Abstract
To a large extent, public health can be prevented from being compromised by having access to potable water whose physicochemical fingerprints conform to the WHO Guidelines for drinking water. This study was carried out to assess the physicochemical properties and heavy metals speciation of groundwater in Nigeria. Water samples from thirteen sites were collected for three months and analysed for levels of pH, conductivity, nitrate, sulphate, phosphate, and heavy metals using standard methods. The results of the analysis revealed that the respective mean temperature, conductivity, pH, total dissolved solids, and oxygen reduction potential of Ifetedo sites were 27.57 ± 0.66 °C, 318.93 ± 108.58 µS/cm, 5.29 ± 0.20, 207.40 ± 73.58 mg/L, and 265.17 ± 11.29 mV, while those of Garage Olode were 28.53 °C, 275.04 ± 133.75 µS/cm, 5.23 ± 0.04, 177.66 ± 89.37 mg/L, and 270.43 ± 37.75 mV. Total mean levels (µg/mL) of heavy metals ranged from 0.04 ± 0.01 Cd to 26.21 ± 3.63 Mn at Ifetedo, while at Garage Olode, the range was between 0.02 ± 0.00 Cd and 14.49 ± 5.70 Mn. The respective ranges of mean values (µg/mL) of exchangeable metals were 0.01 ± 0.00 Cd to 10.64 ± 1.20 Mn and 0.01 ± 0.00 to 7.53 ± 2.57 Mn at Ifetedo and Garage Olode. Going by WHO provision for drinking water, the study concluded that the levels of heavy metals in the potable water samples could constitute serious health hazards over prolonged usage unless some water treatment protocols are adopted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is perhaps the most essential resource on the earth required for drinking, cooking, tourism, and cultural purposes; it is also required for sanitation and sustaining of the earth ecosystem in addition to having a critical input in the industry (Mark et al., 2002; Mishra & Pandey, 2008). Water is also found to play a key role in climate and co-evolution of life on Earth (Bhoemick et al., 2017). Despite the abundance of water on earth, about 0.3% is usable (Bibi et al., 2016) and it is on record that about 30% of the entire global population have no access to potable water (WHO, 2017). A study has revealed that about 320 million people in the African continent were excluded from drinking water services (Bazié, 2014), while drinking water availability in West Africa has been estimated to be below 60.00% (CMAE, 2008).
Groundwater (from wells and boreholes) is the main source of potable water in rural West Africa (Adamou et al., 2020; Raphael et al., 2018) as indicated by several studies that were carried out to investigate the quality of drinking water in rural areas (Barakat et al., 2018; Khatri et al., 2016; Malek et al., 2019; Mulamattathil et al., 2015; Raphael et al., 2018). Also, risk assessment for consuming unhealthy water has been carried out in West Africa, particularly the challenges that the rural dwellers are facing (Adamou et al., 2020; Degbey et al., 2011; Dougna et al., 2015; Raji & Ibrahim, 2011; Raphael et al., 2018). Some studies conducted in Nigeria to determine the potability of various water sources inferred that anthropogenic activities are the primary cause of pollution of these water sources (Egbueri, 2018; Ekere et al., 2019; Egbueri & Mgbeonu, 2020; Ukah et al., 2020).
According to WHO analysis, about 80% of diseases are caused by consumption of unhealthy water in developing countries (Khan et al., 2013) and about 3.1% of deaths occurred as a result of unhygienic and poor quality water (Pawari & Gawande, 2015). Water pollution is thought to be one of the primary causes of sickness, with over fourteen thousand people dying every day around the world (Egbueri, 2018; Egbueri et al., 2020a; Ukah et al., 2020). Trace metals occurring as part of the contents of inorganic compounds have been recognized as substantial pollutants in many drinking water sources (Egbueri, 2020a, b). Heavy metals penetrate the soil/water system and eventually pollute the food chain due to their nondegradable nature (Azimi et al., 2017; Pratush et al., 2018). Sometimes, groundwater contamination occurs naturally, but pollution is usually the result of human activities on the land surface. The source of heavy metals and other contaminants could be from waste generated mainly from households, markets, offices, farmland, spoilt electronic, and vehicle parts. These contaminants could penetrate the soil and contaminate the groundwater overtime.
Groundwater serves as the major source of water for the people of the study area. Up till now, there is a paucity of data on groundwater resources of Ifetedo and Garage Olode particularly in the area of physicochemical composition. The two towns are the economic hubs of Ife-South Local Government Area (LGA), actively involved in all-year-round agricultural practices, diverse commercial activities and semi-mega industrial engagements in water packaging, and artisan works that involve metals and wood. This study investigated the physicochemical properties and heavy metal speciation analysis of groundwater of Ifetedo and Garage Olode in Ife-South LGA of Osun State. This was to evaluate the extent to which the water conformed to World Health Organization (WHO) safety guidelines.
Methodology
Study area
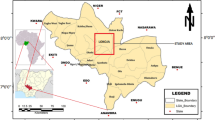
Ife-South, a local government area in Osun State, Nigeria, has its headquarters in the town of Ifetedo on latitude 7.183°N and longitude 4.7°E with an area of 1.948 km2 (Omotola & Ofoize, 2019). The groundwater sampling regime covered the two major towns, Ifetedo and Garage Olode, in Ife-South LGA of Osun State. The two towns are relatively close to each other and are moderately populated; the population of Ifetedo, using the annual 26.8% increase, has been estimated to be 39,491 as at 2020. The geographical locations of the sampling sites are presented in Table 1.
Sterilization of apparatus
The newly purchased plastic bottles used for sampling were first washed with liquid detergent solution, rinsed properly with tap water, and then with distilled water. They were then soaked in 10% HNO3 for 48 h. This was followed by vigorous triplicate rinsing with distilled water. The bottles were then rinsed with the water samples at the sampling site three times before being filled to the brim with the sample to preclude trace metal contamination from any other source (Fig. 1).
