Abstract
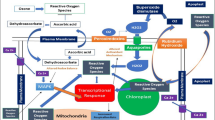
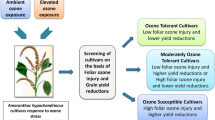
The plant response to elevated ozone stress reveals inter-species and intra-species disparity. Ozone-induced crop yield loss is predicted to increase in the future, posing a threat to the world economy. This study aims to evaluate the cultivar specific variation in rice exposed to elevated ozone. Fifteen short-duration rice cultivars were exposed to 50 ppb ozone for 30 days at reproductive stage. The physiological, biochemical, growth and yield traits of all test cultivars were significantly affected in response to elevated ozone. On an average, ozone stress decreased the tiller number by 22.52%, number of effective tillers by 30.43%, 1000 grain weight by 0.62% and straw weight by 23.83% over control. Spikelet sterility increased by 19.26% and linear multiregression 3D model significantly fits the spikelet sterility and photosynthetic traits with the R2 of 0.74 under elevated ozone. Principal Component Analysis with total variance of 57.5% categorized 15 rice cultivars into four major groups, i.e., ozone sensitive (MDU6, TRY(R)2 and ASD16), moderately ozone sensitive (ASD18, ADT43, and MDU5), moderately ozone tolerant (ADT37, ADT(R)45, TPS5, Anna(R)4, PMK(R)3, and ADT(R)48), and ozone tolerant (CO51, CO47, and ADT36). This study indicates that the different responses of rice cultivars to elevated ozone stress through a change in plant physiology, biochemical, growth, and yield traits and the results directed to provide scientific information on plant adaptations to ozone stress and helps in efforts to search ozone tolerant gene for plant breeding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tropospheric ozone (O3) is the third most important contributor of greenhouse radiative forcing (+0.41 Wm–2) after carbon dioxide (CO2) and methane (CH4) (IPCC, 2013). Right from the industrial revolution until today, there is a continuous alarming rise in ozone forming precursors like oxides of nitrogen (NOx) and volatile organic compounds (VOCs) in the atmosphere, which in turn increases the tropospheric ozone (Monks et al., 2015). The ozone-forming precursor, NOx showed a strong positive trends over India (+29%), China (+26%), and the Middle East (+20%), and the negative trends over the USA (−29.4%), southern Africa (−8.2%), and Western Europe (−8.8%) per decade over a period of 2005–2014 (Miyazaki et al., 2017). According to Kumari et al. (2020), NOx level showed an increasing trend of 0.9 ppb per year from 2010 to 2015 over Indo Gangetic plains, India. This increase in NOx emissions from industries and motor vehicle raises tropospheric ozone concentration.
Since the mid-1990s, tropospheric ozone trends has increased in the northern mid-latitude regions (2–7%; 1–4 ppb) and tropics (2–14%; 1–5 ppb) with the largest increases of 8–14% (3–6 ppb) per decade in the southern Asian region (IPCC, 2021). The average ozone concentration varied from 45 to 65 ppb over Indian regions (David & Nair, 2013; Deb Roy et al., 2009). An increasing trend of ozone by 3–5.6 and 1.2–2% per decade were observed over Indo Gangetic plains and southern regions of India, respectively over the study period of 2005–2010 (Lal et al., 2012). According to Kumari et al. (2020), the annual mean ozone concentration increased by 19.2% from 2010 to 2015 over Indo Gangetic plains. Moreover, in southern regions of India, the maximum ozone concentration reached up to 56 ppb at Kanyakumari (Krishna Sharma & Nagaveena, 2016) and 62 ppb at high altitude region of Western Ghats, Ooty, Tamil Nadu (Udayasoorian et al., 2013). Hence, the current level of ozone concentration has crossed the standard permissible limit for vegetation (40 ppb) and severely affects the forest (Feng et al., 2019), agricultural (Fischer, 2019; Shao et al., 2020) and horticultural crop species (Singh et al., 2018; Yang et al., 2017).
Rice, an important food crop of the world is susceptible to many pollutants particularly air pollutants. In Asian regions, rice is cultivated almost throughout the year with two major rice growing seasons (rabi and kharif) which generally overlaps with the peak ambient ozone concentration (Frei, 2015; Ziemke et al., 2019). Several studies on rice crop revealed that elevated tropospheric ozone (eTO3) reduced the photosynthetic rate, photosynthetic pigments, oxidative stress induced bronzing symptoms and altered antioxidant metabolism (Li et al., 2017; Peng et al., 2018). These physiological and biochemical stress leads to reduction in growth, biomass, tiller numbers, spikelet numbers, and grain yield (Akhtar et al., 2010; Jing et al., 2016; Shao et al., 2020).
The tropospheric ozone causes considerable yield loss in rice (Pandey et al., 2015, 2018; Singh et al., 2018). Van Dingenen et al. (2009) reported 3.7% yield loss in rice at global level. Ozone-induced damage to rice in India is estimated to be 2.1 ± 0.8 Mt which was sufficient to feed roughly 35% of population in India (Ghude et al., 2014). Furthermore, in India annual loss of 0.3–6.7 million ton (0.3–6.3%) for rice crop is estimated based on the accumulated ozone over a threshold of 40 ppb (AOT40) and mean ozone for 7 h during the day (M7) (Lal et al., 2017). The crop economic loss simulated using Weather Research and Forecasting-Chemistry (WRF-Chem) model showed 8% relative yield loss for rice crop in Indian region (Sharma et al., 2019).
Tropospheric ozone-induced yield loss estimation is important for countries like India with increased population and urbanization, which needs to be addressed urgently to maintain the food security. Only very few studies has been reported so far in India and there is no information on elevated tropospheric ozone (eTO3) impact on rice in southern part of India. Since rice yield loss to ozone stress is one aspect of global climate change issue, cultivar sensitivity and tolerance need to be thoroughly studied. Hence, an experimental study was conducted to explore the intra-specific variations of rice cultivars exposed to elevated ozone stress. Further, better understanding of cultivar specific variations in rice plants under ozone stress shall provide scientific information about cultivar adaptations to environmental condition. The outcome of the work would throws light on the significance of implementing ozone tolerant plant breeding programs and formulation of ozone reduction policies to protect rice productivity at global scale.
