Abstract
The variability in zooplankton density and species composition in response to tidal oscillations were investigated in the lesser saline upper reaches (E1) and higher saline lower reaches (E2) of the Mahanadi Estuary, located at the mouth of the third largest river in Indian Peninsula. This is the first of its kind high frequency observation over the tidal cycle ranging from highest high tide to lowest low tide in this estuary revealing dynamic variability of zooplankton assemblages. Zooplankton abundance was higher during high tide in comparison to low tide, irrespective of salinity regimes. On the diurnal scale, it was higher at night in comparison to the day at both E1 and E2. The higher abundance of zooplankton groups such as Copepoda, Cladocera, and planktonic larvae during the night at E1 as well as E2 indicating an upward migration. Many of the zooplankton taxa (e.g., Pseudodiaptomus serricaudatus, Pseudodiaptomus sp., Acartia danae, Acrocalanus longicornis, Oithona sp., Corycaeus andrewsi) migrated towards E1 due to tidal effect during high tide and maintained their position even during low tide. In contrast, the prevalence of limnetic taxa (e.g., Brachionus rubens, Polyarthra vulgaris, Bosminopsis deitersi, Moina micrura, Heliodiaptomus sp.) at E2 during low tide indicated a predominant riverine source. The tidal variability of Brachyura (zoea and megalopa) revealed different emergence times that indicated dispersal of zoeas to the adjacent Bay of Bengal and the return of magalopa to the Mahanadi Estuary. Species diversity index was higher during high tide, and prominent at E2. At E1, marine, fresh, marine-brackish, and marine-brackish-fresh zooplankton species dominated during high tide, while brackish-fresh taxa dominated during low tide. Differently, E2 was enriched with marine, marine-brackish, and marine-brackish-fresh taxa during high tide, whereas fresh, brackish-fresh, and marine-fresh dominated during low tide. Salinity and suspended matter influenced the dominant zooplankton taxa at E2 and E1, respectively. Zooplankton assemblages exhibited a pattern of prominent diurnal-spatial variation in comparison to the tidal scale in the Mahanadi Estuary. Overall, this study documented a very high zooplankton diversity (92 taxa belonging to 13 groups) and significant variations in species abundance which highlighted the importance of carrying out sampling over the tidal cycle at contrasting salinity regimes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries are transition zones that link rivers to the sea where freshwater mixes with marine water (Gazeau et al., 2005). In general, the estuaries are highly productive zones attributed to variable degrees of marine and freshwater ingress that makes the water quality highly dynamic resulting in spatial and temporal hydro-biological patterns. The nutrient trapping efficiency and short nutrient regeneration time of estuaries support a wide array of biota forming ecological niches (Leandro et al., 2007; Menendez et al., 2012). The faunal planktonic component, zooplankton, prey upon phytoplankton exerting top-down control on their ecology (Micheli, 1999; Winder & Jassby, 2011). On the other hand, zooplankton act as a trophic level intermediate by serving as a major diet source for fishes. And thereby, zooplankton regulate fish migration, control the survival of fish larvae, and support coastal water fisheries (Abo-Taleb, 2019; Ayon et al., 2008). In addition, zooplankton perform a major ecological role in the aquatic environment by predominantly contributing to organic matter flux and nutrient recycling (Steinberg & Landry, 2017). Therefore, understanding the distribution and variability of zooplankton community composition is important in evaluating ecosystem health (Marques et al., 2009).
Unlike other aquatic ecosystems, estuarine zooplankton assemblages are closely coupled with fluctuation in the ambient physico-chemical parameters over the tidal and diurnal cycle (Marques et al., 2009). Estuarine zooplankton performs migration and exhibits adaptation mechanisms in response to tidal and diurnal dynamics. Tidal oscillation in estuaries results in the significant movement of water layers resulting in highly dynamic salinity regimes that influence the distribution of zooplankton populations (Chew & Chong, 2011). Several studies have reported relatively higher abundances of zooplankton during low tide in comparison to high tide (Davies & Ugwumba, 2013; Gao et al., 2011; Jessopp & McAllen, 2008). In contrast, some studies have also shown maximum zooplankton abundances during high tide (da Costa et al., 2013; Gajbhiye et al., 1984). In regard to the diurnal cycle of zooplankton, solar insolation plays an important role in the variability of estuarine zooplankton community (Chew et al., 2015).
In the context of Indian estuaries, spatio-temporal studies have been carried out at regular intervals on estuarine zooplankton ecology (Nandy & Mandal, 2020; Srichandan et al., 2021; Venkataramana et al., 2017). However, high-resolution studies at the diurnal and tidal scales are very scanty, especially in estuaries of the Indian east coast. Mahanadi Estuary formed at the mouth of Indian Peninsula’s 3rd largest river system Mahanadi on the east coast, which hosts a semi-diurnal tidal ecosystem (Srichandan et al., 2019). This estuary owes significant ecological and socio-economic importance. Mahanadi Estuary and adjoining coastal waters of the Bay of Bengal are major fishing grounds on the east coast of India. In addition, the coastal waters off Mahanadi Estuary host mass-mating of olive ridley sea turtles. On the other hand, rapid urbanization and industrialization in nearby areas of the Mahanadi Estuary pose an ecological risk for the inhabiting biota (Samantray et al., 2009; Sundaray et al., 2009). Since zooplankton plays a pivotal role in estuarine and coastal biogeochemistry, understanding the variability in community composition of these organisms will help in ecosystem health assessment. In this context, the tidal and diurnal distributions of the zooplankton community structure need to be understood, but remain unexplored in the Mahanadi Estuary. On this backdrop, the present study was carried out with an aim to investigate the effect of tidal and diurnal scale on the zooplankton distribution and community structure over a tidal cycle in the Mahanadi Estuary.