Chemicals used and their sources
All chemical used were of analytical grade: HNO3, HClO4 (Riedel–de Haen, Germany), HCl (Sigma-Aldrich, Germany), NaOH pellets, and NaCl (British Drug House, BDH, Chemical Ltd, Poole England). From these, reagent I (0.12 M HCl containing 100 g of NaCl in 500 mL solution) and reagent II (2.0 M NaOH and 0.34 M NaCl in 1 L of water) were prepared using doubly distilled water.
Sample collection, handling, and storage
About 2 L of the water sample for metal and physicochemical parameters analyses was collected at various sampling sites. The samples were immediately transported to the laboratory for immediate speciation and anion analyses, while 2 mL of conc. HNO3 was added to the samples meant for total metal analysis at the sampling site before being stored in the refrigerator at about 4 °C. This was done to prevent microbial degradation.
Physicochemical study
The physicochemical parameters were carried out in situ with the aid of a calibrated water instrument analyser (Ultra Meter II™6Psi Serial 620,769, USA). The samples collected were analysed for temperature, conductivity, pH, total dissolved solids, and oxidation–reduction potential. In addition, concentrations of NO3−, SO42−, and PO43− were also determined using appropriate standard techniques.
Preparation of the samples for heavy metal speciation study
The method described by Ogunfowokan et al. (2013) for speciation analysis of heavy metals was adopted in this study.
Digestion of water samples for total metal determination
The water samples were digested using the method described by APHA (2005) in which case 20 mL aliquot of water sample was transferred into a Teflon beaker to which about 5 mL of conc. HNO3 was added along with a few Hengar granules. This was brought to a gentle boiling on a thermostated hot plate for about 45 min while replenishing with conc. HNO3 to avoid total dryness. Thereafter, 5 mL mixture of conc. HClO4 and conc. HNO3 in the ratio 1:1 was introduced into the sample (Christian, 2004) and then heated again to a slow-boiling for about 30 min making sure that evaporation to dryness was prevented as more of the acid mixture was added when necessary. The digested sample was quantitatively transferred into 25 mL volumetric flask and diluted to volume with doubly distilled water. A blank determination was carried out. Metals in the worked-up samples were directly quantified by AAS (PG 990 model) available at the Department of Geology, Obafemi Awolowo University, Ile-Ife, Nigeria.
Quality control measures adopted
Blank determination
Alongside each determination was a blank to check for background levels of the heavy metals in the reagents used particularly for the digestion procedures.
Recovery analysis
This was conducted to ascertain the precision of the analytical instrument used in this study since standard reference water material was not available when this study was carried out. Accurately measured 25 mL water sample was put into a Teflon beaker and was spiked with 25 mL of 50 µg/mL of the standard heavy metal solution mixture. Also, another 25 mL water sample was put into a Teflon beaker and 25 mL of doubly distilled water was added. Both samples, after digestion protocols, were taken through instrumental analysis. The results obtained were compared, and percentage recoveries were evaluated. The percentage recovery (%R) was evaluated from the relationship:
where A is the metal concentration in the spiked sample and B is the metal concentration in the unspiked sample.
Replicate analysis
Triplicate analysis was carried out to further check the reproducibility of the methods used in this study along with routine analysis of the prepared standards and the blanks. The instrument was calibrated using the method of a standard addition to reduce the matrix effect.
Exposure and risk assessment
To estimate the daily exposure of an individual, USEPA suggests the lifetime average daily dose (LADD) as the exposure metric. The following equation is a similar representation of the daily exposure route modified from Chrowtoswski (1994) and adopted by Kavacar et al. (2009):
where CDI is the chronic daily intake (mg kg−1 day−1), C is the drinking water contaminant concentration (mg L−1), DI is the average intake rate of drinking water (3.7 L day−1 for male and 2.7 L day−1 for female), and BW is the body weight (i.e. 65 kg). The hazard quotient (HQ) is calculated using the following equation (USEPA, 1992; Kavacar et al., 2009):
where RfD is the reference dose (mg kg−1 day−1). RfD values used in this study were obtained from USEPA IRIS (2010). Health risk assessment of the metals was interpreted based on the values of HQ and THI. Values less than 1 (HQ or THI < 1) means no risk.
Hazardous index
To estimate the risk to human health through more than one heavy metal (HM), the hazard index (HI) has been developed. The hazard index is the sum of the hazard quotients for all heavy metals which were calculated by the equation below (Guerra et al., 2010).
It assumes that the magnitude of the adverse effect will be proportional to the sum of multiple metal exposures.
Statistical method used
Analyses of variance (ANOVA), correlation, and cluster analyses were used to interpret and determine the relationships existing among the results of the heavy metals obtained. Correlation and cluster analyses provide information on the interactions and sources of heavy metals in the groundwater.
Results and discussion
Validation of analytical procedure
The reliability of the analytical procedure adopted in this study was tested in terms of percentage recovery of the analysed metals under experimental conditions used. The percentage recoveries obtained, with a range between 83.78% Cd2+ and 98.2% Mn2+ (Table 2), fell within the 70–110% recovery range stipulated by the EU (1999) as the acceptable limit within which the analytical procedure is adjudged to be reliable. Thus, the measured values of metals obtained in this study are adjudged reliable and acceptable.
Physicochemical parameters of the groundwater quality
The mean values of physicochemical properties of the groundwater are presented in Table 3. The values obtained were compared with that of the World Health Organization (WHO, 2017) and the Standard Organization of Nigeria (SON, 2015) guidelines for drinking water.
Temperature
The temperature values of Ifetedo water samples ranged from 28.10 ± 0.10 °C at Oke-Awona to 29.00 ± 0.10 °C at Otutu with a mean value of 28.53 ± 0.31 °C, while those of Garage Olode samples, with a mean value of 27.57 ± 0.66 °C, ranged from 26.90 ± 0.20 °C at Oluweri II to 28.30 ± 0.10 °C at Iyodun. The coefficients of variations (CV) values are 1.08 and 2.39 respectively for Ifetedo and Garage Olode. Thus, there was no significantly different variability in the factors that could alter the temperature values of the studied areas. The metabolic rates of most groundwater organisms are controlled by temperature; warm water aids microbial metabolism and reduces oxygen solubility. These two opposing factors would naturally favour the multiplication of anaerobic microorganisms leading to their increased activities at releasing more wastes into the groundwater at higher rates than expected. In the process, groundwater becomes unsafe for domestic uses, and consumption of such polluted groundwater could cause gastroenteritis, diarrhoea, and diseases such as cholera (Mbanugo et al., 1990). These temperatures were all above the WHO maximum limit of 25 °C (WHO, 2011a; Sixtus et al., 2016). It is desirable to have the temperature of drinking water to be moderate (not too hot or cold) as this enhances its palatability.