Materials and methods
Experimental site and ozone treatment
The 15 short-duration rice cultivars viz., ADT36, ADT37, ADT43, ADT(R)45, ADT(R)48, Anna(R)4, ASD16, ASD18, CO47, MDU5, PMK(R)3, Rice MDU6, Rice TPS5, Rice CO51, and TRY(R)2, which are popularly cultivated in and around Tamil Nadu region were chosen for this study. The specific characteristics of chosen rice cultivars developed in various research stations of Tamil Nadu Agricultural University are described in Table 1. In order to maintain the elevated ozone concentration, the experimental study was carried out in Open Top Chambers (3.5 × 3.5 m) located in the wetlands (11.00° N, 76.92° E) of Tamil Nadu Agricultural University (TNAU), Coimbatore, India, during January to May 2019. During the experimental period, monthly mean maximum and minimum temperature ranged between 32.7 and 17.8 °C, respectively, while the maximum and minimum relative humidity varied from 98 to 19%, respectively. Experimental soil chosen for the study has a clay loam texture with slightly alkaline pH (8.38) and an electrical conductivity (EC) of 0.32 dS m−1. Furthermore, the soil had an organic carbon content of 0.53% organic carbon and 226, 12.4, and 287 kg ha−1 of available nitrogen (N), phosphorous (P) and potassium (K), respectively. All the crop management practices were followed as per the recommendation of crop production guide (TNAU, 2019). Seeds of 15 rice cultivars were soaked in water for 24 h to induce sprouting. The sprouted seeds were sown uniformly in the well prepared nursery bed by maintaining a thin film of water for 20 days under natural light and ambient temperature. Three-week old seedlings were transferred into two Open Top Chambers, one for control (n = 90) and another for ozone treatment (n = 90) with six replications for each cultivar (N = 180). The factorial completely randomized block design was followed in control and ozone chambers. Three days after transplanting, pre-emergence herbicide butachlor (1.25 kg/ha) was applied and seventh day after transplanting, gap filling was carried out to maintain equal number of seedlings in each treatment. To provide adequate nutrient supply, the fertilizer recommendation of 150:50:50 kg N:P:K ha−1 were supplied as four equal split. The split application of N and K were followed at basal, tillering, panicle initiation, and heading stages, while P was applied as basal alone. Submergence of 5 cm water level was maintained from seventh day after transplanting to throughout the crop period.
Ozone generator (A4G, Faraday, India) was used for ozone production and the ozone emission was set at a distance of 30 cm above the plant canopy. The ozone concentration inside the chambers was monitored using ambient ozone monitor (G09-O3-3121). The plants were exposed to ozone fumigation for 30 days (10.00 h–17.00 h) from 51 days after sowing to 80 days after sowing (reproductive stage). The daily average ozone concentration in ozone treatment chamber ranged from 46 to 56 ppb, to achieve the target level of 50 ppb O3 (AOT40 = 2.1 ppm h) (Table S1, Supplementary Information). In control chamber, plants were grown equivalently without ozone and the concentration was < 10 ppb.
Leaf visible symptom
The ozone exposed plants depicting injury symptoms on leaves like necrotic dark brown spots, chlorotic, and pale color of leaves and they were evaluated based on certain criteria. After 30 days of ozone exposure, the whole plant with all leaves of each cultivar and treatment were observed for evaluating injury symptoms. A non-destructive visible scoring method was followed by assigning a leaf injury percentage (LIP) from 0 to 100 (Chaudhary & Agrawal, 2013) and leaf bronzing score (LBS) from 0–10 (Ueda et al., 2015) to all rice cultivars. The criteria for evaluating leaf visible symptom are given in Table 2.
Physiological traits
A non-destructive physiological measurement was performed on three randomly selected rice plants (n = 3) within each cultivar and treatment (N = 90). A portable photosynthesis system (ADC BioScientific LCpro-SD System, UK) was utilized to quantify photosynthetic rate (A) and stomatal conductance (gs) while chlorophyll content meter (CCM-200+, USA) was used to assess chlorophyll content (Chl). After 30 days of ozone exposure, measurement was taken at three different points of third youngest fully expanded leaf of each cultivar in treatment and the average of three points were calculated.
Biochemical traits
The destructive biochemical measurements were performed on three randomly chosen rice plants (n = 3) of each cultivar and treatment (N = 90). After 30 days of ozone exposure, third youngest fully expanded leaf of selected plants were collected and frozen at –80 °C until further analysis of malondialdehyde (Heath & Packer, 1968), proline (Bates et al., 1973), and ascorbic acid (Keller & Schwager, 1977).
Growth and yield traits
All the growth and yield traits of rice cultivars, i.e., root length (RL), shoot length (SL), panicle length (PL), number of tillers (NTP), number of effective tillers per plant (NETP), number of spikelets per panicle (NSPi), number of filled spikelets per panicle (NFSPi), thousand grain weight (1000 GW), and straw weight (SWP) were measured at crop maturity stage. Three selected plants (n = 3) of each cultivar and treatment (N = 90) which were not destructed earlier for any of the analysis were chosen for observation to obtain precise yield results. Grains were separated from the panicles manually and then the whole plant was dried at 80 °C for 72 h to measure the straw weight. Grains were soaked in water and numbers of floating and sunken spikelets were counted manually to determine filled and unfilled spikelets. Twenty randomly chosen filled grains were weighed and the value was multiplied by 50 to obtain the thousand grain weight. Spikelet sterility (SS) was calculated as the number of sterile spikelets relative to the total number of spikelets.
Statistical analysis
All the statistical analysis was performed using the SPSS statistical package (SPSS Inc., version 16.0.0). One-way ANOVA (Analysis of variance) was used to test the effect of ozone on physiological, biochemical, growth and yield traits of 15 rice cultivars. Two-way ANOVA was used to test the treatment; cultivar and their interaction effect of various plant traits and Tukey method was used to identify difference among treatment means. The ozone-induced percentage reduction of yield traits over control was estimated by the following formula, 100-[(ozone/control) × 100]. Shapiro–Wilk test was used to determine the normality of the data and linear multiregression analysis was used to fit the 3D model of the plant traits (Urban et al., 2017). The regression equation used in the 3D model is described in Table 3. The degree of correlation between leaf injury percentage, photosynthetic and yield traits were determined based on Pearson’s correlation coefficient. P values less than 0.05 (P < 0.05) is considered as significant. Principal component analysis (PCA) was performed in R software (Version 3.5.1) using all observed physiological, biochemical, growth, and yield traits. SigmaPlot 14 and OriginPro 2019 (Version 9.6.5) were used to plot the graphs.
