Abstract
Milk is a complete food useful to promote growth and development of the infant mammals as it contains vital nutrients (proteins, essential fats, vitamins, and minerals) in a balanced proportion. It is also a nutritious food for adults too. Milk can also contain hazardous chemicals and contaminants including heavy metals which can be a risk for health. This study was aimed at determining the level of heavy metals in cow’s milk collected from Butajira and Meskan districts, south Ethiopia. Cows’ milk was collected from 193 healthy lactating cows. Samples were digested by optimized microwave digestion method using HNO3 and H2O2. Analysis was done using ICP-OES for Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn levels. MP-AES was used for Ca, Mg, K, and Na. Ni was not detected in all samples. Concentrations of heavy metals in the studied samples were Cd (0.0–0.03), Cr (0.0–0.4), Cu (0.03–1.1), Fe (0.0–1.9), Mn (0.0–0.7), Pb (0.0–12.3), Zn (0.0–8.2), Ca (380.1–532.4), Mg (159.6–397.9), K (1114.2–1685.8), and Na (495.9–1298.3) ppm. These values were compared with permissible values prescribed by different international organizations and available literatures. Cd, Cr, Cu, Mn, Pb, Zn, and Mg levels were found above the permissible limits. Thus, special attention needs to be given to the level of heavy metals in cows’ milk, as they are difficult to remove from the body. Their accumulation to a level greater than their permissible limit could be deleterious to the health of the user.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Milk is a complex mixture of bioactive substances helpful to promote growth and development of infants in mammals (Qin et al., 2009). It is widely consumed by children and adults as it is an important food item. It is beneficial for health and normal growth. Some important constituents of milk are fat, proteins, carbohydrates, vitamins, and minerals (Santos et al., 2015). According to Tetra Pak Dairy Index, 2015 global milk consumption has grown from 212 billion liters in 2013 to 223 billion liters in 2016. The significant increase in demand for dairy products is being fueled primarily by emerging markets in China, India, South and South East Asia, Africa, as well as the Greater Middle East due to economic growth and urbanization (Steyn, 2015).
The potential for future expansion of dairy consumption remains significant, as income levels are expected to grow continuously in developing countries, especially in countries where levels of per capita consumption are still relatively low (Gerosa & Skoet, 2012). For example, from 1990 to 2000, total milk production in Ethiopia grew at an estimated rate of 3% compared to 1.8% during the period from 1975 to 1990 (Ahmed et al., 2000). The consumption level is significantly correlated with household income, family size, education level, and location of the household (Melesse & Beyene, 2009).
Milk must be of satisfactory quality to contribute to the health of the community. It should be obtained from healthy animals. And collection and storage processes should be done under hygienic conditions (Qin et al., 2009).
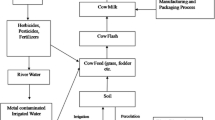
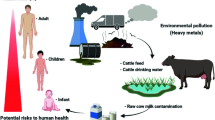
Currently, contamination of milk with toxic elements is becoming a global concern (Arianejad et al., 2015). Heavy metal is a general collective term used for those elements with a density at least five times that of water. Metals included under this category include cadmium (Cd), copper (Cu), lead (Pb), zinc (Zn), chromium (Cr), and iron (Fe). Among these Zn and Fe are essential trace elements to be consumed at low concentration. Metal ions such as Cd, Cu, Zn, Cr, and Fe are released into the biosystem mainly from industries in simple cationic forms (Volesky & May-Phillips, 1995). In addition to the increasing number of industries, vehicles are known to increase level of toxic heavy metals in air, water, soil, and plants (Yahaya et al., 2010)
Heavy metals such as Cd and Pb are common air pollutants and are emitted into the air as a result of various industrial activities. Although their atmospheric level is low, it contributes to deposition and build-up in soil, water, and plants and leads to their introduction into the food chain. This imposes threats to human and animal health (Bilandžić et al., 2011).
Ability of heavy metals to bio-accumulate in the environment makes them health hazard to living organisms. They cannot be degraded and are very toxic even at low concentration. Living organisms, when exposed to these compounds through food such as milk, they get accumulated in the body at a rate higher than their metabolism and excretion (Tsoumbaris & Papadopoulou, 1994).
Accumulation of toxic heavy metals in the body leads to metabolic disorders and serious health problems such as weakness, heart failure, cancer, and kidney problem (Licata et al., 2004). Toxic effect of heavy metals depends on the total amount absorbed, route of exposure, duration of exposure, and age of the individual. For example, young children are more susceptible to the effects of Pb exposure because they absorb higher percentage of what is ingested and their system is not developed enough to eliminate what is absorbed compared with adults (Tunegová et al., 2016).
Possible causes for the presence of heavy metals in cow’s milk could be through feed sources like grass, drinking water, and dust that settle on the grass (Amponsah, 2014; Singh et al., 2010).
Information on the level of heavy metals in cow’s milk can help in assessing risk to human health. Currently, many analytical methods are being developed and validated to perform accurate measurements of heavy metals in environmental matrices in order to assess compliance with national and international standards. Those standards specify permissible concentration of such pollutants in various food inputs.
Some modern analytical methods currently in use for accurate determination and quantification of metals in different samples include flame atomic absorption spectrometry (Alem et al., 2015; Eka et al., 2012), graphite furnace atomic absorption spectroscopy (Arianejad et al., 2015), atomic emission/fluorescence spectrometry (Abdelkhalek et al., 2015), inductively coupled plasma mass spectrometry (Tanase et al., 2012), inductively coupled plasma optical emission spectrometry (Canbay & Doğantürk, 2017), and microwave plasma atomic emission spectroscopy (Kamala et al., 2014).