Materials and methods
Study site
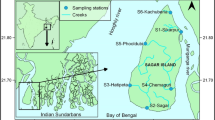
The present study was carried out in the Mahanadi River Estuary connected to the Bay of Bengal on the east coast of India at Paradip (Fig. 1). Mahanadi River flows through hilly terrain in its upper reaches and a low-lying deltaic coastal plain before draining into the bay (Borole et al., 1979; Panda et al., 2006). It is one of the major Indian rivers in terms of water potential and flood producing capability (Chakrapani & Subramanian, 1990). The river runs a total length of ~ 851 km and a peak discharge of ~ 44,740 m3 s−1 during monsoon season (Ganguly et al., 2011; Raj et al., 2013). The basin is characterized by a tropical climate and receives 90% annual rainfall during the south-west monsoon that sets in between June-July and withdraws in October (Bastia & Equeenuddin, 2016; Chakrapani & Subramanian, 1990; Mishra et al., 2009). The annual river runoff follows the rainfall pattern (Rao et al., 2004). The estuarine system is classified as a tide-dominated and partially mixed coastal plain estuary on the basis of physical characteristics (Panda et al., 2006; Rao et al., 2004). It is a micro-tidal estuary and experiences semi-diurnal tides, and the tidal water influence could be observed up to 40 km upstream (Murali et al., 2015; Naik et al., 2020; Panda et al., 2006). The estuarine flux signature was traced to 43 km off the coast in the Bay of Bengal (Mishra et al., 2009). The water column of the estuary varies from 2 to 12 m (Rao et al., 2004). Mahanadi Estuary supports livelihood of thousands of fisher folks by facilitating hundreds of fishing fleets for capture fishery in the estuarine region as well as in the coastal waters of the Bay of Bengal. The average annual fish landing is 25,000—30,000 MT in Mahanadi Estuary fishing harbor at Paradip (Acharyya et al., 2021; Baliarsingh et al., 2021a, b; Paradeep Fishing Harbour, 2020). Agricultural run-off and industrial-urban effluents drain into the river, and the amount is very large (Sundaray et al., 2009).
Map showing sampling stations in the upper (E1) and lower (E2) Mahanadi Estuary, on the east coast of India. (Image reprinted by permission from Springer Nature, Bulletin of Environmental Contamination and Toxicology, Baliarsingh et al., 2021a)
Methodology
Field surveys were carried out from 3rd to 9th October of 2018 at upper (E1) and lower (E2) reaches of Mahanadi Estuary (Fig. 1). E1 represents a low saline regime, while E2 represents a high salinity regime. E1 is located at a distance of ~ 12 km upstream from E2 (Baliarsingh et al., 2021b). This estuary experiences semi-diurnal tides with two high and two low tides in a 24-h cycle. Surface water samples for analysis of physical, chemical, and biological parameters were collected during each high as well as low tide over the study period on board a mechanized boat (Fig. 2). The specific duration of the survey was fixed to collect samples within the highest high tide to lowest low tide of a tidal cycle. Tide height data was obtained from the Survey of India, and accordingly, the aforementioned dates for tidal cycle were selected. Zooplankton samples were collected from the surface using a plankton net (mesh size: 120 µm, make: KC Denmark) equipped with a flow meter (make: KC Denmark). The plankton net was towed horizontally by a mechanized boat. The net towing speed was maintained at ~ 1–2 knot and lasted for 5–10 minutes per sample. The volume of water filtered through the net was calculated using the following equation (Srichandan et al., 2021).
NRF = number of revolution of flow meter, PI = pitch of impeller (m), NOA = net opening area (m2).
The average filtered volume of water was 72 m3. Zooplankton samples were immediately preserved with 5% formaldehyde. Qualitative and quantitative analyses were carried out in the laboratory using a trinocular compound microscope (make: Labomed; model: LX-400). Taxonomic keys were referred for zooplankton species identification (Al-Yamani et al., 2011; Battish, 1992; Conway et al., 2003). The zooplankton species habitat was defined based on WoRMS (World Register of Marine Species, 2021) and above-mentioned standard taxonomic literature.
Salinity and temperature were recorded with the aid of a CTD Profiler (make: Sea-Bird Electronics, model: 19Plus). Dissolved oxygen (DO) and biochemical oxygen demand (BOD) were estimated using the Winkler’s method as described by Grasshoff et al. (1999). Total suspended matter (TSM) was analyzed gravimetrically by filtering a known volume of the water sample through a 0.45-µm filter. Chlorophyll-a (chl-a) concentration was measured by adopting 90% acetone extraction method with aid of a UV-Visible spectrophotometer (make: Shimadzu, model: UV2600). Colored dissolved organic matter (CDOM) was measured with aid of a fluorometer (make: Turner Designs, model: 7200–000). The water sample (1 l) for phytoplankton analysis were fixed with formalin-Lugol’s iodine solution and concentrated by a gravity sedimentation method. Phytoplankton cells were enumerated using a 1-ml Sedgwick-Rafter counting cell under a trinocular compound microscope (Labomed; model: LX-400).