Electrical conductivity
The electrical conductivity of Ifetedo water samples ranged from 108.00 ± 0.80 µS/cm at Okesoda to 503.00 ± 1.30 µS/cm at Baba Akodi with a mean value of 275.04 ± 133.75 µS/cm, while that of Garage Olode samples, with a mean value of 318.57 ± 108.58 µS/cm, ranged from 192.70 ± 0.20 µS/cm at Abanise to 476.00 ± 0.10 µS/cm at Abacele. The CV values were 48.63 and 34.04 respectively. The EC values in the groundwater samples were below the maximum permissible standard of 1000 μS/cm set for drinking water (SON, 2015; WHO, 2017). Therefore, with respect to EC values, the groundwater samples of the studied communities were safe for domestic use such as drinking.
pH
The pH values for Ifetedo water samples ranged from 5.19 ± 0.23 at Ayesan to 5.30 ± 0.04 at Alagbado with a mean value of 5.29 ± 0.20, while that of Garage Olode samples, with a mean value of 5.23 ± 0.04, ranged from 5.01 ± 0.05 at Abacele to 5.55 ± 0.21 at Oluweri II. These values fell below the range of 6.5–8.5 recommended by WHO and SON for drinking water. Considering these pH values, the water samples were considered to be slightly acidic to be used as potable water. Some health-related issues in humans associated with exposure to extremely low pH values are an increase in eye and skin, irritation (HC, 2016). There could also be an increase in the solubility of metallic xenobiotics where water pH is consistently within acidic range.
Total dissolved solid
The TDS of Ifetedo water samples ranged from 68.21 ± 0.10 mg/mL at Okesoda to 332.00 ± 0.20 mg/L at Baba Akodi with mean values of 177.66 ± 89.37 mg/mL, while that of Garage Olode samples, with a mean value of 207.40 ± 73.58 mg/mL, ranged from 123.30 ± 0.10 mg/mL at Abanise to 314.50 ± 0.20 mg/mL at Abacele. The TDS results obtained in this study were within the acceptable limit of 1000 mg/L for domestic use (WHO, 2017). Relying on water sources with high TDS levels can increase the chances of having kidney stones (Sharma & Bhattachatya, 2016). In addition, using water with elevated levels of TDS for drinking purposes could cause some health challenges for both humans and fowls especially where anions such as Cl− are responsible for the high levels of TDS in the water (Weltzien, 2002).
Oxidation reduction potential
The oxygen reduction potential in Ifetedo water samples had a mean value of 270.40 ± 37.75 (mV) and ranged from 253.90 ± 0.10 (mV) at Ayesan to 259.90 ± 0.10 (mV) at Isale-Ife, while those of Garage Olode samples had a mean value of 265.17 ± 11.29 (mV) with values that ranged from 254.98 ± 0.02 (mV) at Abanise to 284.99 ± 0.01 (mV) at Abacele. There was no significantly different variability among the oxygen-reduction potential values of the studied areas. Oxidation–reduction potential (ORP) simply means a determination of water’s capability to oxidize or reduce contaminants. When the ORP levels of a particular water sample ranged between 300 and 500 mV, the water is considered healthy (Wetzel, 1983). However, when the ORP value of water is low, the toxicity of certain metals and contaminants can increase, thereby increasing the amount of dead and decaying materials in the water that cannot readily clear off (Horne & Goldman, 1994).
Levels of metals in the water samples
The results in Table 4 revealed that the mean amount of total metals (Mn, Cu, Zn, Cd, and Pb) in the groundwater of Ifetedo and Garage Olode towns was significantly higher than the guideline limits stipulated by World Health Organisation (WHO, 2017) for drinking water. The respective total metal burden (TMB) (in µg/mL) with respect to Mn, Cu, Zn, Cd, and Pb in Ifetedo was of the order: Okesoda (3.80) < Otutu (23.78) < Alagbado (24.63) < Baba Akodi (26.22) < Oke-Awona (29.22) < Isale-Ife (31.41) < Ayesan (32.31), while that of Garage Olode was of the order: Abanise (39.56) < Abacele (39.88) < Iyodun (40.52) < Oluweri II (45.19) < Gbaro (47.32) < Oluweri I (50.10). The high metal load observed at Ayesan area in Ifetedo and Oluweri I in Garage Olode could be a result of the geochemical composition of the underlying rocks around the well and aerial deposition of dust which might contain some of the metals. It could also be due to the slight acidic pH values which enhanced the solubility and mineralization of the metals from their underlying rocks and compounds.
The total Zn metal analysed in Ifetedo water samples with a mean of 8.23 ± 3.49 µg/mL ranged from 0.84 ± 0.28 µg/mL at Okesoda to 11.02 ± 2.30 µg/mL at Ayesan, while that of Garage Olode having a mean of 13.57 ± 2.39 ranged from 10.33 ± 1.99 µg/mL at Iyodun to 15.77 ± 4.62 µg/mL at Oluweri II. The exchangeable fractions at Ifetedo and Garage Olode respectively gave mean values of 5.67 ± 2.41 µg/mL and 6.70 ± 0.43 µg/mL, and a range of 0.90 ± 0.05 µg/mL at Okesoda to 7.10 ± 0.04 µg/mL at Oke-Awona for Ifetedo samples, while the range at Garage Olode was from 6.00 ± 0.02 µg/mL at Gbaro to 7.10 ± 0.03 µg/mL at Iyodun (Table 5). The percentage of the exchangeable fraction at Ifetedo and Garage Olode were 68.69% and 41.78% respectively (Table 6). The Zn values obtained in this study were lower than either the 147.3 ± 30.4 µg/mL reported for hand-dug wells at Ile-Ife (Oluyemi et al., 2010) or the 45–350 µg/mL for boreholes and wells in Makurdi metropolis (Nsi and Ogori., 2005). However, the values were much higher than that reported by Oyeku and Eludoyin for Ojota area in Lagos water samples with values that ranged from 0 to 0.23 µg/mL (Oyeku & Eludoyin, 2010). These discrepancies might be a result of geological differences, and to a lesser extent, from other anthropological influences. Most of the total and exchangeable levels of Zn detected in the current study exceeded the WHO guideline limit of 3 µg/mL (WHO, 2017).
