Abstract
Organochlorine pesticides (OCPs) are widely used in certain countries. We determined atmospheric concentrations, distribution patterns, and seasonal variations of OCPs at four sites in South Korea for 1 year. Samples of 22 OCPs were collected using a high-volume air sampler, and measured via the isotope dilution method with HRGC/HRMS. In South Korea, pentachlorobenzene (PeCB), hexachlorocyclohexane (HCB), and endosulfan (EnSF) were dominant, accounting for > 87% of total OCPs. Spatial distributions showed significant differences and the highest levels were observed in Seosan (295.2 pg·m−3), indicating the compounding potential of diverse sources as Seosan has concentrated large-scale industrial complexes and agricultural activity (Seoul: 243.6 pg·m−3 > Jeju: 193.5 pg·m−3 > Baengnyeong: 178.2 pg·m−3). The isomeric ratios of OCPs in the South Korean atmosphere indicated that the dominant sources of HCB and dichlorodiphenyltrichloroethane were primarily used in the past; meanwhile, chlordane (CHL) and EnSFs were derived from recent material inputs. Seasonally, OCP concentrations largely peaked in summer with minimum values in winter. This apparent temperature dependence suggests the re-volatilization of accumulated chemicals into the atmosphere. Additionally, an air mass back trajectory indicated the influence of pollutants released from a reservoir through long-range atmospheric transport in the summer. In particular, restricted OCPs are primarily released into the atmosphere by inadvertent sources, such as industrial activities and volatilization from contaminated areas. Thus, severe OCP pollution in Korea is due to the mobile nature of the particles. These data can be useful for the continuous monitoring of long-range transported air pollutants that are transferred between countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organochlorine pesticides (OCPs) are a primary group of persistent organic pollutants (POPs), and have been used extensively worldwide over the previous decades to increase agricultural productivity and control insect damage (Luo et al., 2019). Moreover, OCP toxicity, persistence, and biological accumulation have been found in various studies, leading to global concerns over correlated damage to the environment and human health (Choi et al., 2018; Wang et al., 2018a). Increasing concern over these adverse characteristics led to the adoption of the Stockholm Convention on POPs in 2001 (implemented in 2004), which was aimed at minimizing the damage from hazardous chemicals such as OCPs (Alshemmari, 2021). Under the convention, pesticides including hexachlorobenzene (HCB), aldrin, dieldrin, endrin, chlordane (CHL), dichlorodiphenyltrichloroethane (DDT), mirex, and toxaphene were listed as the “dirty dozen.” Notably, HCB was also listed as a harmful industrial chemical and by-product. Since then, pentachlorobenzene (PeCB), hexachlorocyclohexane (HCH), lindane (also known as γ-HCH), chlorodecone, and later endosulfan (EnSF) were added to the list of OCPs in 2009 (Jennings & Li, 2015; Lammel & Lohmann, 2012). Most recently, dicofol was added to the list of POPs in 2019 (Liu et al., 2021).
As OCPs exhibit semi-volatile features and a strong resistance to degradation, they can be easily released into the air and travel though long-range atmospheric transport (LRAT) from a contamination source area (Li et al., 2020). Until the 1980s, about 3600 tons of OCPs (except for mirex and HCB) had been used for agricultural activities in Korea (Kim & Smith, 2001). Although their use and production has been either restricted or banned since the Korean government implemented the Stockholm Convention in 2008, they are still being detected within the environment (Kim et al., 2019; Lee et al., 2014). This persistence can be attributed to various factors, such as re-emissions of historically used pesticides in the atmosphere and the application of current used pesticides (Barron et al., 2017; Wang et al., 2018b). Research has found that OCPs are still being produced and used in some countries, including China and India where high concentrations have recently been observed (Khuman et al., 2020; Zhou et al., 2013). Spatiotemporal distributions and transportation patterns within a given area are closely correlated with meteorological conditions; thus, it is important to understand the role of the atmosphere in the long-range transport of source emissions (Kim et al., 2005, 2021b). Indeed, a number of monitoring-based studies have focused on the sources, transport, and behaviors of pesticides via examinations of regional LRAT (Gao et al., 2020; Li et al., 2020; Rimondino et al., 2018). As these processes are difficult to capture through LRAT alone, multiple other factors and approaches have been assessed in this study, including isomer patterns, principal component analysis, and spatiotemporal distribution variations. In particular, monitoring of OCP concentration trends according to seasonal and regional differences can confirm the influence of air pollution between countries; hence, this is necessary for national measurement network data.
The purpose of this study was to understand the behavior and origins of OCP pollutants found within the atmosphere of South Korea by (1) investigating the spatial and seasonal distribution patterns, (2) comparing OCP concentrations with those of other Asian nations, and (3) exploring the efficacy of various factors for influencing contamination.
Materials and methods
Chemicals and reagents
Standard solutions (native standard, ES-5464-A; clean-up standard, ES-5465-A; internal standard, EC-5350) were acquired for the analysis of OCPs (PeCB, HCB, cis-/trans-CHL [CC/TC], cis-/trans-nonachlor, oxy-CHL, o,p′-/p,p′-DDE, o,p′-/p,p′-DDD, o,p′-/p,p′-DDT, heptachlor [HEPT], cis-/trans-HEPT epoxide, α-/β-/γ-/δ-HCH, and EnSF 1/2) (Cambridge Isotope Laboratories, MA, USA). Florisil SPE cartridges (InertSep FL, 5 g·20 mL−1) were obtained for clean-up procedures from GL Science, Inc., Tokyo, Japan. Meanwhile, acetone, n-hexane, dichloromethane, and toluene (for pesticide residue and polychlorinated biphenyl (PCB) analysis grade) were purchased from Fujifilm Wako Pure Chemical Co., Inc., Osaka, Japan, and anhydrous sodium sulfate (for pesticide residue and PCB analysis grade) was obtained from Kanto Chemical Co., Inc., Osaka, Japan, respectively.
Sample collection
Sampling was conducted at four sites in South Korea (Table S1). Jeju is representative of the background site, as monitoring has been continuously carried out there since 2009 after the Stockholm Convention implementation; Baengnyeong is located at the westernmost point of the country, and is representative of the background levels near northeastern China; Seoul is the largest residential site, with abundant traffic and a substantial floating population; and Seosan comprises an active agricultural area and is a heavily industrialized city where severe air pollution has recently become an issue. Accordingly, each site was classified as either background, urban, or industrial according to their environment and geography.
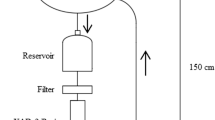
Daily air samples were collected for three consecutive days from January to December 2020 (winter [December–February], spring [March–May], summer [June–August], and fall [September–November]) using a high-volume air sampler (HV-1000F, Sibata Scientific Technology, Saitama, Japan), whose flow rate was set to 700 L·min−1. The total volume of each collected sample was 1007.9 m3. Then, 3-day averages were calculated and used for subsequent analysis. All field blanks were performed by exposure to the surroundings during sampling. To collect both the gas- and particle-phase substances, a pair of polyurethane foam disks (PUF; 84-mm diameter × 50-mm height; Sibata Scientific Technology, Saitama, Japan), piece of activated carbon felt (ACF; 84 mm; Sibata Scientific Technology, Saitama, Japan), and quartz fiber filter (QFF; 203 × 254 mm; Whatman, Buckinghamshire, UK) were used per sample. Prior to use, the PUFs and ACFs were cleaned with acetone using Soxhlet extractors for 24 h, and then dried completely; the QFFs were placed in an oven at 600 °C for 5 h to remove any organic residue. The pre-treated materials were individually packed in an aluminum foil and stored in a desiccator. After sampling, the used materials were rewrapped with aluminum foil, placed in ziplock bags, and stored in a refrigerator at −4 °C until extraction commenced.
