161. https://doi.org/10.1016/j.pbi.2016.04.006
Ernst, W. (1998). Sulfur metabolism in higher plants: Potential for phytoremediation. Biodegradation, 9, 311–318. https://doi.org/10.1023/A:1008250827209
Fernandez, Y. T., Diaz, O., Acuna, E., Casanova, M., Salazar, O., & Masaguer, A. (2016). Phytostabilization of arsenic in soils with plants of the genus Atriplex established in situ in the Atacama Desert. Environmental Monitoring and Assessment, 188(2016), 235. https://doi.org/10.1007/s10661-016-5247-x
Fravolini, A., Williams, D. G., & Thompson, T. L. (2002). Carbon isotope discrimination and bundle sheath leakiness in three C4 subtypes grown under variable nitrogen, water and atmospheric CO2 supply. Journal of Experimental Botany, 53, 2261–2269. https://doi.org/10.1093/jxb/erf084
Gil-Loaiza, J., White, S. A., Root, R. A., Solís-Dominguez, F. A., Hammond, C. M., Chorover, J., & Maier, R. M. (2016). Phytostabilization of mine tailings using compost-assisted direct planting: Translating greenhouse results to the field. Science of the Total Environment, 565, 451–461. https://doi.org/10.1016/j.scitotenv.2016.04.168
Gouveia, C. S. S., Ganança, J. F. T., Slaski, J., Lebot, V., & Pinheiro de Carvalho, M. Â. A. (2019). Stable isotope natural abundances (δ13C and δ15N) and carbon-water relations as drought stress mechanism response of taro (Colocasia esculenta L. Schott). Journal of Plant Physiology, 232, 100–106. https://doi.org/10.1016/j.jplph.2018.11.024
Havlin, J., Tisdale, S., Nelson, W., & Beaton, J. (2014). Soil Fertility and Fertilizers: An Introduction to Nutrient Management (8th ed.). Pearson.
Hooda, P. (2010). Trace Elements in Soils. Wiley.
Hossner, L., & Shahandeh, H. (2005). Rehabilitation of minerals processing residue (tailings). In R. Lal (Ed.), Encyclopedia of Soil Science (pp. 1450–1455). Marcel Dekker.
Kabata-Pendias, A. (2011). Trace elements in soil and plants (4th ed.). CRC Press.
Kamari, A., Yusoff, S. N. M., Putra, W. P., Ishak, C. F., Hashim, N., Mohamed, A., & Phillip, E. (2014). Metal uptake in water spinach grown on contaminated soil amended with chicken manure and coconut tree sawdust. Environmental Engineering & Management Journal, 13(9), 2219–2228.
Kang, J., Zhang, Z., & Wang, J. (2011). Influence of humic substances on bioavailability of Cu and Zn during sewage sludge composting. Bioresource Technology, 102, 8022–8026. https://doi.org/10.1016/j.biortech.2011.06.060
Khan, M. J., & Jones, D. L. (2009). Effect of composts, lime, and diammonium phosphate on the phytoavailability of heavy metals in a copper mine tailing soil. Pedosphere, 19(5), 631–641. https://doi.org/10.1016/S1002-0160(09)60158-2
Knicker, H., & Lüdemann, H. (1995). N-15 and C-13 CPMAS and solution NMR studies of N-15 enriched plant material during 600 days of microbial degradation. Organic Geochemistry, 23, 329–341. https://doi.org/10.1016/0146-6380(95)00007-2
Koning, C., Barekatain, R., Singh, M., & Drake, K. (2019). Saltbush (Atriplex nummularia and A. amnicola) as potential plants for free-range layer farms: consequences for layer performance, egg sensory qualities, and excreta moisture. Poultry Science, 98(10), 4555–4564. https://doi.org/10.3382/ps/pez294
Kuo, S. (1996). Phosphorus. pp. 869–920. In D. L. Sparks, et al. (Eds.), Methods of soil analysis. Part 3. Soil Science Society of America Book Series 5. Soil Society of America, USA, pp. 1390.
