Abstract
Microplastics are a widespread environmental contaminant that raises serious concern for aquatic organisms. Hence, the present study was conducted to investigate the spatial and seasonal variation of microplastics, their characteristics, polymer types and the risk assessment caused by the microplastics in six sampling sites along the Thoothukudi region. The average microplastic abundance ranged from 32 ± 26 to 232 ± 229 items/kg and 54 ± 41 to 619 ± 377 items/l in sediment and surface water, respectively, and they exhibited a significant spatial difference among the sampling sites. The microplastic abundance also showed a significant difference among the seasons with the monsoon significantly recording the highest mean microplastic abundance in sediment (160 ± 130 items/kg) and surface water (454 ± 374 items/l). In sediment and surface water, fragment (sediment: 52.72%, surface water: 40.89%), 0.5–1 mm (sediment: 43.96%, surface water: 31.11%) and blue-coloured (sediment: 52.33%, surface water: 41.85%) microplastics were dominant with no significant difference both spatially and seasonally. Polyethylene, the dominant polymer, was observed in both the sediment and surface water, accounting for about 47.58% and 49.83%, respectively, and it showed no significant difference among the selected sites. This signifies that they are homogenously distributed along the coast and further suggests that these particles persisted in the sediment and surface water for a longer period of time. The results of the polymer hazard index show that the sediment (PHI = 1181.63) and surface water (PHI = 1018.66) were severely contaminated (hazard level V) with microplastic polymers such as PE, PP, PS, PET and PA. It was also found that the degree of the microplastic contamination in sediment (PLI = 3.57) and surface water (PLI = 3.84) was lower (hazard level I). The overall risk index (RI) for sediment (253.48) and surface water (444.74) falls under the higher risk category. From the correlation analysis, a significantly positive relationship was observed between microplastics in sediment and surface water based on each classification (abundance, shape, size, colour and polymer). This suggests that microplastics rejoin the water column from the sediment through resuspension, which occurs due to the circulation, tides and sedimentation rate. This might be the reason for the higher microplastic abundance in the surface water than in the sediment. As a result, proper management measures to reduce plastic waste disposal in the marine environment should be implemented to lessen the effects of microplastics on marine biota and on public health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growth of plastic production has increased exponentially due to various factors, including low cost, corrosion resistance, high strength and insulation (Gao et al., 2021). Globally, the annual production of plastic has increased to about 367 million tonnes (Europe, 2021). Plastic, which accounts for up to 80% of litter, is primarily derived from land-based sources. In addition to this, the aquaculture and fishing sectors are also attributed to plastic debris in the marine environment (Radhakrishnan et al., 2021). Due to improper waste management practices and marine and coastal activities, these plastic particles find their way into the marine environment, where they pose a severe threat to the marine biota (Veerasingam et al., 2016).
The larger size of plastic debris in the marine environment gets fragmented due to various factors, including physical, chemical and biological processes, resulting in a huge amount of minute plastic fragments. Of these smaller plastics, particles less than 5 mm in size are called microplastics and they are classified as primary and secondary microplastics based on their origin (Veerasingam et al., 2021b). Microplastics are ubiquitous in the marine environment and exist in various shapes such as fragments, fibres, foam, film and irregular. They are made up of different polymers including polyethylene (PE), polypropylene (PP), polystyrene (PS), polyamide (PA), polyethylene terephthalate (PET), polyvinyl chloride (PVC), etc. (Chen & Chen, 2020). Microplastic accumulation in the marine ecosystem may vary with the proximity of urban activities, coastal water usage and different patterns of the ocean and winds (James et al., 2021). Fishing significantly contributes to high microplastic abundance through deliberate or accidental release of fishing materials, including ropes, floats, nets, lines, etc., and they are added up in the marine environment in the form of fragments and fibres (Nithin et al., 2022). Due to their smaller size, shape, colour, size and chemical composition, these microplastics are carried through the food chain and result in physical damage, exposure to pathogens, oxidative stress, DNA damage, neurological damage, reduction in survival and reproduction (Liu et al., 2019; Prata et al., 2020; Sánchez-Hernández et al., 2021). Microplastics have the potential to adsorb persistent organic pollutants, which in turn affect the environment and organisms (Lima et al., 2015).
Microplastics are widely distributed in the oceans, beaches and seafloor (Jualaong et al., 2021). Beaches containing microplastics are mainly from land-based sources, tourism, shipping, fishing and aquacultural activities. The microplastic prevalence on beaches is also affected by the various environmental factors, which include tides, currents, rainfall, winds, etc. (Gao et al., 2021; Karthik et al., 2018). Once microplastics have entered the environment, they may either suspend in the water column or sink to the bottom based on their density (Yona et al., 2019). The density of the microplastics ranges from < 0.05 g/cm3 (polystyrene foam) to 2.1–2.3 g/cm3 (polytetrafluoroethylene). The density of the microplastics influences their distribution in which lower density microplastics (with a density less than seawater) either float in the surface layer or suspend in the water column (Li et al., 2018) and are easily available to the pelagic and planktivorous fishes (Kalaiselvan et al., 2022), whereas the higher density microplastics are deposited in the marine benthic environments and easily accumulated by the detritivores and benthic feeders (Li et al., 2020).
The buoyant microplastics on the beaches deposit and finally sink into the sediment, causing the accumulation of microplastics (Wu et al., 2020). The microplastics generated from terrestrial sources reach the ocean through rivers, streams, stormwater drains and sewage systems (Rahman et al., 2020). In addition to this, rainfall in the areas near the mouth of the river is also a possible source of microplastics in coastal areas (Castro et al., 2020). The microplastic abundance shows spatial and seasonal variation in beaches and coastal waters, and their distribution was affected by various factors, which included river input (Selvam et al., 2021), currents, wind, local population and tourism in recreational beaches (Patterson et al., 2020; Sathish et al., 2019). The risk assessment caused by microplastics in the marine environment has received attention. Hence, to assess it, three methods are adopted, which include the pollution load index (PLI), polymer hazard index (PHI) and risk index (RI) (Peng et al., 2018; Ranjani et al., 2021; Wang et al., 2021; Xu et al., 2018).
Thoothukudi (08° 45′ 51.12″ N and 78° 08′ 5.29″ E) is an industrial city situated on the south eastern coast of Tamil Nadu, bordered by the Gulf of Mannar region on the east and south east. This region centres on the fishing port, fishing, tourism, recreational, cultural and religious activities. Unmonitored dumping of plastic waste, sewage effluents containing plastic waste, river runoff, and industrial and commercial waste are all sources of litter inputs on land. This puts tremendous pressure on coastal beaches and may result in an increase in plastic litter, which is then degraded or fragmented into microplastics. Hence, the present study was conducted to analyse the microplastic abundance and characteristics in both sediment and surface water. The present study aims to study the (i) spatial and seasonal distribution (post-monsoon, summer, pre-monsoon and monsoon) of microplastics in abiotic matrices (sediment and water); (ii) microplastic characteristics (shape, size and colour) and their distribution in abiotic matrices both spatially and seasonally; (iii) polymer types and ecological risk assessment of microplastics and (iv) relationship between microplastics in sediment and water in terms of abundance, shape, size, colour and polymers along the coast of Thoothukudi, south Tamil Nadu, south east India. This study provides the baseline information on microplastic contamination in abiotic matrices both spatially and seasonally along the Thoothukudi coast.
