Abstract
The current study was focused on hydrogeochemistry of Ambagarh chowki groundwater. The main aim of the study was to evaluate the water quality for drinking and irrigation uses, hydrogeochemistry of groundwater. For this purpose, various physicochemical parameters like pH, electrical conductivity (EC), total hardness (TH), chloride (Cl−), fluoride (F−), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), bicarbonate (HCO3−), and sulfate (SO42−) were analyzed. The major dominating ions in groundwater was found as cation Ca2+ > Mg2+ > Na+ > K+ and as anions in order of HCO3− > Cl− > SO42− > F−. The pH of sampled groundwater was ranged from neutral to alkaline in nature (7.0–8.8). Among, all-analyzed parameters in groundwater were safe for drinking purpose except few ions and bicarbonate content was also exceeding the permissible limit which was not suitable for drinking purpose. The potential health hazard element fluoride was varied from 0.1 to 2.2 mg/l and positively associated with the pH, Na+, and HCO3− content in groundwater. The drinking water quality index (WQI) was fluctuated between 12.22 and 185.56 and reported that most of the groundwater was suitable for drinking purpose except only one sample was unsuitable for drinking. Irrigation water quality assessment of the area was performed by evaluating as sodium adsorption ratio (SAR), permeability index (PI), Kelly ratio (KR), magnesium hazard ratio (MHR), percent sodium (%Na), potential salinity (PS), and residual sodium carbonate (RSC). Whereas most of the groundwater were good and suitable for irrigation use excluding PI (3.70%), MHR (40.74%), RSC (22.22%) and PS (7.41%) were unsuitable for continuous irrigation. Hydrogeochemistry of groundwater evaluated with correlation, Piper, Gibbs, and other geochemical analysis. The Piper trilinear diagram reflects the Ca (Mg)-HCO3− type water was mainly contributed approximately 90% of entire sampled groundwater. The different ions in groundwater were originated from the rock water interaction through silicate and carbonate weathering of minerals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water covers approximately three fourth of the earth’s surface and plays a pivotal role in all living beings by fulfilling their fundamental needs of the globe (Kumbhar & Salkar, 2014). However, 96.5% of global water belongs to seawater system which is not suitable for use due to having high total dissolved solid (TDS) value about > 35,000 ppm. Only 2.5% of water is freshwater which can be used for consumption (Atab et al., 2018). In India, about 4000 billion cubic meters water was annual precipitated. Of which, 75% of precipitation occurs from June to September during the south west monsoon season. Where approximately 1986.5 billion cubic meter volumes of water are lost through runoff. A total of 433 billion cubic meters are a total utilizable groundwater resource in India. Similarly, 411 billion cubic meters of water is having groundwater potential to irrigate 64 Mha area of land (CWC, 2019). The per capita water availability in India is about 1720.29 m3 (CWC, 2019). Water is utilized for many different purposes ranging from drinking, irrigation, industrial, and other allied sectors (Upadhay, 2013). Approximately 70% of the freshwater is used by humans for agriculture sectors and 80% groundwater is needed for the rural population to fulfillment their domestic use in India (Karthikeyan et al., 2010; Kumbhar & Salkar, 2014).
In the present world, the groundwater level is depleting and polluted day by day due to huge pressure of urbanization and industrialization. Most of the water is not fit for plant and human consumption; hence, the scarcity of good quality water is an emerging problem (Aboelnga et al., 2020; Hassan Rashid et al., 2018). The ionic concentration of EC, TH, Ca2+, Mg2+, Na+, K+, Cl−, SO42−, CO32−, HCO3−, nitrate (NO3−), and F− were increasing in different groundwater (Ali et al., 2020; Cao et al., 2019; Khan & Jhariya, 2018; Khan et al., 2021; Kumar et al., 2018; Lal et al., 2022; Moussa et al., 2020; Mustafa et al., 2019; Nematollahi et al., 2018). Declining water and its quality degradation is a major challenge for today due to various natural and anthropogenic activities. For example, hydro-geological, atmospheric, climatic, topographic, and litho-logical factors are covered under natural means (Uddin et al., 2021). Anthropogenic activities involve the intensive application of fertilizer and pesticides, mining, effluents from industrial waste, and chemical spills (Chuah et al., 2016; Meena et al., 2020). In this context, assessing the chemical properties of water can help in understanding the usefulness of water for a specific need. Determination of water quality concerning their physicochemical parameters is a good measure for alarming human health and other problems (Ewaid & Abed, 2017). In this context, various workers have reported the standard methods for assessing the water quality and its properties. For example, the method of water quality index (WQI) is utilized for assessing the drinking water suitability for humans. Moreover, various geogenic factors are responsible for poor water quality and its unfit use (Gupta & Misra, 2018). Moreover, the groundwater was tested for various parameters pH, EC, TDS, CaCO3, phosphate (PO43−), NO3−, Cl−, F−, Ca2+, Mg2+, Na+, K+, and HCO3− for calculating the water quality index and drinking water quality (Brhane, 2018; Zakir et al., 2020). Irrigation water suitability was determined by using SAR, RSC, MHR, PI, PS, and EC (Bilali & Taleb, 2020). Sustainable agriculture needs a good quality of irrigation water for generating quality food for the rising population (Jhariya et al., 2019, 2021; Kumar et al., 2020). Similarly, the application of quality irrigation water does not degrade the soil quality of other natural resources that implies a better agriculture production system and environmental quality (Pereira et al., 1996; Uthes & Matzdorf, 2013; Banerjee et al., 2020, 2021; Raj et al., 2020; Haj-Amor et al., 2022). The study area mainly consists of four geological units namely Dongargarh granite, Bailadila group, Nandgaon Bijli Rhyolite, and Nandgaon Pitepani group. Nandgaon Bijli Rhyolite is dominating geology in the study area than the Dongargarh granite and other groups. Pandey et al. (2002, 2004, 2006) found the Lower-Middle Proterozoic age in the area, Dongargarh granite, Nandgaon Pitepani, and Nandgaon Bijli Rhyolite reflected Palaeo Proterozoic age while the Bailadila group was Archaean—Palaeo Proterozoic age. The Nandgaon group comprises with lower Bijli Rhyolite and Pipepani intrusive volcanic and Bailadila group is having phyllitic shales and haematite quartzites. However, Dongargarh granite is overlaying rocks of the area with intrusion of dolerite, dykes, and quarts. The Proterozoic age of the area is having mainly Chhattisgarh supergroup Chilpi, Kotri, Dongargarh, and Iron ore supergroup formation with lithology of limestone, shale arkose, conglomerate, sand stone, shale, schist, phyllite, slate, gneiss, and marble (Shukla et al., 2010). The Chowki area comes under Kotri lineament of Baster in which volcanic rhyolite was dominant which also reported by Prithviraj et al. (2021) where pegmatitic, rhyolite, granite, basalt, dolerite, and pegmatite intrusions rocks were dominant with biotite, chlorite, illite, kaolinite, and goethite clay minerals are predominant (Acharyya & Shah, 2007; Acharyya et al., 2005; Bhattacharya et al., 2012). The study area is covered with laterite loam and alluvium (Singh et al., 2021).
The present study has analyzed the groundwater quality of different farmer fields of the Ambagarh Chowki, Chhattisgarh, India. This study was concerned with groundwater quality because most of the farmer families belong to the rural area and they use groundwater for drinking and irrigation uses. Therefore, this study would help to explore water quality and hydrogeochemistry of groundwater. Furthermore, this can be possible through better research, development, and technological inputs which must be included in current policymakers and planners. Thus, quality water ensures healthy and productive lives for a better sustainable world.