The heavy metal concentration values determined in a study need to be compared with recommended dietary allowance (RDA) values of national and international standards to assess safety. It is also advisable to compare with the corresponding values for milk in different countries as reported in different literatures for root cause analysis and assessment of deviations. In this study, cow’s milk was collected from Butajira and Meskan districts, south central Ethiopia, an area where there is a habit of milk serving along meals and a community health surveillance site for the Addis Ababa University, College of Health Sciences as Butajira Health and Demographic Surveillance Site (BHDSS) to determine its heavy metal levels using inductively coupled plasma-optical emission spectrometry and microwave plasma atomic emission spectroscopy.
Materials and methods
Description of the study area
A cross-sectional study was conducted from July to August 2017 with respect to sample collection to determine the level of heavy metals in cow’s milk in Butajira and Meskan districts. Butajira is a town and separate district in south central Ethiopia located at the base of the Zebider massif in the Gurage zone of the Southern Nations, Nationalities, and People’s Region (SNNPR). The town of Butajira is surrounded by Meskane and located about 130 km south of Addis Ababa, Ethiopia, as shown in Fig. 1 (Sinaga et al., 2015). According to Butajira’s livestock and fish development office, there are about 14,000 milking cows, and daily milk production is estimated to be greater than 3500 l. The district consists of 30 peasant associations, 65 private herders, and two agro-industries. The total population of the district is estimated to be 78,000. Four out of five kebeles (lower level of village administrative district in Ethiopia) (named kebeles 01, 02, 03, and 04) were purposively selected from Butajira district.
According to the district’s livestock and fish development office, there are about 34,198 milking cows in Meskan. The total population of the Meskan district is estimated to be 196,000. Three kebeles were selected from Meskan district based on their climatic condition to accommodate the three agro climatic conditions of Ethiopia. The selected sites were Enseno town (lowland), Mekicho (middle land), and Huletenya Wolensho (highland) (Berhane & Byass, 2002).
Inhabitants in Butajira and Meskan districts support their livelihood by farming in agro-pastoral settings. They grow cereals, false banana (enset) and Catha edulis Forsk (Khat) (Berg et al., 2009; Nana, 2016). In the study area, there is a practice of serving milk with meals. The daily milk consumption for Ethiopia was reported to be 53 g/day (Belete et al., 2014). So, the study areas were chosen in expectation of high milk consumption.
Sample collection
Polyethylene sterile screw cupped sampling bottles soaked in HNO3 and rinsed with deionized water were used for collection of fresh cow’s milk.
For this study, a total of one hundred ninety-three healthy and lactating cows were randomly selected from Butajira and Meskan districts: eighty-five and one hundred eight cows respectively in each district. The health of the selected lactating cows was ascertained by veterinarians practicing in the area. Samples were labeled into 17 different codes, 8 of which were unmixed (taken from single cow), and the remaining 9 were mixed (prepared by taking about 6 ml from 20 cows). The purpose of collecting mixed samples was to minimize cost for determination of heavy metals, i.e., analytical work. Eight unmixed samples were prepared from randomly selected four cows at Butajira and four cows at Meskan; 125 ml milk was taken from each cow and put directly into eight sterile screw–capped bottles. Nine homogenized samples were also prepared from randomly selected eighty-one cows at Butajira and one hundred four cows at Meskan; about 6 ml milk was taken from each cow directly into nine sterile screw–capped bottles. Twenty cows were included in the preparation of each homogenized sample. These samples were collected manually at any time of the day from both districts by the farmers. The quality of sample collection process was directly observed. The udder of each cow was washed with tap water and then rinsed thoroughly with distilled and deionized water before milking. The samples were kept in an ice box and were maintained under refrigeration for 9 days until collection was completed. Finally, seventeen samples were transported to Addis Ababa and immediately kept in a deep freezer (−20 °C) until microwave digestion was carried out.
Chemicals and reagents
All the chemicals used were of analytical reagent grade. Nitric acid (68%), hydrogen peroxide (32%) (Sigma-Aldrich St. Louis, USA), and deionized water (having a resistivity of 18.2 mega ohm-cm (Μ Ω-cm) and conductivity of 0.055 micro Siemens (µS/cm) were used for cleaning sample holders and in the preparation of all solutions. Nitric acid (68%) and hydrogen peroxide (32%) were used for digesting milk samples throughout this work. The standard solutions (99.99 %, ICP grade) of Ca (Lot BCB-L3192V), K (Lot GD16060759), Mg (Lot GD16060760), Na (Lot BCB-L2315V), Ni (Lot BCB-K4387V), Fe (Lot GD16060755), Cu (Lot BCB-M1539V), Cr (Lot BCB-K3741V), Mn (Lot BCB-R4235V), Pb (Lot GD16060764), Cd (Lot GD16060750), and Zn (Lot BCB-N8385V) corresponding to 1000 mg/L of each metal were obtained from Sigma-Aldrich (St. Louis, USA) as standards/certified reference materials (CRMs).