Sample pre-treatment
Pre-treatment and analytical procedures were carried out in accordance with the United States Environmental Protection Agency (US EPA) methods (US EPA, 1999, 2007) and Korean Official Method of Organochlorine Pesticides in Ambient Air (ES 10,900.1a). PUF, ACF, and QFF were Soxhlet extracted with 500 mL of dichloromethane for 24 h. The extract was dehydrated with an anhydrous sodium sulfate, concentrated to 0.5 mL using a rotary evaporator (N-1300, EYELA, Tokyo, Japan) and solvent exchanged into 5 mL hexane. The solvent was again concentrated to 1 mL hexane under a nitrogen concentrator (N-EVAP 112, Organomation, MA, USA) (Kim et al., 2020; Tasdemir et al., 2004). Within this step, the isotopically labeled standard (ES-5465-A, Cambridge Isotope Labs, MA, USA) was spiked into the concentrate to ensure the accuracy of overall experimental procedures (Jin et al., 2013; Kim et al., 2020). Prior to cleanup, 2 g of anhydrous sodium sulfate was added to the top layer of the Florisil SPE cartridge, and the cartridge was then conditioned with 20 mL of hexane. Once activated, the sample was loaded into the cartridge and eluted with 100 mL of hexane (1st fraction); subsequently, it was eluted with 100 mL of hexane/dichloromethane (v/v 3:1) (2nd fraction). The eluent was concentrated using a rotary evaporator, and purged again with a nitrogen concentrator. Finally, the internal standard (EC-5350, Cambridge Isotope Labs, MA, USA) was spiked into the resultant solution, and the volume was reduced to 100 μL under a gentle stream of nitrogen for instrumental analysis.
Instrumental analysis
Target components were analyzed using a gas chromatographer (7890B, Agilent Technologies, CA, USA), which was coupled with a high-resolution mass spectrometer (JMS-800D, JEOL, Tokyo, Japan) and a fused silica capillary column (Rtx-CLPestides 2, 30-m length, 0.25 mm i.d., 0.25-μm film thickness, Restek, PA, USA). The mass spectrometer was operated in the electron ionization mode, with an applied energy of 38 eV, a current of 500 μA, and the selected ion monitoring mode with > 10,000 resolutions. Samples of 1 μL were injected through the splitless mode using helium (99.9999%) as a carrier gas, with a constant flow rate of 1 mL·min−1. The inlet and source temperatures were maintained at 260 and 280 °C, respectively. The initial oven temperature was maintained at 100 °C for 1 min, which was then increased to 180 °C, 200 °C, 220 °C, and 300 °C at a rate of 20 °C·min−1, 1.4 °C·min−1, 5 °C·min−1 (held for 0.314 min), and 40 °C·min−1 (held for 4.4 min), respectively. Quantification of each OCP component was carried out via the isotope dilution method.
Quality assurance/quality control
Field blanks (once per site, per month), solvent-only method, and spiked blanks (standards injected into the solvent) were processed and analyzed to assess the procedure quality. Laboratory blank samples showed either negligible or non-quantifiable levels of contaminating compounds. Method detection limits (MDLs) were derived from a Student’s t-value of 3.14 (99% confidence level, n = 7) multiplied by the standard deviation of seven replicates of the spiked blank samples (injection volume for all components, 0.5 ng), and ranged from 0.037 to 0.269 pg·m−3 (Table S2). A certified reference material (CRM818-50G, Sigma-Aldrich, MO, USA) was analyzed and confirmed that the results corresponded to true values (Table S3). The recoveries of 13C-labeled internal standards for the blank and real samples ranged from 40 to 120%. Concentrations < MDLs were assigned a value of zero.
Air mass backward trajectories and statistical analyses
Air mass backward trajectories were conducted using the hybrid single-particle Lagrangian integrated trajectory (HYSPLIT 4) model developed by the US National Oceanic and Atmospheric Administration (NOAA) to assess the origins and transport of OCPs from potential source areas. The trajectories for each sampling site were conducted in 6-h intervals (00:00 UTC, coordinated universal time) at an altitude of 500 m above ground level. Scatterplots of the spatial and seasonal concentration distributions of OCPs were created in SigmaPlot v.12.0 (Systat Software Inc., CA, USA). All statistical analyses were performed in SPSS v.20.0 (IBM, NY, USA). Pearson correlation coefficients were calculated to assess the relationships between OCP concentration levels and seasons (i.e., changes in local temperature). A principal component analysis (PCA) was also conducted to identify the relationship between each component and potential source. For both statistical analyses, p < 0.05 was considered to be statistically significant.
Results and discussion
Spatial distribution and OCP levels
Atmospheric concentrations of OCPs measured at each site are shown in Table 1, and individual OCP values are presented in Table S4. Although the use and production of OCPs in South Korea has been banned since 2008, most of these chemicals are still detected in the present-day atmosphere.
PeCB and HCB
In the past, PeCB has been used as an intermediate in the manufacturing process of pentachloronitrobenzene, an organochlorine fungicide, and a chlorobenzene mixture for products containing PCBs (Mitra et al., 2019). Meanwhile, HCB has served as a precursor for pentachlorophenol production and as a material component for flame retardants, plasticizers, and synthetic rubbers (Dhaibar et al., 2021; Pan et al., 2020). Production and release volumes of PeCB and HCB peaked from the late 1970s to the early 1980s (Su et al., 2006); however, since it was banned under the Stockholm Convention, there are currently no known manufacturing sites or commercial uses of these chemicals anywhere in the world (Bailey et al., 2009; Stockholm Convention, 2021c, d).
PeCB concentrations ranged from 42.4 to 150.3 pg·m−3, with the following site averages (± 1 SD): Jeju, 62.4 ± 14.5 pg·m−3; Baengnyeong, 74.2 ± 36.3 pg·m−3; Seoul, 80.7 ± 27.7 pg·m−3; and Seosan, 84.5 ± 19.9 pg·m−3. HCB concentrations ranged from 49.1 to 202.4 pg·m−3, with the following site averages: Jeju, 104.2 ± 29.3 pg·m−3; Baengnyeong, 101.5 ± 35.8 pg·m−3; Seoul, 109.3 ± 24.2 pg·m−3; and Seosan, 129.5 ± 46.9 pg·m−3. The levels of both substances tended to be higher in the residential and industrial areas than those in the background sites. Furthermore, these two chemicals were distributed at high concentrations, accounting for up to 80% of the total OCPs across all sites (Fig. 1; PeCB, 28–38%; HCB, 44–50%).
As mentioned above, although PeCB and HCB were not previously used as pesticides in South Korea, they were still detected at fairly high concentrations. These results were likely caused by primary emissions from the unintentional formation of by-products through diverse processes (e.g., industrial fields, use of chlorinated compounds, combustion), and secondary emissions from accumulated chemicals in the environment (Liu et al., 2018; Wang et al., 2010). Table 2 presents previously reported atmospheric concentrations of individual OCPs for other Asian nations. In particular, the atmospheric levels of PeCB reported in the present study were comparable to those of Kuwait and Japan, whereas HCB concentrations were much lower than those of all other countries that were examined (except Pakistan). Accordingly, it is proposed here that these chemicals may be entering Korea from polluted sites via LRAT.
HCHs
HCH was broadly used and manufactured for agricultural applications in two forms: technical mixture and lindane. Technical HCH was mainly used as an insecticide in the 1940s; however, when crop problems were observed, it was replaced with a more effective insecticidal product, lindane. It was reported that both HCH forms were used in Asian regions including India, China, and Vietnam, as well as in South America up until the 1990s; their illegal use still persists in some countries to the present day (Pozo et al., 2004; Vijgen et al., 2019). Since, HCH isomers were registered under the Stockholm Convention in 2009, and lindane has been strictly controlled, with specific exemptions for medical and pharmaceutical uses on head lice and scabies in China until 2019 (Stockholm Convention, 2021a). In South Korea, the use of technical HCH and lindane as pesticides was banned in 1979 and 1969, respectively, with an estimated pre-ban consumption of 6153 and 209 tons, respectively (Hong et al., 2006).