Materials and methods
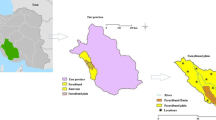
The experiment was carried out in different farmer fields of the Ambagarh Chowki, Chhattisgarh, India. The study sites were situated at 20°35–20°55 N latitude and 80°35–80°50 E longitude. Approximately < 1200 mm annual rainfall was observed and most of the rainfall comes from the southwest monsoon (June to September). The study area is comprising with four types of geology, i.e., Dongargarh granite, Bailadila group, Nandgaon Bijli Rhyolite, and Nandgaon Pitepani group indicated in Fig. 1. Bhata (Entisol), Matasi (Inceptisol), Dorsa (Alfisol), and Kanhar (Vertisol) types of soil were prevalent in this region. Climate is hot moist to dry sub-humid types and comes under Chhattisgarh plain zone of eastern plateau and hill region (class VII) of agro-climatic zone of India as per the planning commission (NBSS&LUP, 2018; Agricoop, 2019). The major dominating rock in the study area is Dongargarh granite and rhyolite which contains minerals like quartz, feldspar (plagioclase and orthoclase), ferromagnesium (biotite and muscovite), and rarely pyroxene and iron oxide (Shukla et al., 2010). The district has a 354.21 (000 ha) net sown area and 89.43 (000 ha) is the gross irrigated area (Agricoop, 2019). The groundwater levels and annual groundwater recharge values of Ambagarh chowki were 5–10 m below ground level and 46,385,300 m3, respectively (Verma, 2013).
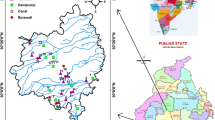
Different tube wells water sources of farmer fields were collected during June month 2016 from 27 groundwater samples in the Ambagarh Chowki (Fig. 2). The samples were collected in a 500-ml capacity of high density cleaned polyethylene plastic bottle rinsing with double distilled water by using handheld Global Positioning System (GPS). Bottles were rinsed properly 2–3 times with running water before samples collection. The samples were tested for various hydro-chemical parameters including pH, EC, TDS, TH as CaCO3, Na+, K+, Cl−, Ca2+, Mg2+, SO42−, HCO3−, CO3−, and F− respectively. The samples were transported immediately into the laboratory where pH and EC were measured by using systronics pH meter model-362 and systronics conductivity meter model-304.The standard ethylenediaminetetraacetic acid (EDTA) titrimetric method was used for the determination of TH and Ca2+, whereas the magnesium ion and TDS was calculated from the obtained TH, Ca2+, and EC, respectively. A flame photometer (Systronics flame photometer model-128, India) was used for determining alkali metals such as Na+ and K+, respectively. Chloride (Cl−) content in groundwater was analyzed by the AgNO3 titrimetric method. The value of bicarbonate (HCO3−) and carbonate (CO32−) were determined by titration with sulphuric acid. Colorimetry method with a UV–visible double beam spectrophotometer (Systronics model – 2201, India) was used for estimating sulfate (SO42−) in the water samples. Similarly, fluoride (F−) was estimated by the SPADNS method by using systronics UV–VIS double beam spectrophotometer model-2201. All the parameters in groundwater were analyzed as per the APHA standards methods (APHA, 1999). The spatial distribution of water quality and study location with geological map were prepared with the help of ArcGIS-10.4, and Pearson correlation study and data visualization R software were used.
The water quality index (WQI) of the collected sample was calculated by using the weighted arithmetic water quality index method (Brown et al., 1970; Horton, 1965). The standard value of the parameters was used from Bureau of Indian Standards (BIS, 2012) and World Health Organization (WHO, 2011) to assess the water quality indices. The relative weight and standard value used for calculation are in Supplementary Table 1.
where \(Wi\) is the unit weight of each parameter used in water quality analysis, which was calculated as follows:
where \(K\) represents a constant value of proportionality and \({S}_{std}\) shows a standard value (BIS and WHO) for different parameters.
Similarly, \(Qi\) represents the quality rating scale of the ith parameters for all n water quality parameters. It was computed as follows:
\(Qa\) represents an actual detected value of the parameters whereas, \(Qi\) showed an ideal value of the parameters in pure water. The symbol \({S}_{std}\) is the standard value of the parameters.
The ideal value was used for pH = 7 and zero for the other parameters (Khatri et al., 2020; Salam & Salwan, 2017). Some studied on WQI had conducted in abroad and India was also reported by various workers (Adimalla et al., 2018; Ahmed et al., 2020; Fahad et al., 2017; Karunanidhi et al., 2020; Kumar et al., 2015; Lal et al., 2022; Tripathi & Singal, 2019; Varol, 2020).
Similarly, various irrigation water quality parameters were assessed. For example, the value of SAR by Richards (1954), Na% by Wilcox (1955), RSC by Eaton (1950), PS by Doneen (1964), KR by (Kelly, 1963), MHR by Raghunath (1987), and PI by Doneen (1962) were adopted for evaluating the irrigation water suitability. However, the computation formulas for irrigation water quality are given below:
The calculated values are expressed in meq/l (meq/l is obtained by mg/l divided by an equivalent weight of the respective elements). Piper diagrams was also calculated for understanding the irrigation suitability of groundwater in the study sites (Piper, 1953). The correlation and dendrogram plots were determined for assessing the interrelationship among different attributes (Jhariya & Singh, 2021a, b).
Results and discussion
Groundwater chemistry
The hydro-chemical analysis was performed for different sampled groundwater in the study areas is depicted in Tables 1 and 3. From this table, the value of pH, EC (μS/cm), F− (mg/l), TH (mg/l), HCO3− (mg/l), Ca2+ (mg/l), Mg2+ (mg/l), Na+ (mg/l), K+ (mg/l), Cl− (mg/l), and SO42− (mg/l) are varied from 7.0 to 8.8, 390 to 1350, 0.1 to 2.2, 72.79 to 300.15, 213.56 to 500.34, 21.04 to 274.27, 6.30 to 48.64, 6.10 to 29.40, 0.03 to 2.15, 35.16 to 170.80, and 5.0 to 45.0, respectively. This highest pH value of groundwater was reported in Sonsaytola C, whereas the least value was observed in Dongaghat. Therefore, the pH value of water samples was observed neutral to alkaline that represents the presence of very weak basic salts of calcium and magnesium ions in groundwater (Adhikari et al., 2014; Xu et al., 2013).
The value of EC was found maximum in Biharikala and the least in Atargaon village. The mean value of total hardness as CaCO3 was reported as 145.08 mg/l in entire villages of study sites. Groundwater was also classified on basis of EC and total hardness in the study area which is depicted in Table 2. From this table, based on EC, most of the groundwater samples were fall under the permissible water type (74.08%) while remaining samples were good (22.22%) and brakish (3.70%) category (Langenegger, 1990). However, all samples were suitable for drinking purposes because they fall beyond the permissible limit as described by the water quality standards (WHO, 2011). Similarly, based on CaCO3 (mg/l) presence the total hardness of groundwater was classified into soft water (7.41%), moderately hard water (55.56%), hard water (33.33%), and only one sample (3.70%) fall under the very hard category (Sawyer & McCarthy, 1967). The hard water may be caused health problems such as cardiovascular disorders, prenatal mortality, urolithiasis, and some cancer when it consumes continuously for a long time (Ahamed et al., 2015).