Univariate diversity indices, viz., species richness, species diversity index, and species evenness were computed following Margalef (1958), Shannon and Weaver (1963), and Pielou (1966), respectively. Similarity percentage (SIMPER) analysis and canonical correspondence analysis (CCA) were carried out using the PAST statistical package (version 4.03) (Hammer et al., 2001). Group average Bray–Curtis dendrogram and non-metric multi-dimensional scaling (NMDS) were constructed with normalized data using PRIMER (version 6.1.7) (Clarke & Warwick, 2001). One-way analysis of variance (ANOVA) was carried out using Microsoft Excel Spread sheet function, and the probability (p) values < 0.05 were considered to explain the significant variability of parameters. Graphical representations were prepared using Grapher (version 8.8).
Results and discussion
Tidal-diurnal variability in zooplankton species diversity
A total of 39 species of holoplankton represented by 6 diverse categories of organisms, namely, Ciliophora (2 species), Rotifera (15 species), Cladocera (3 species), Copepoda (16 species), Ostracoda (1 species), and Malacostraca (2 species) and 9 types of planktonic larvae were observed at E1 (Table 1). The zooplankton population was observed to be highly diverse at E2 (holoplankton: 69 species, planktonic larvae: 14 types) in comparison to E1 (Table 1). Holoplankton assemblages were distributed into 12 diverse taxa, namely, Radiozoa (1 species), Ciliophora (5 species), Rotifera (16 species), Hydrozoa (3 species), Ctenophora (1 species), Gastropoda (4 species), Cladocera (4 species), Copepoda (28 species), Malacostraca (2 species), Chaetognatha (2 species), and Chordata (3 species) at E2. Among the 39 identified taxa of holoplankton at E1, 18 were fresh, 9 marine, 6 marine-brackish-fresh, 5 marine-brackish, and 1 brackish-fresh habitat zooplankton. Contrastingly, at E2, a higher number of marine taxa (35 species) was observed, followed by fresh (17 species), marine-brackish (9 species), marine-brackish-fresh (6 species), brackish-fresh (1 species), and marine-fresh (1 species). Among the total identified taxa over the study period, 5 eutrophication indicator species of zooplankton were also noticed (Table 1).
The species diversity indices serve as important indicators of ambient water quality and species homogeneity among the populations (Lopes et al., 2009; Thakur et al., 2013). The species diversity and evenness index did not show prominent variation with tides at E1 (Fig. 3). However, the richness index was higher during high tide in comparison to low tide. This might be attributed to the transport of marine species to the upstream during high tide. At E2, the species diversity along with richness and evenness indices showed higher values during high tide in comparison to low tide. During high tide, the intrusion of marine species from the adjacent Bay of Bengal could have triggered species diversity and richness indices. Zooplankton species diversity was higher in E2 as compared to E1, which could be attributed to the contribution of both incursive marine and estuarine species that contribute to the overall population (Wooldridge, 1999). The diversity indices also highlighted the differences between day and night periods in the Mahanadi Estuary. Species diversity index was significantly higher at night in comparison to the day at E1, suggesting the occurrence of vertical migration (Marques et al., 2009). Concerning the diurnal cycle at E2, higher values for species diversity and evenness indices were observed during the day in comparison to the night and could be attributed to the diurnal variation of zooplankton with diverse assemblages.
Zooplankton abundance and community structure in response to tidal cycle
The tidal oscillation largely determines zooplankton distribution pattern and plays a significant role in the dynamics of resident zooplankton through inflow and outflow of stocks (Krumme & Liang, 2004; Villate, 1997). In order to determine the degree of similarity, NMDS was applied to the zooplankton abundance. NMDS technique has been proved to be useful in ecological studies of plankton in identifying spatial heterogeneity (Alfonso et al., 2010; Anandavelu et al., 2020; Connelly et al., 2020). NMDS prominently differentiated zooplankton abundance at E1 and E2 with respect to tides demonstrating spatial heterogeneity in zooplankton distribution (Fig. 4).
Non-metric multi-dimensional scaling (NMDS) showing tidal (left panel) and diurnal (right panel) similarity based on zooplankton species abundance at the upper and lower Mahanadi Estuary. Larger distance between the symbols denotes lesser similarity (E1: upper estuary, E2: lower estuary, HT: high tide, LT: low tide, D: day, N: night)
The zooplankton abundance varied in response to tidal oscillations at both E1 and E2. The zooplankton abundance was relatively higher during high tide (avg. 1430 org m−3) in comparison to low tide (avg. 718 org m−3) at E1 (Fig. 5). Similarly, E2 also showed a higher abundance of zooplankton during high tide (avg. 456 org m−3) as compared to low tide (avg. 401 org m−3) (Fig. 5). However, there was no statistically significant variation between the tides at both E1 (p = 0.28) and E2 (p = 0.61). The higher abundances during high tide, irrespective of sites, could be attributed to the high inflow of seawater from the adjacent Bay of Bengal. Similarly, higher abundances of zooplankton at high tide have been reported earlier from the Han River Estuary (Youn & Choi, 2008). In terms of the different forms of zooplankton, according to the habitat, at E1, a higher abundance of marine, fresh, marine-brackish, and marine-brackish-fresh species was observed during high tide, while a higher abundance of brackish-fresh species was observed during low tide (Fig. 6). At E2, relatively larger proportions of marine, marine-brackish, and marine-brackish-fresh were observed during high tide, while fresh, brackish-fresh, and marine-fresh dominated mostly during low tide (Fig. 6).