Technical HCH is usually composed of α-HCH (60–70%), β-HCH (5–12%), γ-HCH (10–12%), and δ-HCH (3–4%), whereas lindane is nearly pure γ-HCH (> 99%) (Mishra et al., 2013). Because technical HCH contains a high proportion of α-HCH, the ratio of the two isomers (α-HCH/γ-HCH) is used to evaluate usage patterns. It has been generally observed that the ratio ranges between 4.0 and 7.0 in technical HCH (Yu et al., 2019); therefore, without any recent input of technical HCH, this value should decrease with time. The measured concentrations of ∑HCHs ranged from 5.2 to 80.4 pg·m−3, with the following site averages (± 1 SD): Jeju, 8.2 ± 2.9 pg·m−3; Baengnyeong, 10.3 ± 3.3 pg·m−3; Seoul, 16.0 ± 12.0 pg·m−3; and Seosan, 13.1 ± 7.3 pg·m−3 (Fig. 2a). HCH concentrations were relatively high in the residential and industrial regions, with maximum levels being observed in Seoul; however, these concentration levels were much lower than those observed in other Asian nations. The range of the α-HCH/γ-HCH ratios observed in the present study was 1.94 to 3.84 (mean: 3.03 ± 0.70), with the following site averages: Jeju, 3.38 ± 0.86; Baengnyeong, 3.84 ± 0.89; Seoul, 1.94 ± 0.73; and Seosan, 2.96 ± 0.42. Since all observed values were below 4 across all sites, it indicates that modern-day HCH concentrations in the Korean atmosphere predominantly originated from lindane. Notably, the ratios here show a significant reduction compared to those observed during a previous monitoring study in South Korea that was conducted less than a decade ago (Jin et al., 2013).
CHLs
CHL was largely applied as a pesticide for a range of agricultural crops (mostly corn and citrus), management of home lawns and gardens, and termite control in housing foundations. Due to its hazardous features, most countries prohibited the use of CHL in the late 1980s (Becker et al., 2012); however, information on global use indicated that about 58 tons of chlordane were imported into Venezuela up until 2003, and that China consumed about 745 tons from 1998 to 2008 (Girones et al., 2020; Niu et al., 2016). Further, its production and use as a termiticide continued in China, Zambia, and Botswana until 2009, well after its registration under the Stockholm Convention (Stockholm Convention, 2021c). CHL has been banned in South Korea since early 1969, prior to which about 3 tons was used over the previous 4 years (Park et al., 2011). Figure 2c shows the mean concentrations of ∑CHLs and ratios of geometric isomers for each site. The concentration of ∑CHLs ranged from 1.8 to 14.1 pg·m−3, with the following site averages (± 1 SD): Jeju, 5.6 ± 2.7 pg·m−3; Baengnyeong, 4.1 ± 1.2 pg·m−3; Seoul, 4.5 ± 2.0 pg·m−3; and Seosan, 6.1 ± 3.4 pg·m−3; no significant differences were observed in these concentrations, according to spatial variation in South Korea. The CHL levels observed in the present study were analogous to the atmospheric concentrations of Hong Kong, and much lower than those observed in other Asian sites (Table 2).
Technical CHL, a mixture composed of more than 140 compounds, contains TC (~13%), CC (~11%), and HEPT (~5%). The ratio of the two isomers (TC/CC) is a recognized indicator of CHL age (typically ranging from 1.16 to 1.56), with values < 1.56 resulting from the more rapid decomposition rate of TC than that of CC (Yang et al., 2008). The observed TC/CC ratios ranged from 1.67 to 2.21 (1.88 ± 0.20), with the following site averages: Jeju (1.85 ± 1.40), Baengnyeong (1.67 ± 0.41), Seoul (1.78 ± 0.77), and Seosan (2.21 ± 0.82); these results indicate the influence of recent CHL inputs. As only a relatively small amount of chlordane was used in South Korea over 50 years ago, its relative levels are quite low compared to other OCPs; however, there is still evidence of its current use within the South Korean atmosphere. Recently, high chlordane levels and corresponding proportions of TC have been reported in several Asian regions, including Pakistan and China (Aslam et al., 2021; Huang et al., 2019); the direct cause for this result remains unknown, although it may be related to meteorological conditions and geographical positioning.
DDTs
DDT was used extensively to prevent the spread of insect-borne diseases (e.g., malaria and typhus) and increase agricultural efficiency; however, DDT was banned in many countries in the 1970s after its true toxicity to humans and animals was discovered (Van Dyk et al., 2010). In 2004, DDT officially became one of the initial members listed by the Stockholm Convention; however, use and production of this chemical persisted in China and India until the specific exemption on the manufacture of dicofol expired in 2009 (Stockholm Convention, 2021d). Estimates of the production and import of DDT for agricultural purposes across its 30-year history in South Korea were 758 and 1320 tons, respectively, until 1971 (Kim et al., 2009). Technical DDT commonly consists of p,p′-DDT (75%), o,p′-DDT (15%), p,p′-DDE (5%), and others forms (< 5%). DDT metabolizes to dichlorodiphenyldichloroethylene (DDE) under aerobic (or oxidizing) conditions, and degrades to dichlorodiphenyldichloroethane (DDD) through the dechlorination process under anaerobic (or reducing) conditions; thus, the ratio of DDT isomers (DDT/[DDD + DDE]) can be a useful indicator of environmental input, indicating that present atmospheric concentrations are a result of current DDT use when the ratio is > 1 (Kim et al., 2002; Zheng et al., 2020).
Dicofol (trade name: Kelthane) was used to control crop mites following the ban of technical DDT due to its acaricidal activation. Because dicofol is normally synthesized through chlorination and hydrolysis reactions of technical DDT, high DDT and related-DDT contents remain as impurities in dicofol. It has been totally banned without exemption by the Stockholm Convention since 2019 (Turgut et al., 2009). From 1972 to 2002, approximately 1515 tons of dicofol was used for the management of citrus and gerbera in South Korea (Baek et al., 2013). Although both technical DDT and dicofol mainly comprise p,p′-DDT, there is a difference in their distribution. For this reason, the o,p′-DDT/p,p′-DDT ratio appears to be different for each type of DDT (technical DDT, 0.2–0.3; dicofol-type DDT, 1.3–9.3) (Zhang et al., 2009).
Figure 2b shows mean ∑DDT concentrations, and the corresponding isomer ratios at each site. Concentrations of ∑DDTs ranged from 1.9 to 22.0 g·m−3, with the following site averages (± 1 SD): Jeju, 4.0 ± 2.6 g·m−3; Baengnyeong, 4.4 ± 1.1 g·m−3; Seoul, 7.9 ± 5.7 g·m−3; and Seosan, 4.2 ± 1.3 g·m−3. The highest concentrations were recorded in Seoul, and no significant difference was found among the other locations. DDT levels detected in other Asian nations were much higher than those in South Korea, with maximum concentration distributions in Vietnam and China being about 250 and 150 times greater, respectively. The DDT ratios measured at each site revealed similar patterns (Fig. 3). In the present study, the DDT/(DDE + DDD) ratio ranged from 0.55 to 0.72 (0.68 ± 0.05), with the following site averages: Jeju, 0.62 ± 0.22; Baengnyeong, 0.69 ± 0.31; Seoul, 0.65 ± 0.27; and Seosan, 0.77 ± 0.52. Moreover, the o,p′-DDT/p,p′-DDT ratio ranged from 0.62 to 0.96 (0.79 ± 0.13), with the following site averages: Jeju, 0.96 ± 0.48; Baengnyeong, 0.62 ± 0.35; Seoul, 0.85 ± 1.17; and Seosan, 0.73 ± 0.41. Accordingly, DDT/(DDE + DDD) indicated that past DDT use is a predominant source of present-day atmospheric DDT concentrations. This result suggested that the decomposition of DDT to its metabolites during atmospheric exposure had an effect on the distribution of DDTs, along with the use of dicofol.