Materials and methods
Water and sediment sample collection
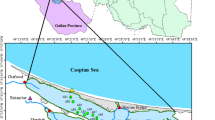
Sediment and surface water samples were collected from the six sampling sites (Fig. 1), i.e. Vembar (VE), Vellapatti (VP), Threspuram (TH), Spic Nagar (SN), Punnakayal (PN) and Thiruchendur (TC), during the post-monsoon (Jan–Mar), summer (Apr–May), pre-monsoon (Jul–Sept) and monsoon (Oct–Dec) of 2021. The six sampling sites are located in the Thoothukudi region and the characteristics of each location are given in Table 1. Surface water samples were collected at each sampling site in triplicate by towing the plankton net (mesh size: 48 μm), which was operated at the minimum speed to prevent damage. The samples settled in the cod end were washed with seawater and transferred to the pre-cleaned glass bottle, fixed in formalin at 5% and taken to the laboratory. Sediment from the sampling sites was collected in triplicate from a foreshore area by placing a quadrant of 1 m2 size. A shovel was used for collecting the sediment samples of about 3 kg at a depth of 4 cm, and they were taken in a pre-cleaned glass bottle. The collected sediment and water samples were taken to the laboratory for the extraction of microplastics.
Extraction, quantification and characterisation of microplastics
The extraction of microplastics from the sediments and surface water samples was carried out by adopting the methods that were published previously (Qiu et al., 2016; Sathish et al., 2020b). The sampled sediment was dried in a hot air oven and sieved with a 500-µm sieve to remove the larger particles. After sieving, 100 g of sediment was taken in a 500-ml beaker and it was digested with 30% hydrogen peroxide for the digestion of organic matter. Then, a saturated sodium chloride solution was added to the digested sample for the floatation of low-density polymers. The supernatant was filtered by the vacuum filtration unit with the Whatman filter paper 1 with a pore size of 11 μm. This was followed by the addition of saturated zinc chloride for the floatation of high-density polymers. Again, the supernatant was filtered with the same vacuum filtration unit as earlier.
The preserved water samples were digested with 30 ml of 30% H2O2 in 1 l of water. It was then left for 24 h for the digestion of organic matter in the water samples. After digestion, the samples were subjected to density separation with super saturated sodium iodide and left overnight. The supernatant was then filtered with the same vacuum filtration unit as mentioned above. The filter papers obtained from the filtering of sediment and water samples were dried at 40 °C in a hot air oven. The extraction procedure for each water and sediment samples was analysed in triplicates for better extraction.
The dried filter papers were observed under the stereomicroscope (40 ×) with an eyepiece to observe, measure and quantify microplastics. Under the stereomicroscope, microplastics were quantified and classified into different shapes (fragment, fibre, foam, film and irregular), sizes (0.5–1 mm, 1–2 mm, 2–3 mm, 3–4 mm and 4–5 mm) and colours (green, blue, transparent, red, black, yellow and white) (Hidalgo-Ruz et al., 2012). The polymer composition of the microplastics was investigated using attenuated total reflectance (ATR)–Fourier transform infrared spectroscopy (FTIR) in the 4000–400 cm−1 spectral range, with 32 scans at a resolution of 4 cm−1. The identification of polymers was done based on comparing the obtained ATR-FTIR spectrum with the standard reference spectra of the polymers given in earlier reports (Jung et al., 2018; Veerasingam et al., 2021a).
Ecological risk assessment of microplastics
The ecological risk assessment methods, which include polymer hazard index (PHI), potential load index (PLI) and potential ecological risk index (PERI), were used for assessing the risk of microplastics in sediments and water (Pan et al., 2021; Peng et al., 2018; Ranjani et al., 2021; Xu et al., 2018). The PHI values were calculated based on Eq. (1).
where PHI denotes the polymer hazard index, which is calculated based on the proportion of microplastic polymers in sediment and water. Pn is the polymer percentage observed at each sampling site. Sn is the hazard score of the microplastic polymers. According to Lithner et al. (2011), the hazard scores of the PE, PP, PS, PET and PA were found to be 11, 1, 30, 4 and 47, respectively.
The degree of microplastic pollution in sediments and water between the different areas is calculated from Eqs. (2)–(4).
where CFi is the microplastic concentration factor calculated from the ratio of Ci (microplastic abundance at each site) and Coi is the lowest microplastic abundance observed in the water and sediments, which in this study were taken as the background concentration of microplastics. The square root of CFi gives the PLI (pollution load index) values. The microplastic pollution load index (PLIZone) for the sediments and water of the Thoothukudi region was obtained from the 6th root of PLI, which was calculated from the 6 sampling sites multiplied together.
The PERI involves the assessment of ecological hazard from single (Ei) and combined polymer (RI) and their calculation methods are given in Eqs. (5)–(7).
where Ti is the toxicity response factor, calculated from the percent composition of polymer types observed at each sampling site (Pn) multiplied with the hazard score of each polymer (Sn) as given by Lithner et al. (2011). The sum of ecological hazard from the single polymer (Ei) gives the ecological risk (RI). The value Ei is obtained from the Ti and CFi values multiplied together.
Quality control
To reduce the background contamination of microplastics during the analysis, blank experiments were carried out with the deionised water in the pre-cleaned beaker. They were then analysed for the presence of airborne contamination during the investigation, and the results showed the absence of microplastics in the blank samples. To avoid cross contamination, lab coats and surgical gloves were worn during the analysis. The glasswares and equipment were washed with distilled water at the start and end of the analysis.
Statistical analysis
The microplastic abundance was given in an item ± SD/kg of the dry weight sediment and items ± SD/l for water. One-way analysis of variance was conducted to find the statistical difference between the microplastic abundance and their characteristics (shape, size and colour) in surface water and sediments according to the sampling sites and season. This test was also carried out to determine the differences among sampling sites in the composition of microplastic polymers in sediment and surface water. If significant differences were observed, the Tukey post hoc test was done for pairwise comparisons. The Pearson correlations were used to compare the relationship between sediment and water in terms of abundance, shape, size and polymer. All analyses were set at a significance level of < 0.05.
Result and discussion
Microplastic abundance in abiotic matrices
Spatial variation
The mean microplastic abundance in the sediment samples ranged from 32 ± 26 to 232 ± 229 items/kg, whereas in surface water it ranged between 54 ± 41 and 619 ± 377 items/l. The average abundance of microplastics in each sampling site followed a descending order: TH > PN > VP > SN > VE > TC in sediments; PN > TH > SN > TC > VP > VE in surface water. The ANOVA test revealed that the abundance of microplastics showed significant spatial variation among sampling sites in sediments (F = 6.421; p = 0.000) and in surface water (F = 12.634; p = 0.000).