Tidal and diurnal variation of zooplankton abundance in the upper (E1) and lower (E2) Mahanadi Estuary. D1 to D7 denote the sampling dates as mentioned in Fig. 2
A distinct variation in the abundance of different zooplankton groups was evident at the tidal scale in the Mahanadi Estuary. Prominent increment of Copepoda and planktonic larvae abundances was observed during high tide at E1 (Fig. 7). This pattern could be attributed to the migration of non-resident species of Copepoda and planktonic larvae from sea to E1. This observation is in agreement with previous studies in tropical coastal waters that reported the higher abundance of Copepoda during high tide (Robertson et al., 1988). In addition, the presence of marine zooplankton taxa belonging to Malacostraca at E1 during high tide revealed tidal migration. At E2, Gastropoda, Copepoda, and Malacostraca showed prominent differences in abundances with higher values during high tide. On the other hand, Rotifera, Cladocera, and planktonic larvae dominated during low tide (Fig. 7). Zooplankton groups, namely, Radiozoa, Hydrozoa, Ctenophora, Chaetognatha, and Chordata were encountered only at high tide, which could be related to their high-saline condition affinity and intrusion from the adjacent Bay of Bengal.
In terms of species composition, zooplankton showed pronounced changes across tidal scale in the Mahanadi Estuary (Table 1). At E1, the most abundant zooplankton taxa were Asplanchna brightwelli, Asplanchna sp., Moina micrura, Pseudodiaptomus serricaudatus, Pseudodiaptomus sp., Mesocyclops sp., and Gastropoda veligers. The occurrence of large numbers of P. serricaudatus and Pseudodiaptomus sp. at high tide could have resulted from strong downstream flushing. However, these species were also observed during low tide with lower abundances than during high tide. This type of observation could be attributed to the retention mechanism of these species which were imported into E1 during high tide and possibly sank towards bottom preventing horizontal export out of E1 during low tide (Krumme & Liang, 2004). Literature also suggests that P. annandalei of the family Pseudodiaptomidae exhibits rheotaxis abilities to resist water currents and maintain its position in the estuary against the net seaward flow by adopting mechanisms like vertical migration and attachment to the bottom sediments (Shang et al., 2008). Other high saline preferring taxa such as Acartia danae, Acrocalanus longicornis, Oithona sp., and Corycaeus andrewsi were also observed during high tide. This was in agreement with studies which have shown that zooplankton taxa belonging to order Calanoida and Poecilostomatoida were maximum during high tide (Ali et al., 2011). The freshwater zooplankton species such as Asplanchna brightwelli, Asplanchna sp., and Moina micrura were abundant during low tide in comparison to high tide and could be sourced from river discharge.
At E2, a prominent variation in species composition was evident. Zooplankton taxa such as Acartia erythraea, Acrocalanus longicornis, Bivalvia veligers, and protozoea of Lucifer were more abundant during high tide, which could be attributed to the intrusion of marine taxa to the estuary. Brachionus rubens, Mesocyclops sp., Brachyura zoea larvae, and caridean larvae were observed with higher abundances during low tide. In addition, maximum Bivalvia veligers abundance coincided with high tide at E2, indicating its coastal origin. The more frequent presence of B. rubens and Mesocyclops sp. during low tide in comparison to high tide might have resulted from the strong riverine freshwater influx, which brings limnetic zooplankton species.
Interestingly, when larvae of Brachyura (zoea and megalopa) and Gastropoda veligers were analyzed at E1 and E2, prominent variations were evident at the tidal scale. Brachyura zoea larvae were significantly abundant during low tide in comparison to high tide at both E1 and E2. In contrast, the abundance of Brachyura megalopa larvae was higher during high tide at both sites. It is well known that the early larval stages of Brachyura crab such as zoea migrates seaward to spend most of their time in the sea to avoid high osmotic-thermal stress and intense pelagic predation in the estuarine environment (Anger et al., 1998; Hovel & Morgan, 1997; Lopez-Duarte et al., 2011). In addition, it has been shown in previous studies that low tide may effectively transport zoea of estuarine crabs to the sea. While megalopa is transported into the estuary during high tide attributed to their weak swimming relative to the current speed (Dittel & Epifanio, 1990; Oishi & Saigusa, 1997; Paula et al., 2004). In the present study, E2 showed five times greater abundance of Brachyura zoea larvae during low tide (avg. 115 org m−3) than high tide (avg. 21 org m−3). Gastropoda veligers abundance during high tide (avg. 449 org m−3) was six times greater than low tide (avg. 76 org m−3) at E1. In contrast, Gastropoda veligers were more abundant during low tide than high tide at E2. The distribution pattern of Gastropoda veligers in response to the tidal rhythm indicated that it is the resident zooplankton taxa in the Mahanadi Estuary.
In order to find out the discriminating species among a biological community, SIMPER analysis has been proved very useful (Vineetha et al., 2015). SIMPER analysis based on the Bray Curtis dissimilarity between groups revealed species that contribute to the maximum dissimilarity between the assemblages. The SIMPER analysis identified the discriminating species between high tide and low tide at both E1 and E2 (Fig. 8). The overall average dissimilarity between high tide and low tide were 73.8 and 88.0 at E1 and E2, respectively. The species responsible for the maximum dissimilarity on the tidal scale at E1 were Mesocyclops sp., Gastropoda veligers, Pseudodiaptomus sp., Asplanchna sp., Pseudodiaptomus serricaudatus, Brachionus rubens, Moina micrura, Brachionus sp., Asplanchna brightwelli, and Anuraeopsis sp. While Brachyura zoea larvae, caridean larvae, Acrocalanus longicornis, Mesocyclops sp., Brachionus rubens, protozoea of Lucifer, Acartia erythraea, Gastropoda veligers, Bivalvia veligers, and Lucifer hanseni displayed dissimilarity in their abundance on the tidal scale at E2.