EnSFs
EnSF is an insecticide used until recently for preventing damages from tsetse flies, mites, and other pests. From 1950 to 2000, an estimated 310 ktons was used worldwide, with about 5,400 tons and 2,800 tons being produced annually in India and China, respectively (Mitton et al., 2016; Weber et al., 2010). EnSF was added to the Stockholm Convention list in 2011; however, it was used in Guatemala and China (where it was also produced) until 2019 because of its specific exemptions for crop-pest complexes (Stockholm Convention, 2021b). In South Korea, it is estimated that 900–1000 tons·yr−1 of EnSF was applied as a pesticide from 1971 until it was outlawed in 2010 (Choi & Chun, 2007). Technical EnSF is a racemic mixture comprising EnSF 1 (α-form) and EnSF 2 (β-form) in proportions of 2:1–7:3 (i.e., 2.0–2.3), with values < 2.3 indicating that the primary emission sources are related to past use (Kim et al., 2020). Figure 2d shows the mean ∑EnSF concentrations of two stereoisomers for each site. The concentrations of ∑EnSFs ranged from 1.9 to 199.8 pg·m−3, with the following site averages (± 1 SD): Jeju, 19.5 ± 17.0 pg·m−3; Baengnyeong, 5.0 ± 1.9 pg·m−3; Seoul, 14.8 ± 17.0 pg·m−3; and Seosan, 55.0 ± 63.8 pg·m−3. Accordingly, EnSF at most of the sites was detected at relatively high concentrations; indeed, it was the most abundant OCP observed in this study, excluding unintentional POPs (UPOPs: PeCB and HCB in this study), accounting for up to 18% of total OCPs (followed by ∑HCHs [~6.5%] > ∑DDTs [~2.9%] > ∑CHLs [~2.7%] > ∑HEPTs [~0.5%]). In particular, ∑EnSF levels in Seosan were three times higher than those recorded in Vietnam, although lower than those observed in Lahore, Pakistan. The recorded EnSF 1/EnSF 2 ratios ranged between 5.85 and 6.60 (6.15 ± 0.30), with the following site averages: Jeju, 6.60 ± 3.91; Baengnyeong, 6.13 ± 3.28; Seoul, 6.01 ± 3.96; and Seosan, 5.85 ± 2.49. The likely sources of EnSF can be explained in two ways: first, there is evidence of atmospheric input from recent use, and second, EnSF 2 is converted to EnSF 1 during its atmospheric transport due to its low stability. However, the higher isomer ratio than that of technical EnSF and the low EnSF 2 concentrations indicate that the dominant source of EnSF in South Korea may be related to LRAT inputs from other sites (Miglioranza et al., 2021; Shunthirasingham et al., 2010).
HEPT
HEPT was a widely used cyclodiene insecticide for controlling crop pests from the 1950s to 1970s, ever since it was isolated from chlordane in 1946 (Guida et al., 2018). HEPT was included as a POP without exemptions under the Stockholm Convention in 2004, and since then there have been no confirmed reports of its use or production. In South Korea, total agricultural consumption was roughly ~16,617 tons until it was banned in 1979 (Chung et al., 2001). The concentrations of ∑HEPTs ranged from 0.3 to 4.7 pg·m−3, with the following site averages: Jeju, 0.8 ± 0.4 pg·m−3; Baengnyeong, 0.5 ± 0.2 pg·m−3; Seoul, 1.1 ± 0.5 pg·m−3; and Seosan, 1.7 ± 1.2 pg·m−3. Accordingly, although larger past amounts of HEPT were comparable to those of other analytes, it maintained the lowest concentration distributions (< 1% of total OCPs) and no significant spatial differences were observed. Furthermore, HEPT concentrations were the lowest than those reported in other Asian nations, implying that this occurred due to the use of chlordane, as the technical type comprises 10% HEPT (Pokhrel et al., 2018).
Seasonal variation and OCP levels
Figure 4 shows the seasonal distributions of total OCPs (excluding UPOPs) during the four seasons (additional details on seasonal OCP concentrations for each site are shown in Table S5). The atmospheric OCP concentrations in summer (34.8–213.9, 103.2 ± 68.5 pg·m−3) were remarkably higher than those in spring (25.8–49.2, 37.3 ± 8.9 pg·m−3), fall (26.6–48.8, 38.5 ± 9.9 pg·m−3), and winter (19.4–27.1, 21.9 ± 3.0 pg·m−3). This may be related to the re-volatilization of historically used and/or recent applications of OCPs from the soil and sediment as ambient temperature increases (Sanlı & Tasdemir, 2020). These observed seasonal characteristics are distinct to South Korea due to its temperate climate (Fig. 5). It has also been shown that atmospheric OCP distributions can vary with meteorological conditions (Kim et al., 2021a). In particular, summer concentrations in Seosan (213.1 pg·m−3) increased much more sharply than the other sampling sites (Jeju, 60.5 pg·m−3; Baengnyeong, 41.3 pg·m−3; and Seoul, 107.1 pg·m−3). These results may be caused by their regional large-scale industrial facilities, such as petrochemical and steel complexes and coal-fired power plants, in addition to various agricultural activities; however, the precise set of emission sources remains unknown. The correlations between atmospheric OCPs and seasonal variability for specific regions have been frequently reported in previous studies (Chakraborty et al., 2019; Romanić et al., 2018). Figure 5 shows the average temperatures and OCP concentrations for each season, which mostly follow the pattern of summer maximums and winter minimums, particularly in Seoul and Seosan. Similarly, a Pearson correlation analysis between OCP concentrations and local temperatures was performed, revealing that most OCPs were positively related to temperatures (HCHs, r > 0.7, p < 0.01; DDTs, r > 0.4, p < 0.01; CHLs, r > 0.7, p < 0.01; and EnSFs, r > 0.6, p < 0.01). HEPTs also showed a positive correlation with temperature (r > 0.6, p < 0.01), but their extremely low concentrations indicate that further analyses are needed to confirm this trend.
Alternatively, no seasonal or temperature-based variations were observed for PeCB and HCB (p > 0.05), indicating that their dominant source is not historical use, but the unintentional sustained releases from industrial activities and/or atmospheric inputs (Moon et al., 2009). To investigate the possibility of LRAT from potential sources, air mass backward trajectories were performed for each site during the 3 days with maximum summer concentration distributions (Fig. 6). In this period, air masses in the background sites primarily originated from the East China Sea and China; however, for Seoul and Seosan, inputs from the Philippines Sea passing through southwestern Japan were found along with those from the east coast of China. These results indicate that the primary mechanism behind the high summer contamination levels may be the LRAT, which occurs due to meteorological conditions. The winter back trajectories of the low concentration levels indicated that the air masses at all sites originated from northeast China, Mongolia, and Russia (Fig. S1).