Lachica, M., Aguilar, A., & Yañez, J. (1973). Análisis foliar. Métodos analíticos utilizados en la Estación Experimental del Zaidín. Anales De Edafologia y Agrobiologia, 32, 1033–1047.
Lam, E., Gálvez, M., Cánovas, M., Montofré, I., & Keith, B. (2018). Assessment of the adaptive capacity of plant species in copper mine tailings in arid and semiarid environments. Journal of Soils and Sediments, 18, 2203–2216. https://doi.org/10.1007/s11368-017-1835-9
Lam, E. J., Cánovas, M., Gálvez, M. E., Montofré, Í. L., Keith, B. F., & Faz, Á. (2017). Evaluation of the phytoremediation potential of native plants growing on a copper mine tailing in northern Chile. Journal of Geochemical Exploration, 182, 210–217. https://doi.org/10.1016/j.gexplo.2017.06.015
Li, C. L., Ji, F., Wang, S., Zhang, J. J., Gao, Q., Wu, J. G., & Zheng, L. R. (2015). Adsorption of Cu (II) on humic acids derived from different organic materials. Journal of Integrative Agriculture, 14(1), 168–177. https://doi.org/10.1016/S2095-3119(13)60682-6
Lin, Z., Li, Y., Tang, C., Luo, Y., Fu, W., Cai, X., Li, Y., Yue, T., Jiang, P., Hu, S., et al. (2018). Converting natural evergreen broadleaf forests to intensively managed moso bamboo plantations affects the pool size and stability of soil organic carbon and enzyme activities. Biology and Fertility of Soils, 54, 467–480. https://doi.org/10.1007/s00374-018-1275-8
Lottermoser, B. G. (2010). Mine wastes. Characterization, treatment, and environmental impacts (3rd ed.). Berlin, Germany: Springer. 400 p.
Mahipala, M. R. P., Krebs, G. L., McCafferty, P., & Gunaratne, L. H. P. (2009). Chemical composition, biological effects of tannin and in vitro nutritive value of selected browse species grown in the West Australian Mediterranean environment. Animal Feed Science and Technology, 153, 2013–2215. https://doi.org/10.1016/j.anifeedsci.2009.06.014
Manzano, R., Silvetti, M., Garau, G., Deiana, S., & Castaldi, P. (2016). Influence of iron-rich water treatment residues and compost on the mobility of metal(oid)s in mine soils. Geoderma, 283, 1–9. https://doi.org/10.1016/j.geoderma.2016.07.024
Mariotte, P., Vandenberghe, C., Kardol, P., Hagedorn, F., & Buttler, A. (2013). Subordinate plant species enhance community resistance against drought in semi-natural grasslands. Journal of Ecology, 101(3), 763–773. https://doi.org/10.1111/1365-2745.12064
Marques, A. P., Rangel, A. O., & Castro, P. M. (2009). Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Critical Reviews in Environment Science and Technology, 39(8), 622–654. https://doi.org/10.1080/10643380701798272
Marschner, H. (2012). Marschner's mineral nutrition of higher plants. Academic press.