The spatial variation of microplastics in sediment and surface water is given in Fig. 2a and b, respectively. The post hoc comparison results revealed that the TH registered the highest microplastic abundance in the sediment (232 ± 229 items/kg) than other sites and also showed significantly higher microplastic abundance in surface water (409 ± 275 items/l) than VE and VP. Direct discharge of sewage and industrial effluents along with plastic waste reaches the sea through the openings of Buckle canal are the probable sources that might be attributed to the high abundance of microplastics in the Threspuram region (Kumar et al., 2018). PN located in the lower stretches of Thamirabharani river may receive the plastic waste through the river discharges from the land-based activities like urbanisation, industrial and recreational activities (Karthik et al., 2018) and unmonitored dumping (Kumar et al., 2018). These are the probable sources that resulted in the significantly high concentration of microplastics in surface waters from the PN region (619 ± 377 items/l). Nithin et al. (2022) also reported similar results in which river inputs play a major role in transporting the microplastics from the terrestrial to the marine environment. TC beach receives the huge amounts of litter due to the pilgrimage and tourism activities (Edward et al., 2021) which resulted in an increase in microplastic abundance in this region (Vanapalli et al., 2021). But in the present study, the significantly lowest microplastic abundance in sediments was recorded in the TC region (32 ± 26 items/kg). The probable reasons attributed to the lowest microplastic abundance in the TC region are the land-based sources of the microplastics that enter the ocean due to the waves, tidal actions and floods during heavy rainfall (Pavithran, 2021). In water, significantly lower microplastics were observed in the VE region (54 ± 41 items/l) when compared with the sampling sites TH and PN regions. This might be due to the seasonal flow of Vembar river (Peter et al., 2014), minimal influence of human activities in this site and fewer domestic discharges into the sea (Cordova et al., 2019; Nithin et al., 2022). However, in sediment, there was no significant difference between the microplastic abundance at PN and the other sampling sites (p > 0.05) except at TH. Also in surface water, no significant difference was observed between the VE, VP, SN and TC regions (p > 0.05). From the above studies, it can be suggested that the discharge of sewage without treatment (Vidyasakar et al., 2020), plastic waste released unintentionally or escaped from wastewater treatment or the large volumes of plastic waste generated on the land that are transported to the marine environment through river runoff (Tiwari et al., 2019) and these plastic waste are dispersed by the wind, wave and currents that moving along south to south west direction (Gowthaman et al., 2013; Karthik et al., 2018) which are then fragmented or degraded into microplastics by various weathering processes such as UV radiation, fluctuating temperatures, microbial degradation and biofilm formation (Jahnke et al., 2017). This could be the possible sources which influenced significant differences in microplastic pollution among the selected sampling sites along the Thoothukudi region.
Box plot of spatial and seasonal variation of microplastics in sediment (a, c) and surface water (b, d) along the Thoothukudi coast. Different letters (a, b, c)—significant variation, same letters—insignificant variation among sites or seasons according to Tukey’s post hoc comparison. The symbols (**p < 0.01; ***p < 0.001) indicate the significant difference based on comparison among groups
The microplastic abundance in sediment and surface water from other regions worldwide is given in Table S2. The variation of microplastics in sediment and surface water between various studies (Table S2) might be due to the dissimilarity between the sampling sites, differences in the source of pollution and depth, sampling and its processing (Liu et al., 2019). The mean microplastic abundance in sediment (80 ± 72 items/kg) observed at the Thoothukudi coast was relatively lower in the present study when compared with the earlier reports. The probable reasons for the lower microplastic abundance in the sediments are that the land-based sources of the microplastics are carried to the ocean by the surface currents, winds, waves and rainfall (Pavithran, 2021). The mean microplastic abundance in the surface waters (263 ± 243 items/l) was moderate compared with the worldwide studies. Furthermore, the high abundance of microplastics in the surface waters was observed in the current study rather than in the earlier reports from the Thoothukudi region, suggesting that microplastic levels in surface waters are increasing among the selected sampling sites.
Seasonal variation
The overall minimum abundance of microplastics in sediment and surface water was observed at VE during the summer season (May, 1.33 ± 0.57 items/kg) and post-monsoon season (March, 23 ± 15 items/l), respectively. Overall, the highest microplastic abundance was recorded at Threspuram with 700 ± 81 items/kg (December) and at Punnakayal (1360 ± 256 items/l, December) during the monsoon season in sediments (Fig. S1a) and surface water (Fig. S1b), respectively. The rainfall induces the high input of plastics waste into the Thamirabharani river from the land-based activities during the monsoon season, which might be the reason for the higher abundance of microplastics in the PN region. Resuspension of the microplastics from the sediments during monsoon season and direct discharge of effluents through the Buckle canal could be the possible sources for the highest abundance in the sediments of the TH region (Nithin et al., 2022; Park et al., 2020; Ramirez et al., 2019). The lowest abundance in the VE during the summer and post-monsoon season might be due to the seasonal flow of the Vembar river (Peter et al., 2014) or due to the less influence of human activities on this site (Nithin et al., 2022).
The abundance of microplastics in water and sediment is strongly influenced by the seasons (Castro et al., 2020). In the present study, the mean microplastic concentration observed in sediment during post-monsoon (PO-M), summer (S), pre-monsoon (PR-M) and monsoon (M) registered 42 ± 59, 25 ± 15, 92 ± 80 and 160 ± 130 items/kg (Fig. 2c), respectively, whereas in surface water, the microplastics showed a mean abundance of 142 ± 102, 157 ± 126, 298 ± 286 and 454 ± 374 items/l (Fig. 2d), respectively. The seasonal variation of microplastic abundance in sediments was M > PR-M > PO-M > S (Fig. 2d); M > PR-M > S > PO-M > S in surface water (Fig. 2d). Based on the ANOVA F test, the distribution of microplastics showed a significant variation among seasons in sediment (F = 4.754, p = 0.005) and water (F = 6.032; p = 0.001). The post hoc comparison results for the seasonal distribution of microplastics revealed that the M season significantly recorded the highest abundance of microplastics in sediments and water. This was in agreement with other studies that reported the maximum microplastic concentration in the sediment during the monsoon season along the south east coast of India (Dhineka et al., 2022; Ranjani et al., 2022). James et al. (2020) highlighted that the relative concentration of microplastics in the Kochi region was highest during the monsoon season. Previous studies also revealed that the abundance of microplastics in sediments from the coasts of Mexico (Ramirez et al., 2019) and Portugal (Prata et al., 2020) was higher during the rainy season.
Resuspension of microplastics from sediment by the action of winds, currents and rainfall that occurred during the monsoon season might be the reason for the highest abundance of microplastics in sediment along the Thoothukudi coast (Dhineka et al., 2022; James et al., 2021). Veerasingam et al. (2016) reported that west or south westerly winds combined with the currents play a major role in the transportation and deposition of microplastics on beaches along the Goa coast. Subsequently, these factors might also lead to microplastic suspension in the water column, which then results in an increase in the microplastic concentration in the surface waters (James et al., 2021). Also, the plastic debris that was accumulated on the beaches might be transported to the coastal water due to the waves, currents, tides, coastal erosion and heavy rainfall. These are possible reasons which might contribute to the highest microplastic concentration in surface waters during the monsoon (Jualaong et al., 2021; Park et al., 2020) season. Liu et al. (2020) recorded the increase in microplastics in the seawater of Jiaozhou Bay due to the action of rainfall, wind and runoff.