Principal component analysis
PCA was performed to estimate the sources of individual OCPs, and Fig. 7 shows their distributions according to the first three components, namely: PC1, PC2, and PC3 (detailed results are presented in Tables S6 and S7). PC1 (42.8% variance explained), which exhibits the highest proportion of all the components, had a strong positive correlation with o,p'-DDT, p,p'-DDT, o,p'-DDD, p,p'-DDD, o,p'-DDE, and p,p'-DDE; this indicated that DDTs and its metabolites were originated from similar sources. PC2 (16.1% variance explained) was highly correlated to HEPT, CC, TC, trans-nonachlor, EnSF 1, and EnSF 2, suggesting the similarity of their origin sources. Furthermore, this result was indicative of the intimate relationship between HEPT and TC. In fact, technical chlordane and technical HEPT comprise a certain quantity of each other (technical chlordane contains ~5% of HEPT, and technical HEPT contains ~20% of TC) (Qu et al., 2019). PC3 (11.4% variance explained) was strongly correlated with HCH isomers (α-, β-, γ-, and δ-), which indicated their similar sources based on the effects of both technical HCH and lindane and the isomerization reactions of γ-HCH (Devi et al., 2015). PC4 (8.4% variance explained) had a high positive correlation with only PeCB and HCB, indicating that they were originated by inflows of diverse routes and had similar sources as those of UPOPs. PC5, PC6, and PC7 held eigenvalues < 1, indicating that the origins of oxychlordane, HEPT epoxide, and cis-nonachlor were non-distinct (Lv et al., 2020); this was likely due to their low concentrations and detection rates in South Korea. Ultimately, most OCP compound concentrations were closely correlated with similar congeners.
Conclusion
The atmospheric OCP concentrations of the sampling sites demonstrated that even though all OCPs examined are currently listed under the Stockholm Convention and have been completely banned in South Korea, the majority of them are still being observed. OCP levels in the urban and industrial locations were relatively higher than those in the background sites, especially in Seosan, where concentrations about 1.6 times higher than those of other sites indicated the possibility of various local emission sources. Among OCPs, PeCB, HCB, and EnSFs were detected at much higher concentrations than other components and were comparable to (or higher than) values reported in other nearby Asian countries. Indeed, isomeric ratios of the South Korean atmosphere indicate the current use of CHLs and EnSFs. Seasonal variations in OCPs appeared to reach peak concentrations in the summer, and dropped to their lowest concentrations in the winter. HCHs, HEPTs, CHLs, DDTs, and EnSFs were positively related to local temperatures, while no such correlations were observed for PeCB and HCB. The elevated concentration levels and isomeric ratios indicate the continued effects of volatile chemicals used in the past, or an increase in recent use; these impacts increased with temperature and LRAT from contaminated areas. The PCA results revealed that similar substances had a strong correlation with each other, implying that they share similar origin sources and behaviors.
The drawback of monitoring in this study was that air samples were collected only 3 days per month. The average concentrations of individual OCP may not be representative of annual concentrations due to seasonal variation. Although it is desirable to consider more realistic variables for regional temporal factors and seasonal influences, we speculate that the main findings and overall conclusions of this study will not change significantly. This national monitoring study of atmospheric OCPs can facility various related forms of research in the future, and assist with decreasing the atmospheric concentrations of these hazardous chemicals as a part of the Stockholm Convention implementation.
References
Alshemmari, H. (2021). Inventories and assessment of POPs in the State of Kuwait as a basis for Stockholm Convention implementation. Emerging Contaminants, 7, 88–98. https://doi.org/10.1016/j.emcon.2021.02.003
Aslam, I., Mumtaz, M., Qadir, A., Jamil, N., Baqar, M., Mahmood, A., Ahmad, S. R., & Zhang, G. (2021). Organochlorine pesticides (OCPs) in air-conditioner filter dust of indoor urban setting: Implication for health risk in a developing country. Indoor Air, 31, 807–817. https://doi.org/10.1111/ina.12772
Baek, S.-Y., Jurng, J., & Chang, Y.-S. (2013). Spatial distribution of polychlorinated biphenyls, organochlorine pesticides, and dechlorane plus in Northeast Asia. Atmospheric Environment, 64, 40–46. https://doi.org/10.1016/j.atmosenv.2012.09.015
Bailey, R. E., van Wijk, D., & Thomas, P. C. (2009). Sources and prevalence of pentachlorobenzene in the environment. Chemosphere, 75, 555–564. https://doi.org/10.1016/j.chemosphere.2009.01.038
Barron, M. G., Ashurova, Z. J., Kukaniev, M. A., Avloev, H. K., Khaidarov, K. K., Jamshedov, J. N., Rahmatullova, O. S., Atolikshoeva, S. S., Mamadshova, S. S., & Manzenyuk, O. (2017). Residues of organochlorine pesticides in surface soil and raw foods from rural areas of the Republic of Tajikistan. Environmental Pollution, 224, 494–502. https://doi.org/10.1016/j.envpol.2017.02.031
Becker, S., Halsall, C. J., Tych, W., Kallenborn, R., Schlabach, M., & Manø, S. (2012). Changing sources and environmental factors reduce the rates of decline of organochlorine pesticides in the Arctic atmosphere. Atmospheric Chemistry and Physics, 12, 4033–4044. https://doi.org/10.5194/acp-12-4033-2012
Chakraborty, P., Zhang, G., Li, J., Sampathkumar, P., Balasubramanian, T., Kathiresan, K., Takahashi, S., Subramanian, A., Tanabe, S., & Jones, K. C. (2019). Seasonal variation of atmospheric organochlorine pesticides and polybrominated diphenyl ethers in Parangipettai, Tamil Nadu, India: Implication for atmospheric transport. Science of the Total Environment, 649, 1653–1660. https://doi.org/10.1016/j.scitotenv.2018.07.414
Choi, M., & Chun, M.-Y. (2007). Organochlorine pesticides concentration in soil. Journal of the Korean Society for Environmental Analysis, 10, 169–174.
Choi, M. P. K., Kang, Y. H., Peng, X. L., Ng, K. W., & Wong, M. H. (2009). Stockholm Convention organochlorine pesticides and polycyclic aromatic hydrocarbons in Hong Kong air. Chemosphere, 77, 714–719. https://doi.org/10.1016/j.chemosphere.2009.08.039
Choi, S., Kim, H.-J., Kim, S., Choi, G., Kim, S., Park, J., Shim, S.-S., Lee, I., Kim, S., Moon, H.-B., Choi, K., Lee, J. J., & Kim, S. Y. (2018). Current status of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) exposure among mothers and their babies of Korea-CHECK cohort study. Science of the Total Environment, 618, 674–681. https://doi.org/10.1016/j.scitotenv.2017.07.232
Chung, R.-P., Choi, M.-K., Yeo, H.-G., & Chun, M.-Y. (2001). Seasonal variations in the concentration of persistent organochlorine pesticides in atmosphere. The Korean Society of Environmental Agriculture, 20, 79–85.