By implication, anyone who drinks up to 1 L of the water per day would have exceeded the 15–20 mg recommended daily intake of Zn (World Health Organization, 2008), and hence, may suffer from Zn induced health problems such as nausea, dizziness, gastric ulcer, muscle pain, impairment of immune function, headaches, fatigue, possible renal failure, and increased blood level of insulin-like growth factor and testosterone, both of which are related to prostate cancer (Gonzalez et al., 2009; Jamesone, 1997; Michael & Stanford, 2003). The CV values of 40.40 and 17.39 were recorded for Ifetedo and Garage Olode respectively, indicated a significant difference (at p ≤ 0.05). This implies that the Zn level was differently distributed at the two locations. However, from site to site within a location, there was no significant difference in distribution. Hence, the factors such as lithogenic characteristics of the bedrocks that accounted for the presence of Zn in the water samples were not subjected to sudden noticeable changes as could be witnessed in the case of anthropogenic factors. Zinc has also been found to be useful in the body for the healing process after injury, normal growth and development during pregnancy, childhood, and adolescence also for proper sense of taste and smell (Cohen & David, 2007; Elinder, 1986); prostate enlargement and cancer in humans have been linked to low levels or excess of zinc in human body (Gonzalez et al., 2009).
Lead (Pb), with no known health benefit, had significant amounts of it detected in all groundwater samples of the areas under study. The mean total Pb analysed in Ifetedo water samples with mean value of 0.36 ± 0.16 µg/mL ranged from 0.07 ± 0.05 µg/mL at Okesoda to 0.50 ± 0.15 µg/mL at Otutu, while those of Garage Olode samples with a mean value of 0.94 ± 0.17 µg/mL ranged from 0.64 ± 0.01 µg/mL at Abacele to 1.16 ± 0.33 µg/mL at Oluweri II. The exchangeable fractions of Pb at Ifetedo and Garage Olode respectively gave mean values of 0.16 ± 0.06 µg/mL and 0.12 ± 0.03 µg/mL, and a range from 0.04 ± 0.01 at Okesoda to 0.20 ± 0.03 µg/mL at Baba Akodi for Ifetedo samples, while that of Garage Olode ranged from 0.05 ± 0.03 µg/mL at Gbaro to 0.15 ± 0.02 µg/mL at Iyodun. The percentages of exchangeable fractions at Ifetedo and Garage Olode were 44.44% and 17.02% respectively (Table 6). The Pb values obtained in this study were lower than the 4.01 ± 3.82 µg/mL reported by Oluyemi et al. (2010) for water samples of Ife North Local Government of Osun State and 4.9 ± 0.18 µg/mL reported by Abiola (2010) for groundwater in Ibadan. The elevated level of groundwater Pb observed in this study could be an indication of surface pollution resulting from automobile combustion of petrol containing organo-lead additives (that are still widespread in the environment due to the indestructible nature of metals), disposal of used lead-acid batteries, alloys, soldering metals, and so on (Oluyemi et al., 2010). Both the total and exchangeable levels of Pb detected in the current study exceeded WHO guideline limit of 0.01 µg/mL (WHO, 2017). By implication, anyone who drinks up to 1 L of the water per day would have exceeded the recommended daily intake of Pb. Hence, from the perspectives of their Pb contents, the water samples are unfit for long-term human consumption to avoid Pb-induced health problems such as the increased risk of hypertension and promotion of birth defects in pregnant women (Amadi et al., 2017).
The CV values recorded for Ifetedo and Garage Olode are 44.44 and 18.09 respectively. The Pb distribution indicated a significant difference (at P ≤ 0.05) implying that the Pb level was not evenly but differently distributed at the two locations. There was no significant difference in distribution from site to site within a location. Hence, the factors such as lithogenic characteristics of the bedrocks that accounted for the presence of Pb in the water samples were not subjected to sudden noticeable changes as could be witnessed in the case of anthropogenic factors such as combustion of fuel containing lead compound as anti-knocking agents. It has been reported that lead interferes in the biosynthesis of porphyrins and heme (Hodgson, 2010; Nuwayhid et al., 2003). A high concentration of lead in the body affects the nervous system, proper functioning of kidneys, and blood circulation (Assi et al., 2016).
In the case of Cd, the levels detected in the water samples of Ifetedo had a mean value of 0.02 ± 0.00 µg/mL. The values ranged from 0.01 ± 0.00 µg/mL at Isale-Ife to 0.02 ± 0.00 µg/mL at Okesoda, while those of Garage Olode samples, with a mean value of 0.04 ± 0.01 µg/mL, ranged from 0.02 ± 0.01 µg/mL at Iyodun to 0.05 ± 0.03 µg/mL at Oluweri II. The exchangeable fractions at Ifetedo and Garage Olode respectively gave mean values of 0.01 ± 0.00 µg/mL at Baba Akodi for Ifetedo samples, while that of Garage Olode ranged from 0.01 ± 0.00 µg/mL at Gbaro to 0.01 ± 0.00 µg/mL at Abacele. The percentages of the exchangeable fractions at Ifetedo and Garage Olode were 50% and 25% respectively (Table 6) which implies that factors contributing to Cd levels at the two sampling areas differed from each other. The values of Cd obtained in this study were lower than the values (0.98 ± 0.67) µg/mL reported by Oluyemi et al. (2010). The total and exchangeable levels of Cd detected in the current study exceeded the WHO guideline limit of 0.003 µg/mL (WHO, 2017). Considering the Cd levels, the water samples were unfit for drinking, and long-term reliance of consumers on the untreated water for drinking purposes may portend Cd-induced health problems manifesting as cancer and weakness of bones (WHO, 2011b; Rahimzdeh et al., 2017).