Apparatus
All glass wares were washed before use with deionized water soaked in nitric acid and rinsed with deionized water. Thoroughly cleaned glasses were dried in an oven. Glass wares after drying were stored until used in a desiccator. Milk samples were digested by using milestone start D microwave digester (START D, Milestone, Basel, Switzerland). ICP-OES (Agilent 700 Series, CA, USA) and MP-AES (Agilent 4200 Series, CA, USA) were used in determining level of heavy metals in the milk samples.
Sample digestion and preparation of analyte solution for ICP-OES and MP-AES
Optimized microwave digestion procedure was selected based on the clarity of digests, minimal digestion time, and reagent volume, absence of undigested milk samples, simplicity, and low heating temperature. Three milliliters of cow’s milk from each sample was mixed with 9 ml 68% nitric acid and 2 ml 32% hydrogen peroxide in dried digestion vessels. The digestion vessels were placed in a microwave digestion system at 180 °C (30 min) until no more brown fume is produced, indicating completion of oxidation of organic matter. The digestion vessels were then cooled to room temperature to avoid foaming and splashing on opening. The digestion vessels were opened carefully in a fume hood. The resulting cold and clear solution was filtered in to 100 ml volumetric flask using Whatman filter paper (0.45-µm pore diameter ) to remove any suspended residues. Fourteen milliliter, 1% nitric acid was added to the filtrate and diluted up to the mark with deionized water. Blank sample preparation was performed following the same procedure.
Determination of metal content of digested samples by ICP-OES and MP-AES
Measurement of levels of nickel (Ni), zinc (Zn), iron (Fe), copper (Cu), chromium (Cr), manganese (Mn), lead (Pb), and cadmium (Cd) were carried out using inductively coupled plasma optic emission spectroscopy (ICP-OES). Magnesium (Mg), sodium (Na), potassium (K), and calcium (Ca) were determined by microplasma atomic emission spectroscopy (MP-AES). Prior to analysis of the samples, both MP-AES and ICP-OES were optimized using standard solutions of the metals to get maximum signal strength by adjusting parameters such as wavelength, nebulizer flow, pump speed, and lamp current for each element as shown in Table 1.
Calibration curves were drawn for Ni, Zn, Fe, Cu, Cr, Mn, Pb, and Cd using linear regression analysis of the concentrations of the standard solutions versus emission values. Series of working standard solutions of metals were prepared by diluting the intermediate standard solution with deionized water and sample recovery solution in matrix matching way. The optimized procedure was then employed for the determination of the elements in digested, spiked, and blank samples. Three measurements were taken for each sample, and the mean was plotted. Concentration of the metal ions present in the cow’s milk was determined by reading their emission using ICP-OES and comparing it with the respective standard calibration curve. Correlation determination coefficient between concentrations and emission of the metals were done (Belete et al., 2014).
The correlation coefficients of calibration curves for elements investigated are given in Table 2. All the metallic elements exhibited linear relationships of the instrumental response and the solutions containing the metals with insignificant intercepts and coefficients of determination (0.994 or better). Correlation coefficient values of greater than 0.994 was found that indicated strong linear relationship between concentrations and emission intensity. Solutions of Ni, Zn, Fe, Cu, Cr, Mn, Pb, and Cd were aspirated into the plasma of the ICP-OES instrument, and the emission value of the metals was recorded. The solutions of Mg, Na, K, and Ca were aspirated into MP-AES instrument and then emission value of the metals was recorded. Both the ICP-OES and MP-AES methods were observed to exhibit good linearity, confirming its reliability for determining trace levels of the heavy metals in cow’s milk. Concentrations of each metal was determined from the emission versus concentration plotted calibration curves. For each sample, triplicate determinations were performed, and average results were reported.
Method validation
Precision and accuracy
Analytical results must be evaluated to decide on the best values to report and to establish the probable limits of errors of these values (Kikuchi et al., 2002). In this study, the precision of the results were evaluated by percentage relative standard deviation of the results of three samples (N=3) and triplicate readings for each sample giving a total of nine measurements for a given bulk sample. Accuracy and validity of the measurements were determined by analyzing samples spiked with 1mL of 100 ppm metal standard solution to 3 ml cow’s milk and digested as explained earlier. Analysis was done in triplicates.
Percentage recovery (% R) was calculated for each element using the following equation:
Percentage recoveries of Ca (98. 3%), Cd (99.4%), Cr (97.4%), Cu (96.6%), Fe (98.1%), K (98.8%), Mg (97.1%), Mn (95.6%), Na (98.5%), Ni (96.9%), Pb (97.6%), and Zn (97.3%) were found in this study.
In this study, the variability in the method between the runs on the same day (intra-day variation) and the variability between runs on different days (inter-day variation) was examined by using samples from a standard reference. In addition, reproducibility of the method was also checked by using samples from a standard reference.
Method detection and quantification limits
For determination of the detection limits of the method, blank sample was digested following the same procedure as the milk samples, and each of the blank samples was analyzed for Ni, Zn, Fe, Cu, Cr, Mn, Pb, and Cd using ICP-OES and for K, Na, Mg, and Ca using MP-AES. The method detection and quantification limits for MP-AES and ICP-OES are shown in Table 3. Standard deviation for each element was calculated from the blank measurements to determine method detection and quantification limits of the instrument.
Statistical analyses
Microsoft Office Excel 2016 was used to calculate the descriptive statistics (like mean, standard deviation) and draw calibration graphs. One-way analysis of variance (ANOVA) was performed to compare the differences among samples using SPSS 20.0. Origin was used to draw graph for each metal in all samples (in mg/L). p < 0.05 was considered significant.