Devi, N. L., Yadav, I. C., Raha, P., Shihua, Q., & Dan, Y. (2015). Spatial distribution, source apportionment and ecological risk assessment of residual organochlorine pesticides (OCPs) in the Himalayas. Environmental Science and Pollution Research, 22, 20154–20166. https://doi.org/10.1007/s11356-015-5237-5
Dhaibar, H. A., Patadia, H., Mansuri, T., Shah, R., Khatri, L., Makwana, H., Master, S., & Robin, P. (2021). Hexachlorobenzene, a pollutant in hypothyroidism and reproductive aberrations: A perceptive transgenerational study. Environmental Science and Pollution Research, 28, 11077–11089. https://doi.org/10.1007/s11356-020-11278-x
Dien, N. T., Hirai, Y., Koshiba, J., & Sakai, S.-I. (2021). Factors affecting multiple persistent organic pollutant concentrations in the air above Japan: A panel data analysis. Chemosphere, 277, 130356. https://doi.org/10.1016/j.chemosphere.2021.130356
Gao, Y., Zheng, H., Xia, Y., & Cai, M. (2020). Global scale distribution, seasonal changes and long-range transport potentiality of endosulfan in the surface seawater and air. Chemosphere, 260, 127634. https://doi.org/10.1016/j.chemosphere.2020.127634
Gevao, B., Porcelli, M., Rajagopalan, S., Krishnan, D., Martinez-Guijarro, K., Alshemmari, H., Bahloul, M., & Zafar, J. (2018). Spatial and temporal variations in the atmospheric concentrations of “Stockholm Convention” organochlorine pesticides in Kuwait. Science of the Total Environment, 622–623, 1621–1629. https://doi.org/10.1016/j.scitotenv.2017.10.036
Girones, L., Oliva, A. L., Marcovecchio, J. E., & Arias, A. H. (2020). Spatial distribution and ecological risk assessment of residual organochlorine pesticides (OCPs) in South American marine environments. Current Environmental Health Reports, 7, 147–160. https://doi.org/10.1007/s40572-020-00272-7
Guida, Y. D. S., Meire, R. O., Torres, J. P. M., & Malm, O. (2018). Air contamination by legacy and current-use pesticides in Brazilian mountains: An overview of national regulations by monitoring pollutant presence in pristine areas. Environmental Pollution, 242, 19–30. https://doi.org/10.1016/j.envpol.2018.06.061
Hong, S., Yim, U., Shim, W., Li, D., & Oh, J. (2006). Nationwide monitoring of polychlorinated biphenyls and organochlorine pesticides in sediments from coastal environment of Korea. Chemosphere, 64, 1479–1488.
Huang, H., Ding, Y., Chen, W., Zhang, Y., Chen, W., Chen, Y., Mao, Y., & Qi, S. (2019). Two-way long-range atmospheric transport of organochlorine pesticides (OCPs) between the Yellow River source and the Sichuan Basin, Western China. Science of the Total Environment, 651, 3230–3240. https://doi.org/10.1016/j.scitotenv.2018.10.133
Jennings, A. A., & Li, Z. (2015). Residential surface soil guidance applied worldwide to the pesticides added to the Stockholm Convention in 2009 and 2011. Journal of Environmental Management, 160, 226–240. https://doi.org/10.1016/j.jenvman.2015.06.020
Jin, G.-Z., Kim, S.-M., Lee, S.-Y., Park, J.-S., Kim, D.-H., Lee, M.-J., Sim, K.-T., Kang, H.-G., Kim, I.-G., Shin, S.-K., Seok, K.-S., & Hwang, S.-R. (2013). Levels and potential sources of atmospheric organochlorine pesticides at Korea background sites. Atmospheric Environment, 68, 333–342. https://doi.org/10.1016/j.atmosenv.2012.10.036
Khuman, S. N., Vinod, P. G., Bharat, G., Kumar, Y. S. M., & Chakraborty, P. (2020). Spatial distribution and compositional profiles of organochlorine pesticides in the surface soil from the agricultural, coastal and backwater transects along the south-west coast of India. Chemosphere, 254, 126699. https://doi.org/10.1016/j.chemosphere.2020.126699
Kim, H.-J., Jeon, J.-W., Hwang, S.-M., Chu, K.-I., Cha, Y.-H., Kwak, Y.-D., Kim, Y.-H., Choi, S.-D., Aslam, M., & Kim, C.-G. (2021a). Long-term nationwide assessment of polychlorinated dibenzo-p-dioxins/dibenzofurans and dioxin-like polychlorinated biphenyls ambient air concentrations for ten years in South Korea. Chemosphere, 263, 127903. https://doi.org/10.1016/j.chemosphere.2020.127903
Kim, I., Lee, K., Lee, S., & Kim, S. D. (2019). Characteristics and health effects of PM2.5 emissions from various sources in Gwangju, South Korea. Science of The Total Environment, 696, 133890. https://doi.org/10.1016/j.scitotenv.2019.133890
Kim, J.-H., & Smith, A. (2001). Distribution of organochlorine pesticides in soils from South Korea. Chemosphere, 43, 137–140. https://doi.org/10.1016/S0045-6535(00)00281-2
Kim, J., Yoon, S.-C., Jefferson, A., Zahorowski, W., & Kang, C.-H. (2005). Air mass characterization and source region analysis for the Gosan super-site, Korea, during the ACE-Asia 2001 field campaign. Atmospheric Environment, 39, 6513–6523. https://doi.org/10.1016/j.atmosenv.2005.07.021
Kim, K.-S., Lee, S. C., Kim, K.-H., Shim, W. J., Hong, S. H., Choi, K. H., Yoon, J. H., & Kim, J.-G. (2009). Survey on organochlorine pesticides, PCDD/Fs, dioxin-like PCBs and HCB in sediments from the Han river, Korea. Chemosphere, 75, 580–587. https://doi.org/10.1016/j.chemosphere.2009.01.075
Kim, L., Jeon, J.-W., Son, J.-Y., Kim, C.-S., Ye, J., Kim, H.-J., Lee, C.-H., Hwang, S.-M., & Choi, S.-D. (2020). Nationwide levels and distribution of endosulfan in air, soil, water, and sediment in South Korea. Environmental Pollution, 265, 115035. https://doi.org/10.1016/j.envpol.2020.115035
Kim, S.-W., Heo, J., & Park, J.-U. (2021b). Relationship between submicron particle formation and air mass history observed in the Asian continental outflow at Gosan, Korea, during 2008–2018. Air Quality, Atmosphere & Health, 14, 291–300. https://doi.org/10.1007/s11869-020-00934-3
Kim, S. K., Oh, J. R., Shim, W. J., Lee, D. H., Yim, U. H., Hong, S. H., Shin, Y. B., & Lee, D. S. (2002). Geographical distribution and accumulation features of organochlorine residues in bivalves from coastal areas of South Korea. Marine Pollution Bulletin, 45, 268–279. https://doi.org/10.1016/S0025-326X(01)00279-X
Lammel, G., & Lohmann, R. (2012). Identifying the research needs in the global assessment of toxic compounds 10 years after the signature of the Stockholm Convention. Environmental Science and Pollution Research, 19, 1873–1874. https://doi.org/10.1007/s11356-012-0967-0
Lee, S.-H., Ra, J.-S., Choi, J.-W., Yim, B.-J., Jung, M.-S., & Kim, S.-D. (2014). Human health risks associated with dietary exposure to persistent organic pollutants (POPs) in river water in Korea. Science of the Total Environment, 470–471, 1362–1369. https://doi.org/10.1016/j.scitotenv.2013.08.030
Li, Y., Lohmann, R., Zou, X., Wang, C., & Zhang, L. (2020). Air-water exchange and distribution pattern of organochlorine pesticides in the atmosphere and surface water of the open Pacific ocean. Environmental Pollution, 265, 114956. https://doi.org/10.1016/j.envpol.2020.114956
Liu, L., Wu, G., Ren, Y., Chen, Y., & Feng, Q. (2021). China’s synergy in the implementation of international conventions on chemicals and waste. Emerging Contaminants, 7, 132–138. https://doi.org/10.1016/j.emcon.2021.03.002