Similarity percentage (SIMPER) analysis based on the Bray–Curtis dissimilarity of zooplankton taxa between (a) high and low tide in upper estuary (E1), (b) high and low tide in lower estuary (E2), (c) day and night in upper estuary (E1), and (d) day and night in lower estuary (E2). MES Mesocyclops sp., GAV Gastropoda veligers, PSP Pseudodiaptomus sp., PSS Pseudodiaptomus serricaudatus, ASS Asplanchna sp., MOM Moina micrura, BRR Brachionus rubens, ASB Asplanchna brightwelli, BRS Brachionus sp., ACD Acartia danae, ACE Acartia erythraea, ACL Acrocalanus longicornis, ANS Anuraeopsis sp., BIV Bivalvia veligers, BZL Brachyura zoea larvae, CAL Caridean larvae, LUH Lucifer hanseni, PRL Protozoea of Lucifer
Zooplankton abundance and community structure in response to diurnal cycle
NMDS based on zooplankton abundance significantly differentiated E1 and E2 with respect to the diurnal cycle (Fig. 4). Accordingly, zooplankton abundance also showed a prominent diurnal pattern at E1, with higher abundance during the night (avg. 1654 org m−3) and lower abundance during the day (avg. 583 org m−3). However, diurnal differences in zooplankton abundances were not statistically significant which could be due to the dynamic variability. At E2, significant variation (p < 0.01) in zooplankton was evident at diurnal scale with higher abundance during the night (avg. 550 org m−3) than during the day (avg. 326 org m−3) (Fig. 5). Similar diurnal vertical migration has also been reported earlier from other tropical estuaries (Vineetha et al., 2015). The reason for this vertical migration could be attributed to adaptive behavior to avoid visual predators (Loose & Dawidowicz, 1994; Vineetha et al., 2015; Williamson et al., 2011). In addition, all forms of holoplankton communities such as marine, fresh, brackish-fresh, marine-fresh, marine-brackish, and marine-brackish-fresh were higher during the night at both E1 and E2 (Fig. 6).
The zooplankton group composition in the Mahanadi Estuary appeared to be largely influenced by the diurnal cycle (Fig. 7). For instance, the abundance of Copepoda, Cladocera, and planktonic larvae was relatively higher during the night as compared to the day at E1. In contrast, Ciliophora, Rotifera, and Ostracoda did not exhibit diurnal vertical migration. At E2, holoplanktonic groups such as Gastropoda, Cladocera, Copepoda, Malacostraca, and Chordata exhibited a clear diurnal pattern in abundance, with higher peaks during the night. Similarly, planktonic larvae showed a prominent diurnal pattern, with higher abundance during the night. However, higher densities of some groups (Radiozoa, Ciliophora, Rotifera, Hydrozoa, Chaetognatha) were observed during the day. On the contrary, only one group, i.e., Ctenophora was observed only during the night. The observed vertical migration rhythm of copepods could be attributed to their swimming speed as they can migrate with a speed of 20–30 m per hour (Hattori, 1989).
With regard to species composition, the dominant holoplankton taxa such as Moina micrura, Pseudodiaptomus serricaudatus, Pseudodiaptomus sp., and Mesocyclops sp. were clearly more abundant during the night, while Asplanchna sp. and Brachionus sp. were more abundant during the day at E1 (Table 1). Several other holoplankton species (Brachionus rubens, Bosminopsis deitersi, Acartia danae, Heliodiaptomus sp., Pseudodiaptomus aurivilli, P. annandalei, and Lucifer hanseni) also exhibited a clear diurnal pattern in abundance, with higher peaks during the night. This type of nocturnal migration of Pseudodiaptomus has been reported in many tropical estuarine ecosystems (Kouassi et al., 2001). Literature also suggests that Pseudodiaptomus are predominantly epibenthic and exhibits diurnal upward migration (Hart & Allanson, 1976; Walter, 1987). Furthermore, a laboratory study has shown vertical migration of P. annandalei and their attachment to the bottom (Shang et al., 2008). Chew et al. (2015) have also observed a clear diurnal pattern of Acartia in the Matang Estuary (Malaysia). The nocturnal migration tendency of Lucifer hanseni has been observed many times in the Cochin backwaters (India) (Madhupratap & Rao, 1979; Vineetha et al., 2015). In the present study, all planktonic larvae (Bivalvia veligers, Brachyura megalopa larvae, Brachyura zoea larvae, caridean larvae, cirripede cypris, Copepoda nauplii, fish larvae, Gastropoda veligers, protozoea of Lucifer) showed the diurnal pattern, with higher abundances during the night. However, Gastropoda veligers and Brachyura zoea larvae were the main planktonic larvae in terms of abundance during the night in comparison to the day.