Results and discussion
Average heavy metal concentrations of seventeen cow milk samples collected from Butajira and Meskan districts, south central Ethiopia is presented in Tables 4 and 5, respectively.
Analysis was done for twelve elements consisting of toxic metals (Mn, Pb, and Cd) and essential mineral elements (Ca, K, Mg, Na, Ni, Zn, Fe, Cu, and Cr). Concentrations of these metals in milk samples were recorded in mg/l (ppm) after calibration as presented in Tables 4 and 5. Ni was not detected in all the milk samples. Concentrations of metals in the studied milk samples were Cd (0.0–0.03), Cr (0.0–0.4), Cu (0.03–1.1), Fe (0.0–1.9), Mn (0.0–0.7), Pb (0.0–12.3), Zn (0.0–8.2), Ca (380.1–532.4), Mg (159.6–397.9), K (1114.2–1685.8), and Na (495.9–1298.3) ppm. Values obtained for Cd, Cr, and Pb in this study were compared with the permissible limit set by Codex Alimentarius, 2012. Those of Fe, Zn, Ca, K, Mg, and Na were compared with the permissible limit set by Chinese foods standard, 2009. The Mn value was compared with the permissible limit set by Japanese foods standard, 2015. Cu values obtained were compared with Egyptian standard, 2010, as shown in Table 6. Cd, Cr, Cu, Mn, Pb, Zn, and Mg were found to be over the permissible limits in 12%, 6%, 53%, 65%, 82%, 6 %, and 100 % of the samples, respectively. The values over permissible limits of heavy metals in the study areas could be due to the commonly used underground water and feed that the cows consume.
It is known that heavy metal contents in milk can vary widely due to many factors such as its secretion from the mammary gland, feeding, use of different water sources, and industrial pollutions (Moreno et al., 1993, Caggianoa et al., 2005; Younus et al., 2016). As shown in Fig. 2, Ni was not detected in all milk samples though 0.036 ppm and 0.045–0.311 ppm were reported by Abou-Arab et al. (2008) in Egypt and Arianejad et al. (2015) in Iran, respectively. This could be as a result of low exposure of cows to Ni in the study areas. Except cow milk samples (CM16 & CM17), the results of ANOVA analysis showed that there are no significant differences among the Cd, Cr, Cu, Mn, Pb, Fe, and Zn level within samples and among the unmixed and homogenized samples collected from the two districts. The same is true for the Ca, Na, K, and Mg (p = 0.05). Figure 2a shows the metals which were found in a concentration greater than 245.5235 mg/L in all milk samples, and Fig. 2b stands for metals which were found to be in concentrations less than 2 mg/L. The concentration of Cu in this study ranged from 0.03 to 1.1 ppm. The average concentration in nine (53%) milk samples was found to exceed the maximum permissible limit of Cu for cow’s milk and dairy product taken as 0.4 mg/L. The concentration of Cu obtained in the present study is comparable to values reported by Abdulkhaliq et al.. (2012) in Palestine, Muhib et al. (2016) in Bangladesh, and Mohammed et al. (2017) in Nigeria 0.62–0.85, 0.04–1.778, and 0.17–1.79 ppm, respectively. Lower levels 0.087–0.122, 0.181–0.229, and 0.203–0.251 ppm were reported by Belete et al. (2014) in Ethiopia, Alem et al. (2015) in Ethiopia, and Maheswara and Murthy (2017) in Tanzania, respectively. Cu is an essential trace element that plays a vital role in the physiology of animals for fetal growth and early post-natal development. Excess Cu in the body leads to Wilson’s disease which is characterized by abdominal pain and fluid build-up in the legs or abdomen. It also causes problems with speech (Hassan & Masood, 2004; Lawal & Mohammed, 2006).
In this study, the concentration of Pb ranged from 0.00 for samples CM3, CM5, and CM8 to 12.30 ppm for sample CM16. Among the seventeen milk samples, 14 (82%) were found to contain Pb above the recommended dietary allowance for adult which is 0.02 ppm. Except for samples CM16 & CM17, the concentrations of Pb obtained in the present study were comparable to what is reported by Mohammed et al. (2017) in Nigeria (1.850–4.404 ) and Malhat et al. (2012) in Egypt (1.87–2.34 ppm). The Pb content found in this study was observed to be higher than what is reported by Ahmad et al. (2016) in Bangladesh (0–0.93 ppm) and Abdulkhaliq et al. (2012) in Palestine (0.0–0.39 ppm). Like Cu, high concentration of Pb in milk may result from consumption of contaminated feed and the common use of underground water in the districts. Pb has no beneficial biological function and is known to accumulate in the body. Pb exposure can cause adverse health effects, especially in young children and pregnant women. Pb is a neurotoxin that permanently interrupts normal brain development (Duruibe et al., 2007).
Results presented in Tables 4 and 5 show that the average Cr concentration in cow’s milk analyzed ranged from 0.00 to 0.4 ppm which is lower than values indicated in previous studies Belete et al. (2014) (0.845–0.895 ppm), Akele et al. (2017) (0.468–0.828 ppm), and Alem et al. (2015) (0.055–0.075 ppm) in Ethiopia and Ahmad et al. (2016) (0.0–0.11 ppm) in Bangladesh. Cr content in only one sample (CM13) exceeded the permissible limit. Like other microelements, Cr is essential to maintain the metabolic systems of the human body, but at higher level, it causes stomach upsets and ulcers, convulsions, kidney and liver damage, and even death (Qin et al., 2009).