Liu, X., Fiedler, H., Gong, W., Wang, B., & Yu, G. (2018). Potential sources of unintentionally produced PCB, HCB, and PeCBz in China: A preliminary overview. Frontiers of Environmental Science & Engineering, 12, 1. https://doi.org/10.1007/s11783-018-1036-9
Luo, Y., Yang, R., Li, Y., Wang, P., Zhu, Y., Yuan, G., Zhang, Q., & Jiang, G. (2019). Accumulation and fate processes of organochlorine pesticides (OCPs) in soil profiles in Mt. Shergyla, Tibetan Plateau: A comparison on different forest types. Chemosphere, 231, 571–578. https://doi.org/10.1016/j.chemosphere.2019.05.181
Lv, M., Luan, X., Guo, X., Liao, C., Guo, D., Miao, J., Wu, X., Zhou, R., Liu, D., Wang, D., Zhao, Y., & Chen, L. (2020). A national-scale characterization of organochlorine pesticides (OCPs) in intertidal sediment of China: Occurrence, fate and influential factors. Environmental Pollution, 257, 113634. https://doi.org/10.1016/j.envpol.2019.113634
Miglioranza, K. S. B., Ondarza, P. M., Costa, P. G., de Azevedo, A., Gonzalez, M., Shimabukuro, V. M., Grondona, S. I., Mitton, F. M., Barra, R. O., Wania, F., & Fillmann, G. (2021). Spatial and temporal distribution of persistent organic pollutants and current use pesticides in the atmosphere of Argentinean Patagonia. Chemosphere, 266, 129015. https://doi.org/10.1016/j.chemosphere.2020.129015
Mishra, K., Sharma, R. C., & Kumar, S. (2013). Contamination profile of DDT and HCH in surface sediments and their spatial distribution from North-East India. Ecotoxicology and Environmental Safety, 95, 113–122. https://doi.org/10.1016/j.ecoenv.2013.05.029
Mitra, S., Corsolini, S., Pozo, K., Audy, O., Sarkar, S. K., & Biswas, J. K. (2019). Characterization, source identification and risk associated with polyaromatic and chlorinated organic contaminants (PAHs, PCBs, PCBzs and OCPs) in the surface sediments of Hooghly estuary, India. Chemosphere, 221, 154–165. https://doi.org/10.1016/j.chemosphere.2018.12.173
Mitton, F. M., Gonzalez, M., Monserrat, J. M., & Miglioranza, K. S. B. (2016). Potential use of edible crops in the phytoremediation of endosulfan residues in soil. Chemosphere, 148, 300–306. https://doi.org/10.1016/j.chemosphere.2016.01.028
Moon, H.-B., Kim, H.-S., Choi, M., Yu, J., & Choi, H.-G. (2009). Human health risk of polychlorinated biphenyls and organochlorine pesticides resulting from seafood consumption in South Korea, 2005–2007. Food and Chemical Toxicology, 47, 1819–1825. https://doi.org/10.1016/j.fct.2009.04.028
Murayama, H., Takase, Y., Mitobe, H., Mukai, H., Ohzeki, T., Shimizu, K.-I., & Kitayama, Y. (2003). Seasonal change of persistent organic pollutant concentrations in air at Niigata area, Japan. Chemosphere, 52, 683–694. https://doi.org/10.1016/S0045-6535(03)00105-X
Nasir, J., Wang, X., Xu, B., Wang, C., Joswiak, D. R., Rehman, S., Lodhi, A., Shafiq, S., & Jilani, R. (2014). Selected organochlorine pesticides and polychlorinated biphenyls in urban atmosphere of Pakistan: Concentration, spatial variation and sources. Environmental Science & Technology, 48, 2610–2618. https://doi.org/10.1021/es404711n
Niu, L., Xu, C., Zhu, S., & Liu, W. (2016). Residue patterns of currently, historically and never-used organochlorine pesticides in agricultural soils across China and associated health risks. Environmental Pollution, 219, 315–322. https://doi.org/10.1016/j.envpol.2016.10.060
Pan, X., Wei, J., Qu, R., Xu, S., Chen, J., Al-Basher, G., Li, C., Shad, A., Dar, A. A., & Wang, Z. (2020). Alumina-mediated photocatalytic degradation of hexachlorobenzene in aqueous system: Kinetics and mechanism. Chemosphere, 257, 127256. https://doi.org/10.1016/j.chemosphere.2020.127256
Park, J. S., Shin, S. K., Kim, W. I., & Kim, B. H. (2011). Residual levels and identify possible sources of organochlorine pesticides in Korea atmosphere. Atmospheric Environment, 45, 7496–7502. https://doi.org/10.1016/j.atmosenv.2010.10.030
Pokhrel, B., Gong, P., Wang, X., Khanal, S. N., Ren, J., Wang, C., Gao, S., & Yao, T. (2018). Atmospheric organochlorine pesticides and polychlorinated biphenyls in urban areas of Nepal: Spatial variation, sources, temporal trends, and long-range transport potential. Atmospheric Chemistry and Physics, 18, 1325–1336. https://doi.org/10.5194/acp-18-1325-2018
Pozo, K., Harner, T., Shoeib, M., Urrutia, R., Barra, R., Parra, O., & Focardi, S. (2004). Passive-sampler derived air concentrations of persistent organic pollutants on a north−south transect in Chile. Environmental Science & Technology, 38, 6529–6537. https://doi.org/10.1021/es049065i
Qu, C., Albanese, S., Li, J., Cicchella, D., Zuzolo, D., Hope, D., Cerino, P., Pizzolante, A., Doherty, A. L., Lima, A., & De Vivo, B. (2019). Organochlorine pesticides in the soils from Benevento provincial territory, southern Italy: Spatial distribution, air-soil exchange, and implications for environmental health. Science of the Total Environment, 674, 159–170. https://doi.org/10.1016/j.scitotenv.2019.04.029
Rimondino, G. N., Pepino, A. J., Manetti, M. D., Olcese, L., & Argüello, G. A. (2018). Latitudinal distribution of OCPs in the open ocean atmosphere between the Argentinian coast and Antarctic Peninsula. Environmental Science and Pollution Research, 25, 13004–13013. https://doi.org/10.1007/s11356-018-1572-7
Romanić, S. H., Vuković, G., Klinčić, D., & Antanasijević, D. (2018). Self-organizing maps for indications of airborne polychlorinated biphenyl (PCBs) and organochlorine pesticide (OCPs) dependence on spatial and meteorological parameters. Science of the Total Environment, 628–629, 198–205. https://doi.org/10.1016/j.scitotenv.2018.02.012
Sanlı, G. E., & Tasdemir, Y. (2020). Seasonal variations of organochlorine pesticides (OCPs) in air samples during day and night periods in Bursa, Turkey. Atmospheric Pollution Research, 11, 2142–2153. https://doi.org/10.1016/j.apr.2020.06.010
Shunthirasingham, C., Oyiliagu, C. E., Cao, X., Gouin, T., Wania, F., Lee, S.-C., Pozo, K., Harner, T., & Muir, D. C. G. (2010). Spatial and temporal pattern of pesticides in the global atmosphere. Journal of Environmental Monitoring, 12, 1650–1657. https://doi.org/10.1039/C0EM00134A
Srimurali, S., Govindaraj, S., Krishna Kumar, S., & Babu Rajendran, R. (2015). Distribution of organochlorine pesticides in atmospheric air of Tamilnadu, southern India. International Journal of Environmental Science and Technology, 12, 1957–1964. https://doi.org/10.1007/s13762-014-0558-3
Stockholm Convention. (2021a). Register of specific exemptions: Lindane, Stockholm Convention. Retrieved 8 June 2021, from http://chm.pops.int/Implementation/Exemptions/SpecificExemptions/LindaneRoSE/tabid/5036/Default.aspx
Stockholm Convention. (2021b). Register of specific exemptions: Technical endosulfan and its related isomers, Stockholm Convention. Retrieved 10 June 2021, from http://chm.pops.int/Implementation/Exemptions/SpecificExemptions/TechnicalendosulfanRoSE/tabid/5037/Default.aspx