The above studies suggest that microplastic contamination in sediment and surface waters along the Thoothukudi region was highly influenced by the seasonal and spatial variation. The high abundance of microplastics was reported in surface waters during the monsoon season, which may result in the high accumulation of microplastics by marine organisms. Sathish et al. (2020b) reported that microplastic abundance in fish was significantly affected by the microplastic pollution in the surrounding water, disregarding the fish feeding habitat, length and weight. James et al. (2020) found out the increase in the microplastic abundance in the commercially important fishes of Kochi during the monsoon season, which shows that microplastic abundance in fishes greatly depends on the contamination of microplastics from the surrounding environment. From this present investigation, it shows that microplastic accumulation in abiotic matrices is one of the major factors influencing microplastic ingestion in marine biota. Further, the usage of brine water from this polluted marine or estuarine water may contribute to the microplastic contamination in table salts, which raises a serious threat to public health because table salt is one of the common food commodities that humans consume (Selvam et al., 2020). For this reason, Nithin et al. (2021) recommended the utilisation of groundwater instead of marine or estuarine water as the source for the production of table salts. Hence, proper management measures to reduce the disposal of plastic waste in the marine environment should be implemented to lessen the effects of microplastics on marine biota and on public health.
Characteristics of microplastics in abiotic matrices (shape, size, colour and polymer type)
The observed shapes in the abiotic matrices along the Thoothukudi coast were fragment, fibre, foam, irregular and film in which the fragments were dominant in both the sediment and water, accounting for about 52.72% and 40.89%, respectively. This was followed by fibre, irregular, foam and film shaped microplastics, which contributed 22.48%, 11.88%, 8.88% and 4.04% in sediment (Fig. 3a) and 22.89%, 20.1%, 9.01% and 4.08% in surface water (Fig. 3a). The microplastic shape that was predominant in all the seasons was fragment, which varied between 43.99 and 62.44% in sediment (Fig. 3b, p > 0.05) and 38.96 and 41.80% in surface water (Fig. 3c, p > 0.05). Spatially, their contribution was higher at PN (70.46%) in sediment (Fig. 3b) and at TH in water with 63.17% (Fig. 3c) (p > 0.05). The result of this study shows that fragments are the most abundant microplastics in sediment and surface water, which is in line with the studies that were reported earlier. For example, the studies conducted in the beach sediment of Cox’s Bazar, Bangladesh (Rahman et al., 2020) and in the coastal ecosystems of the Gulf of Mannar and Palk Bay, south east India (James et al., 2021) found the higher dominance of fragment microplastics in the abiotic matrices. In contrast to the present study, fibres were the predominant type of microplastics in the sediment and surface waters of the Tuticorin region (Jeyasanta et al., 2020; Patterson et al., 2020; Sathish et al., 2020b). The sampling site and season of the sample collection, degradation of the plastic litter and proximity to industrial and tourism activities (Maharana et al., 2020; Xu et al., 2018) could be the possible reasons for the diverse distribution of plastic shapes between different locations. The observed microplastic shapes during the present study indicate that they are of secondary origin (Rahman et al., 2020). The higher dominance of fragments in the sediment and surface water is most likely due to the fragmentation or weathering of larger plastic pieces, including plastic bags, discarded plastic bottles and packaging materials (Jiang et al., 2022; Prata et al., 2020), which are then transported long distances through municipal and sewage effluents (Robin et al., 2020).
The sizes of the microplastics that were observed in sediment and surface water are classified into 5 classes which include 0.5–1 mm, 1–2 mm, 2–3 mm, 3–4 mm and 4–5 mm. Among all the classes, 0.5–1 mm sized microplastics were highly prevalent in sediment (43.96%) followed by 1–2 mm (21.71%), 2–3 mm (16.66%), 3–4 mm (10.81%) and 4–5 mm (6.11%) (Fig. 4a). In surface water, 0.5–1 mm size dominated with 31.11%. The other sizes observed in surface water are shown in descending order as 2–3 mm, 1–2 mm, 3–4 mm and 4–5 mm, which accounted for about 21.74%, 20.93%, 17.11% and 9.12%, respectively (Fig. 4a). Microplastics with a size of 0.5–1 mm highly contributed to about 48.09% in PR-M and 35.95% in M in sediments (Fig. 4b, p > 0.05) and surface water (Fig. 4c, p > 0.05), respectively. These sized microplastics were mostly detected at all the sampling sites in both sediment (except in VE and TC) and water (except in VE, SN and TC) which are given in Fig. 4b and c (p > 0.05), respectively. The present results were in agreement with the earlier studies that have reported the dominance of 0.5–1 mm from the Tuticorin coast (Patterson et al., 2019; Sathish et al., 2020a), Surabaya, Indonesia (Cordova et al., 2019) and Chabahar Bay in the Oman Sea (Hosseini et al., 2020). Fragmentation of larger particles into smaller ones could be the probable reason for the dominance of 0.5–1 mm sized microplastics in sediment and surface water. These smaller sized particles are similar to the size of the zooplankton and there is the greatest possible ingestion of these particles by the aquatic organisms (Park et al., 2020) and may be transported into the soft tissue of the organisms that pose the greatest risk to the aquatic organisms (Triebskorn et al., 2019). Robin et al. (2020) and Rasta et al. (2021) reported that microplastics of the size range of 0.3–5 mm has a larger surface area and may result in the adsorption of pollutants from the environment. Ingestion of these particles may increase bioaccumulation and can cause a larger impact on aquatic organisms.
The collected microplastics were categorised by colour and their categories include transparent, white, blue, green, yellow, red and black. Blue-coloured microplastics registered the highest dominance in sediment and surface water with proportions of 52.33% and 41.85%, respectively. The other colours observed are shown in descending order: green > white > red > transparent > yellow > black in sediments (Fig. 5a); white > green > transparent > yellow > red > black in surface water (Fig. 5a). Among all the seasons, the higher dominance of blue-coloured microplastics was recorded during the monsoon in sediment and surface water, which contributed about 62.41% (Fig. 5b) and 50.37% (Fig. 5c), respectively (p > 0.05). They were also largely registered in sediment (Fig. 5b, except in VE, SN and TC, p > 0.05) and water (Fig. 5c, except in TC, p > 0.05) in almost all the sites.
The occurrence of various colours indicates that their origin is from various sources and their presence in the marine environment may influence their uptake by the marine organisms (Sathish et al., 2019) due to their resemblance with the plankton (Patchaiyappan et al., 2020). The high prevalence of blue-coloured microplastics in the present study was in line with the previous studies that were conducted along the Tuticorin coast (Sathish et al., 2019; Sathish 2020a), Punnakayal estuary (Selvam et al., 2021), Kerala (Pavithran, 2021), south western Caspian Sea (Rasta et al., 2021) and the west coast of Ireland (Pagter et al., 2020). Blue-coloured microplastics are mainly from fishing activities (Martin et al., 2017) and their dominance in sediment and surface water might influence their uptake by shrimps (Daniel et al., 2020) and fishes since it resembles with the plankton (Ory et al., 2018). The colour of the microplastics is from the synthetic colourants that may leach into the marine environment and pose a risk to aquatic organisms (Wicaksono et al., 2021).