The CV values of 0.00 and 25.00 for Cd distribution at Ifetedo and Garage Olode respectively indicated a significant difference (at P ≤ 0.05), implying that Cd was not evenly distributed at the two locations. There was no significant difference in the distribution of Cd from site to site within a given location. High levels of Cd in humans have been linked skeletal deformities, reproductive system disturbances, and grave embryonic developmental challenges (Hodgson, 2010). Acute exposure to Cd could lead to nausea, vomiting, and abdominal pain (Hodgson, 2010; Jãrup, 2003; Thompson & Bannigan, 2008).
The total mean level of Cu metal in Ifetedo water samples was 1.39 ± 0.62 µg/mL, having a range of 0.56 ± 0.39 µg/mL at Okesoda to 2.16 ± 1.10 µg/mL at Oke-Awona, while that of Garage Olode samples with a mean value of 2.90 ± 0.56 µg/mL ranged between 2.27 ± 1.07 µg/mL at Abanise to 3.83 ± 0.81 µg/mL at Oluewri I. The exchangeable Cu fractions at Ifetedo and Garage Olode respectively had mean values of 0.37 ± 0.15 µg/mL and 0.40 ± 0.02 µg/mL respectively with a range of 0.30 ± 0.06 µg/mL at Isale-Ife to 0.50 ± 0.01 µg/mL at Baba Akodi for Ifetedo samples, while that of Garage Olode ranged from 0.30 ± 0.08 µg/mL at Oluweri I to 0.40 ± 0.02 µg/mL at Oluweri II. The percentage of exchangeable fractions at Ifetedo and Garage Olode were 26.62% and 12.76% respectively (Table 6). Both the total and exchangeable levels of Cu detected in the current study exceeded WHO guideline limit of 2 µg/mL (WHO, 2017) which implies that those drinking up to 1 L of the water per day would have exceeded the 2–3 mg recommended daily intake of Cu (WHO, 2008), hence may suffer from Cu induced health challenges which include inflammation in the brain tissue, fatigue, hair loss, acne, allergies, depression, premenstrual syndrome, migraines, anxiety, panic attacks, kidney and liver dysfunctions, adrenal hyperactivity and insufficiency, learning disorders, autism, cancer, and severe neurological defects (Ackah et al., 2014; Uriu-Adams & Keen, 2005). It has been reported that a moderate level of copper in the body could serve as an antioxidant and helps the body to remove free radicals and prevent cell structure damage (Salama & Radwan, 2005).
The CV values of 46.60 and 18.98 respectively for Cu distribution at Ifetedo and Garage Olode indicated a significant difference (at P ≤ 0.005) implying that the Cu level was not evenly distributed at the two locations. Hence, factors such as lithogenic characteristics of the bedrocks that accounted for the presence of Cu in the water samples were not subject to sudden noticeable changes, but anthropogenic factors that might include unguarded disposal of copper-containing materials could be equally responsible.
The mean total Mn analysed in Ifetedo water samples has a mean value of 14.49 ± 5.70 µg/mL ranged from 2.31 ± 0.76 µg/mL at Okesoda to 18.68 ± 1.51 µg/mL at Ayesan, while those at Garage Olode samples has a mean value 26.21 ± 3.63 µg/mL ranged between 20.51 ± 5.59 at Abanise to 31.45 ± 3.54 µg/mL at Oluweri I. The exchangeable fractions at Ifetedo and Garage Olode respectively had 7.53 ± 2.57 µg/mL and 10.64 ± 1.20 µg/mL as mean values. The percentage of the exchangeable metal fractions at Ifetedo and Garage Olode were 22.02% and 40.59% respectively (Table 6). The Mn values obtained in this study were lower than the 93.65 ± 0.16 µg/mL for hand-dug well and 99.14 ± 0.23 µg/mL for boreholes of Ile-Ife reported by Oluyemi et al., (2010). However, the values obtained were relatively high when compared with the concentration of 0.005–0.0055 mg/L in well water and 0.14—0.15 mg/L in boreholes of Makurdi reported by Nsi and Ogori (2005). The total and exchangeable levels of Mn detected in the current study exceeded WHO guideline limit of 0.15 µg/mL (WHO, 2017). The recommended daily intake of Mn is 11 mg/L (WHO, 2008). Drinking just 1 L of the water per day would mean exceeding the recommended quantity. A higher concentration of Mn in the body could lead to neurological toxicity characterized by facial muscle spasms, irritability, aggressiveness, hallucinations, and tremors (Iweala et al., 2014).
The CV values of 39.33 and 13.84 respectively for Mn distribution at Ifetedo and Garage Olode indicated a significant difference (at P ≤ 0.05) implying that there was high disparity in the factors responsible for the occurrence of Mn at the two locations probably as a result of different lithogenic characteristics of the bedrocks and anthropogenic activities. It has been reported that ingestion of oxides of manganese and its compounds could lead to central nervous system disorder, a permanent neurological crippling disorder of the extrapyramidal system similar to Parkinson’s diseases and a diet deficient in Mn could lead to poor growth and impaired reproduction (Hidiroglou, 1979; Keen & Ziderberg-Cher, 1996).
Trends of coefficient of variation of metals in the water samples
Generally, coefficient of variation (CV) is a useful statistical tool that can be employed to interpret the temporal and spatial distribution and variability pattern of pollutants in an environmental matrix. According to the charts in Figs. 2 and 3, the trends of Mn, Zn, and Cu followed the same patterns for the sample locations, while the general trend was of the order Mn ˃ Zn ˃ Cu ˃ Pb ˃ Cd. This suggests that the metals may have originated from a common source and the ratio of their composition is relatively constant. The geology of Garage Olode and Ifetedo relative to soil structure and mineral composition of the soil formed mainly from igneous rock weathering might have a link to the trend and the high content of these metals in groundwater serves as transporting medium of the minerals in the second deposition process.