Mårtensson, L.-M., Carlsson, G., Prade, T., Kørup, K., Lærke, P. E., & Jensen, E. S. (2017). Water use efficiency and shoot biomass production under water limitation is negatively correlated to the discrimination against 13C in the C3 grasses Dactylis glomerata, Festuca arundinacea and Phalaris arundinacea. Plant Physiology and Biochemistry, 113, 1–5. https://doi.org/10.1016/j.plaphy.2017.01.021
Mayans, B., Pérez-Esteban, J., Escolástico, C., Eymar, E., & Masaguer, A. (2019). Evaluation of commercial humic substances and other organic amendments for the inmobilization of copper trough 13C CPMAS NMR, FT-IR, and DSC analyses. Agronomy, 9, 762. https://doi.org/10.3390/agronomy9110762
Meeinkuirt, W., Kruatrachue, M., Pichtel, J., Phusantisampan, T., & Saengwilai, P. (2016). Influence of organic amendments on phytostabilization of Cd-contaminated soil by Eucalyptus camaldulensis. ScienceAsia, 42(2), 83–91. https://doi.org/10.2306/scienceasia1513-1874.2016.42.083
Meinzer, F. C., & Zhu, J. (1999). Efficiency of C4 photosynthesis in Atriplex lentiformis under salinity stress. Functional Plant Biology, 26(1), 79–86. https://doi.org/10.1071/PP98143
Menares, F., Carrasco, M., González, B., Fuentes, I., & Casanova, M. (2017). Phytostabilization ability of Baccharis linearis and its relation to properties of a tailings-derived technosol. Water, Air, and Soil Pollution, 228(5), 182. https://doi.org/10.1007/s11270-017-3348-y
Méndez, M. O., & Maier, R. M. (2008). Phytostabilization of mine tailings in arid and semiarid environments—an emerging remediation technology. Environmental Health Perspectives, 116(3), 278–283. https://doi.org/10.1289/ehp.10608
Merchant, A., Buckley, T. N., Pfautsch, S., Turnbull, T. L., Samsa, G. A., & Adams, M. A. (2012). Site-specific responses to short-term environmental variation are reflected in leaf and phloem-sap carbon isotopic abundance of field grown Eucalyptus globulus. Physiologia Plantarum, 146(4), 448–459. https://doi.org/10.1111/j.1399-3054.2012.01638.x
Midhat, L., Ouzzani, N., Hejjaj, A., Ouhammou, A., & Mandi, L. (2019). Accumulation of heavy metals in metallophytes from three mining sites (Southern Centre Morocco) and evaluation of their phytoremediation potential. Ecotoxicology and Environmental Safety, 169, 150–160. https://doi.org/10.1016/j.ecoenv.2018.11.009
Mileusnić, M., Siyowi, M. B., Fred, K. A., Ružičić, S., Mapaure, I., & Chimwamurombe, P. M. (2014). Assessment of agricultural soil contamination by potentially toxic metals dispersed from improperly disposed tailings, Kombat mine, Namibia. Journal of Geochemical Exploration, 144, 409–420. https://doi.org/10.1016/j.gexplo.2014.01.009
Monneveux, P., Sheshshayee, M. S., Akhter, J., & Ribaut, J. M. (2007). Using carbon isotope discrimination to select maize (Zea mays L) inbred lines and hybrids for drought tolerance. Plant Science, 173(4), 390–396. https://doi.org/10.1016/j.plantsci.2007.06.003
Moreno-Jiménez, E., Esteban, E., & Peñalosa, J. (2012). The fate of arsenic in soils-plants system. Reviews of Environmental Contamination and Toxicology, 215, 1–37. https://doi.org/10.1007/978-1-4614-1463-6_1
Mulvaney, R. L. (1996). Nitrogen-inorganic forms. In D. L. Sparks (Ed.), Methods of soil analysis. Part 3. Soil Science Society of America Book Series 5 (pp. 11123–12000). Soil Society of America, USA, pp. 1390.
Nelson, D. W., & Sommers, L. E. (1982). Total carbon, and organic matter. In A. L. Page (Ed.), Methods of soil analysis (pp. 571–573). American Society of Agronomic Inc.
Norman, H., Freind, C., Masters, D., Rintoul, A., Dynes, R., & Williams, I. (2004). Variation within and between two saltbush species in plan composition and subsequent selection by sheep. Australian Journal of Agricultural Research, 55, 999–1007.
NRC (National Research Council). (2005). Mineral tolerance of animals (second revised). National Academies Press.