ATR-FTIR analysis confirmed the presence of PE, PP, PS, PA and PET and their composition abiotic matrices are given in Fig. 6a. PE dominated in sediments (p < 0.05) followed by PP, PS and PET with proportions of 47.58%, 27.07%, 20.37% and 4.95%, respectively. Also, PE significantly dominated in surface water (p < 0.05) with 49.83% followed by PP (37.33%), PS (10%) and PA (2.83%). This is in line with the studies in which PE is found to be the most dominant polymer in the abiotic matrices (Patterson et al., 2020; Sathish et al., 2019; Veerasingam et al., 2016). Their dominance was observed in all the sampling sites, which varied between 38.50 and 69.35% in sediment (Fig. 6b) and 34.65 and 68% in surface water (Fig. 6c).
The PE and PP polymers are derived from plastic bags, single-use plastics, cosmetic products, packaging materials, fishing ropes and nets. PS may be derived from floats and fishing and aquaculture activities, as well as cups and disposable cutlery, whereas PA may be derived from fibre-based plastics and synthetic fibres derived from the fishing industry. The potential sources of PET are mainly derived from packaging, bottles and containers, etc. (Schwarz et al., 2019; Wu et al., 2020). The above-mentioned polymers may reach the environment through dumping, discharge of sewage effluents, mishandling or unintentional release of plastics (Gao et al., 2021) which results in the distribution of these polymers in the sediment and water. In the present investigation, PE is the low-density and dominant polymer, and its density ranged between 0.91 and 0.97 g/cm3. Due to the high consumption rate and higher global production, these polymers reach the marine environment through run off and inflow of sewage into the sea (Tiwari et al., 2019). These polymers have the potential to float in the surface waters due to their low density, travel long distances by the action of wind and current, and result in the wide distribution of polymers in the water bodies (Ranjani et al., 2021). In addition to this, their densities may increase due to weathering and biofouling and result in an increase in density (may be > 1.2 g/cm3) being deposited in the sediment from the water column, which results in the high predominance of PE polymers in sediments (Rahman et al., 2020). This action may influence the uptake of low-density PE polymers by both pelagic and benthic organisms (Kalaiselvan et al., 2022) which can cause intestinal damage, changes in the intestinal microbiota in these fishes (Varo et al., 2021).
The statistical analysis revealed that microplastic characteristics (shape, size and colour) exhibited no significant difference in sediment and surface water samples, both spatially and seasonally (p > 0.05). The polymers of the microplastics also showed no significant difference among the selected sites. This signifies that microplastic shape, size, colour and polymers were homogenously distributed in abiotic matrices along the Thoothukudi coast (Prata et al., 2020). The present result is in congruence with the earlier reports in which a unique composition of microplastic characteristics (size, shape and colour) was observed due to their no significant differences among sampling sites on the Silver Beach (Vidyasakar et al., 2020). Further, this result suggests that microplastic characteristics (shape, size, colour and polymers) did not vary with sampling sites and seasons, which shows that these particles persist in the sediment and water for a longer period of time and this may cause a serious threat to the marine environment (Wang et al., 2021).
Ecological risk assessment of microplastics
Risk assessment of microplastics (PHI, PLI and RI)
The ecological risk caused by microplastic pollution in sediments and water was evaluated by adopting the ecological risk assessment models, including PHI, PLI and RI. Based on these values, the risk caused by microplastics was categorised and presented in Table S1.
The PHI values for each site in sediment and surface water were classified under the hazard level IV and their values at each sampling site are given in Fig. 7a. The overall PHIsediments and PHIwater were calculated from the average polymer composition of microplastics in the six sampling sites and their respective hazard scores yielded values of 1181.63 and 1018.66, respectively, which exhibited the hazard level V. This shows that microplastic polymers such as PE, PP, PS, PET and PA were severely contaminated in sediment and surface waters. From Fig. 7a, it can be seen that PHI values are found to oscillate for the six sampling sites, which ranged between 130.42 and 258.44 in sediment, and in surface water, it ranged between 107 and 259. The varying PHI values were mainly due to the differences in the microplastic polymer composition of sediment and surface water. For example, the higher PHI in sediment and surface water was observed in the TH (258.44) and TC (259) regions, respectively, which was more likely due to the high composition of PE (sediment—38.50%; water—42.27%) and PS (sediment—23.60%; water—24.39%). These polymers are discharged through the inflow of sewage into the sea, accidental loss, coastal and fishing activities, which contribute to microplastic pollution in the marine environment (Sathish et al., 2019). An insignificant correlation was observed between microplastic abundance and PHI values, but microplastic pollution in the sampling sites can cause severe potential risk (Pan et al., 2021).
From Fig. 7b, the PLI values derived from the microplastic concentrations in the sediments and surface water at each sampling site were all at the hazard level I. Overall PLIzone values for the sediment (3.57) and surface water (3.84) along the Thoothukudi coast yielded a hazard level of I. This indicates that the degree of the microplastic contamination in sediment and surface water was lower. Significantly, a very strong positive correlation was observed between the microplastic abundance and the PLI, which suggests that changes in the microplastic abundance along the Thoothukudi coast will affect the PLI values. The microplastic abundance along the Thoothukudi coast is attributed to the release of sewage and domestic effluents without treatment, fishing activities, boat building, waste from the fishing processing industry, tourism, pilgrimage and recreational activities (Jeyasanta et al., 2020). The RI value calculated in sediments and water (Fig. 7c and Table S3) for each sampling site falls under the minor risk category. The overall RIzone along the Thoothukudi coast exhibited values of 253.48 and 444.74 in sediments and surface water, respectively, which falls under the higher risk category.
The correlation analysis revealed a significant relationship between microplastic abundance in surface water and sediments (p < 0.05). Even though a close relationship exists between the concentration of microplastics in water and sediments, there is no significant relationship between the risk levels in sediments and water (p > 0.05). The probable reason for the insignificant difference is most likely due to the Ei (ecological hazard from the single polymer), which is influenced by land- and sea-based sources along the Thoothukudi region (Prarat & Hongsawat 2022). The present study provides preliminary results on the level of microplastic pollution from the values of the polymer hazard index (PHI), pollution load index (PLI) and risk index (RI) of the sediment and water along the Thoothukudi region.
Correlation analysis
The statistical results revealed that there was a significant, strong positive relationship between the microplastic abundance in sediment and surface water (r = 0.574; p = 0.000). Also, a significantly very strong positive relationship was observed between the microplastic types in sediments and surface water based on their shapes (r = 0.740; p = 0.000) and size (r = 0.744; p = 0.000). A significantly positive relationship was detected between microplastic colour and polymers in sediments and surface water (r = 0.654; p = 0.000). The results showed that resuspension of microplastics from sediment, caused by nearshore circulation, tides and sedimentation rate, causes the microplastics to return to the water column. This action might result in the high microplastic abundance in the water compared to the sediment during the present study (Takarina et al., 2022).