Health risk index
Table 7 presents the health risk index parameter obtained for the total mean of the metals in the water sample obtained from the two towns. The hazard quotient of Mn, Zn, Cu, Pb, and Cd in water from the study area were 82.742, 2.068, 3.053, 9.250, 1.780, and 60.379, 1.509, 2.228, 6.750, 1.246 for male and female respectively. The fact that these values were greater than 1 implies that they pose a health risk to regular consumers of the water samples being investigated.
Concentration of anion levels in the water samples
The concentration of nitrate in Ifetedo ranged from 20.35 ± 0.25 µg/mL at Ayesan to 79.32 ± 0.23 µg/mL at Okesoda with a mean value of 79.32 ± 19.99 µg/mL, while that of Garage Olode has a mean value of 41.41 ± 7.65 µg/mL, ranged from 31.95 ± 2.07 µg/mL at Iyodun to 53.35 ± 1.06 µg/mL at Gbaro. These values fell within 50–100 µg/mL recommended SON standard of underground water (well) waters. By implication, the water is free of Algae cover which may result from eutrophication and consequently suffers no depletion in the dissolved oxygen. The CV values of 25.20 and 18.47 recorded for Ifetedo and Garage Olode respectively show that there was no high variability among the nitrate values of the studies area.
The sulphate ion recorded in Ifetedo with a mean of 150.37 ± 96.61 µg/mL ranged from 20.34 ± 0.56 µg/mL at Baba Akodi to 270.34 ± 20.11 µg/mL at Isale-Ife, while that of Garage Olode with a mean value of 250.25 ± 3.05 µg/mL ranged from 10.23 ± 1.01 µg/mL at Gbaro to 250.25 ± 3.05 µg/mL at Oluweri I. These values fell below 250 µg/mL SON standard of groundwater. A high concentration of sulphate ions can cause scale build-up in water pipes and may be associated with a bitter taste in water, and can have a laxative effect on humans and young livestock (Atoyebi, 2010). With CV values of 62.24 and 67.75, there was no high variability among the sulphate values of the studied areas.
The phosphate in Ifetedo has a mean value of 0.04 ± 0.03 µg/mL ranged from 0.01 ± 0.00 µg/mL at Otutu to 0.08 ± 0.02 µg/mL at Ayesan, while those of Garage Olode sample has a mean value of 0.02 ± 0.01 µg/mL ranged from 0.001 ± 0.01 µg/mL at Oluweri I and Abanise to 0.03 ± 0.03 µg/mL at Abacele fell below 5 µg/mL SON recommended standard for groundwater. The phosphate levels were generally low hence could not support the presence of blue algae growth on the walls of most of the sampled wells. The CV values of 75.00 and 50.00 recorded for.both towns indicated that there were relatively high variabilities among the phosphate values.
Cluster analysis of heavy metals in the ground water
The cluster analysis of the heavy metals in the groundwater is presented in Figs. 4 and 5. At both sites, the cluster analysis showed that a relationship existed among Mn, Zn, Cu, Pb, and Cd which meant that the presence of these metals might have been contributed from the same anthropogenic activities that occurred around the sampling sites (Table 8).
Conclusion
This study determined the physicochemical parameters, anions, total, and exchangeable heavy metals in groundwaters of Ifetedo and Garage Olode in the South Western part of Nigeria. The anions determined in the groundwater revealed that the values recorded were within the limits stipulated by national and international regulatory bodies for drinking and domestic waters. The values recorded for most of the physicochemical parameters determined were above the limits stipulated by national and international regulatory bodies for drinking and domestic waters, thus making the untreated waters unfit for domestic purposes. In addition, the groundwater samples were contaminated with Mn, Zn, Cu, Pb, and Cd in their different species and at levels significantly above the recommended limit in drinking and domestic water by regulatory bodies. Thus, groundwater samples of the two towns required some levels of treatment to qualify them for potability purposes.
Data availability
All data generated or analysed during this study are included in this published article.
References
Abiola, O. P. (2010). Lead and coliform contaminants in potable groundwater sources in Ibadan, South-West Nigeria. Journal of Environmental Chemistry and Ecotoxicology, 2(5), 79–83.
Ackah, M., Anim, A. K., Zakaria, N., Osei, J., Saah-Nyarko, E., & GyamfiE, T. (2014). Determination of some heavy metal levels in soft drinks on the Ghanaian market using atomic absorption spectrometry method. Environmental Monitoring and Assessment, 186, 8499–8507.
Adamou, H., Ibrahim, B., Salack, S., Adamou, R., Sanfo, S., & Liersch, S. (2020). Physico-chemical and bacteriological quality of groundwater in a rural area of Western Niger: A case study of Bonkoukou. Journal of Water and Health, 18(1), 77–90. https://doi.org/10.2166/wh.2020.082
Amadi, C. N., Igweze, Z. N., & Orisakwe, O. E. (2017). Review: Heavy metals in miscarriages and stillbirths in developing nations. Middle East Fertility Society Journal., 22, 91–100.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). American Public Health Association/American Water Works Association/Water Environment Federation.
Assi, M. A., Mohd, N. M., Mohd, N., Abd, W. H., Mohd, Y., & Mohd, A. R. (2016). The detrimental effects of lead on human and animal health. Veterinary World, 9, 660.
Atoyebi, A. O. (2010). Determination of the levels of total dissolved heavy metals in 1999, University of Missouri Press. Columbia., 593, 81–89.
Azimi, A., Azri, A., Rezkazemi, M., & Ansarpour, M. (2017). Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Reviews, 4, 37–59.
Barakat, A., Meddah, R., Afdali, M., & Touhami, F. (2018). Physicochemical and microbial assessment of spring water quality for drinking supply in Piedmont of Béni-Mellal Atlas (Morocco). Physics and Chemistry of the Earth, 104, 39–46.
Bazié, J. B. (2014). Accès à l’eau : L’Afrique entre abondance et pénurie (Access to Water: Africa Between Abundance and Scarcity). Revue Après-Demain, 32, 28–29.
Bhomick, P. C., Supong, A., & Sinha, F. (2017). Organic pollutants in water and its remediation using biowaste-activated carbon as greener adsorbent. International Journal of Hydology., 1(3), 91–92.
Bibi, S., Khan, R. L., & Nazir, R. (2016). Heavy metals in drinking water of Lakki Marwat District, KPK. Pakistan. World Applied Science Journal, 34(1), 15–19.