At E2, the most abundant zooplankton taxa that exhibited upward migration during the night were Acrocalanus longicornis, Mesocyclops sp., Brachyura zoea larvae, caridean larvae, and protozoea of Lucifer. In addition, some other holoplanktonic species such as Janthina sp., Moina micrura, Subeucalanus subcrassus, Thermocyclops sp., Oithona nana, and Lucifer hanseni also showed upward migration during the night. A similar diurnal pattern of Oithona sp., with higher abundance during the night, was also reported earlier from the Moreton Bay (Jacoby & Greenwood, 1989). In concomitant to the present study, a higher abundance of Thermocyclops during the night has been reported from other tropical systems (Arcifa et al., 2016). In general, marked pigmentation reduces the photo-damage to Subeucalanus and makes them easily visible to predators (Raymont, 1983). The reason for the higher abundance of Subeucalanus subcrassus during the night could be related to the avoidance from visual predators during the day. It has been also reported that Calanoida are the most targeted prey in the Indian Ocean (Ali, 2015; Ali et al., 2019).
The prominent dissimilarity between the day and night abundance of several zooplankton taxa evidenced through the SIMPER analysis supported the premise of diurnal variability of the zooplankton community at E1 (overall average dissimilarity 75.2) and E2 (overall average dissimilarity 82.5) (Fig. 8). At E2, zooplankton taxa such as caridean larvae, Acrocalanus longicornis, Mesocyclops sp., Brachyura zoea larvae, protozoea of Lucifer, Brachionus rubens, Acartia erythraea, Bivalvia veligers, Lucifer hanseni, and Gastropoda veligers were characteristic species on the diurnal scale. Furthermore, SIMPER analysis revealed prominent variation of Mesocyclops sp., Gastropoda veligers, Pseudodiaptomus sp., Pseudodiaptomus serricaudatus, Asplanchna sp., Moina micrura, Brachionus rubens, Asplanchna brightwelli, Brachionus sp., and Acartia danae on the diurnal scale at E1.
Tidal-diurnal interactions among zooplankton taxa
In general, zooplankton communities were highly variable, and their clustering appeared to be governed by many factors such as distance, site-specific environmental variables (e.g., salinity), tide (high and low), and photoperiod (day and night). Thus, based on the abundance of major zooplankton species on the diurnal and tidal scale, four clusters were identified (Fig. 9). The zooplankton taxa (Moina micrura, Pseudodiaptomus sp., Gastropoda veligers, and Mesocyclops sp.) that had higher abundance during the night at E1 were grouped in a single cluster (Cluster I). The zooplankton taxa such as caridean larvae and Brachionus rubens showed higher similarities with each other and were clustered (Cluster II) together in one group. The reason for this clustering could be related to their higher abundance during low tide as compared to high tide, especially at E2. Cluster III consisted of zooplankton species such as Pseudodiaptomus serricaudatus and Brachionus sp. The similarity in the distribution of these two species at E1 was observed to be controlled by the effect of high tide. Cluster IV was represented by five zooplankton taxa, namely, Acartia erytharea, Acrocalanus longicornis, protozoea of Lucifer, Lucifer hanseni, and Bivalvia veligers. This cluster could be related to the noticeably higher abundance of these species during high tide at E2. The cluster analysis also clearly showed that Brachyura zoea larvae were well separated from other taxa and did not form a distinct cluster. This observation might be related to the synchronization of peak Brachyura zoea larvae accumulation in the surface waters with the low tide period and nighttime.
Dominant zooplankton taxa in relation to hydro-biological variables
Zooplankton dynamics in estuarine ecosystems are greatly influenced by the hydrological conditions. Among the most important hydrological variables, salinity showed very low fluctuations at lower magnitude irrespective of the tidal influence at E1. Therefore, E1 can be considered as a low saline regime (Table 2; Baliarsingh et al., 2021a). In contrast, variation in salinity levels were observed with rising and fall during high and low tides, respectively at E2. The average water temperature was similar between the tides at E1, whereas relatively higher during low tide at E2 (Table 2). DO concentrations were higher at E1 in comparison to E2 during low tide. During high tide, DO concentrations at both stations were almost similar. The average concentration of BOD was similar at both reaches of the estuary over the tidal cycle. TSM concentration was higher during low tide at both E1 and E2. The variability of CDOM showed higher values during low tide suggesting riverine source and resuspension from the bottom (Menon et al., 2011) (Table 2). There was no large difference in phytoplankton abundance between the tides at both stations. The average abundance of phytoplankton was relatively higher during high tide in comparison to low tide at E1, while E2 showed the opposite pattern. The chl-a concentration increased during low tide and decreased during high tide (Table 2).
In general, interpretation of large hydro-biological data requires appropriate statistical analysis to discern influencing factors. Multivariate statistical analysis such as CCA has been proved to yield satisfactory results in tracing out latent interrelationships among a large set of hydro-biological variables (Tackx et al., 2004). CCA ordination delineated a cluster for E1 showing close association of zooplankton taxa such as Mesocyclops sp., Asplanchna brightwelli, and Moina micrura indicating their tidal variability (Fig. 10). This is supported by the highest abundance of these species during low tide at night, while lowest abundance was observed during high tide in daytime at E1. In addition, species of the family Pseudodiaptomidae, i.e., Pseudodipatomus sp. showed a close association with TSM, which could be due to an advantage of detritivorous feeding habits to higher TSM concentration at E1 during low tide. Literature also suggests that turbid condition helps to reduce visual ranges of predators (Grecay & Targett, 1996). In addition, higher turbidity linked to detritus and associated microbes provide an important source of energy for copepods, especially Pseudodiaptomus (Morgan et al., 1997). The CCA ordination showed grouping of dominant zooplankton taxa such as Acrocalanus longicornis, and Acartia erythraea with salinity at E2, where tidal influence was relatively higher than at E1 (Fig. 10).