Conclusion
This study reports the comprehensive results of the spatial and seasonal distribution of microplastics, their characteristics, chemical composition and the risk assessment of microplastics in abiotic matrices (surface water and sediment). The microplastic abundance in surface water and sediment showed a significant difference among sampling sites and seasons. Spatially, Threspuram and Punnakayal were most polluted with microplastics in sediment (232 ± 229 items/kg) and surface water (619 ± 377 items/l), respectively. The probable reasons for the high concentration of microplastics in the Threspuram region are the direct release of effluents along with the plastic waste through the Buckle canal, whereas in Punnakayal, the source of microplastics is attributed to unmonitored dumping and river discharges from land-based activities like urbanisation, industrialisation and recreational activities. The microplastic abundance in the sediment and surface water is greatly influenced by the season with the highest being reported in the monsoon season, which accounted for about 160 ± 130 items/kg and 454 ± 374 items/l, respectively. Fragment, 0.5–1 mm and blue-coloured microplastics were dominant in sediments and surface water. Polyethylene, the low-density and dominant polymer in sediment and surface water, reaches the marine environment through run off and the inflow of sewage into the sea. Due to their low density, they float and are widely distributed in the surface waters. Their densities may increase due to weathering and biofouling, and they deposit in the sediment from the water column result in a high dominance of polyethylene polymer in the sediment. The characteristics of the microplastics (shape, size and colour) exhibited no significant difference both spatially and seasonally. The polymers of microplastics also showed no significant difference among the sampling sites. This signifies that they are homogenously distributed along the coast and further suggests that these particles persisted in the sediment and surface water for a longer period of time. The preliminary risk assessment results revealed that the risk index (RI) of the Thoothukudi region ended up in the higher risk category in sediment (253.48) and surface waters (444.74). The overall polymer hazard index show that the sediment (PHI = 1181.63) and surface water (PHI = 1018.66) were severely contaminated (hazard level V) with microplastic polymers such as PE, PP, PS, PET and PA. It was also found that the degree of the microplastic contamination in sediment (PLI = 3.57) and surface water (PLI = 3.84) was lower (hazard level I). A positive correlation was observed between the microplastics in sediment and water based on the abundance, shape, size, colour and polymers of the microplastics. This indicates that microplastics rejoin the water column from the sediment because of resuspension, which occurs due to the tide, circulation and sedimentation rate. This might explain the higher concentration of microplastics in the surface water. The higher abundance of microplastics in the surface waters may raise serious concerns for the marine organisms. Hence, proper management measures to reduce plastic waste disposal in the marine environment should be implemented to lessen the effects of microplastics on marine biota and on public health.
Availability of data and materials
All data generated or analysed during this study are included in this published articles (and its supplementary information files).
References
Castro, R. O., da Silva, M. L., Marques, M. R., & de Araújo, F. V. (2020). Spatio-temporal evaluation of macro, meso and microplastics in surface waters, bottom and beach sediments of two embayments in Niterói, RJ Brazil. Marine Pollution Bulletin, 160, 111537.
Chen, M. C., & Chen, T. H. (2020). Spatial and seasonal distribution of microplastics on sandy beaches along the coast of the Hengchun Peninsula Taiwan. Marine Pollution Bulletin, 151, 110861.
Cordova, M. R., Purwiyanto, A. I. S., & Suteja, Y. (2019). Abundance and characteristics of microplastics in the northern coastal waters of Surabaya, Indonesia. Marine Pollution Bulletin, 142, 183–188.
Daniel, D. B., Ashraf, P. M., & Thomas, S. N. (2020). Abundance, characteristics and seasonal variation of microplastics in Indian white shrimps (Fenneropenaeus indicus) from coastal waters off Cochin, Kerala India. Science of the Total Environment, 737, 139839.
Dhineka, K., Sambandam, M., Sivadas, S. K., Kaviarasan, T., Pradhan, U., Begum, M., & Murthy, M. V. (2022). Characterization and seasonal distribution of microplastics in the nearshore sediments of the south-east coast of India, Bay of Bengal. Frontiers of Environmental Science & Engineering, 16(1), 1–11.
Edward, J. P., Jayanthi, M., Malleshappa, H., Jeyasanta, K. I., Laju, R. L., Patterson, J., & Grimsditch, G. (2021). COVID-19 lockdown improved the health of coastal environment and enhanced the population of reef-fish. Marine Pollution Bulletin, 165, 112124.
Europe, P. (2021). Plastics—The facts 2021. An analysis of European latest plastics production, demand and waste data.
Gao, F., Li, J., Hu, J., Sui, B., Wang, C., Sun, C., & Ju, P. (2021). The seasonal distribution characteristics of microplastics on bathing beaches along the coast of Qingdao China. Science of the Total Environment, 783, 146969.
Gowthaman, R., Kumar, V. S., Dwarakish, G. S., Mohan, S. S., Singh, J., & Kumar, K. A. (2013). Waves in Gulf of Mannar and Palk Bay around Dhanushkodi, Tamil Nadu, India. Current Science, 1431–1435.
Hidalgo-Ruz, V., Gutow, L., Thompson, R. C., & Thiel, M. (2012). Microplastics in the marine environment: A review of the methods used for identification and quantification. Environmental Science & Technology, 46(6), 3060–3075.
Hosseini, R., Sayadi, M. H., Aazami, J., & Savabieasfehani, M. (2020). Accumulation and distribution of microplastics in the sediment and coastal water samples of Chabahar Bay in the Oman Sea Iran. Marine Pollution Bulletin, 160, 111682.
Jahnke, A., Arp, H. P. H., Escher, B. I., Gewert, B., Gorokhova, E., Kühnel, D., Ogonowski, M., Potthoff, A., Rummel, C., Scmitt-Jansen, M., Toorman, E., & MacLeod, M. (2017). Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment. Environmental Science and Technology Letters, 4(3), 85–90.
James, K., Vasant, K., Padua, S., Gopinath, V., Abilash, K. S., Jeyabaskaran, R., Babu, A., & John, S. (2020). An assessment of microplastics in the ecosystem and selected commercially important fishes off Kochi, south eastern Arabian Sea India. Marine Pollution Bulletin, 154, 111027.
James, K., Vasant, K., SM, S. B., Padua, S., Jeyabaskaran, R., Thirumalaiselvan, S., & Benjamin, L. V. (2021). Seasonal variability in the distribution of microplastics in the coastal ecosystems and in some commercially important fishes of the Gulf of Mannar and Palk Bay, Southeast coast of India. Regional Studies in Marine Science, 41, 101558.
Jeyasanta, K. I., Sathish, N., Patterson, J., & Edward, J. P. (2020). Macro-, meso-and microplastic debris in the beaches of Tuticorin district, southeast coast of India. Marine Pollution Bulletin, 154, 111055.
Jiang, F., Wang, M., Ding, J., Cao, W., & Sun, C. (2022). Occurrence and seasonal variation of microplastics in the effluent from wastewater treatment plants in Qingdao, China. Journal of Marine Science and Engineering, 10(1), 58.
Jualaong, S., Pransilpa, M., Pradit, S., & Towatana, P. (2021). Type and distribution of microplastics in beach sediment along the coast of the Eastern Gulf of Thailand. Journal of Marine Science and Engineering, 9(12), 1405.
Jung, M. R., Horgen, F. D., Orski, S. V., Rodriguez, V., Beers, K. L., Balazs, G. H., & Lynch, J. M. (2018). Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Marine Pollution Bulletin, 127, 704–716.
Kalaiselvan, K., Pandurangan, P., Velu, R., & Robinson, J. (2022). Occurrence of microplastics in gastrointestinal tracts of planktivorous fish from the Thoothukudi region. Environmental Science and Pollution Research, 1–9.
Karthik, R., Robin, R. S., Purvaja, R., Ganguly, D., Anandavelu, I., Raghuraman, R., & Ramesh, R. (2018). Microplastics along the beaches of southeast coast of India. Science of the Total Environment, 645, 1388–1399.