Orrego, F., Ortiz-Calderón, C., Lutts, S., & Ginocchio, R. (2020). Growth and physiological effects of single and combined Cu, NaCl, and water stresses on Atriplex atacamensis and A. halimus. Environmental and Experimental Botany, 169, 103919. https://doi.org/10.1016/j.envexpbot.2019.103919
Oyarzún, J., Oyarzun, R., Lillo, J., Higueras, P., Maturana, H., & Oyarzún, R. (2016). Distribution of chemical elements in calc-alkaline igneous rocks, soils, sediments and tailings deposits in northern central Chile. Journal of South American Earth Sciences, 69, 25–42. https://doi.org/10.1016/j.jsames.2016.03.004
Paetsch, L., Mueller, C. W., Rumpel, C., Houot, S., & Kogel-Knabner, I. (2016). Urban waste composts enhance OC and N stocks after long-term amendment but do not alter organic matter composition. Agriculture, Ecosystems & Environment, 223, 211–222. https://doi.org/10.1016/j.agee.2016.03.008
Pardo, T., Bernal, M. P., & Clemente, R. (2017). Phytostabilisation of severely contaminated mine tailings using halophytes and field addition of organic and inorganic amendments. Chemosphere, 178, 556–564. https://doi.org/10.1016/j.chemosphere.2017.03.079
Parraga-Aguado, I., González-Alcaraz, M. N., Álvarez-Rogel, J., & Conesa, H. M. (2014). Assessment of the employment of halophyte plant species for the phytomanagement of mine tailings in semiarid areas. Ecological Engineering, 71, 598–604. https://doi.org/10.1016/j.ecoleng.2014.07.061
Peel, M. C., Finlayson, B. L., & McMahon, T. A. (2007). Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences, 11, 1633–1644. https://doi.org/10.5194/hess-11-1633-2007
Pérez, R., Tapia, Y., Antilén, M., Casanova, M., Vidal, C., Santander, C., Aponte, H., & Cornejo, P. (2021). Interactive effect of compost application and inoculation with the fungus Claroideoglomus claroideum in Oenothera picensis plants growing in mine tailings. Ecotoxicology and Environmental Safety., 208, 111495. https://doi.org/10.1016/j.ecoenv.2020.111495
Pérez-de-Mora, A., Madejón, P., Burgos, P., Cabrera, F., Lepp, N. W., & Madejón, E. (2011). Phytostabilization of semiarid soils residually contaminated with trace elements using by-products: Sustainability and risks. Environmental Pollution, 159, 3018–3027. https://doi.org/10.1016/j.envpol.2011.04.015
Rabier, J., Laffont-Schwob, I., Pricop, A., Ellili, A., D’Enjoy-Weinkammerer, G., Salducci, M. D., Prudent, P., Lotmani, B., Tonetto, A., & Masotti, V. (2014). Heavy metal and arsenic resistance of the halophyte Atriplex halimus L. along a gradient of contamination in a French Mediterranean spray zone. Water, Air, & Soil Pollution, 225(7), 1993. https://doi.org/10.1007/s11270-014-1993-y
RE 7849. (2020). Resolución exenta No: 8132/2020. Servicio Agrícola y Ganadero. SAG. Ministerio de Agricultura, Chile. Retrieved September 14, 2022, from https://www.sag.gob.cl/ambitos-de-accion/programa-de-recuperacion-de-suelos-degradados
Robinson, D., Handley, L. L., Scrimgeour, C. M., Gordon, D. C., Forster, B. P., & Ellis, R. P. (2000). Using stable isotope natural abundances (δ15N and δ13C) to integrate the stress responses of wild barley (Hordeum spontaneum C. Koch.) genotypes. Journal of Experimental Botany, 51(342), 41–50. https://doi.org/10.1093/jexbot/51.342.41
Romero, R., Plaza, C., Senesi, N., Nogales, R., & Polo, A. (2007). Humic acid-like fractions in raw and vermicomposted winery and distillery wastes. Geoderma, 139, 397–406. https://doi.org/10.1016/j.geoderma.2007.03.009
Ruiz, J. M., López-Cantero, I., Rivero, R. M., & Romero, L. (2003). Sulphur phytoaccumulation in plant scpecies characteristic of gypsiferous soils. International Journal of Phytoremediation, 5(3), 203–210. https://doi.org/10.1080/713779220
Sanchez-Lopez, A. S., Carrillo-Gonzalez, R., Gonzalez-Chavez, M. D. C. A., Rosas-Saito, G. H., & Vangronsveld, J. (2015). Phytobarriers: Plants capture particles containing potentially toxic elements originating from mine tailings in semiarid regions. Environmental Pollution, 205, 33–42. https://doi.org/10.1016/j.envpol.2015.05.010
Santibañez, C., de la Fuente, L. M., Bustamante, E., Silva, S., León-Lobos, P., & Ginocchio, R. (2011). Potential use of organic- and hard-rock mine wastes on aided phytostabilization of large-scale mine tailings under semiarid mediterranean climatic conditions: Short-term field study. Applied and Environmental Soil Science, 2012, 1–15. https://doi.org/10.1155/2012/895817
Santibañez, F. (2017). Atlas Agroclimatico de Chile. Tomo III. ISBN 978-956-19-1047-8. Universidad de Chile.Fundación para la Innovación Agraria.