All the average values of Fe in milk samples were found to be below permissible limit which ranged from 0.0 to 1.9 ppm. These values were lower than 10.95–16.38 ppm and 3.21–12.91 ppm as reported by Malhat et al. (2012) in Egypt and Abdulkhaliq et al. (2012) in Palestine, respectively. The mean concentrations of Zn ranged from 0.0 to 8.2 ppm. In the present study, the highest level of Zn was found in sample CM12 only. This result agrees with results (5.003–6.218 ppm) reported by Belete et al. (2014) in Ethiopia, higher result 4.770–10.75 ppm was reported by Malhat et al. (2012) in Egypt, while lower results (1.96–3.640 ppm) were reported by Bano et al. (2015) in Pakistan.
As indicated in Tables 4 and 5, the Cd level ranged from 0.0 to 0.03 ppm. The average concentrations of Cd observed in this study is in agreement with the report made by Abou-Arab et al. (2008) (0.016–0.04 ppm) in Egypt and Abdulkhaliq et al. (2012) (0.022–0.057 ppm) in Palestine, but it is lower than what is reported by Malhat et al. (2012) (0.200–0.288 ppm) in Egypt. The level of Mn in milk sample from the two sites ranged from 0.0 to 0.7 ppm, and 65% of the milk samples were observed to be above the limit. Lower levels were reported by Belete et al. (2014) (0.411–0.441 ppm) in Ethiopia and Bano et al. (2015) (0.37–0.52 ppm) in Pakistan. Exposure to high concentration of Mn is associated with mental and emotional disturbances, impaired male fertility, birth defects, and impaired bone development (Santamaria, 2008).
Magnesium was detected in all samples as shown in Tables 4 and 5. The levels of Mg in cow’s milk in this study ranged from 159.6 to 397.9 ppm which is lower than the report made by Birghila et al. (2008) (919.8 ppm) in Romania. The mean concentration of Mg in this study is higher than the maximum set for food as shown in Table 6. Tables 4 and 5 indicated that the concentration of K in this study ranged from 1114.2 to 1685.8 ppm, and this is higher than the value reported by Qin et al. (2009) in China (1000 ppm) and agrees with the report by Zamberlin et al. (2012) (1440–1780 ppm) in Croatia and Pereira (2014) (1510–1660 ppm) in Portugal. The mean concentration of K in this study is lower than the maximum standard indicated for food composition as shown in Table 6.
The mean values of Ca in this study ranged from 380.1 to 532.4 ppm that was found to be below the permissible limit set by Chinese Food Guidelines (820–1130 ppm) and which is also much lower than the concentration reported by Qin et al. (2009) in China (1000 ppm) and agree with the result by Bano et al. (2015) (195–1528 ppm) in Pakistan. The level of Na in samples from all the sites ranged from 495.9 to 1298.3 ppm, and this is higher than the value reported by Qin et al. (2009) (361–574 ppm) in China and by Zamberlin et al. (2012) (400–580 ppm) in Croatia. The mean concentration of Na in this study is lower than the maximum standard indicated for food composition as shown in Table 6.
The high level of heavy metals in these districts could be from the contaminated feeding hay, an agricultural left over locally referred to as geleba hay from maize plantations, or Chid (Teff (Ergratois teff) straw) and the commonly used underground water.
Assuming a 53 g/day for milk consumption in Ethiopia as mentioned by Belete et al. (2014), the daily intake of these metals was determined and presented in Table 7. The estimated daily intake for each metal was calculated to assess the health risks associated with trace metals due to milk consumption. The last column shows the RDA values as set by institute of medicine (2011), FAO and WHO. The density of whole, full-fat milk is very close to the density of water, which is 1.0002 g/ml. Therefore, it is possible to assume that 53 ml is equal to 53 g of milk.
where EDI is estimated daily intake, Mc is metal concentrations in milk (mg/L), and 53 g/day = daily milk consumption rate for Ethiopia (Belete et al., 2014).
The daily intake of the metals depends on both the concentration and the amount of food consumed (Farid, 2004). Since the estimated daily intake ( EDI) values of all the analyzed metals are below the RDA values, milk consumption at this level is safe in the study area even for children. This could be due to the very low daily milk consumption in Ethiopia compared with many countries, for example, 113 g/day in Mumbai (Tripathi et al., 1997), 124 g/day in Spain (Schuhmacher et al., 1993), and 224 g/day in USA (Lopez et al., 1995).
Conclusions
In this study, seventeen samples of cow’s milk, collected from Butajira and Meskan districts, south central Ethiopia, were analyzed for their heavy metal levels using ICP-OES (Cd, Cr, Cu, Fe, Mn, Pb, Ni, and Zn) and MP-AES (Ca, K, Mg, and Na). The concentrations of Cd, Cr, Cu, Fe, Mn, Pb, Zn, Ca, Mg, K, and Na in the studied milk samples were found to be in the ranges of 0.0–0.03, 0.0–0.4, 0.03–1.1, 0.0–1.9, 0.0–0.7, 0.0–12.3, 0.0–8.2, 380.1–532.4, 159.6–397.9, 1114.2–1685.8, and 495.9–1298.3 ppm, respectively. Ni was not detected in all milk samples. The average concentrations of heavy metals observed were comparable with values reported in different scientific literatures. It also complies with guidelines of different international organizations for food. However, this study gives a clear picture of high concentrations of toxic metals (Pb, Cd, Mn) in cow’s milk consumed and supplied to the market by the inhabitants of the two districts. This high level could be due to cows’ exposure to contaminated feed and/or underground water in these areas. These demand cautious attention that needs to be given to the level of heavy metals in cows’ milk, as they are difficult to remove from the body due to their accumulative nature and having deleterious health effects.