Kumar, V. E., Ravikumar, G., & Jeyasanta, K. I. (2018). Occurrence of microplastics in fishes from two landing sites in Tuticorin, south east coast of India. Marine Pollution Bulletin, 135, 889–894.
Li, L., Li, M., Deng, H., Cai, L., Cai, H., Yan, B., Hu, J., & Shi, H. (2018). A straightforward method for measuring the range of apparent density of microplastics. Science of the Total Environment, 639, 367–373.
Li, Y., Zhang, H., & Tang, C. (2020). A review of possible pathways of marine microplastics transport in the ocean. Anthropocene Coasts, 3(1), 6–13.
Lima, A. R. A., Barletta, M., & Costa, M. F. (2015). Seasonal distribution and interactions between plankton and microplastics in a tropical estuary. Estuarine, Coastal and Shelf Science, 165, 213–225.
Lithner, D., Larsson, Å., & Dave, G. (2011). Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Science of the Total Environment, 409(18), 3309–3324.
Liu, S., Jian, M., Zhou, L., & Li, W. (2019). Distribution and characteristics of microplastics in the sediments of Poyang Lake China. Water Science and Technology, 79(10), 1868–1877.
Liu, T., Zhao, Y., Zhu, M., Liang, J., Zheng, S., & Sun, X. (2020). Seasonal variation of micro-and meso-plastics in the seawater of Jiaozhou Bay, the Yellow Sea. Marine Pollution Bulletin, 152, 110922.
Maharana, D., Saha, M., Dar, J. Y., Rathore, C., Sreepada, R. A., Xu, X. R., Koongolla, J. B., & Li, H. X. (2020). Assessment of micro and macroplastics along the west coast of India: Abundance, distribution, polymer type and toxicity. Chemosphere, 246, 125708.
Martin, J., Lusher, A., Thompson, R. C., & Morley, A. (2017). The deposition and accumulation of microplastics in marine sediments and bottom water from the Irish continental shelf. Scientific Reports, 7(1), 1–9.
Nithin, A., Sundaramanickam, A., & Sathish, M. (2022). Seasonal distribution of microplastics in the surface water and sediments of the Vellar estuary, Parangipettai, southeast coast of India. Marine Pollution Bulletin, 174, 113248.
Nithin, A., Sundaramanickam, A., Surya, P., Sathish, M., Soundharapandiyan, B., & Balachandar, K. (2021). Microplastic contamination in salt pans and commercial salts–A baseline study on the salt pans of Marakkanam and Parangipettai, Tamil Nadu India. Marine Pollution Bulletin, 165, 112101.
Ory, N., Chagnon, C., Felix, F., Fernández, C., Ferreira, J. L., Gallardo, C., & Thiel, M. (2018). Low prevalence of microplastic contamination in planktivorous fish species from the southeast Pacific Ocean. Marine Pollution Bulletin, 127, 211–216.
Pagter, E., Frias, J., Kavanagh, F., & Nash, R. (2020). Differences in microplastic abundances within demersal communities highlight the importance of an ecosystem-based approach to microplastic monitoring. Marine Pollution Bulletin, 160, 11644.
Pan, Z., Liu, Q., Jiang, R., Li, W., Sun, X., Lin, H., & Huang, H. (2021). Microplastic pollution and ecological risk assessment in an estuarine environment: The Dongshan Bay of China. Chemosphere, 262, 127876.
Park, T. J., Lee, S. H., Lee, M. S., Lee, J. K., Park, J. H., & Zoh, K. D. (2020). Distributions of microplastics in surface water, fish, and sediment in the vicinity of a sewage treatment plant. Water, 12(12), 3333.
Patchaiyappan, A., Ahmed, S. Z., Dowarah, K., Jayakumar, S., & Devipriya, S. P. (2020). Occurrence, distribution and composition of microplastics in the sediments of South Andaman beaches. Marine Pollution Bulletin, 156, 111227.
Patterson, J., Jeyasanta, K. I., Sathish, N., Edward, J. P., & Booth, A. M. (2020). Microplastic and heavy metal distributions in an Indian coral reef ecosystem. Science of the Total Environment, 744, 140706.
Patterson, J., Jeyasanta, K. I., Sathish, N., Booth, A. M., & Edward, J. P. (2019). Profiling microplastics in the Indian edible oyster, Magallana bilineata collected from the Tuticorin coast, Gulf of Mannar, Southeastern India. Science of the Total Environment, 691, 727–735.
Pavithran, V. A. (2021). Study on microplastic pollution in the coastal seawaters of selected regions along the northern coast of Kerala, southwest coast of India. Journal of Sea Research, 173, 102060.
Peng, G., Xu, P., Zhu, B., Bai, M., & Li, D. (2018). Microplastics in freshwater river sediments in Shanghai, China: A case study of risk assessment in mega-cities. Environmental Pollution, 234, 448–456.
Peter, T. S., Chandrasekar, N., Selvakumar, S., Kaliraj, S., Magesh, N. S., & Srinivas, Y. (2014). Tidal effects on estuarine water quality through a sandymarine beach: A case study in Vembar estuary, southeast coast of Tamil Nadu India. Journal of Coastal Science, 1(1), 6–14.
Prarat, P., & Hongsawat, P. (2022). Microplastic pollution in surface seawater and beach sand from the shore of Rayong province, Thailand: Distribution, characterization, and ecological risk assessment. Marine Pollution Bulletin, 174, 113200.
Prata, J. C., Reis, V., Paço, A., Martins, P., Cruz, A., da Costa, J. P., & Rocha-Santos, T. (2020). Effects of spatial and seasonal factors on the characteristics and carbonyl index of (micro) plastics in a sandy beach in Aveiro Portugal. Science of the Total Environment, 709, 135892.
Qiu, Q., Tan, Z., Wang, J., Peng, J., Li, M., & Zhan, Z. (2016). Extraction, enumeration and identification methods for monitoring microplastics in the environment. Estuarine, Coastal and Shelf Science, 176, 102–109.
Radhakrishnan, K., Sivapriya, V., Rajkumar, A., Akramkhan, N., Prakasheswar, P., Krishnakumar, S., & Hussain, S. M. (2021). Characterization and distribution of microplastics in estuarine surface sediments, Kayamkulam estuary, southwest coast of India. Marine Pollution Bulletin, 168, 112389.
Rahman, S. M. A., Robin, G. S., Momotaj, M., Uddin, J., & Siddique, M. A. M. (2020). Occurrence and spatial distribution of microplastics in beach sediments of Cox’s Bazar Bangladesh. Marine Pollution Bulletin, 160, 111587.
Ramirez, M. M. B., Caamal, R. D., & von Osten, J. R. (2019). Occurrence and seasonal distribution of microplastics and phthalates in sediments from the urban channel of the Ria and coast of Campeche, Mexico. Science of the Total Environment, 672, 97–105.
Ranjani, M., Veerasingam, S., Venkatachalapathy, R., Mugilarasan, M., Bagaev, A., Mukhanov, V., & Vethamony, P. J. M. P. B. (2021). Assessment of potential ecological risk of microplastics in the coastal sediments of India: A meta-analysis. Marine Pollution Bulletin, 163, 111969.