Canonical correspondence analysis (CCA) ordination of water quality parameters and dominant zooplankton taxa in the upper (E1) and lower (E2) Mahanadi Estuary. (WT water temperature, TSM total suspended matter, DO dissolved oxygen, BOD biochemical oxygen demand, Chl chlorophyll-a, PA phytoplankton abundance, CDOM colored dissolved organic matter, ASB Asplanchna brightwelli, ASS Asplanchna sp., BRR Brachionus rubens, BRS Brachionus sp., MOM Moina micrura, ACE Acartia erythraea, ACL Acrocalanus longicornis, PSS Pseudodiaptomus serricaudatus, PSP Pseudodiaptomus sp., MES Mesocyclops sp., LUH Lucifer hanseni, BIV Bivalvia veligers, BZL Brachyura zoea larvae, CAL Caridean larvae, GAV Gastropoda veligers, PRL Protozoea of Lucifer
Conclusion
The estuarine zooplankton community distribution exhibited spatial, tidal, and diurnal patterns. However, diurnal and spatial variations of zooplankton were relatively prominent than variation due to tidal oscillation. Zooplankton assemblages exhibited a prominent diurnal upward migration pattern, with higher abundance during night irrespective of sites. Zooplankton also followed the tidally driven migration pattern with higher abundance during high tide at both sites. With regard to the nature of the habitat, the zooplankton communities were mainly comprised of marine, fresh, brackish-fresh, marine-fresh, marine-brackish, and marine-brackish-fresh. Pseudodiaptomus serricaudatus, Pseudodiaptomus sp., Brachionus rubens, Mesocyclops sp., Brachyura zoea larvae, and caridean larvae significantly varied in response to tidal oscillations. Pseudodiaptomus serricaudatus and Pseudodiaptomus sp. were the most abundant taxa during high tide at E1, while Brachionus rubens, Mesocyclops sp., Brachyura zoea larvae, and caridean larvae contributed with larger proportions to the zooplankton community during low tide at E2. Over the diurnal scale, the abundance of zooplankton taxa such as Moina micrura, Pseudodiaptomus serricaudatus, Pseudodiaptomus sp., and Mesocyclops sp. increased during the night compared to the day at E1, showing evidence of its upward movements. At E2, Acrocalanus longicornis, Mesocyclops sp., Brachyura zoea larvae, caridean larvae, and protozoea of Lucifer increased during the night, demonstrating upward migration. Diversity indices such as species diversity, richness, and evenness were higher during high tide at E2, indicating an intrusion of several marine species from the adjacent Bay of Bengal. The occurrence of dominant detritivorous species Pseudodipatomus during low tide at E1 suggested a pivotal role of hydrological conditions, especially suspended matter, in its distribution. At E2, dominant zooplankton taxa such as Acrocalanus longicornis, and Acartia erythraea were driven mostly by salinity. This study reinforces the importance of tidal and diurnal cycles in understanding zooplankton community distribution in tropical estuaries. Therefore, future studies on zooplankton research in this ecologically important estuary should consider the entire tidal cycle ranging from highest high tide to lowest low tide in different seasons with an additional observation in the coastal waters to gain a better understanding of the zooplankton migration pattern and population dynamics.
Data Availability
The datasets used and/or analyzed are included in the manuscript.
References
Abo-Taleb, H. (2019). Importance of plankton to fish community. In Y. Bozkurt (Eds.), Biological research in aquatic science. Intech Open.
Acharyya, T., Sudatta, B. P., Raulo. S., Singh, S., Srichandan, S., Baliarsingh, S. K., Samanta, A., & Lotliker, A. A. (2021). A systematic review of biogeochemistry of Mahanadi River estuary: Insights and future research direction. In S. Das, & T. Ghosh, (Eds.), Estuarine biogeochemical dynamics of the east coast of India. Springer.
Alfonso, G., Belmonte, G., Marrone, F., & Naselli-Flores, L. (2010). Does lake age affect zooplankton diversity in Mediterranean lakes and reservoirs? A case study from southern Italy. Hydrobiologia, 653, 149–164.
Ali, M. A. (2015). Arabian Gulf food web and the effect of salinity on feeding and growth rate of young threespine sticklebacks (Gasterosteus aculeatus). Doctoral dissertation, University of Sheffield.
Ali, M., Al-Mutairi, M., Subrahmanyam, M. N. V., Isath, S., AlAwadi, M. A., Kumar, P. N., Al-Hebini, K., & Omar, S. A. S. (2019). Temporal variations in abundance and species richness of zooplankton with emphasis on ichthyoplankton in the subtidal waters of Umm Al-Namil Island, Northwestern Arabian Gulf of the ROPME Sea Area. American Journal of Environmental Science, 15, 188–204.
Ali, M., Al-Yamani, F., & Polikarpov, I. (2011). The effect of tidal cycles on the community structure of plankton (with emphasis on copepods) at AFMED Marina in winter (a preliminary study). Crustaceana, 84(5–6), 601–621.
Al-Yamani, F. Y., Skryabin, V., Gubanova, A., Khvorov, S., & Prusova, I. (2011). Marine zooplankton practical guide for the Northwestern Arabian Gulf. (Vol. 1)Kuwait Institute for Scientific Research.
Anandavelu, I., Robin, R. S., Purvaja, R., Ganguly, D., Hariharan, G., Raghuraman, R., Prasad, M. H. K., & Ramesh, R. (2020). Spatial heterogeneity of mesozooplankton along the tropical coastal waters. Continental Shelf Research, 206, 104193.