Result and discussion
Tropospheric ozone-induced loss on rice production is still unknown in southern parts of India and hence it is important to generate data with reference to tropospheric ozone-induced impact on rice cultivars. In this study, an average ozone concentration of 50 ppb was fixed to mimic current ozone level over southern India with popularly growing 15 short-duration rice cultivars.
Leaf visible symptom
The leaf bronzing score and leaf injury percentage were given out based on bronzing injury symptom which varied from 3 to 6 and 23.3 to 51.7%, respectively. Among the cultivars, MDU6 and TRY(R)2 showed high leaf injury percentage (51.7%) and leaf bronzing score of 6, while less leaf injury percentage (23.3%) and leaf bronzing score (3) were noticed in Anna(R)4 and PMK(R)3 (Table 4). The ozone-induced leaf injury symptom in this study may be due to entry of O3 into the plant system via gas exchange during photosynthesis and breakdown of ozone into reactive oxygen species (ROS) in the apoplast which might have caused cell death and the development of necrotic symptoms (Baier et al., 2005). Few cultivars, Anna(R) 4 and PMK(R) 3 showed less injury symptoms which might be related to enhanced antioxidant system compared to MDU6 and TRY(R)2. This result is consistent with the study by Wang et al. (2014) who reported that lesser bronzing symptoms were associated with improved antioxidant system like lipid peroxidation in ozone tolerant rice and also presence of quantitative trait loci OzT9, which is responsible for leaf bronzing formation under ozone stress.
Physiological response
A reduction in stomatal conductance was observed between 8.62% (Anna(R)4) and 29.31% (TRY(R)2) (Fig. 1). On average across all test cultivars, 21.35% reduction in stomatal conductance under elevated ozone stress would be related to controlling gas influx in leaf mesophyll region and closure of stomata with response to ozone stress (Fiscus et al., 2005). This stomatal closure is also related with the production of reactive oxygen species under ozone stress, which controls the activity of guard cell ion channels and protein kinase activity in stomata, resulting in the reduced stomatal conductance (Vainonen & Kangasjarvi, 2015). Similar results were found by Pang et al. (2009) who reported that rice cultivars, Shanyou63 and Wuyunjing3 experienced a significant decreased in stomatal conductance up to 36.70% under elevated ozone which were mediated via stomatal closure with response to elevated ozone stress.
The stomatal response to elevated ozone alters the photosynthetic capacity of the rice cultivars (Chen et al., 2011; Pandey et al., 2018). In photosynthetic rate, highest percentage reduction was observed in ASD16 (26.78%) and lowest in PMK(R)3 (11.11%) (Fig. 1). The reduction in stomatal conductance by ozone was proportional to the decline in photosynthetic rate by 19.23% which was related to the stomatal limitation in all rice cultivars that inhibited the photosynthetic CO2 fixation in the plant system. It is also correlated with the report of Masutomi et al. (2019) who confirmed the fact that elevated ozone altered the linear relationship between stomatal conductance and net photosynthetic rate. Consistently, the observation by Akhtar et al. (2010) confirmed a significant reduction in photosynthetic rate with the maximum of 66.3% in Bangladeshi rice cultivars under 100 ppb ozone which was not only attributed to damage of photosynthetic enzyme but also due to ozone-induced stomatal closure.
As a consequence of lipid peroxidation in PSII reaction center undoubtedly affecting the light harvesting efficiency of all test cultivars (Jing et al., 2016; Ueda et al., 2015), this would result in the reduction of chlorophyll content between 17.04% (Anna(R)4) and 35.08% (Rice MDU6) (Fig. 1). Moreover, on an average across 15 rice cultivars, chlorophyll content reduced by 27.19% was associated with decreased photosynthetic pigments especially chlorophyll located in the thylakoids. Similar findings were reported by Li et al. (2017) wherein both SY63 and Bt-SY63 rice cultivars showed a reduction in chlorophyll content under elevated ozone stress that was associated with degenerated chloroplasts which mediates the changes in carbon assimilation cycle. This observed physiological disorder under elevated ozone stress causes photosynthetic instability which results in alteration at sub-cellular, cellular, plant organ, and whole plant level. Thirty days of ozone exposure at reproductive stages significantly reduced all physiological traits. A significant treatment and cultivar interaction effect were noticed in stomatal conductance and chlorophyll content; while there was no interaction effect observed in photosynthetic rate.
Biochemical response
Oxidative stress leads to damage in membrane lipids of plant system measured by malondialdehyde concentration (Li et al., 2017; Ueda et al., 2013) which is used as an important parameter for assessing ozone stress by measuring lipid peroxidation. The percentage increment of MDA content was highest in MDU6 (191.62%) and lowest in CO51 (50.00%) (Fig. 2). MDA content increased by 121.43% over control indicating an altered reactive oxygen species metabolism in ozone exposed plants. Consequently, higher concentration of reactive oxygen species generated in ozone exposed plant leads to membrane damage and cell death (Frei, 2015). Ashrafuzzaman et al. (2017) also reported that Nipponbare rice variety exhibited an increase in MDA content under elevated ozone, thereby validating a continuous accumulation of reactive oxygen species mediated lipid peroxidation.
A non-enzymatic antioxidant, proline acts as a scavenger of singlet oxygen and hydroxyl radicals in response to environmental stress (Rejeb et al., 2014). In this study, a hike in proline content varied between 50.15% (PMK(R)3) to 145.15% (MDU6) (Fig. 2) over control might be correlated with participation of proline in reactive oxygen species scavenging mechanism in plant tissues that would be beneficial for its tolerance to environmental stress (Gill & Tuteja, 2010). This result coincides with the observation of Kibria et al. (2017) and Nahar et al. (2018) who reported that rice cultivars accumulate free proline under biotic and abiotic stresses. Similarly, Upadhyaya et al. (2007) reported that increased proline concentration in rice cultivars under hydrogen peroxide treatment results from defense response of plant system to oxidative stress.