Mahud village with having highest value of calcium ion concentration in groundwater while least value was observed in Aatargaon village. Most of the tested water samples were having Ca2+ content below the permissible limits as prescribed by BIS standards. The percolating water consists of CO2 which produced H2CO3 and reacted with CaCO3 containing minerals it formed Ca2+ in the groundwater. This process has enriched the value of Ca2+ in the groundwater of the study sites. Similarly, magnesium hazard was negligible in the groundwater samples, where all samples below the permissible limit were observed which ranged from 6.3 to 48.64 mg/l, whereas sodium and potassium ion concentrations in groundwater were also estimated under the permissible limit as described by the drinking water quality standards (WHO). However, the presence of silicate clay minerals may increase the K+ concentration in groundwater but lower potassium was detected due to more resistance of K-bearing minerals (feldspars) to chemical weathering (Mondal et al., 2013; Rao et al., 2015). Bicarbonate content in groundwater was the most dominating anions it might be released through organic matter decomposition and silicate rocks weathering process. The minimum value of HCO3− was reported in Jadutola B village whereas the maximum in Hoditola village. Moreover, chloride (Cl−) was also reported in water samples as an ionic form of chlorine. In this study, groundwater samples were safe for drinking uses with respect to the presence of Cl− under the permissible limit as per the standards of BIS. Sulfate is also a major anion that was observed in the water samples it entered in groundwater through the natural process of weathering of S-bearing and other fertilizer sources. All the groundwater samples were observed below the permissible limit with respect to sulfate ion in groundwater and safe for drinking purposes.
The fluoride (F−) content in groundwater was analyzed with lowest in Aatargaon village to highest in Hoditola village, respectively. Fluoride (F−) is the toxic elements when it presents in higher concentration in drinking water and produce toxicity symptoms on human and animals since it continuously consumes for a longer period. The acceptable range of F− in drinking water is 0.5–1.5 mg/l and dental cavity occur < 0.5 mg/l F− in drinking water. However, the F− content in drinking water is > 1.5 mg/l because dental fluorosis and having > 4 mg/l are also responsible to cause skeletal fluorosis. However, various possible sources of F− in groundwater from minerals such as apatite, biotite, muscovite, hornblende, and fluorite. Phosphatic fertilizers are also having F− content but it negligible contributed particularly it may be possible source of heavy P-fertilization zone. Furthermore, most of the analyzed groundwater sample (77.78%) was fall under 0.5 mg/l of F− concentration, whereas 18.52% of water sample fallen between 0.5 to 1.0 mg/l while only 3.70% of water sample was having above the permissible limit (> 1.5 mg/l) of fluoride content in groundwater (Table 4 and Fig. 4). The higher value of fluorides in groundwater is due to prevailing hydro-geological conditions and the process of weathering of fluoride-bearing minerals (Arveti et al., 2011; Brahman et al., 2014; Nirmala et al., 2012).
A cluster diagram of different sampled groundwater of the study area is indicated in Fig. 3. This hierarchical cluster analysis (HCA) was evaluated for eleven water quality parameters namely pH, EC, TH, Na+, K+, Ca2+, Mg2+, HCO3−, F−, Cl−, and SO42− of collected groundwater samples from a different village. Four villages were separated as the contaminated village namely Atargaon (8), Teliatola (13), Hoditola (20), and Mangatola (21), whereas Hoditola was separated for higher fluoride content (2.2 mg/l) in groundwater while the other village Atargaon, Teliatola, and Mangatola were due to high content of EC and TH in groundwater. These water samples were not recommended for long-term consumption or unsafe for drinking water and it may create many problems in the future.
Drinking water quality index (WQI)
Water quality index (WQI) is a quantitative measure to assess the quality of water for its suitability for drinking purposes. WQI is used to classifying different groundwater and assessing their fitness for drinking purposes based on comparison with BIS and WHO guidelines (Tables 3, 4 and 5). From this table, groundwater was classified into five different categories as excellent, good, poor, very poor, and unsuitable for drinking based on WQI values as 0–25, 25–50, 50–75, 75–100, and > 100, respectively as indicated in Fig. 4. The WQI value of groundwater samples was ranged from 12.22 in Jadutola B village to 185.56 in Hoditola village, respectively. Moreover, 44.44%, 29.63%, and 22.22% groundwater were reported as excellent, good, and poor water categories among all the estimated WQI of selected groundwater samples in study sites. The sample from Hoditola village was reported unsuitable for drinking purposes due to scoring more WQI with having elevated fluoride content.
Groundwater suitability for irrigation purpose
Assessing groundwater is a key step for determining irrigation water suitability for cultivating crops in the study areas. Farmers of the study area were cultivating many crops like rice, wheat, mustard, chickpea, lentil, lathyrus, and other vegetable crops like tomato, brinjal, Knolkhol, and cauliflower in these regions. Therefore, the purpose of assessing irrigation water quality in the study areas was important for understanding water quality and its suitability for crops in this region. Thus, irrigation water quality can be assessed by using various standards calculative methods such as SAR, PI, KR, MHR, %Na, and RSC along with the well-established method of United State Salinity Laboratory (USSL, 1954).
The water levels declined due to evaporations in the summer season that increased the solute concentrations of various chemical elements in the groundwater.
The calculated sodium adsorption ratio varied from 0.2 to 0.72 with an average value of 0.36, whereas the salinity hazard as EC ranged between 390 and 1350 μS/cm in groundwater. SAR and EC values were used for classifying irrigation water which is illustrated in Table 6. These both the parameters were used for USSL (1954) diagram are in Fig. 5. Moreover, 81.48% of groundwater samples were under the low sodium hazard (C2S1) and medium value of salinity. Only 18.52% of samples were reported under the low sodium hazard (C3S1) category and high value of salinity. The high salinity irrigation water was unsuitable for continuous irrigation and it may responsible for poor soil quality and other drainage problems due to heavily accumulation of soluble salts in soil when this salty water applies for long period. Assessing the sodium ion concentration in groundwater is another important step that determines the quality of irrigation water. Na+ acts as deflocculating/dispersing agents which is able to destroy the soil aggregation and affect the various physicochemical properties of soil. In this context, irrigation water quality parameters and their percent contribution of groundwater are depicted in Table 7.