Availability of data and materials
All data generated in the study are included in the manuscript.
References
Abdelkhalek, A., Elsherbini, M., & Gunbaej, E. E. (2015). Assessment of heavy metals residues in milk powder and infant milk formula sold in Mansoura City. Egypt. Alexandria Journal of Veterinary Sciences, 47(1), 71–77. https://doi.org/10.5455/ajvs.200728
Abdulkhaliq, A., Swaileh, K., Hussein, M., & Matani, M. (2012). Levels of metals (Cd, Pb, Cu and Fe) in cow’s milk, dairy products and hen’s eggs from the West Bank, Palestine. International Food Research Journal, 19, 1089–1094. http://www.ifrj.upm.edu.my/19%20(03)%202012/(41)%20IFRJ%2019%20(03)%202012%20Saileh.pdf
Abou-Arab, A., Abou-Donia, M., & Enb, A. (2008). Chemical composition, metals content and pesticide residues in raw, pasteurized and UHT milk and their dietary intake. Journal of the Egyptian Society of Parasitology, 39, 111–121.
Ahmad, M., Roy, S., Sarwar, N., Morshed, S., AlamM, M. A., & Kobra, T. (2016). Contamination of raw milk, market pasteurized milk and powdered milk by toxic heavy metals in Bangladesh. Scientific Research Journal, 4, 19–24.
Ahmed, M., Jabbar, M., & Ehui, S. (2000). Household-level economic and nutritional impacts of market-oriented dairy production in the Ethiopian highlands. Food and Nutrition Bulletin, 21, 460-465. https://doi.org/10.1177/156482650002100423
Akele, M., Abebe, D., Alemu, A., Assefa, G., & Madhusudhan,A. (2017). Analysis of trace metal concentrations in raw cow’s milk from three dairy farms in North Gondar, Ethiopia: Chemometric approach. Environment Monit Assess, 3, 499–511. https://doi.org/10.1007/s10661-017-6203-0Alem
Alem, G., Tesfahun, K., & Kassa, B. (2015). Quantitative determination of the level of selected heavy metals in the cows’ milk from the dairy farm of the Haramaya University, Eastern Ethiopia. International Journal of Chemical and Natural Science, 3, 240–248. http://ijcns.aizeonpublishers.net/content/2015/1/ijcns240-248.pdf
Alimentarius, C. (2012). FAO/WHO food standards. Codex standard for milk powders and cream powders. Codex Stan, 207–1999.
Amponsah, D. (2014). Determination of levels of heavy metals (arsenic, lead, cadmium and mercury) in tin milk produced in Ghana. International Journal of Advancements in Research & Technology, 3, 129–133.
Arianejad, M., Alizadeh, M., Bahrami, A., & Arefhoseini, R. (2015). Levels of some heavy metals in raw cow’s milk from selected milk production sites in Iran: Is there any health concern?. Health promotion perspectives, 5, 176–182. https://doi.org/10.15171/hpp.2015.021
Bano, H., Mahmood, A., & Ain, U. (2015). Comparative study of zinc, manganese and calcium in commercially available milk samples. International Journal of Advanced Research, 3, 982–993.
Belete, T., Hussen, A., & Rao, M. (2014). Determination of concentrations of selected heavy metals in cow’s milk: Borena Zone, Ethiopia. Journal of Health Science, 4, 105–112. https://doi.org/10.5923/j.health.20140405.01
Berg, S., Firdessa, R., Habtamu, M., Gadisa, E., Mengistu, A., Yamuah, L., Ameni, G., Vordermeier, M., Robertson, D., & Smith, H. (2009). The burden of mycobacterial disease in Ethiopian cattle: Implications for public health. PLoS ONE, 4(4), e5068. https://doi.org/10.1371/journal.pone.0005068
Berhane, Y., & Byass, P. (2002). Butajira DSS Ethiopia. Population and health in developing countries, 1, 1–10. http://indepth-network.org/dss_site_profiles/butajira.pdf
Bilandžić, N., Đokić, M., Sedak, M., Solomun, B., Varenina, I., Knežević, Z., & Benić, M. (2011). Trace element levels in raw milk from northern and southern regions of Croatia. Food Chemistry, 127, 63–66. https://doi.org/10.1016/j.foodchem.2010.12.084
Birghila, S., Dobrinas, S., Stanciu, G., & Soceanu, A. (2008). Determination of major and minor elements in milk through ICP-AES. Environmental Engineering & Management Journal, 7, 805–808. http://www.eemj.eu/index.php/EEMJ/article/view/2495
Caggianoa, M., Sabiaa, M., D’emiliob, A., Ragostad, M., & Painoe, S. (2005). Metal levels in fodder, milk, dairy products, and tissues sampled in ovine farms of Southern Italy. Environmental Research, 99, 48–57. https://doi.org/10.1016/j.envres.2004.11.002
Canbay, S., & Doğantürk, M. (2017). Metals determination by microwave digestion ICP-OES of some dietary supplements and diet products in Turkey. Eurasian Journal of Analytical Chemistry, 12, 45–53. https://doi.org/10.12973/ejac.2017.00143a
Duruibe, S., Ogwuegbu, R., & Egwurugwu, M. (2007). Heavy metals pollution and human toxic effects. International Journal of Physical Science, 2, 112–118. https://doi.org/10.5897/IJPS.9000289
Egyptian Standard. (2010). Essential requirements for milk and milk products, ES: 7123/2010. Egyptian organization for standardization and quality control.