Ascorbic acid, a low molecular weight antioxidant showed defense against reactive oxygen species (ROS) by detoxification mechanism (Kao, 2015). Reduction in ascorbic acid content was observed between 10.19% (ADT37) and 28.75% (ASD18) (Fig. 2). A decrease in ascorbic acid content by 20.99% was observed in this study, which might be due to continuous ozone exposure gradually depleting the ascorbic acid pool of plant system by O3-derived ROS. This inability of ascorbic acid regeneration in ozone exposed plant suggests the plant’s susceptibility towards ozone stress. In the same way, Wang et al. (2013) reported that ascorbic acid content in rice cultivar, Shanyou63 decreased by 22.75% under 250 ppb ozone which was associated with the accumulation of O3-derived ROS degraded ascorbic acid scavenging system thereby leading to increased sensitivity of the plants when it encounters ozone stress. On contrary, Ashrafuzzaman et al. (2018) observed no changes in ascorbic acid content even at 108 ppb ozone in few rice cultivars (BINA11, BR28, NB, and L81). Furthermore, the relationship between ascorbic acid and O3 remains unclear (Bellini & De Tullio, 2019).
A significant increment in the level of malondialdehyde and proline content was noticed in all rice cultivars; while ascorbic acid content decreased. In all biochemical traits, significant cultivar and treatment effect was observed. An interaction effect was significant in malondialdehyde and proline content; whereas ascorbic acid content showed no significant variation.
Growth response
The ozone-induced reduction in biomass and yield has been well documented for a wide range of crop species (Lal et al., 2017; Shi et al., 2009; Zheng et al., 2013). In terms of growth traits, the cultivar ASD16 showed maximum reduction (23.58%) in root length, while minimum reduction was observed in ADT(R)48 (1.71%). The maximum reduction in shoot length was noticed in MDU5 (11.25%) while slight increment was observed in TRY(R)2 (2.88%) and number of tillers showed maximum reduction in MDU6 (33.33%) and minimum in CO51 (9.09%) (Fig. 3). In this study, on an average, elevated ozone stress decreased the growth traits namely root length by 11.99%, shoot length by 2.11% and tiller numbers by 22.52%, which might be attributed to loss of photosynthetic capacity directly affecting the foliar carbon assimilation rate, which in turn inhibits the growth and development of rice cultivars (Jing et al., 2016; Wang et al., 2012). Similar results have been noticed in indica, japonica, and Bangladeshi rice cultivars with reduced plant height and tillering at elevated ozone stress indicating ozone damages in leaves thereby preventing the plant growth and development (Akhtar et al., 2010; Frei et al., 2008; Shao et al., 2020).
A significant cultivar and treatment effect were noticed in root length, shoot length and number of tillers. An interaction effect was significant in shoot length and number of tillers, while root length showed no interaction effect (Table 5).
Yield response
All yield parameters were significantly influenced by elevated ozone treatment. In this study, a maximum reduction in number of effective tillers were noticed in MDU6 (50.00%) and minimum in Anna(R)4 (15.38%). Panicle length showed a maximum reduction in ASD18 (21.05%) while slight increment was observed in TRY(R)2 (0.22%) (Fig. 4). On an average, decrease in number of effective tillers by 30.43% and panicle length by 11.69% was primarily caused by the inhibition of tillering formation and reduced plant height which leads to reduction in effective tillers and panicle size. Similar results were observed by Shao et al. (2020) who reported that smaller panicle size was coincides with reduced plant height and effective tillers under ozone stress.
Cultivar ADT43 depicted a maximum reduction (38.52%) and ADT(R)45 showed minimum reduction (13.56%) in number of spikelets per panicle. The maximum reduction in number of filled spikelets per panicle was noticed in ADT43 (50.99%) and minimum in Anna(R)4 (22.87%) (Fig. 4). Decrease in yield traits, i.e., number of spikelets per panicle by 23.32% and number of filled spikelets per panicle by 35.27% had confirmed the negative effects of ozone on yield. This indicates that under ozone stress assimilate allocation to panicles were significantly reduced due to more utilization for plant respiration and regulating antioxidant metabolism. Similar observations were reported by Wang et al. (2014) under 100 ppb ozone in ozone sensitive Nipponbare rice variety. Further, Terao et al. (2010) reported that a gene APO1 located at the end of Chromosome 6 was affected due to ozone stress.
On an average, the percentage of spikelets sterility per panicle increased by 19.26% (Fig. 5) revealed that reduction in availability of carbohydrate might have limited the grain filling process eventually decreasing the grain yield to a larger extent as reported by Lin et al. (2014) and Jing et al. (2016).
The maximum reduction in 1000 grain weight were noticed in ADT37 (4.01%) while slight increment were observed in ASD18 (1.05%), ADT(R)45 (1.61%), and ADT36 (1.74%) (Fig. 4). A decrease in 1000 grain weight by 0.62% coincides with O3-induced reduction in net photosynthesis leading to decrease in cumulative carbon gain. Correspondingly, ozone-derived reduction in individual grain mass was reported in Bangladeshi rice varieties (Ashrafuzzaman et al., 2017), transgenic Bt-SY63 (Li et al., 2017) and modern indica and japonica rice cultivars (Shao et al., 2020) under elevated ozone stress proving the negative impact of ozone on pollen fertilization efficiency and weaken sink strength due to limited CO2 assimilation rate.
The cultivar, CO47 showed maximum reduction (35.90%), while minimum reduction was observed in ADT43 (18.52%) (Fig. 4). On an average, the straw weight decreased by 23.83% across all rice cultivars which was attributed to reduction in net photosynthetic rate and modification in phloem loading and/or translocation (Ainsworth, 2008; Akhtar et al., 2010; Frei et al., 2008). Similarly, total dry weight was considerably reduced in Nipponbare and Indica rice cultivars under elevated ozone (Chen et al., 2011; Peng et al., 2018).
A significant cultivar and treatment effect were noticed in number of effective tillers, number of spikelets and filled spikelets per panicle, percentage of spikelets sterility per panicle, 1000 grain weight and straw weight. All the observed yield traits showed a significant interaction effect except for 1000 grain weight and straw weight (Table 6).