As table suggested that the value of sodium percent in groundwater was fluctuated between 4.68 and 18.2% with a mean value of 11.11%. Therefore, all calculated % Na was under the excellent and safe water category which is depicted in Fig. 6 (Eaton, 1950; Wilcox, 1955). The permeability index was also calculated by the developed equation of Doneen (1964) and used for assess the water permeability in soil which directly related to drainage activity. As per this, the value of PI was varied from 23.49 (minimum) to 92.40% (maximum). Similarly, 70.37% of groundwater samples fall in good water and 25.93% are recorded as excellent water while only one sample falls in unsuitable groundwater for irrigation. The selected groundwater samples were suitable for irrigation purposes as per calculation made under KR (Kelly, 1963). Magnesium hazard ratio is also a key parameter used for evaluating the irrigation quality of the groundwater. The MHR values of the study region were 3.65–68.93 meq/l with an average value of 43.90 meq/l. However, 59.26% of groundwater samples were suitable for irrigation (MHR < 50 meq/l) and 40.74% water sample unsuitable for irrigation (MHR > 50 meq/l) (Raghunath, 1987). When carbonate content in groundwater exceed, it combines with calcium and magnesium ions which precipitated and solid material is formed. The residual sodium carbonate in groundwater was ranged from − 8.61 to 3.19 meq/l. The 40.74% of total groundwater samples fall under the doubtful water category (1.25–2.25), 37.07% in good water (< 1.25), and 22.22% groundwater samples were unsuitable for irrigation concerning RSC (> 2.25) as described by Eaton (1950). The negative value of RSC was also observed in the few groundwater samples of the study area which was confirmed by Adimalla and Venkatayogi (2018) due to incomplete precipitation of Ca2+ and Mg2+ content in groundwater. Potential salinity is an indication of the salinity hazard of groundwater-related to Cl− and SO42− content. The results ranged from 1.15 to 5.29 meq/l with a mean value of 2.90 meq/l. Moreover, 62.96% of groundwater samples were suitable for irrigation and 29.63% fell under the good to injurious water category. Only two samples were found in the injurious to unsatisfactory water category (Doneen, 1962). When high PS irrigation water is applied for a long time to field, it may cause accumulation of soluble salts and become salt-affected soil. However, irrigation water applied with high sodium and bicarbonate content may lead to deterioration of soil health and quality in terms of various physicochemical and biological properties of soil. This has also affected overall nutrient availability to plant and their production. Thus, poor water quality affects both water use efficiency (WUE) and nutrient use efficiency (NUE) severely which affects overall plant health and productivity.
Hydrogeochemistry of groundwater
For the hydrogeochemical evaluation of different sampled groundwater correlation, Piper, Gibbs, and other bivariate plot were performed in the study area. Correlation analysis was also performed among the tested parameters in groundwater is represented in Fig. 7. The correlation matrix showed correlation coefficient (r) among the various eleven parameters namely pH, EC, TH, HCO3−, Na+, K+, Ca2+, Mg2+, Cl−, SO42−, and F− in groundwater. The relationship between the parameters were classify into three different categories according to the obtained value of correlation coefficient (r), i.e., strong relationship (1 > r > 0.75), moderate relationship (0.5 < r < 0.75), and weak relationship (0.25 < r < 0.5). As figure suggested that the value of pH was weak but significantly correlated with F− (r = 0.39, p = 0.05) and HCO3− (r = 0.46, p = 0.05). EC was strongly correlated with TH (r = 0.89, p = 0.001) and Ca2+ (r = 0.80, p = 0.001), whereas moderately correlated with Na+ (r = 0.73, p = 0.001), Cl− (r = 0.52, p = 0.05), and SO42− (r = 0.70, p = 0.001). Total hardness of the groundwater was also having moderately relation with HCO3− (r = 0.51, p = 0.05), Ca2+ (r = 0.73, p = 0.001), Na+ (r = 0.50, p = 0.05), SO42− (r = 0.56, p = 0.05), and weak association with Mg2+ (r = 0.49, p = 0.05) and Cl− (r = 0.47, p = 0.05). Calcium ions have significantly positive relation with Na+ (r = 0.70, p = 0.001), SO42− (r = 0.59, p = 0.001), and Cl− (r = 0.47, p = 0.05) in groundwater. Sodium was significant correlated with SO42− (r = 0.61, p = 0.001), HCO3− (r = 0.39, p = 0.05), and Cl− (r = 0.47, p = 0.05), respectively. Similarly, a weak relationship was reported between HCO3− and Mg2+ (r = 0.48, p = 0.05) and Cl− and SO42− (r = 0.46, p = 0.05) while other parameters were observed as non-significant relationship. Therefore, a major variable constituting ions in groundwater is related to the hydro-chemical variables originating from mineralization of groundwater (Kumar et al., 2017; Patel et al., 2015; Usman et al., 2014).
The fluoride ion in groundwater was having positive correlation with pH (r = 0.39), Na+ (r = 0.44), and HCO3− (r = 0.36), whereas negative relation was also observed among the TH, EC, Ca2+, and Mg2+. The alkaline pH of water enhanced the fluoride content in groundwater. The alkaline pH of water was due to high HCO3− content in water and having ability to mobilize the F− ion from the F− bearing minerals by the replacement of OH− to the groundwater (Jia et al., 2019; Li et al., 2018; Rao, 2009; Singh et al., 2019). Moreover, clay minerals were also responsible for F− concentration in the groundwater sample due to the process of occurring ion exchange where the F− were replaced by OH− ions with supported by high HCO3− content in groundwater.
The possible source of HCO3− content in groundwater was enriched with production of carbonic acid (Eq. 1) and weathering of silicate minerals (Eqs. (5) and (6)).
High pH and HCO3− involves in the following hydrolysis of minerals by replacement of OH−
Biotite
Muscovite
Fluorite
The negative relation of F− and Ca2+ was also confirmed by Liu et al. (2021) and Rao et al. (2016), whereas Ca2+ was reduced with the increasing pH because precipitation of calcite was also explaining the positive relation between F− and HCO3−. Therefore, higher concentration of HCO3− in groundwater was possible source of high F− in Hoditola village. The positive relationship with F− and pH, Na+, and HCO3− were also reported by Reddy et al. (2010), Adimalla and Venkatayogi (2017), and Su et al. (2019).
Equation (5) suggested that weathering of hornblende mineral was responsible for the different ions source in groundwater and Eq. (6) explains the possible source for releasing fluoride and other ions in groundwater.
Hornblende
Biotite
The Piper trilinear diagram (Piper, 1953) plotted to know the different cation and anion concentration in groundwater of the study area is depicted in Fig. 8. The cation concentration in groundwater was having sequence of Ca2+ > Mg2+ > Na+ > K+ and anions in order of HCO3− > Cl− > SO42− > F−. The major cations in groundwater were clustered within the plot was approximately 40–50% of Ca2+, 30–40% no dominant type, and 10–20% of Mg2+ dominant type water, whereas anion concentration was majorly contributed by the HCO3− (90–95%) and remaining were no dominant type 5–10% in sampled groundwater. The groundwater was mainly dominated with Ca(Mg)-HCO3− and mixed type water. Ca(Mg)-HCO3− type groundwater was dominated which contributed approximately 90% of entire sampled water. The source of calcium and magnesium in groundwater was assumed from the weathering of different calcium and magnesium bearing minerals in soil. The possible ionic group in mixed type of water was mainly associated with Mg-Ca-Na-HCO3-Cl−, Ca-Mg-Na-HCO3−, Na-Ca-Mg-HCO3−, Na-Mg-Ca-HCO3−, Na-Mg-Ca-K-HCO3−, Na-Ca-HCO3−, Ca–Mg–K-HCO3-Cl−, Mg–K-Ca-Na-HCO3−, and K-Mg-Na-HCO3−. The Ca-Mg-HCO3− and Cl− type water may able to dissolve the silicate minerals with presence of CO2, dissolution of calcite and weathering of silicate minerals were enriched with Ca2+, Mg2+, Na+, and HCO3− (Kumar & Singh, 2015; Kumar et al., 2017) content in groundwater. The possible source of Na+, K+, Ca2+, Mg2+, and HCO3− from the weathering and exchange phenomena of different clay minerals.