Stockholm Convention. (2021c). Specific exemptions for Annex A chemicals: Expired in 2009, Stockholm Convention. Retrieved 10 June 2021, from http://chm.pops.int/Implementation/Exemptions/SpecificExemptions/AnnexAchemicalsexpiredin2009/tabid/5032/Default.aspx
Stockholm Convention. (2021d). Specific exemptions for Annex B chemicals: Expired in 2009, Stockholm Convention. Retrieved 9 June 2021, from http://chm.pops.int/Implementation/Exemptions/SpecificExemptions/ChemicalistedinAnnexBRoSE/AnnexBchemicalsexpiredin2009/tabid/5048/Default.aspx
Su, Y., Hung, H., Blanchard, P., Patton, G. W., Kallenborn, R., Konoplev, A., Fellin, P., Li, H., Geen, C., Stern, G., Rosenberg, B., & Barrie, L. A. (2006). Spatial and seasonal variations of hexachlorocyclohexanes (HCHs) and hexachlorobenzene (HCB) in the Arctic atmosphere. Environmental Science & Technology, 40, 6601–6607. https://doi.org/10.1021/es061065q
Syed, J. H., Malik, R. N., Liu, D., Xu, Y., Wang, Y., Li, J., Zhang, G., & Jones, K. C. (2013). Organochlorine pesticides in air and soil and estimated air–soil exchange in Punjab, Pakistan. Science of the Total Environment, 444, 491–497. https://doi.org/10.1016/j.scitotenv.2012.12.018
Tasdemir, Y., Vardar, N., Odabasi, M., & Holsen, T. M. (2004). Concentrations and gas/particle partitioning of PCBs in Chicago. Environmental Pollution, 131, 35–44. https://doi.org/10.1016/j.envpol.2004.02.031
Turgut, C., Gokbulut, C., & Cutright, T. J. (2009). Contents and sources of DDT impurities in dicofol formulations in Turkey. Environmental Science and Pollution Research, 16, 214–217. https://doi.org/10.1007/s11356-008-0083-3
US EPA. (1999). Compendium of methods for the determination of toxic organic compounds in ambient air, United States Environmental Protection Agency. Retrieved 3 May 2022, from https://www.epa.gov/sites/default/files/2015-08/documents/to-4ar2r.pdf
US EPA. (2007). SW-846 test method 8081B: Organochlorine pesticides by gas chromatography, United States Environmental Protection Agency. Retrieved 3 May 2022, from https://www.epa.gov/sites/default/files/2015-12/documents/8081b.pdf
Van Dyk, J. C., Bouwman, H., Barnhoorn, I. E. J., & Bornman, M. S. (2010). DDT contamination from indoor residual spraying for malaria control. Science of the Total Environment, 408, 2745–2752. https://doi.org/10.1016/j.scitotenv.2010.03.002
Vijgen, J., de Borst, B., Weber, R., Stobiecki, T., & Forter, M. (2019). HCH and lindane contaminated sites: European and global need for a permanent solution for a long-time neglected issue. Environmental Pollution, 248, 696–705. https://doi.org/10.1016/j.envpol.2019.02.029
Wang, D., Wang, Y., Singh, V. P., Zhu, J., Jiang, L., Zeng, D., Liu, D., Zeng, X., Wu, J., Wang, L., & Zeng, C. (2018a). Ecological and health risk assessment of PAHs, OCPs, and PCBs in Taihu Lake basin. Ecological Indicators, 92, 171–180. https://doi.org/10.1016/j.ecolind.2017.06.038
Wang, G., Lu, Y., Han, J., Luo, W., Shi, Y., Wang, T., & Sun, Y. (2010). Hexachlorobenzene sources, levels and human exposure in the environment of China. Environment International, 36, 122–130. https://doi.org/10.1016/j.envint.2009.08.005
Wang, S., Salamova, A., Hites, R. A., & Venier, M. (2018b). Spatial and seasonal distributions of current use pesticides (CUPs) in the atmospheric particulate phase in the Great Lakes Region. Environmental Science & Technology, 52, 6177–6186. https://doi.org/10.1021/acs.est.8b00123
Wang, W., Wang, Y., Zhang, R., Wang, S., Wei, C., Chaemfa, C., Li, J., Zhang, G., & Yu, K. (2016). Seasonal characteristics and current sources of OCPs and PCBs and enantiomeric signatures of chiral OCPs in the atmosphere of Vietnam. Science of the Total Environment, 542, 777–786. https://doi.org/10.1016/j.scitotenv.2015.10.129
Weber, J., Halsall, C. J., Muir, D., Teixeira, C., Small, J., Solomon, K., Hermanson, M., Hung, H., & Bidleman, T. (2010). Endosulfan, a global pesticide: A review of its fate in the environment and occurrence in the Arctic. Science of the Total Environment, 408, 2966–2984. https://doi.org/10.1016/j.scitotenv.2009.10.077
Yang, Y., Li, D., & Mu, D. (2008). Levels, seasonal variations and sources of organochlorine pesticides in ambient air of Guangzhou, China. Atmospheric Environment, 42, 677–687. https://doi.org/10.1016/j.atmosenv.2007.09.061
Yu, S. Y., Liu, W. J., Xu, Y. S., Zhao, Y. Z., Cai, C. Y., Liu, Y., Wang, X., Xiong, G. N., Tao, S., & Liu, W. X. (2019). Organochlorine pesticides in ambient air from the littoral cities of northern China: Spatial distribution, seasonal variation, source apportionment and cancer risk assessment. Science of the Total Environment, 652, 163–176. https://doi.org/10.1016/j.scitotenv.2018.10.230
Zhang, L., Dong, L., Shi, S., Zhou, L., Zhang, T., & Huang, Y. (2009). Organochlorine pesticides contamination in surface soils from two pesticide factories in Southeast China. Chemosphere, 77, 628–633. https://doi.org/10.1016/j.chemosphere.2009.08.055
Zhang, L., Dong, L., Yang, W., Zhou, L., Shi, S., Zhang, X., Niu, S., Li, L., Wu, Z., & Huang, Y. (2013). Passive air sampling of organochlorine pesticides and polychlorinated biphenyls in the Yangtze River Delta, China: Concentrations, distributions, and cancer risk assessment. Environmental Pollution, 181, 159–166. https://doi.org/10.1016/j.envpol.2013.06.033
Zheng, H., Gao, Y., Xia, Y., Yang, H., & Cai, M. (2020). Seasonal variation of legacy organochlorine pesticides (OCPs) from East Asia to the Arctic Ocean. Geophysical Research Letters, 47, e2020GL089775. https://doi.org/10.1029/2020GL089775
Zheng, X., Chen, D., Liu, X., Zhou, Q., Liu, Y., Yang, W., & Jiang, G. (2010). Spatial and seasonal variations of organochlorine compounds in air on an urban–rural transect across Tianjin, China. Chemosphere, 78, 92–98. https://doi.org/10.1016/j.chemosphere.2009.10.017
Zhou, Q., Wang, J., Meng, B., Cheng, J., Lin, G., Chen, J., Zheng, D., & Yu, Y. (2013). Distribution and sources of organochlorine pesticides in agricultural soils from central China. Ecotoxicology and Environmental Safety, 93, 163–170. https://doi.org/10.1016/j.ecoenv.2013.03.029
Acknowledgements
The authors would like to thank Dr. Gwang-Ryul Lee and Hee-Young Kim at the National Institute of Environmental Research (NIER), and Prof. Sung-Deuk Choi at Ulsan National Institute of Science and Technology (UNIST) for their sample collection efforts to perform this study.
Funding
This work was supported by the National Institute of Environmental Research, funded by the Ministry of Environment of the Republic of Korea (grant number NIER-2020–01-01–023). The funding agency had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, M., Lee, S., Noh, S. et al. Assessment of organochlorine pesticides in the atmosphere of South Korea: spatial distribution, seasonal variation, and sources. Environ Monit Assess 194, 754 (2022). https://doi.org/10.1007/s10661-022-10335-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10335-x