Plant trait relationship
A positive relationship between photosynthetic rate and chlorophyll content existed (r = 0.87***) while both had a significantly negative correlation with spikelet sterility (r = −0.81*** and r = −0.84**) (Fig. 6). A regression analysis used in 3D model clearly depicted the relationship of photosynthetic traits with spikelet sterility, i.e., decrease in photosynthetic rate and chlorophyll content increased spikelet sterility (R2 = 0.74***) (Fig. 7). This significant negative correlation between plant physiological traits and spikelet sterility might be due to sink limitations to the plant economic parts. Further, altered carbon assimilation rate would limit the grain yield rather than storage and phloem loading (Ueda et al., 2013). The above correlation suggests that the plants were exposed to ozone during heading and flowering stages and hence the grain yield was affected. Correspondingly, leaf injury percentage showed a positive correlation with spikelet sterility (r = 0.85***) while weaker association was observed with straw weight (Fig. 8). This results confirmed the alteration in source-sink regulation and alteration in assimilate partitioning (Crous et al., 2006). Similarly, Ueda et al. (2015) showed a weak relation between injury percentage and biomass production.
Principal component analysis (PCA) has been used to identify ozone responsive parameters in European beech (Fagus sylvatica) (Löw et al., 2012) and wheat cultivars (Fatima et al., 2019). In this study, principal component analysis was performed to categorize the principal components of plant physiological, biochemical, growth, and yield traits of 15 rice cultivars that best explain the response to ozone stress to identify ozone tolerant cultivars. The first two principal components (PC1 and PC2) accounted for 46.1 and 11.4% among rice cultivars, respectively with a cumulative eigen value of 57.5% and clustered all physiological traits and most of the yield traits as the best descriptors followed by growth and biochemical traits (Fig. 9). PCA analysis revealed a strong correlation between physiological and yield traits (cluster together) more than with biochemical parameters using PC1 and PC2.
Principal component analysis describing the classification of ozone responsive plant traits of 15 rice cultivars. (LIP—leaf injury percentage; LBS—leaf bronzing score; A—photosynthetic rate; gs—stomatal conductance; Chl—chlorophyll content; MDA—malondialdehyde content; Proline—proline content; AsA—ascorbic acid content; RL—root length; SL—shoot length; PL—panicle length; NTP—number of tillers per plant; NETP—number of effective tillers per plant; NSPi—number of spikelets per panicle; NFSPi—Number of filled spikelets per panicle; 1000 GW—100 grain weight; SWP—straw weight per plant)
The first principal component (PC1) correspond to higher values for all physiological, growth and yield traits except for straw weight, but lesser loadings for leaf visible symptoms and biochemical traits except for ascorbic acid content. The second principal component (PC2) explained higher values for leaf visible symptoms, biochemical traits (proline and MDA), growth (shoot length), and all the yield traits except for 1000 grain weight, and lesser loading for all physiological traits, biochemical trait (ascorbic acid), growth (root length and number of tillers per plant), and yield traits (1000 grain weight). This multivariate analysis allows the identification of ozone responsive variables that are best described to ozone tolerance.
Cultivars showing highest values for observed physiological, biochemical, growth and yield traits for PC1 and PC2 located in the positive quadrant were considered as ozone tolerant cultivars. In contrast, cultivars showing the low values for PC1 and PC2 fall in negative quadrant were considered as ozone sensitive cultivars. Cultivars with moderate values for PC1 and PC2 fall in positive and negative quadrant respectively were considered as moderately ozone tolerant cultivars, while PC1 and PC2 with negative and positive quadrant, respectively were considered as moderately ozone sensitive cultivars. Hence, the 15 rice cultivars were categorized into four major groups indicating ozone tolerant (Rice CO51, CO47, and ADT36), moderately ozone tolerant (ADT37, ADT(R)45, Rice TPS5, Anna(R)4, PMK(R)3, and ADT(R)48), moderately ozone sensitive (ASD18, ADT43, and MDU5), and ozone sensitive (Rice MDU6, TRY(R)2, ASD16) cultivars (Fig. 10). Similar to this investigation, Mazid et al. (2013) clustered 41 different rice genotypes for screening bacterial blight resistance genotypes using PCA. Kakar et al. (2019) also clustered 74 rice genotypes for identifying salt tolerance using principal component analysis for accuracy and reliability.
Conclusion
Decline in rice production with increasing tropospheric ozone concentration instigates researchers to select and develop ozone tolerant rice cultivars for retaining global food security. The current investigation revealed that elevated ozone significantly decreased plant photosynthetic traits, altered antioxidant metabolism, reduced tiller numbers and increased spikelet sterility. Ultimately, these altered plant traits led to substantial yield loss suggesting that all 15 rice cultivars are relatively susceptible to elevated tropospheric ozone stress. Nevertheless, the test cultivars with different genetic diversity depicted variations in ozone tolerance under elevated ozone stress. The cultivars under ozone tolerant and moderately tolerant category namely Rice CO51, CO47, ADT36, ADT37, ADT(R)45, Rice TPS5, Anna(R)4, PMK(R)3, and ADT(R)48 would be recommended for cultivation in the region experiencing the ozone concentration around 50 ppb level. Improved agronomic practices and nutrient management would improve crop production and reduce ozone-induced yield loss of rice cultivars grown under elevated ozone stress. Since prevalence of ozone concentration shows seasonal variation, shifting cropping season that records negligible amount of tropospheric ozone would be a possible option for cultivation of ozone sensitive rice cultivars. Moreover, the cultivar specific variations in plant traits under ozone stress could offer the prospect for developing of ozone tolerant rice cultivars through genome mapping and quantitative trait loci (QTL) based approach which would potentially improve the rice production at global scale. A significant genetic variation in ozone sensitivity and tolerance among test cultivars would perhaps provide a vital information for developing models to predict ozone-induced yield loss and adaptation strategies for sustainable rice production.
Availability of data materials
The authors declare that complete data set is provided in the results and supplementary file of this paper.