The Gibbs diagram (Gibbs, 1970) was plotted against the TDS and Na+ + K+/Na+ + K+ + Ca2+ and TDS vs Cl−/Cl− + HCO3− as suggested in Fig. 9. The Gibbs plot define the dominant mechanism of groundwater geochemistry and it basically describes three processes of ion contribution in water (a) evaporation dominance, (b) weathering dominance or rock water interaction, and (c) precipitation dominance which helps to understand the ion sources in groundwater, as plot indicated that the all the sample points of groundwater were aggravated in the rock weathering zone or rock water interaction zone. Moreover, the source different ions including fluoride in groundwater was mainly originated from the weathering/dissolution of minerals through rock water interaction and cation exchange from the adsorbed site of different present clay minerals in soil. The process completed when water molecules contacted with the rock and minerals in soil profile through percolating water, water can leach different ions from soil to groundwater by exchange phenomena.
The plot Ca2+/Na+ against Mg2+/Na+ and Ca2+/Na+ vs HCO3−/Na+ (Gaillardet et al., 1999) was also support to determine the geochemistry of solute contribution in different groundwater sample either by weathering or dissolution of the silicate, carbonate, and evaporite process. As Fig. 10 elaborated that the most of the groundwater samples data were clustered within the silicate weathering site and few were reported under carbonate dissolution, which indicates the origin of different ions in groundwater including fluoride was from weathering of the silicate and carbonate clay minerals present in the soil. The different ions in groundwater were generated by silicate weathering were also confirmed by Adimalla and Qian (2019) and Marghade et al. (2019). However, plot HCO3− + SO42− against Ca2+ + Mg2+ were also supported for ions were majorly oriented from weathering of silicate minerals except few samples were drop under carbonate weathering (Fig. 11).
Hydrogeochemical indices and ratio were also calculated for plotting bivariate plot which helps to understand the various geochemistry processes involved in sampled groundwater. As Fig. 12a was plotted between Ca2+ against HCO3− which indicated that Ca2+ in groundwater was mainly associated with the weathering of calcium bearing silicate mineral, whereas the plot between Na+ vs HCO3− depicted that the occurrence of Na+ in groundwater sample was from weathering of carbonated minerals (Fig. 12b). The plot Ca2+ vs Na+ explains the ion exchange phenomena of by two ways via forward ion exchange which is replacement of Na+ or K+ by Ca2+ or Mg2+ and reverse ion exchange is replacement of Ca2+ or Mg2+ by Na+ or K+. However, Fig. 12c indicates that forward exchange was dominated over reverse ion exchange and it was possible source of cations concentration in groundwater (Eq. (7)), as Fig. 12d plotted against the Na+ and Cl− which suggest Na+ in groundwater was mainly by the action of reverse ion exchange process which was supported by Eq. (8). It is also suggested that Na/Cl ratio, when the ratio exceeds > 1 silicate weathering, is responsible for Na+ in groundwater while it equal to 1 indicates halite dissolution. However, the Na/Cl ratio of all sampled groundwater were reported < 1 which also explains Cl− was dominated over Na+ in groundwater and its Na/Cl ratio < 1 reflects the reduction of Na+ by reverse ion exchange process which is supported by reverse ion equation and possible factor for more Ca2+ content in groundwater (Egbueri et al., 2020; Saha & Rahman, 2020; Su et al., 2021; Xu et al., 2019).
Forward ion exchange
Reverse ion exchange
The lower concentration of Na+ in groundwater with compare to Ca2+ was due to sodium was consumed for the replacement of Ca.2+ ion exchange in groundwater (Eq. (9))
Conclusions
Water is a key natural resource that governs all essential ecosystem services for a sustainable environment. Hydrogeochemical analysis for assessing water quality is a major concern today from environmental management perspective. Water quality assessment is essential for understanding chemical behaviors and geochemistry for drinking and irrigation purposes. Therefore, twenty-seven groundwater samples were collected from the Ambagarh Chowki, Chhattisgarh, India which was used for assessing water quality and hydrogeochemistry of groundwater. The groundwater of the study area was neutral to alkaline in nature with moderately hard-hard water. Calcium was dominating cations among all cations whereas, bicarbonate was revealed most dominant anions in groundwater. The pH, Ca2+, HCO3− and F− were exceeding the permissible limit of Bureau of Indian Standards (BIS, 2012) and World Health Organizations (WHO, 2011) in drinking water. Fluoride was ranging from 0.10 to 2.2 mg/l in groundwater and positively associated with pH, Na+, and HCO3− in groundwater. WQI of the study area is fluctuated from 12.22 to 185.56 and reported most of groundwater fall under excellent (44.44%) and good (29.63%) water while poor (22.22%) and only one sample was unsuitable for drinking use due to heavy loading of fluoride content in groundwater. For irrigation water quality assessment, various indices were used and found that PI, MHR, RSC, and PS exceed the limit which was mainly describe the salinity and magnesium hazards in groundwater. Piper diagrams shows that most of the groundwater was dominated with Ca(Mg)-HCO3− type water in the study area. Gibbs and other bivariate plot explain that different ions in groundwater was originated from silicate weathering through rock water interaction and ion exchange phenomena. Thus, the above study concludes the silicate weathering was main source of ions in groundwater through rock water interaction of the study area. Therefore, monitoring of the groundwater quality is needed and helpful for the local, scientific, and policymaker’s people for the future decisions.
Data availability
Not applicable.
References
Aboelnga, H. T., El-Naser, H., Ribbe, L., & Frechen, F. B. (2020). Assessing water security in water-scarce cities: Applying the Integrated Urban Water Security Index (IUWSI) in Madaba, Jordan. Water, 12(5), 1–22. https://doi.org/10.3390/W12051299
Acharyya, S. K., & Shah, B. A. (2007). Groundwater arsenic contamination affecting different geologic domains in India—A review: Influence of geological setting, fluvial geomorphology and Quaternary stratigraphy. Journal of Environmental Science and Health Part A, 42, 1795–1805.
Acharyya, S. K., Shah, B. A., Ashyiya, I. D., & Pandey, Y. (2005). Arsenic contamination in groundwater from parts of Ambagarh-Chowki block, Chhattisgarh, India: Source and release mechanism. Environmental Geology, 49, 148–158.
Adhikari, P. P., Dash, C. J., Sarangi, A., & Singh, D. K. (2014). Hydrochemical characterization and spatial distribution of fluoride in groundwater of Delhi state, India. Indian Journal of Soil Conservation, 42(2), 170–173.
Adimalla, N., & Qian, H. (2019). Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicology and Environmental Safety, 176, 153–161.
Adimalla, N., Li, P., & Venkatayogi, S. (2018). Hydrochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environmental Processes, 5, 363–383. https://doi.org/10.1007/s40710-018-0297-4
Adimalla, N., & Venkatayogi, S. (2017). Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana State, South India. Environmental Earth Science, 76(45), 1–10. https://doi.org/10.1007/s12665-016-6362-2
Adimalla, N., & Venkatayogi, S. (2018). Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Applied Water Science, 8, 44. https://doi.org/10.1007/s13201-018-0682-1
Agricoop. (2019). Agriculture contingency plan for district Rajnandgaon, Chhattisgarh. 1–27.
Ahamed, A. J., Loganathan, K., & Jayakumar, R. (2015). Hydrochemical characteristics and quality assessment of groundwater in Amaravathi river basin of Karur district, Tamil Nadu, South India. Sustainable Water Resource Management, 1, 273–291.