Christian, G. D. (2004). Analytical chemistry. 6th Edition. John Wiley and Son (Asia) Pte Ltd.
Chrowtoski, P. C. (1994). Exposure assessment principles. In: Patrick DR (ed) Toxic air pollution. Handbook. Van Nostrand Rein Hold, New York, p 154.
CMAE. (2008). Un Aperçu de la situation de l’eau potable et de l’assainissement en Afrique (An overview of the situation of drinking water and sanitation in Africa). Conseil desMinistres Africains de l’Eau, AfricanMinisters’ Council onWater, Sharm El Sheikh, Egypt.
Cohen, A. A., & David, M. O. (2007). Nigerian, NO.1 market for U.S. wheat: Potential for other grains and feeds. USAID Foreign Agricultural Service Bulletin, 1, 39–42.
Degbey, C., Makoutode, M., Agueh, V., Dramaix, M., & De Brouwer, C. (2011). Facteurs associés à la qualité de l’eau de puits et prévalence des maladies hydriques dans la commune d’Abomey-Calavi (Bénin) (Factors associated with well water quality and prevalence of waterborne diseases in the municipality of Abomey-Calavi (Benin)). Santé, 21, 47–55.
Dougna, A. A., Gnazou, M. D. T., Kodom, T., Djaneye-Boundjou, G., & Bawa, M. L. (2015). Physico-chimie et qualité des eaux des forages d’hydraulique villageoise dans la région centrale au Togo (Physico-chemistry and water quality of village hydraulic boreholes in the central region of Togo). International Journal of Biological and Chemical Sciences, 9(4), 2249–2262.
Egbueri, J. C. (2018). Assessment of the quality of groundwaters proximal to dumpsites in Awka and Nnewi metropolises: A comparative approach. International Journal of Energy and Water Resources, 2(1–4), 33–48.
Egbueri, J. C., & Mgbenu, C. N. (2020). Chemometric analysis for pollution source identification and human health risk assessment of water resources in Ojoto Province, southeast Nigeria. Applied Water Science, 10(4), 1–18.
Egbueri, J. C. (2020a). Heavy metals pollution source identification and probabilistic health risk assessment of shallow groundwater in Onitsha, Nigeria. Analytical Letters. https://doi.org/10.1080/00032719.2020.1712606
Egbueri, J. C. (2020b). Signatures of contamination, corrosivity and scaling in natural waters from a fast developing suburb (Nigeria): Insights into their suitability for industrial purposes. Environment, Development and Sustainability. https://doi.org/10.1007/s10668-020-00597-1
Ekere, N. R., Agbazue, V. E., Ngang, B. U., & Ihedioha, J. N. (2019). Hydrochemistry and Water Quality Index of groundwater resources in Enugu north district, Enugu. Nigeria. Environmental Monitoring and Assessment, 191, 150. https://doi.org/10.1007/s10661-019-7271-0
Elinder, C. G. (1986). Handbook of the toxicology of metals (2nd ed., pp. 318–328). Elsevier.
European Union, EU. (1999). European Union Directive 1999/74/EC. Laying down minimum standards for the protection of laying hens.
Gonzalez, A., Peters, U., Lampe, J. W., & White, E. (2009). Zinc intake from supplements and diet and prostate cancer. Nutrition and Cancer, 61(2), 206–215.
Guerra, K., Konz, J., Lis, I. K., & Neebrem, C. (2010). Exposure factors handbook. USEPA.
HC. (2016). Guidelines for Canadian drinking water quality: Guideline technical document — pH. Heath Canada, Ottawa, Ontario, Canada.
Hidiroglou, M. (1979). Trace element deficiencies and fertility in ruminants: A review 1. Journal of Dairy Science, 62(8), 1195–1206.
Hodgson, E. (2010). A textbook of modern toxicology (4th ed., p. 648). Wiley.
Horne, A. J., & Goldman, C. R. (1994). Limnology (2nd ed., p. 576). McGraw-Hill Inc.
Iweala, E. E. J., Olugbuyiro, J. A. O., Durodola, B. M., Fubara-Manuel, D. R., & Okoli, A. O. (2014). Metal contamination of foods and drinks consumed in Ota Nigeria. Research Journal of Environmental Toxicology, 8, 92–97.
Jamesone, S. (1997). Effects of zinc deficiency in human reproduction. Acta Medica Scandinavica Supplementum, 593, 81–89.
Jãrup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68, 1167–1182.
Kavacar, P., Sofuoglu, A., & Sofuoglu, S. C. (2009). A health risk assessment for exposure to trace metals via drinking water igestion pathway. International Journal of Hygiene and Environmental Health, 212, 216–227.
Keen C. L., & Ziderberg-Cher S. (1996). Manganese. In: Ziegler EE Filler LJ Jr (eds) Present knowledge in nutrition. Wiley, Washington, DC, pp 334–343
Khan, N., Hussain, S. T., & Saboor, A. (2013). Physiochemical investigation of the drinking water sources from mardan, khyber Pakhtunkhwa Pakistan. Physical Science International Journal, 8(33), 1661–1671.
Khatri, N., Tyagi, S., & Rawtani, D. (2016). Assessment of drinking water quality and its health effects in rural areas of Harij Taluka, Patan District of Northern Gujarat. Environmental Claims Journal 0, 1–24.
Malek, A., Kaholu, M., & Bouguerra, H. (2019). Groundwater’s physicochemical and bacteriological assessment: Case study of well water in the region of Sedrata, North-East of Algeria. Journal of Water and Land Development, 41, 91–100.
Mark, W. R., Ximing, C., & Sarah, A. C. (2002). World water and food to 2005, dealing with scarcity, IFPRI, Washington, D. C., p. 1.
Mbanugo, J. I., Sridhar, M. K. C., & Anunuso, C. I. (1990). Methaemoglobinlevels in infants receiving low dietary nitrates in Eastern Nigeria. Toxicological and Environmental Chemistry, 25, 195–199.
Michael, F. I., & Stanford, J. (2003). Epidemiological investigation, Fred Hutchison Cancer. Research Centre and National Cancer Institute, Bethsaida.