References
Ainsworth, E. A. (2008). Rice production in a changing climate: A meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Global Change Biology, 14(7), 1642–1650. https://doi.org/10.1111/j.1365-2486.2008.01594.x
Akhtar, N., Yamaguchi, M., Inada, H., Hoshino, D., Kondo, T., Fukami, M., Funada, R., & Izuta, T. (2010). Effects of ozone on growth, yield and leaf gas exchange rates of four Bangladeshi cultivars of rice (Oryza sativa L.). Environmental Pollution, 158(9), 2970–2976. https://doi.org/10.1016/j.envpol.2010.05.026
Ashrafuzzaman, M., Haque, Z., Ali, B., Mathew, B., Yu, P., Hochholdinger, F., de Abreu Neto, J. B., McGillen, M. R., Ensikat, H. J., Manning, W. J., & Frei, M. (2018). Ethylenediurea (EDU) mitigates the negative effects of ozone in rice: Insights into its mode of action. Plant Cell & Environment, 41(12), 2882–2898. https://doi.org/10.1111/pce.13423
Ashrafuzzaman, M., Lubna, F. A., Holtkamp, F., Manning, W. J., Kraska, T., & Frei, M. (2017). Diagnosing ozone stress and differential tolerance in rice (Oryza sativa L.) with ethylenediurea (EDU). Environmental Pollution, 230, 339–350. https://doi.org/10.1016/j.envpol.2017.06.055
Baier, M., Kandlbinder, A., Golldack, D., & Dietz, K. J. (2005). Oxidative stress and ozone: Perception, signalling and response. Plant Cell & Environment, 28(8), 1012–1020. https://doi.org/10.1111/j.1365-3040.2005.01326.x
Bates, L. S., Waldren, S. P., & Teare, I. D. (1973). Rapid determination of proline for water-stressed studies. Plant and Soil, 39, 205–207.
Bellini, E., & De Tullio, M. C. (2019). Ascorbic acid and ozone: Novel perspectives to explain an elusive relationship. Plants, 8(5), 122. https://doi.org/10.3390/plants8050122
Chaudhary, N., & Agrawal, S. B. (2013). Intraspecific responses of six Indian clover cultivars under ambient and elevated levels of ozone. Environmental Science and Pollution Research, 20(8), 5318–5329. https://doi.org/10.1007/s11356-013-1517-0
Chen, C. P., Frei, M., & Wissuwa, M. (2011). The OzT8 locus in rice protects leaf carbon assimilation rate and photosynthetic capacity under ozone stress. Plant Cell & Environment, 34(7), 1141–1149. https://doi.org/10.1111/j.1365-3040.2011.02312.x
Crous, K. Y., Vandermeiren, K., & Ceulemans, R. (2006). Physiological responses to cumulative ozone uptake in two white clover (Trifolium repens L. cv. Regal) clones with different ozone sensitivity. Environmental and Experimental Botany, 58(1–3), 169–179. https://doi.org/10.1016/j.envexpbot.2005.07.007
David, L. M., & Nair, P. R. (2013). Tropospheric column O3 and NO2 over the Indian Region observed by ozone monitoring instrument (OMI): Seasonal changes and long-term trends. Atmospheric Environment, 65, 25–39. https://doi.org/10.1016/j.atmosenv.2012.09.033
Deb Roy, S., Beig, G., & Ghude, S. D. (2009). Exposure-plant response of ambient ozone over the tropical Indian Region. Atmospheric Chemistry and Physics, 9(14), 5253–5260. https://doi.org/10.5194/acp-9-5253-2009
Fatima, A., Singh, A. A., Mukherjee, A., Dolker, T., Agrawal, M., & Agrawal, S. B. (2019). Assessment of ozone sensitivity in three wheat cultivars using ethylenediurea. Plants, 8(4), 80. https://doi.org/10.3390/plants8040080
Feng, Z., De Marco, A., Anav, A., Gualtieri, M., Sicard, P., Tian, H., Fornasier, F., Tao, F., Guo, A., & Paoletti, E. (2019). Economic losses due to ozone impacts on human health, forest productivity and crop yield across China. Environment International, 131, 104966. https://doi.org/10.1016/j.envint.2019.104966
Fischer, T. (2019). Wheat yield losses in India due to ozone and aerosol pollution and their alleviation: A critical review. Outlook on Agriculture, 48(3), 181–189. https://doi.org/10.1177/0030727019868484
Fiscus, E. L., Booker, F. L., & Burkey, K. O. (2005). Crop responses to ozone: Uptake, modes of action, carbon assimilation and partitioning. Plant Cell & Environment, 28(8), 997–1011. https://doi.org/10.1111/j.1365-3040.2005.01349.x
Frei, M. (2015). Breeding of ozone resistant rice: Relevance, approaches and challenges. Environmental Pollution, 197, 144–155. https://doi.org/10.1016/j.envpol.2014.12.011
Frei, M., Tanaka, J. P., & Wissuwa, M. (2008). Genotypic variation in tolerance to elevated ozone in rice: Dissection of distinct genetic factors linked to tolerance mechanisms. Journal of Experimental Botany, 59(13), 3741–3752. https://doi.org/10.1093/jxb/ern222
Ghude, S. D., Jena, C., Chate, D. M., Beig, G., Pfister, G. G., Kumar, R., & Ramanathan, V. (2014). Reductions in India’s crop yield due to ozone. Geophysical Research Letters, 41(15), 5685–5691. https://doi.org/10.1002/2014GL060930
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of biochemistry and biophysics, 125(1), 189–198. https://doi.org/10.1016/0003-9861(68)90654-1
IPCC. (2013) Climate Change 2013: The Physical Science Basis. In: T. F. Stocker, D. Qin, G. K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, & P. M. Midgley (Eds.), Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press
IPCC. (2021). Summary for Policymakers. In: V. Masson-Delmotte, P. Zhai, A. Pirani, S. L. Connors, Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M. I., Huang, M., Leitzell, K., E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, & B. Zhou (Eds.), Climate Change 2021: The Physical Science Basis Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press
Jing, L., Dombinov, V., Shen, S., Wu, Y., Yang, L., Wang, Y., & Frei, M. (2016). Physiological and genotype-specific factors associated with grain quality changes in rice exposed to high ozone. Environmental Pollution, 210, 397–408. https://doi.org/10.1016/j.envpol.2016.01.023
Kakar, N., Jumaa, S. H., Redoña, E. D., Warburton, M. L., & Reddy, K. R. (2019). Evaluating rice for salinity using pot-culture provides a systematic tolerance assessment at the seedling stage. Rice, 12(1), 1–14. https://doi.org/10.1186/s12284-019-0317-7
Kao, C. H. (2015). Role of L-ascorbic acid in rice plants. Crop Environment & Bioinformatics, 12(1), 1–7.
Keller, T., & Schwager, H. (1977). Air pollution and ascorbic acid. European Journal of Forest Pathology, 7(6), 338–350. https://doi.org/10.1111/j.1439-0329.1977.tb00603.x
Kibria, M. G., Hossain, M., Murata, Y., & Hoque, M. A. (2017). Antioxidant defense mechanisms of salinity tolerance in rice genotypes. Rice Science, 24(3), 155–162. https://doi.org/10.1016/j.rsci.2017.05.001
Krishna sharma, R. & Nagaveena, S. (2016). Variation of ozone with meteorology in surface air over two sites of southern Tamil Nadu, India. Indian Journal of Radio & Space Physics (IJRSP), 45(2), 79–86.