Ahmed, J., Wong, L. P., Chua, Y. P., & Channa, N. (2020). Drinking water quality mapping using water quality index and geospatial analysis in primary schools of Pakistan. Water, 12(3382), 2–18.
Ali, M., Mohammad, A. M., Mohammad, M. M., Marzieh, M., & Zahra, Z. (2020). Environmental hydrogeochemistry characteristics, controlling factors and groundwater quality assessment in Herat city, West Afghanistan. Water Resources, 47(2), 325–335.
APHA. (1999). Standard methods for the examination of water and wastewater. 20th Ect. APHA, AWWA, WPCF. 210–500.
Arveti, N., Sarma, M. R. S., Peterson, A., & Sunil, K. (2011). Fluoride incidence in groundwater: A case study from Talupula, Andhra Pradesh, India. Environmental Monitoring Assessment, 172, 427–443.
Atab, M. S., Smallbone, A. J., & Roskilly, A. P. (2018). A hybrid reverse osmosis/adsorption desalination plant for irrigation and drinking water. Desalination, 444, 44–52.
Banerjee, A., Jhariya, M. K., Yadav, D. K., & Raj, A. (2020). Environmental and sustainable development through forestry and other resources. Apple academic press Inc., CRC Press- a Tayler and Francis Group. ISBN: 9781771888110. (pp. 400). https://doi.org/10.1201/9780429276026
Banerjee A, Meena R. S., Jhariya, M. K., & Yadav, D. K. (2021). Agroecological footprints management for sustainable food system. Springer nature Singapore, eBook ISBN: 978–981–15–9496–0, Hardcover: 978–981–15–9495–3. https://doi.org/10.1007/978-981-15-9496-0. (pp. 514).
Bhattacharya, S., Majumdar, T. J., Rajawat, A. S., Panigrahi, M. K., & Das, P. R. (2012). Utilization of hyperion data over Dongargarh, India, for mapping altered/weathered and clay minerals along with field spectral measurements. International Journal of Remote Sensing, 33(17), 5438–5450.
Bilali, A. E., & Taleb, A. (2020). Prediction of irrigation water quality parameters using machine learning models in a semi-arid environment. Journal of the Saudi Society of Agricultural Sciences. https://doi.org/10.1016/j.jssas.2020.08.001
BIS (Bureau of Indian Standards) 10500. (2012). Indian Standard Drinking Water Specification. 1–16.
Brahman, K. D., Kazi, T. G., Afridi, H. I., Rafique, T., Baig, J. A., Arain, S. S., Ullah, N., Panhwar, A. H., & Arain, S. (2014). Evaluation of fresh and stored rainwater quality in fluoride and arsenic endemic area of Thar Desert, Pakistan. Environmental Monitoring Assessment, 186, 8611–8628.
Brhane, G. K. (2018). Characterization of hydro chemistry and groundwater quality evaluation for drinking purpose in Adigrat area, Tigray, northern Ethiopia. Water Science, 32, 213–229.
Brown, R. M., Clelland, Mc., Deininger, R. A., & Tozer, R. G. (1970). A Water Quality Index—Do We Dare? 117 Water Sewage Works, 339–343.
Cao, X., Lu, Y., Wang, C., Zhang, M., Yuan, J., Zhang, A., Song, S., Baninla, Y., Khan, K., & Wang, Y. (2019). Hydrogeochemistry and quality of surface water and groundwater in the drinking water source area of an urbanizing region. Ecotoxicology and Environmental Safety, 186, 109628.
Chuah, C. J., Lye, H. R., Ziegler, A. D., Wood, S. H., Kongpun, C., & Rajchagool, C. (2016). Fluoride: A naturally occurring health hazard in drinking water resources of Northern Thailand. Science of the Total Environment, 545-546, 266–279.
CWC. (2019). Annual report on central water commission of India. 22–30.
Doneen, L. D. (1964). Notes on water quality in agriculture. University of California.
Doneen, L. D. (1962). The influence of crop and soil on percolating water. Proceedings of the Biennial Conference on Groundwater Recharge, 156–163.
Eaton, E. M. (1950). Significance of carbonates in irrigation waters. Soil Science, 69, 123–133.
Egbueri, J. C., Ezugwu, C. K., Unigwe, C. O., Onwuka, O. S., Onyemesili, O. C., & Mgbenu, C. N. (2020). Multidimensional analysis of the contamination status, corrosivity and hydrogeochemistry of groundwater from parts of the Anambra basin, Nigeria. Analytical Letters. https://doi.org/10.1080/00032719.2020.1843049
Ewaid, S. H., & Abed, S. A. (2017). Water quality index for Al-Gharraf river, southern Iraq. Egyptian Journal of Aquatic Research, 43, 17–122.
Fahad, N. A. B., Abdurahman, M. A. J., & Anwar, A. A. (2017). Water quality assessment and hydrochemical characterization of Zamzam groundwater, Saudi Arabia. Applied Water Science, 7, 3985–3996.
Gaillardet, J., Dupre, B., Louvat, P., & Allegre, C. J. (1999). Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chemical Geology, 159, 3–30.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Journal of Sciences, 17, 1088–1090.
Gupta, R., & Misra, A. K. (2018). Groundwater quality analysis of quaternary aquifers in Jhajjar District, Haryana, India: Focus on groundwater fluoride and health implications. Alexandria Engineering Journal, 57(1), 375–381.
Haj-Amor, Z., Araya, T., Kim, D. G., Bouri, S., Lee, J., Ghiloufi, W., Yang, Y., Kang, H., Jhariya, M. K., Banerjee, A., & Lal, R. (2022). Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Science of the Total Environment, 843, 156946. https://doi.org/10.1016/j.scitotenv.2022.156946
Hassan Rashid, M. A. U., Manzoor, M. M., & Mukhtar, S. (2018). Urbanization and its effects on water resources: An exploratory analysis. Asian Journal of Water, Environment Pollution, 15, 67–74.
Horton, R. K. (1965). An index number system for rating water quality. Journal Water Pollution Control Federation, 37, 300–305.
Jhariya, M. K., & Singh, L. (2021a). Effect of fire severity on soil properties in a seasonally dry forest ecosystem of Central India. International Journal of Environmental Science and Technology, 18, 3967–3978. https://doi.org/10.1007/s13762-020-03062-8
Jhariya, M. K., Banerjee, A., Meena, R. S., & Yadav, D. K. (2019). Sustainable agriculture, forest and environmental management. Springer nature Singapore. eISBN: 978–981–13–6830–1, Hardcover ISBN: 978–981–13–6829–5. (pp. 606). https://doi.org/10.1007/978-981-13-6830-1
Jhariya, M. K., Meena, R. S., & Banerjee, A. (2021). Ecological intensification of natural resources for sustainable agriculture. Springer nature Singapore. eISBN: 978–981–334–203–3, Hardcover ISBN: 978–981–334–206–6. https://doi.org/10.1007/978-981-33-4203-3
Jhariya, M. K., & Singh, L. (2021b). Herbaceous diversity and biomass under different fire regimes in a seasonally dry forest ecosystem. Environmental Development & Sustainability, 23(5), 6800–6818. https://doi.org/10.1007/s10668-020-00892-x
Jia, H., Qian, H., Qu, W., Zheng, L., Feng, W., & Ren, W. (2019). Fluoride occurrence and human health risk in drinking water wells from Southern edge of Chinese loess plateau. International Journal of Environmental Research & Public Health, 16, 1683. https://doi.org/10.3390/ijerph16101683
Karthikeyan, K., Nanthakumar, K., Velmurugan, P., Tamilarasi, S., & Lakshmanaperumalsamy, P. (2010). Prevalence of certain inorganic constituents in groundwater samples of Erode district, Tamilnadu, India, with special emphasis on fluoride, fluorosis and its remedial measures. Environmental Monitoring Assessment, 160, 141–155.