Eka, N., Retno, S., & Rohman, A. (2012). Validation and quantitative analysis of cadmium and lead in snake fruit by flame atomic absorption spectrophotometry. International Food Research Journal, 19, 937–940. http://www.ifrj.upm.edu.my/19%20(03)%202012/(22)%20IFRJ%2019%20(03)%202012%20Eka.pdf
Ershow, A., & Wong, K. (1990). Chinese food composition tables an annotated translation of the 1981 edition published by the institute of nutrition and food hygiene, Chinese academy of preventive medicine, Beijing. Journal of Food Composition and Analysis, 3, 191–434. https://doi.org/10.1016/0889-1575(90)90026-I
Farid, M. (2004). Determination of trace elements in cow’s milk in Saudi Arabia. JKAU: Engineering Sciences, 15, 131–140. https://www.kau.edu.sa/Files/135/Researches/55304_25646.pdf
Gerosa, S., & Skoet, J. (2012). Milk availability: trends in production and demand and medium-term outlook. Food and Agriculture Organization of the United Nations, 12, 1–38. https://ageconsearch.umn.edu/record/289000/files/a-an450e.pdf
Hassan, A., & Masood, F. (2004). Wilson’s disease: A review. Journal Pakistan Medical Association, 54, 479–484. https://ecommons.aku.edu/cgi/viewcontent.cgi?article=1106&context=pakistan_fhs_mc_med_intern_med
Institute of Medicine. (2011). Recommended dietary allowances: Calcium, chromium, copper, fluoride, iodine, iron, magnesium, manganese, molybdenum, phosphorus, selenium, zinc, potassium, sodium, chloride. Washington DC: National Academy Press. http://iom.edu/ (Accessed: 12.08.2018.).
Kamala, C., Balaram, V., Dharmendra, V., Satyanarayanan, M., Subramanyam, K., & Krishnaiah, A. (2014). Application of microwave plasma atomic emission spectrometry for environmental monitoring of industrially contaminated sites in Hyderabad City. Environmental Monitoring and Assessment, 186, 7097–7113. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s10661-014-3913-4
Kikuchi, T., Nomiyama, N., Kumagai, T., Uemura, L., & Omae, K. (2002). Cadmium concentration in current Japanese food and beverages. Journal of Occupational Health, 44, 240–247. https://doi.org/10.1539/joh.44.240
Lawal, O., & Mohammed, D. (2006). Assessment of levels of copper, cadmium and lead in secretion of mammary gland of cows grazed on open fields. The Scientific World Journal, 1, 7-10. https://doi.org/10.4314/swj.v1i1.51689
Licata, P., Trombetta, D., Cristani, M., Giofre, F., Martino, D., Calo, M., & Naccari, F. (2004). Levels of “toxic” and “essential” metals in samples of bovine milk from various dairy farms in Calabria, Italy. Environment International, 30, 1–6. https://doi.org/10.1016/S0160-4120(03)00139-9
Lopez, A., Collins, W., & Williams, H. (1995). Essential elements, cadmium, and lead in raw and pasteurized cow and goat milk. Journal of Dairy Science, 68, 1878–1886. https://doi.org/10.3168/jds.S0022-0302(85)81044-4
Maheswara, R., & Murthy, A. (2017). Determination of concentrations of selected heavy metals in cow’s milk: Dodoma Urban District, Tanzaniaa. International Journal of Engineering Research and Application, 7, 76–84. https://doi.org/10.9790/9622-0704067684
Malhat, F., Hagag, M., Saber, A., & Fayz, E. (2012). Contamination of cows milk by heavy metal in Egypt. Bulletin of Environmental Contamination and Toxicology, 88, 611–613. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s00128-012-0550-x
Melesse, K., & Beyene, F. (2009). Consumption pattern of milk and milk products in Ada’aworeda, East Shoa Zone, central Ethiopia. Livestock Research for Rural Development, 21, 1–13. http://www.lrrd.cipav.org.co/lrrd21/4/mele21056.htm
Mohammed, I., Zahra, M., & Zakari, A. (2017). Determination of some heavy metals in milk of cow grazing at selected areas, of Kaduna Metropolis. International Journals of Advanced Research in Computer Science and Software Engineering, 7, 341–346.
Moreno, R., Zurera, G., & Amaro, M. (1993). Micronutrients in natural cow, ewe and goat milk. International Journal of Food Sciences and Nutrition, 44, 137–46.
Muhib, I., Chowdhury, Z., EashaJ, RahmanM., Shammi, M., Fardous, Z., Bari, L., Uddin, K., Kurasaki, M., & AlamK,. (2016). Investigation of heavy metal contents in cow milk samples from area of Dhaka, Bangladesh. International Journal of Food Contamination, 3, 1–10. https://doi.org/10.1186/s40550-016-0039-1
Nana, T. (2016). Prevalence of ovine gastrointestinal nematodes in Meskan district, Gurage zone, Southern Ethiopia. International Journal Multidisciplinary Advanced Research Trends, 3, 22–30.