Mishra, S. P., & Pandey, S. N. (2008). Essential environmental studies (pp. 82–83). Ane Books Pvt. Ltd.
Mulamattathil, S. G., Bezuidenhout, C., & Mbewe, M. (2015). Analysis of physicochemical and bacteriological quality of drinking water in Mafikeng, South Africa. Journal of Water and Health, 13, 1143–1152.
Nuwayhid, I., Nabulsi, M., Muwakkit, S., Kouzi, S., Salem, G., Mikati, M., & Ariss, M. (2003). Blood lead concentrations in 1–3 year old Lebanese children: a cross-sectional study. Journal of Environmental Health 2 (5).
Nsi, E. W., & Ogori, B. O. (2005). A survey of water quality in some areas of Makurdi. Int J SciTechnol, 2, 7–11.
Ogunfowokan, A., Oyekunle, J., Olutona, G., Atoyebi, A., & Lawal, A. (2013). Speciation study of heavy metals in water and sediments from Asunle River of the Obafemi Awolowo University, Ile-Ife. Nigeria. International Journal of Environmental Protection, 3(3), 6–16.
Oluyemi, E. A., Adekunle, A. S., Adenuga, A. A., & Makinde, W. O. (2010). Physico-chemical properties and heavy metal content of water sources in Ife North Local Government Areas of Osun State, Nigeria. African Journal of Environmental Science and Technology, 4, 691–697.
Omotola, O. A., & Ofoezie, I. E. (2019). Prevalence and intensity of soil transmitted helminths among school children in Ifetedo, Osun State Nigeria. Journal of Bacteriology and Parasitology, 10(352), 2.
Oyeku, O. T., & Eludoyin A. O. (2010). Heavy metal contamination of groundwater resources in a Nigerian Urban settlement. African Journal of Environmental Science and Technology 4, 201–214. Present Knowledge in Nutrition, Washington DC. 334 - 343
Pawari, M. J., Gawande, S. (2015). Ground water pollution and its consequence. International 536 Journal of Engineering Research and General Science 3 (4), 773–776.
Pratush, A., Kumar, A., & Hu, Z. (2018). Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: A review. International Microbiology, 21, 97–106. https://doi.org/10.1007/s10123-018-0012-3
Rahimzdeh, M. R., Kazemi, S., & Moghadamnia, A. (2017). Cadmium toxicity and treatment: An update. Caspian Journal of Internal Medicine, 8(3), 135–145.
Raji, M. I. O., & Ibrahim, Y. K. E. (2011). Prevalence of water-borne infections in North Western Nigeria: A retrospective study. The Journal of Public Health and Epidemiology, 3(8), 382–512.
Raphael, O., John, O. O., Sandra, U. I., & Sunday, A. C. (2018). Assessment of borehole water quality consumed in Otukpo and its environs. International Journal of Ecological Science and Environmental Engineering, 5(3), 71–78.
Salama, A. K., & Radwan, M. A. (2005). Heavy metals (Cd, Pb) and trace elements (Cu, Zn) contents in some foodstuffs from the Egyptian market. Emirates Journal of Food and Agriculture, 17, 192–199.
Sharma, S., & Bhattachatya, A. (2016). Drinking water contamination and treatment techniques. Applied Water Science., 7, 1043–10467.
Sixtus, B. B., Samuel, A. F., Gofred, E. S., Napoleon, J., & Thomas, K. (2016). Assessment of the quality of groundwater for drinking purposes in the Upper West and Northern regions of Ghana. Springer plus., 5(1), 2001.
SON (2007). Standard Organization of Nigeria, Nigerian Standard fordrinking water quality, pp. 15-16.
Standard Organization of Nigerian. (2015). Nigerian standard for drinking water quality. NIS-554–2015, ICS 13.060.20.
Thompson, J., & Bannigan, J. (2008). Cadmium: Toxic effects on the reproductive system and the embryo. Reproductive Toxicology, 25(3), 304–315.
Ukah, B. U., Ameh, P. D., Agbueri, J. C., Unigwe, C. O., & Ubido, O. E. (2020). Impact of effluent derived heavy metals on the groundwater quality in Ajao industrial area, Nigeria: An assessment using entropy water quality index (EWQI). International Journal of Energy and Water Resources, 4, 231–244. https://doi.org/10.1007/s42108020-00058-5
United States Environmental Protection Agency. (1992). Guidelines for exposure assessment. EPA/600/2–92/001. US environmental protection Agency Risk Assessment Forum, Washington, DC
Uriu-Adams, J. Y., & Keen, C. L. (2005). Copper, oxidative stress, and human health. Molecular Aspects of Medicine, 26, 268–298.
USEPA. IRIS. (2010). Toxicological Review of 1,4-Dioxane (FinalReport, 2010). U.S. Environmental Protection Agency, Washington, DC, EPA/635/R-09/005F
Weltzien, E. M. (2002). Water quality for poultry. Poultry industry council factsheet 111. Poultry industry council. Guelph. ON.
Wetzel, R. G. (1983) Limnology, 2nd edn. Saunders College Publishing, Philadelphia39.
WHO. (2017). Guidelines for Drinking-Water Quality (4th ed.). Incorporating first addendum. World Health Organization.
World Health Organization. (2008). Guidelines for drinking water quality. Vol 1, Recommendation. 3rd Edition, Geneva.
WHO. (2011a). Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva.http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/index.html.Accessed: 12 Aug 2015
World Health Organization. (2011b). Cadmium in drinking water. Background document for development of WHO Guidelines for Drinking-water Quality
World Health Organization. (2017). Guidelines for drinking-water quality: Fourth edition incorporating the first addendum. Geneva.License: CC BY-NC-SA 3.0 IGO. ISBN 978–92–4–154995–0
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oyekunle, J.A.O., Adekunle, A.S., Durodola, S.S. et al. Evaluation of physicochemical properties and heavy metal speciation of groundwater in Ifetedo and Garage Olode, Osun State, Nigeria. Environ Monit Assess 194, 565 (2022). https://doi.org/10.1007/s10661-022-10191-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10191-9