Karunanidhi, D., Aravinthasamy, P., Subramani, T., & Muthusankar, G. (2020). Revealing drinking water quality issues and possible health risks based on water quality index (WQI) method in the Shanmuganadhi river basin of South India. Environmental Geochemistry & Health. https://doi.org/10.1007/s10653-020-00613-3
Kelly, W. P. (1963). Use of saline irrigation water. Soil Science, 95, 355–391.
Khan, R., & Jhariya, D. C. (2018). Hydrogeochemistry and groundwater quality assessment for drinking and irrigation purpose of Raipur city, Chhattisgarh. Journal of Geological Society of India, 91, 475–482.
Khan, N., Malik, A., & Nehra, K. (2021). Groundwater hydro-geochemistry, quality, microbiology and human health risk assessment in semi-arid area of Rajasthan, India: A chemometric approach. Environmental Monitoring Assessment, 193, 234. https://doi.org/10.1007/s10661-021-08979-2
Khatri, N., Tyagi, S., Rawtani, D., Tharmavaram, M., & Kamboj, R. D. (2020). Analysis and assessment of ground water quality in Satlasana Taluka, Mehsana district, Gujarat, India through application of water quality indices. Groundwater for Sustainable Development. https://doi.org/10.1016/j.gsd.2019.100321
Kumar, S. K., Logeshkumaran, A., Magesh, N. S., Godson, P. S., & Chandrasekar, N. (2015). Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai city, Tamil Nadu, India. Applied Water Science, 5, 335–343.
Kumar, P., Singh, C. K., Saraswat, C., Mishra, B., & Sharma, T. (2017). Evaluation of aqueous geochemistry of fluoride enriched groundwater: A case study of the Patan district, Gujarat, Western India. Water Science, 31(2), 215–229.
Kumar, A. & Singh, C. K. (2015). Characterization of hydrogeochemical processes and fluoride enrichment in groundwater of South-Western Punjab. Water Quality, Exposure & Health. https://doi.org/10.1007/s12403-015-0157-7
Kumar, N., Kumar, D., Kumar, S., Shukla, V., Shukla, P., & Raj, B. (2018). Spatio-temporal variations in hydro-geochemistry of groundwater at rural, urban and industrial areas of Kanpur, India. Environmental Sustainability. https://doi.org/10.1007/s42398-018-0019-x
Kumar, S., Meena, R. S., & Jhariya, M. K. (2020). Resources use efficiency in agriculture. Springer Nature Singapore. eBook ISBN: 978–981–15–6953–1, Hardcover: 978–981–15–6952–4. (pp. 760). https://doi.org/10.1007/978-981-15-6953-1
Kumbhar, V. S., & Salkar, V. D. (2014). Use of PAC as a substitute for alum in Nalgonda technique. International Journal of Emerging Technology & Advanced Engineering, 4(10), 154–161.
Lal, B., Kumar, P., Sengar, S. S., & Singh, R. N. (2022). Hydro-chemical evaluation and fluoride health risk assessment in school children of plain tropical Central India. Geocarto International. https://doi.org/10.1080/10106049.2022.2052975
Langenegger, O. (1990). Ground water quality in rural areas of western Africa. UNDP Project INT/81/026, 10.
Li, P., He, X., Li, Y., & Xiang, G. (2018). Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese loess plateau: A case study of Tongchuan, northwest China. Exposure and Health. https://doi.org/10.1007/s12403-018-0278-x
Liu, J., Peng, Y., Li, C., Gao, Z., & Chen, S. (2021). A characterization of groundwater fluoride, influencing factors and risk to human health in the southwest plain of Shandong Province, north China. Ecotoxicology and Environmental Safety, 207, 111512.
Marghade, D., Malpe, D. B., Rao, N. S., & Sunitha, B. (2019). Geochemical assessment of fluoride enriched groundwater and health implications from a part of Yavtmal district, India. Human and Ecological Risk Assessment: An International Journal. https://doi.org/10.1080/10807039.2018.1528862
Meena, R. S., Kumar, S., Datta, R., Lal, R., Vijaykumar, V., Brtnicky, M., Sharma, M. P., Yadav, G. S., Jhariya, M. K., Jangir, C. K., Pathan, S. I., Dokulilova, T., Pecina, V., & Marfo, T. D. (2020). Impact of agrochemicals on soil microbiota and management: A Review. Land, 9(2), 34. https://doi.org/10.3390/land9020034
Mondal, D., Gupta, S., & Mahato, A. (2013). Fluoride dynamics in the weathered mantle and the saprolitic zone of the Purulia district, West Bengal. Advances in Applied Science Research, 4(6), 187–196.
Moussa, A. B., Chandoul, S., Mzali, H., Salem, S. B. H., Elmejri, H., Zouari, K., Hafiane, A., & Mrabet, H. (2020). Hydrogeochemistry and evaluation of groundwater suitability for irrigation purpose in the Mornag region, northeastern Tunisia. Environment, Development & Sustainability. https://doi.org/10.1007/s10668-020-00696-z
Mustafa, E. R., Esam, I., & Osman, A. (2019). Assessment of groundwater quality using GIS, hydrogeochemistry, and factor statistical analysis in Qena governorate, Egypt. Desalination and Water Treatment, 162, 14–29.
NBSS&LUP. (2018). ICAR-National bureau of soil survey and land use planning- at a glance. p 11–12.
Nematollahi, M. J., Clark, M. J. R., Ebrahimi, P., & Ebrahimi, M. (2018). Preliminary assessment of groundwater hydrogeochemistry within Gilan, a northern province of Iran. Environmental Monitoring Assessment, 190, 242. https://doi.org/10.1007/s10661-018-6543-4
Nirmala, B., Suresh, K. B. V., Suchetan, P. A., & Shet-Prakash, M. (2012). Seasonal variations of physico chemical characteristics of ground water samples of Mysore city, Karanataka, India. International Research Journal of Environmental Science, 1(4), 43–49.
Pandey, P. K., Yadav, S., Nair, S., & Bhui, A. (2002). Arsenic contamination of the environment: A new perspective from central-east India. Environment International, 28, 235–245.
Pandey, P. K., Nair, S., Bhui, A., & Pandey, M. (2004). Sediment contamination by arsenic in parts of central-east India and analytical studies on its mobilization. Current Science, 86(1), 190–197.
Pandey, P. K., Sharma, R., Roy, M., Roy, S., & Pandey, M. (2006). Arsenic contamination in the Kanker district of central-east India: Geology and health effects. Environmental Geochemistry and Health, 28, 409–420. https://doi.org/10.1007/s10653-005-9039-4
Patel, K. S., Sahu, B. L., Dahariya, N. S., Bhatia, A., Patel, R. K., Matini, L., Sracek, O., & Bhattacharya, P. (2015). Groundwater arsenic and fluoride in Rajnandgaon district, Chhattisgarh, northeastern India. Applied Water Science, 7, 1817–1826.