Pereira, C. (2014). Milk nutritional composition and its role in human health. Nutrition, 30, 619–627. https://doi.org/10.1016/j.nut.2013.10.011
Qin, L. Q., Wang, X. P., Li, W., Tong, X., & Tong, W. J. (2009). The minerals and heavy metals in cow’s milk from China and Japan. Journal of Health Science, 55, 300–305. https://doi.org/10.1248/jhs.55.300
Santamaria,. (2008). Manganese exposure, essentiality and toxicity. Indian Journal of MedicalResearch, 128, 484–500.
Santos, C., Oliveira, A., Martins, D., Oliveira, J., Pedro, F., & Villa, R. (2015). Determination of the concentrations of essential and toxic metals in UHT milk produced in Mato Grosso State, Brazil. International Food Research Journal, 22, 981–986.
Schuhmacher, M., Domingo, J., Llobet, J., & Corbella, J. (1993). Dietary intake of copper, chromium and zinc in Tarragona Province, Spain. Science of the Total Environment, 132, 3–10. https://doi.org/10.1016/0048-9697(93)90257-7
Sinaga, M., Mohammed, A., Teklu, N., Stelljes, K., & Belachew, T. (2015). Effectiveness of the population health and environment approach in improving family planning outcomes in the Gurage, Zone South Ethiopia. BMC Public Health, 15, 1123–1135. https://springerlink.bibliotecabuap.elogim.com/article/10.1186/s12889-015-2484-9
Singh, A., Sharma, K., Agrawal, M., & Marshall, M. (2010). Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food and Chemical Toxicology, 48, 611–619. https://doi.org/10.1016/j.fct.2009.11.041
Steyn, N. (2015). Dare to explore: Processing. The Dairy Mail, 22, 106–110.
Tanase, G., Udristioiu, F., Bunaciu, A., & Aboul-Enein, Y. (2012). Trace elements analysis in paper using inductively coupled plasma-mass spectrometry. Gazi University Journal of Science, 25, 843–851.
Tripathi, R., Raghunath, R., & Krishnamoorthy, T. (1997). Dietary intake of heavy metals in Bombay city, India. Science of the Total Environment, 208, 149–159. https://doi.org/10.1016/S0048-9697(97)00290-8
Tsoumbaris, P., & Papadopoulou, H. (1994). Heavy metals in common foodstuff: Quantitative analysis. Bulletin of Environmental Contamination and Toxicology, 53, 61–66. https://doi.org/10.1007/BF00205139
Tunegová, M., Toman, R., & Tančin, V. (2016). Heavy metals-environmental contaminants and their occurrence in different types of milk. Slovak Journal of Animal Science, 49(3), 122-131. https://office.sjas-journal.org/index.php/sjas/article/view/171
Volesky, B., & May-Phillips, H. (1995). Biosorption of heavy metals by Saccharomyces Cerevisiae. Applied Microbiology and Biotechnology, 42, 797-806. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/BF00171964
Watanabe, T. (2015). Food composition tables of Japan and the nutrient table/database. Journal of Nutritional Science and Vitaminology, 61(Supplement), S25–S27.
Yahaya, M., Ezeh, G., Musa, Y., & Mohammad, S. (2010). Analysis of heavy metals concentration in road sides soil in Yauri, Nigeria. African Journal of Pure and Applied Chemistry, 4, 022–030. https://academicjournals.org/journal/AJPAC/article-full-text-pdf/6022F5A2040.pdf
Younus, M., Abbas, T., Zafar, M., Raza, S., Khan, A., Saleem, A., Idrees, M., Nisa, Q., Akhtar, R., & Saleem, G. (2016). Assessment of heavy metal contamination in raw milk for human consumption. South African Journal of Animal Science, 46, 166–169.
Zamberlin, Š., Antunac, N., Havranek, J., & Samaržija, D. (2012). Mineral elements in milk and dairy products. Mljekarstvo/Dairy, 62, 111–125. https://hrcak.srce.hr/file/124024
Acknowledgements
The authors are grateful to the Addis Ababa University for funding. AT acknowledges the Aksum University for the study leave granted to him while pursuing his MSc studies in Pharmaceutical Analysis and quality assurance at the Addis Ababa University.
Funding
The Addis Ababa University thematic project funded this work. However, the funding had no role in the design, experimental work, interpretation, and publication of this study.
Author information
Authors and Affiliations
Contributions
AA conceptualized the study. AT and AA contributed to study design, sampling, instrumental analysis, data analysis, and writing; AH and SHG contributed to the study design, data analysis, and manuscript review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical consideration
Ethical clearance was obtained from the School of Pharmacy Ethical Review Board on July 24, 2017 (Ref. No. ERB/SOP/37/09/2017) and from the Addis Ababa University College of Health Sciences Institutional Review Board on March 8, 2018 (Protocol No. 099/17/SPH). Accordingly the purpose of the study was explained to farmers who took part in the study at Butajira and Meskan districts. Sample collections were performed after verbal consent.
Consent to participate
Milk samples for the study were collected after verbal consent from the farmers and owners. The study was ethically cleared.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teklu, A., Hymete, A., Gebreyesus, S.H. et al. Determination of the level of heavy metals and assessment of their safety in cow’s milk collected from Butajira and Meskan districts, south central Ethiopia. Environ Monit Assess 195, 206 (2023). https://doi.org/10.1007/s10661-022-10824-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10824-z