Kumari, S., Lakhani, A., & Kumari, K. M. (2020). First observation-based study on surface O3 trend in Indo-Gangetic Plain: Assessment of its impact on crop yield. Chemosphere, 255, 126972. https://doi.org/10.1016/j.chemosphere.2020.126972
Lal, D. M., Ghude, S. D., Patil, S. D., Kulkarni, S. H., Jena, C., Tiwari, S., & Srivastava, M. K. (2012). Tropospheric ozone and aerosol long-term trends over the Indo-Gangetic Plain (IGP), India. Atmospheric Research, 116, 82–92. https://doi.org/10.1016/j.atmosres.2012.02.014
Lal, S., Venkataramani, S., Naja, M., Kuniyal, J. C., Mandal, T. K., Bhuyan, P. K., Kumari, K. M., Tripathi, S. N., Sarkar, U., Das, T., & Swamy, Y. V. (2017). Loss of crop yields in India due to surface ozone: An estimation based on a network of observations. Environmental Science and Pollution Research, 24(26), 20972–20981. https://doi.org/10.1007/s11356-017-9729-3
Li, C., Zhu, J., Zeng, Q., Luo, K., Liu, B., Liu, G., & Tang, H. (2017). Different responses of transgenic Bt rice and conventional rice to elevated ozone concentration. Environmental Science and Pollution Research, 24(9), 8352–8362. https://doi.org/10.1007/s11356-017-8508-5
Lin, Z., Zhang, X., Yang, X., Li, G., Tang, S., Wang, S., Ding, Y., & Liu, Z. (2014). Proteomic analysis of proteins related to rice grain chalkiness using iTRAQ and a novel comparison system based on a notched-belly mutant with white-belly. BMC Plant Biology, 14(1), 1–17. https://doi.org/10.1186/1471-2229-14-163
Löw, M., Deckmyn, G., Op de Beeck, M., Blumenröther, M. C., Oßwald, W., Alexou, M., Jehnes, S., Haberer, K., Rennenberg, H., Herbinger, K., & Häberle, K. H. (2012). Multivariate analysis of physiological parameters reveals a consistent O3 response pattern in leaves of adult European beech (Fagus sylvatica). New Phytologist, 196(1), 162–172. https://doi.org/10.1111/j.1469-8137.2012.04223.x
Masutomi, Y., Kinose, Y., Takimoto, T., Yonekura, T., Oue, H., & Kobayashi, K. (2019). Ozone changes the linear relationship between photosynthesis and stomatal conductance and decreases water use efficiency in rice. Science of the Total Environment, 655, 1009–1016. https://doi.org/10.1016/j.scitotenv.2018.11.132
Mazid, M. S., Rafii, M. Y., Hanafi, M. M., Rahim, H. A., & Latif, M. A. (2013). Genetic variation, heritability, divergence and biomass accumulation of rice genotypes resistant to bacterial blight revealed by quantitative traits and ISSR markers. Physiologia Plantarum, 149(3), 432–447. https://doi.org/10.1111/ppl.12054
Miyazaki, K., Eskes, H., Sudo, K., Boersma, K. F., Bowman, K., & Kanaya, Y. (2017). Decadal changes in global surface NOx emissions from multi-constituent satellite data assimilation. Atmospheric Chemistry and Physics, 17(2), 807–837. https://doi.org/10.5194/acp-17-807-2017
Monks, P. S., Archibald, A. T., Colette, A., Cooper, O., Coyle, M., Derwent, R., Fowler, D., Granier, C., Law, K. S., Mills, G. E., & Stevenson, D. S. (2015). Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmospheric Chemistry and Physics, 15(15), 8889–8973. https://doi.org/10.5194/acp-15-8889-2015
Nahar, S., Vemireddy, L. R., Sahoo, L., & Tanti, B. (2018). Antioxidant protection mechanisms reveal significant response in drought-induced oxidative stress in some traditional rice of Assam India. Rice Science, 25(4), 185–196. https://doi.org/10.1016/j.rsci.2018.06.002
Pandey, A. K., Ghosh, A., Agrawal, M., & Agrawal, S. B. (2018). Effect of elevated ozone and varying levels of soil nitrogen in two wheat (Triticum aestivum L.) cultivars: Growth, gas-exchange, antioxidant status, grain yield and quality. Ecotoxicology and Environmental Safety, 158, 59–68. https://doi.org/10.1016/j.ecoenv.2018.04.014
Pandey, A. K., Majumder, B., Keski-Saari, S., Kontunen-Soppela, S., Mishra, A., Sahu, N., Pandey, V., & Oksanen, E. (2015). Searching for common responsive parameters for ozone tolerance in 18 rice cultivars in India: Results from ethylenediurea studies. Science of the Total Environment, 532, 230–238. https://doi.org/10.1016/j.scitotenv.2015.05.040
Pang, J., Kobayashi, K., & Zhu, J. (2009). Yield and photosynthetic characteristics of flag leaves in Chinese rice (Oryza sativa L.) varieties subjected to free-air release of ozone. Agriculture, ecosystems & environment, 132(3–4), 203–211. https://doi.org/10.1016/j.agee.2009.03.012
Peng, B., Wang, Y., Zhu, J., Wang, Y., & Yang, L. (2018). Effects of ozone stress on rice growth and yield formation under different planting densities-a face study. International Journal of Agriculture and Biology, 20(11), 2599–2605. https://doi.org/10.17957/IJAB/15.0846
Rejeb, K. B., Abdelly, C., & Savouré, A. (2014). How reactive oxygen species and proline face stress together. Plant Physiology and Biochemistry, 80, 278–284. https://doi.org/10.1016/j.plaphy.2014.04.007
Shao, Z., Zhang, Y., Mu, H., Wang, Y., Wang, Y., & Yang, L. (2020). Ozone-induced reduction in rice yield is closely related to the response of spikelet density under ozone stress. Science of the Total Environment, 712, 136560. https://doi.org/10.1016/j.scitotenv.2020.136560
Sharma, A., Ojha, N., Pozzer, A., Beig, G., & Gunthe, S. S. (2019). Revisiting the crop yield loss in India attributable to ozone. Atmospheric Environment: X, 1, 100008. https://doi.org/10.1016/j.aeaoa.2019.100008
Shi, G., Yang, L., Wang, Y., Kobayashi, K., Zhu, J., Tang, H., Pan, S., Chen, T., Liu, G., & Wang, Y. (2009). Impact of elevated ozone concentration on yield of four Chinese rice cultivars under fully open-air field conditions. Agriculture, Ecosystems & Environment, 131(3–4), 178–184. https://doi.org/10.1016/j.agee.2009.01.009
Singh, A. A., Fatima, A., Mishra, A. K., Chaudhary, N., Mukherjee, A., Agrawal, M., & Agrawal, S. B. (2018). Assessment of ozone toxicity among 14 Indian wheat cultivars under field conditions: Growth and productivity. Environmental Monitoring and Assessment, 190(4), 1–14. https://doi.org/10.1007/s10661-018-6563-0
Terao, T., Nagata, K., Morino, K., & Hirose, T. (2010). A gene controlling the number of primary rachis branches also controls the vascular bundle formation and hence is responsible to increase the harvest index and grain yield in rice. Theoretical and Applied Genetics, 120(5), 875–893. https://doi.org/10.1007/s00122-009-1218-8
TNAU. (2019). Tamil Nadu Agricultural University-Crop production guide: agriculture-nutrient management for rice crop. Accessed February 1, 2019, from http://agritech.tnau.ac.in/agriculture/agri_nutrientmgt_rice.html
Udayasoorian, C., Jayabalakrishnan, R. M., Suguna, A. R., Venkataramani, S., & Lal, S. (2013). Diurnal and seasonal characteristics of ozone and NOx over a high altitude Western Ghats location in Southern India. Advances in Applied Science Research, 4(5), 309–320.