Pereira, L. S., Gilley, J. R., & Jensen, M. E. (1996). Research agenda on sustainability of irrigated agriculture. Journal of Irrigation & Drainage Engineering, 122(3), 172–177.
Piper, A. M. (1953). A graphic representation in the geochemical interpretation of groundwater analyses. American Geophysical Union Transactions USA, 25, 914–923.
Prithviraj, N., Tripathy, J. K., Panda, S., & Barik, K. K. (2021). Quality assessment of groundwater in the western parts of Chhattisgarh (Rajnandgaon district), India. Pollution Research, 40(1), 97–103.
Raghunath, H. M. (1987). Groundwater (p. 563). Wiley Eastern Ltd.
Raj, A., Jhariya, M. K., Yadav, D. K., & Banerjee, A. (2020). Climate change and agroforestry systems: Adaptation and mitigation strategies. Apple academic press Inc., CRC Press- a Tayler and Francis group. ISBN: 9781771888226. (p. 383). https://doi.org/10.1201/9780429286759
Rao, S. S. (2009). Fluoride in groundwater, Varaha river basin, Visakhapatnam district, Andhra Pradesh, India. Environmental Monitoring Assessment, 152, 47–60.
Rao, N. S., Dinakar, A., Rao, P. S., Rao, P. N., Madhnure, P., Prasad, K. M., & Sudarshan, P. (2016). Geochemical processes controlling fluoride-bearing groundwater in the granitic aquifer of a semi-arid region. Journal Geological Society of India, 88, 350–356.
Rao, S. N., Rao, S. P., Dinakar, A., Rao, N. P. V., & Margrav, D. (2015). Fluoride occurrence in the groundwater in a coastal region of Andhra Pradesh, India. Applied Water Science, 7, 1467–1478.
Reddy, D. V., Nagabhushanam, P., Sukhija, B. S., Reddy, A. G. S., & Smedley, P. L. (2010). Fluoride dynamics in the granitic aquifer of the Wailapally watershed, Nalgonda district, India. Chemical Geology, 269(3–4), 278–289.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils. Agricultural Handbook, 60, 98–99. USDA and IBH Pub. Co. Ltd.
Saha, N., & Rahman, M. S. (2020). Groundwater hydrogeochemistry and probabilistic health risk assessment through exposure to arsenic-contaminated groundwater of Meghna floodplain, central-east Bangladesh. Ecotoxicology and Environmental Safety, 206, 111349.
Salam, H. E., & Salwan, A. A. (2017). Water quality index for Al-Gharraf River, Southern Iraq. Egyptian Journal of Aquatic Research, 2, 23–45.
Sawyer, G. N., & McCarthy, D. L. (1967). Chemistry of sanitary engineers (2nd ed.). McGraw Hill.
Shukla, D. P., Dubey, C. S., Singh, N. P., Tajbakhsh, M., & Chaudhry, M. (2010). Sources and controls of arsenic contamination in groundwater of Rajnandgaon and Kanker district, Chattisgarh, Central India. Journal of Hydrology, 395, 49–66.
Singh, G., Rishi, M. S., & Herojeet, R. (2019). Multivariate analysis and geochemical signatures of groundwater in the agricultural dominated taluks of Jalandhar district, Punjab, India. Journal of Geochemical Exploration. https://doi.org/10.1016/j.gexplo.2019.106395
Singh, R., Gayen, A., Kumar, S., & Dewangan, R. (2021). Geo-spatial distribution of arsenic contamination of groundwater resources in intricate crystalline aquifer system of Central India: Arsenic toxicity manifestation and health risk assessment. Human and Ecological Risk Assessment: An International Journal. https://doi.org/10.1080/10807039.2020.1865787
Su, H., Wang, J., & Liu, J. (2019). Geochemical factors controlling the occurrence of high-fluoride groundwater in the western region of the Ordos basin, northwestern China. Environmental Pollution, 252, 1154–1162.
Su, H., Kang, W., Kang, N., Liu, J., & Li, Z. (2021). Hydrogeochemistry and health hazards of fluoride-enriched groundwater in the Tarim basin, China. Environmental Research, 200, 111476.
Tripathi, M., & Singal, S. K. (2019). Allocation of weights using factor analysis for development of a novel water quality index. Ecotoxicology and Environmental Safety, 183, 109510.
Uddin, M. G., Nash, S., & Olbert, A. I. (2021). A review of water quality index models and their use for assessing surface water quality. Ecological Indicators, 122, 107218.
Upadhay, M. (2013). High fluoride incidence in groundwater and its potential health effects in parts of Sarguja area Chhattisgarh, India. Recent Research in Science & Technology, 5(5), 47–50.
Usman, U. N., Toriman, M. E., Juahir, H., Abdullahi, M. G., Rabiu, A. A., & Isiyaka, H. (2014). Assessment of groundwater quality using multivariate statistical techniques in Terengganu. Science & Technology, 4(3), 42–49.
USSL. (1954). Diagnosis and improvement of salinity and alkaline soil. USDA Hand Book, 60.
Uthes, S., & Matzdorf, B. (2013). Studies on agri-environmental measures: A survey of the literature. Environmental Management, 51, 251–266.
Varol, M. (2020). Use of water quality index and multivariate statistical methods for the evaluation of water quality of a stream affected by multiple stressors: A case study. Environmental Pollution, 266, 115417.
Verma, S. K. (2013). Groundwater brochure of Rajnandgaon district, Chhattisgarh, Government of India - Ministry of water resources, central ground water board. 4–21.
WHO. (2011). Guidelines for drinking-water quality, (4th ed. p. 340). World Health Organization.
Wilcox, L. V. (1955). Classification and use of irrigation waters. USDA, Washington, DC Circular 969.
Xu, F., Maa, T., Shia, L., Zhanga, J. W., Wanga, Y. Y., & Donga, Y. H. (2013). The hydrogeochemical characteristics of high iodine and fluoride groundwater in the Hetao Plain, Inner Mongolia. Procedia Earth Planetary Science, 7, 908–911.
Xu, B., Zhang, Y., & Wang, J. (2019). Hydrogeochemistry and human health risks of groundwater fluoride in Jinhuiqu irrigation district of Wei river basin, China. Human and Ecological Risk Assessment: An International Journal. https://doi.org/10.1080/10807039.2018.1530939
Zakir, H. M., Sharmin, S., Akter, A., & Rahman, M. S. (2020). Assessment of health risk of heavy metals and water quality indices for irrigation and drinking suitability of waters: A case study of Jamalpur Sadar area, Bangladesh. Environmental Advances, 2, 100005.
Acknowledgements
The authors are thankful to the authorities of IGKV, Raipur (CG), for necessary support. BL thanks to the Department of Soil Science and Agricultural Chemistry, IGKV, Raipur. The authors are thankful to editor and reviewers for the closer look into the MS and suggestions to improve the MS.
Author information
Authors and Affiliations
Contributions
B. Lal: data collection, methodology, formal analysis, and writing. S. S. Sengar: conceptualization, supervision, validation. R. N. Singh: conceptualization, supervision, validation. M. K. J.: compilation, review and editing. A. Raj: drafting, literature and review
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
All the authors approved the manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lal, B., Sengar, S.S., Singh, R. et al. Hydrogeochemistry and groundwater quality assessment in Ambagarh Chowki, Chhattisgarh, India. Environ Monit Assess 195, 43 (2023). https://doi.org/10.1007/s10661-022-10650-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10650-3