Schnitzer, M. (1982). Organic matter characterization. In A. L. Page (Ed.), Methods of Soil Analysis (pp. 581–584). American Society of Agronomic Inc.
Senesi, N. (1989). Composted materials as organic fertilizers. Science of the Total Environment, 81(82), 521–542.
Senesi, N. (2018). Metal-humic substance complexes in the environment. Molecular and mechanistic aspects by multiple spectroscopic approach. In D. C. Adriano (Ed.), Biogeochemistry of trace metals (pp. 429–451). Boca Raton, FL, USA: CRC Press, Taylor and Francis Group.
SERNAGEOMIN. Servicio Nacional de Geología y Minería. (2018). Comité Editor: Pablo Rivas, Carlos del Solar, Jorge Vargas, Rodrigo Pincheira, Heidi Cano, Gullibert Novoa, Alfonso Domeyko, Aníbal Gajardo, Carlos Ferraz, Luis Guerra, Matías Tapia, Andrés León, Rodolfo Olivares (Eds.), Anuario de la Minería de Chile 2018, pp. 271 (ISSN: 0066-5096 Inscripción: N° 303652, Chile).
Sing, R., Bhumbla, D. K., & Keefer, R. F. (1995). Recommended soil sulphate-S test. Chapter 7. In M. L. Horton (Ed.), Recommended soil testing procedures for the Northeastern United States (2nd ed.). USA, 493.
Singh, J., & Lee, B. (2015). Reduction of environmental availability and ecological risk of heavy metals in automobile shredder residues. Ecological Engineering, 81, 76–81. https://doi.org/10.1016/j.ecoleng.2015.04.036
Sparks, D. L. (2003). Environmental soil chemistry (2nd ed.). Elsevier.
Stevenson, F. J. (1994). Humus chemistry: Genesis, composition, reactions (2nd ed., p. 496p). John Wiley & Sons, Inc.
Sun, Z., & Chen, J. (2018). Risk Assessment of Potentially Toxic Elements (PTEs) Pollution at a rural industrial wasteland in an abandoned metallurgy factory in North China. International Journal of Environmental Research and Public Health, 15(1), 85. https://doi.org/10.3390/ijerph15010085
Taeprayoon, P., Homyog, K., & Meeinkuirt, W. (2022). Organic amendment additions to cadmium-contaminated soils for phytostabilization of three bioenergy crops. Science and Reports, 12, 13070. https://doi.org/10.1038/s41598-022-17385-8
Tambone, F., Orzi, V., Zilio, M., & Adani, F. (2019). Measuring the organic amendment properties of the liquid fraction of digestate. Waste Management, 88, 21–27. https://doi.org/10.1016/j.wasman.2019.03.024
Tapia, Y., Bustos, P., Salazar, O., Casanova, M., Castillo, B., Acuña, E., & Masaguer, A. (2017). Phytostabilization of Cu in mine tailings using native plant Carpobrotus aequilaterus and the addition of potassium humatess. Journal of Geochemical Exploration, 183(2017), 102–113. https://doi.org/10.1016/j.gexplo.2017.10.008
Tapia, Y., Casanova, M., Castillo, B., Acuña, E., Covarrubias, J., Antilén, M., & Masaguer, A. (2019). Availability of coper in mine tailings with humic substance addition and uptake by Atriplex halimus. Environmental Monitoring and Assessment, 191, 651. https://doi.org/10.1007/s10661-019-7832-2
Tapia, Y., Diaz, O., Pizarro, C., Segura, R., Vines, M., Zúñiga, G., & Moreno-Jiménez, E. (2013). Atriplex atacamensis and Atriplex halimus resist As contamination in Pre-Andean soils (northern Chile). Science of the Total Environment, 450, 188–196. https://doi.org/10.1016/j.scitotenv.2013.02.021