Ueda, Y., Frimpong, F., Qi, Y., Matthus, E., Wu, L., Höller, S., Kraska, T., & Frei, M. (2015). Genetic dissection of ozone tolerance in rice (Oryza sativa L.) by a genome-wide association study. Journal of experimental botany, 66(1), 293–306. https://doi.org/10.1093/jxb/eru419
Ueda, Y., Uehara, N., Sasaki, H., Kobayashi, K., & Yamakawa, T. (2013). Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiology and Biochemistry, 70, 396–402. https://doi.org/10.1016/j.plaphy.2013.06.009
Upadhyaya, H., Khan, M. H., & Panda, S. K. (2007). Hydrogen peroxide induces oxidative stress in detached leaves of Oryza sativa L. General and Applied Plant Physiology, 33(1–2), 83–95.
Urban, J., Ingwers, M. W., McGuire, M. A., & Teskey, R. O. (2017). Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. Journal of Experimental Botany, 68(7), 1757–1767. https://doi.org/10.1093/jxb/erx052
Vainonen, J. P., & Kangasjärvi, J. (2015). Plant signalling in acute ozone exposure. Plant, Cell & Environment, 38(2), 240–252. https://doi.org/10.1111/pce.12273
Van Dingenen, R., Dentener, F. J., Raes, F., Krol, M. C., Emberson, L., & Cofala, J. (2009). The global impact of ozone on agricultural crop yields under current and future air quality legislation. Atmospheric Environment, 43(3), 604–618. https://doi.org/10.1016/j.atmosenv.2008.10.033
Wang, J., Zeng, Q., Zhu, J., Liu, G., & Tang, H. (2013). Dissimilarity of ascorbate–glutathione (AsA–GSH) cycle mechanism in two rice (Oryza sativa L.) cultivars under experimental free-air ozone exposure. Agriculture, Ecosystems & Environment, 165, 39–49. https://doi.org/10.1016/j.agee.2012.12.006
Wang, Y., Yang, L., Höller, M., Zaisheng, S., Pariasca-Tanaka, J., Wissuwa, M., & Frei, M. (2014). Pyramiding of ozone tolerance QTLs OzT8 and OzT9 confers improved tolerance to season-long ozone exposure in rice. Environmental and Experimental Botany, 104, 26–33. https://doi.org/10.1016/j.envexpbot.2014.03.005
Wang, Y., Yang, L., Kobayashi, K., Zhu, J., Chen, C. P., Yang, K., Tang, H., & Wang, Y. (2012). Investigations on spikelet formation in hybrid rice as affected by elevated tropospheric ozone concentration in China. Agriculture, Ecosystems & Environment, 150, 63–71. https://doi.org/10.1016/j.agee.2012.01.016
Yang, N., Wang, X., Zheng, F., & Chen, Y. (2017). The impact of elevated ozone on the ornamental features of two flowering plants (Tagetes erecta Linn. and Petunia hybrida Vilm.). International Journal of Environment and Pollution, 61(1), 29–45. https://doi.org/10.1504/IJEP.2017.10003698
Zheng, F., Wang, X., Zhang, W., Hou, P., Lu, F., Du, K., & Sun, Z. (2013). Effects of elevated O3 exposure on nutrient elements and quality of winter wheat and rice grain in Yangtze River Delta, China. Environmental Pollution, 179, 19–26. https://doi.org/10.1016/j.envpol.2013.03.051
Ziemke, J. R., Oman, L. D., Strode, S. A., Douglass, A. R., Olsen, M. A., McPeters, R. D., Bhartia, P. K., Froidevaux, L., Labow, G. J., Witte, J. C., & Thompson, A. M. (2019). Trends in global tropospheric ozone inferred from a composite record of TOMS/OMI/MLS/OMPS satellite measurements and the MERRA-2 GMI simulation. Atmospheric Chemistry and Physics, 19(5), 3257–3269. https://doi.org/10.5194/acp-19-3257-2019
Acknowledgements
Authors are thankful to Physical Research Laboratory (PRL), Indian Space Research Organisation (ISRO), Ahmadabad, India, and Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India.
Funding
This work was financially supported by the Physical Research Laboratory (PRL), Indian Space Research Organisation (ISRO), Ahmadabad under Atmospheric Trace gases Chemistry Transport Modeling (ATCTM) Scheme granted to Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramya, A., Dhevagi, P., Priyatharshini, S. et al. Response of rice (Oryza sativa L.) cultivars to elevated ozone stress. Environ Monit Assess 193, 808 (2021). https://doi.org/10.1007/s10661-021-09595-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09595-w