Ranjani, M., Veerasingam, S., Venkatachalapathy, R., Jinoj, T. P. S., Guganathan, L., Mugilarasan, M., & Vethamony, P. (2022). Seasonal variation, polymer hazard risk and controlling factors of microplastics in beach sediments along the southeast coast of India. Environmental Pollution, 305, 119315.
Rasta, M., Rahimibashar, M. R., Torabi Jafroudi, H., Fakheri, S., Tagheipour Kouhbane, S., & Taridashti, F. (2021). Microplastics in sediments of southwest Caspian Sea: Characteristics, distribution and seasonal variability. Soil and Sediment Contamination: An International Journal, 1–15.
Robin, R. S., Karthik, R., Purvaja, R., Ganguly, D., Anandavelu, I., Mugilarasan, M., & Ramesh, R. (2020). Holistic assessment of microplastics in various coastal environmental matrices, southwest coast of India. Science of the Total Environment, 703, 134947.
Sánchez-Hernández, L. J., Ramírez-Romero, P., Rodríguez-González, F., Ramos-Sánchez, V. H., Montes, R. A. M., Rubio, H. R. P., & Jonathan, M. P. (2021). Seasonal evidences of microplastics in environmental matrices of a tourist dominated urban estuary in Gulf of Mexico Mexico. Chemosphere, 277, 130261.
Sathish, M. N., Jeyasanta, I., & Patterson, J. (2020a). Occurrence of microplastics in epipelagic and mesopelagic fishes from Tuticorin, southeast coast of India. Science of the Total Environment, 720, 137614.
Sathish, M. N., Jeyasanta, K. I., & Patterson, J. (2020b). Monitoring of microplastics in the clam Donax cuneatus and its habitat in Tuticorin coast of Gulf of Mannar (GoM) India. Environmental Pollution, 266, 115219.
Sathish, N., Jeyasanta, K. I., & Patterson, J. (2019). Abundance, characteristics and surface degradation features of microplastics in beach sediments of five coastal areas in Tamil Nadu, India. Marine Pollution Bulletin, 142, 112–118.
Schwarz, A. E., Ligthart, T. N., Boukris, E., & Van Harmelen, T. (2019). Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Marine Pollution Bulletin, 143, 92–100.
Selvam, S., Jesuraja, K., Venkatramanan, S., Roy, P. D., & Kumari, V. J. (2021). Hazardous microplastic characteristics and its role as a vector of heavy metal in groundwater and surface water of coastal south India. Journal of Hazardous Materials, 402, 123786.
Selvam, S., Manisha, A., Venkatramanan, S., Chung, S. Y., & Paramasivam, C. R. (2020). Microplastic presence in commercial marine sea salts: A baseline study along Tuticorin coastal salt pan stations, Gulf of Mannar South India. Marine Pollution Bulletin, 150, 110675.
Takarina, N. D., Purwiyanto, A. I. S., Rasud, A. A., Arifin, A. A., & Suteja, Y. (2022). Microplastic abundance and distribution in surface water and sediment collected from the coastal area. Global Journal of Environmental Science and Management, 8(2), 183–196.
Tiwari, M., Rathod, T. D., Ajmal, P. Y., Bhangare, R. C., & Sahu, S. K. (2019). Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Marine Pollution Bulletin, 140, 262–273.
Triebskorn, R., Braunbeck, T., Grummt, T., Hanslik, L., Huppertsberg, S., Jekel, M., & Köhler, H. R. (2019). Relevance of nano-and microplastics for freshwater ecosystems: A critical review. TrAC Trends in Analytical Chemistry, 110, 375–392.
Vanapalli, K. R., Sharma, H. B., Ranjan, V. P., Samal, B., Bhattacharya, J., Dubey, B. K., & Goel, S. (2021). Challenges and strategies for effective plastic waste management during and post COVID-19 pandemic. Science of the Total Environment, 750, 141514.
Varó, I., Osorio, K., Estensoro, I., Naya-Catala, F., Sitja-Bobadilla, A., Navarro, J. C., & Piazzon, M. C. (2021). Effect of virgin low density polyethylene microplastic ingestion on intestinal histopathology and microbiota of gilthead sea bream. Aquaculture, 545, 737245.
Veerasingam, S., Ranjani, M., Venkatachalapathy, R., Bagaev, A., Mukhanov, V., Litvinyuk, D., & Vethamony, P. (2021a). Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review. Critical Reviews in Environmental Science and Technology, 51(22), 2681–2743.
Veerasingam, S., Saha, M., Suneel, V., Vethamony, P., Rodrigues, A. C., Bhattacharyya, S., & Naik, B. G. (2016). Characteristics, seasonal distribution and surface degradation features of microplastic pellets along the Goa coast, India. Chemosphere, 159, 496–505.
Veerasingam, S., Vethamony, P., Aboobacker, V. M., Giraldes, A. E., Dib, S., & Al-Khayat, J. A. (2021b). Factors influencing the vertical distribution of microplastics in the beach sediments around the Ras Rakan Island Qatar. Environmental Science and Pollution Research, 28(26), 34259–34268.
Vidyasakar, A., Krishnakumar, S., Kasilingam, K., Neelavannan, K., Bharathi, V. A., Godson, P. S., & Magesh, N. S. (2020). Characterization and distribution of microplastics and plastic debris along Silver Beach Southern India. Marine Pollution Bulletin, 158, 111421.
Wang, G., Lu, J., Li, W., Ning, J., Zhou, L., Tong, Y., & Xiayihazi, N. (2021). Seasonal variation and risk assessment of microplastics in surface water of the Manas River Basin China. Ecotoxicology and Environmental Safety, 208, 111477.
Wicaksono, E. A., Werorilangi, S., Galloway, T. S., & Tahir, A. (2021). Distribution and seasonal variation of microplastics in Tallo River, Makassar Eastern Indonesia. Toxics, 9(6), 129.
Wu, P., Tang, Y., Dang, M., Wang, S., Jin, H., Liu, Y., & Cai, Z. (2020). Spatial-temporal distribution of microplastics in surface water and sediments of Maozhou River within Guangdong-Hong Kong-Macao Greater Bay Area. Science of the Total Environment, 717, 135187.
Xu, P., Peng, G., Su, L., Gao, Y., Gao, L., & Li, D. (2018). Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary, China. Marine Pollution Bulletin, 133, 647–654.
Yona, D., Sari, S. H. J., Iranawati, F., Bachri, S., & Ayuningtyas, W. C. (2019). Microplastics in the surface sediments from the eastern waters of Java Sea, Indonesia. F1000Research, 8.
Acknowledgements
The authors extend their thanks to the Tamil Nadu Dr. J. Jayalalithaa Fisheries University for providing the support and facilities needed to carry out this research.
Author information
Authors and Affiliations
Contributions
Kalaiselvan Keerthika: acquisition, data processing and interpretation, manuscript drafting and editing; Pandurangan Padmavathy: design of the study, revision of the article; Velu Rani: revision of the article and editing; Robinson Jeyashakila: revision of the article; Samraj Aanand: revision of the article; Ranjeet Kutty: revision of the article.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keerthika, K., Padmavathy, P., Rani, V. et al. Spatial, seasonal and ecological risk assessment of microplastics in sediment and surface water along the Thoothukudi, south Tamil Nadu, south east India. Environ Monit Assess 194, 820 (2022). https://doi.org/10.1007/s10661-022-10468-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10468-z