Tapia, Y., Loch, B., Castillo, B., Acuña, E., Casanova, M., Salazar, O., & Antilén, M. (2020). Accumulation of sulphur in Atriplex nummularia cultivated in mine tailings and effect of organic amendments addition. Water, Air, and Soil Pollution, 231(1), 8. https://doi.org/10.1007/s11270-019-4356-x
Teixeira, L. A. J., Berton, R. S., Coscione, A. R., & Saes, L. A. (2011). Biosolids application on banana production: soil chemical properties and plant nutrition. Applied and Environmental Soil Science. https://doi.org/10.1155/2011/238185
Walker, C. D., & Sinclair, R. (1992). Soil salinity is correlated with a decline in 13C discrimination in leaves of Atriplex species. Australian Journal of Ecology, 17(1), 83–88. https://doi.org/10.1111/j.1442-9993.1992.tb00783.x
Walkley, A., & Black, C. A. (1946). Total carbon, organic carbon, and organic matter. Methods of Soil Analysis Part, 3, 1367–1378.
Wang, K., He, C., You, S. J., Liu, W. J., Wang, W., Zhang, R. J., Qi, H. H., & Ren, N. Q. (2015). Transformation of organic matters in animal wastes during composting. Journal of Hazardous Materials, 300, 745–753. https://doi.org/10.1016/j.jhazmat.2015.08.016
Wang, L., Ji, B., Hu, Y., Liu, R., & Sun, W. (2017). A review on in situ phytoremediation of mine tailings. Chemosphere, 184, 594–600. https://doi.org/10.1016/j.chemosphere.2017.06.025
Wu, Y., Li, Y., Zheng, C., Zhang, Y., & Sun, Z. (2013). Organic amendment application influence soil organism abundance in saline alkali soil. European Journal of Soil Biology, 54, 32–40. https://doi.org/10.1016/j.ejsobi.2012.10.006
Xie, L., & van Zyl, D. (2020). Distinguishing reclamation, revegetation and phytoremediation, and the importance of geochemical processes in the reclamation of sulfidic mine tailings: A review. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.126446
Acknowledgements
The authors thank technicians Mary Sol Aravena and Consuelo Aguilera for their valuable contributions to this work.
Funding
This research was partially funded by project PIA ANILLO ACM no 170002 (2018–2020) and REGULAR FONDECYT no. 1210922 (2021–2024) from the National Agency for Research and Development (ANID)—Science and Technology Ministry.
Author information
Authors and Affiliations
Contributions
Benjamín Castillo: conceptualization, methodology, formal analysis, investigation, writing—original draft, visualization. Edouard Acuña: writing—review and editing. Andrea Sánchez: conceptualization, formal analysis, writing—review and editing. Pablo Cornejo: project administration, funding acquisition. Osvaldo Salazar: resources, project administration, funding acquisition. Yasna Tapia: conceptualization, writing—review and editing, resources, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
All authors have read, understood, and have complied as applicable with the statement on "Ethical responsibilities of Authors" as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Castillo, B., Acuña, E., Sánchez, A. et al. Phytostabilization of trace elements and 13C isotope composition of Atriplex atacamensis Phil. cultivated in mine tailings treated with organic amendments. Environ Monit Assess 195, 354 (2023). https://doi.org/10.1007/s10661-023-10973-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-10973-9