Anger, K., Spivak, E., & Luppi, T. (1998). Effects of reduced salinities on development and bioenergetics of early larval shore crab, Carcinus maenas. Journal of Experimental Marine Biology and Ecology, 220, 287–304.
Arcifa, M. S., Perticarrari, A., Bunioto, T. C., Domingos, A. R., & Minto, W. J. (2016). Microcrustaceans and predators: Diel migration in a tropical lake and comparison with shallow warm lakes. Limnetica, 35(2), 281–296.
Ayon, P., Criales-Hernandez, M. I., Schwamborn, R., & Hirche, H. J. (2008). Zooplankton research off Peru: A review. Progresses in Oceanography, 79, 238–255.
Baliarsingh, S. K., Lotliker, A. A., Srichandan, S., Basu, A., Nair, T. M. B., & Tripathy, S. K. (2021a). Effect of Tidal Cycle on Escherichia coli Variability in a Tropical Estuary. Bulletin of Environmental Contamination and Toxicology. https://doi.org/10.1007/s00128-021-03106-w
Baliarsingh, S. K., Lotliker, A. A., Srichandan, S., Roy, R., Sahu, B. K., Samanta, A., Nair, T. M. B., Acharyya, T., Parida, C., Singh, S., & Jena, A. K. (2021b). Evaluation of hydro-biological parameters in response to semi-diurnal tides in a tropical estuary. Ecohydrology and Hydrobiology. https://doi.org/10.1016/j.ecohyd.2021.03.002
Bastia, F., & Equeenuddin, S. M. (2016). Spatio-temporal variation of water flow and sediment discharge in the Mahanadi River, India. Global and Planetary Change, 144, 51–66.
Battish, S. K. (1992). Fresh water Zooplankton of India. Oxford and IBH Publishing Co.
Borole, D. V., Somayajulu, B. L. K., Mohanti, M., & Ray, S. B. (1979). Preliminary investigations on dissolved uranium and silicon and major elements in the Mahanadi estuary. Proceedings of the Indian Academy of Sciences, 88, 161–170.
Chakrapani, G. J., & Subramanian, V. (1990). Factors controlling sediment discharge in the Mahanadi River Basin. India. Journal of Hydrology, 117(1–4), 169–185.
Chew, L. L., & Chong, V. C. (2011). Copepod community structure and abundance in a tropical mangrove estuary, with comparisons to coastal waters. Hydrobiologia, 666, 127–143.
Chew, L. L., Chong, V. C., Ooi, A. L., & Sasekumar, A. (2015). Vertical migration and positioning behavior of copepods in a mangrove estuary: Interactions between tidal, diel light and lunar cycles. Estuaries Coastal and Shelf Science, 152, 142–152.
Clarke, K. R., & Warwick, R. M. (2001). Change in marine communities: An approach to statistical analysis and interpretation. (p. 144). PRIMER-E.
Connelly, K. A., Rollwagen-Bollens, G., & Bollens, S. M. (2020). Seasonal and longitudinal variability of zooplankton assemblages along a river-dominated estuarine gradient. Estuaries Coastal and Shelf Science, 245, 106980.
Conway, D. V., White, R. G., Hugues-Dit-Ciles, J., Gallienne, C. P., & Robins, D. B. (2003). Guide to the coastal and surface zooplankton of the south-western Indian Ocean. Occasional Publication of the Marine Biological Association of the United Kingdom No. 15, Plymouth.
da Costa, K. G., Bezerra, T. R., Monteiro, M. C., Vallinoto, M., Berredo, J. F., Pereira, L. C. C., & da Costa, R. M. (2013). Tidal-induced changes in the zooplankton community of an Amazon estuary. Journal of Coastal Research, 29(4), 756–765.
Davies, O. A., & Ugwumba, O. A. (2013). Effects of tide on zooplankton community of a tributary of upper bonny estuary, Niger Delta, Nigeria. International Journal of Scientific Research in Knowledge, 1(9), 325–342.
Dittel, A. I., & Epifanio, C. E. (1990). Seasonal and tidal abundance of crab larvae in a tropical mangrove system, Gulf of Nicoya. Costa Rica. Marine Ecology Progress Series, 65(1), 25–34.
Gajbhiye, S. N., Nair, V. R., & Desai, B. N. (1984). Diurnal variation of zooplankton in Malad creek, Bombay. Indian Journal of Marine Sciences, 13, 75–79.
Ganguly, D., Dey, M., Chowdhury, C., Pattnaik, A. A., Sahu, B. K., & Jana, T. K. (2011). Coupled micrometeorological and biological processes on atmospheric CO2 concentrations at the land-ocean boundary. NE coast of India. Atmospheric Environment, 45(23), 3903–3910.
Gao, X., Song, J., & Li, X. (2011). Zooplankton spatial and diurnal variations in the Changjiang River estuary before operation of the Three Gorges Dam. Chinese Journal of Oceanology and Limnology, 29(3), 591–602.
Gazeau, F., Gattuso, J. P., Middelburg, J. J., Brion, N., Schiettecatte, L. S., Frankignoulle, M., & Borges, A. V. (2005). Planktonic and whole system metabolism in a nutrient-rich estuary (the Scheldt estuary). Estuaries, 28, 868–883.
Grasshoff, K., Kremling, K., & Ehrhardt, M. (1999). Methods of seawater analysis. (3rd ed.). Verlag Chemie GmbH. Wiley-VCH, New York.
Grecay, P. A., & Targett, T. E. (1996). Effects of turbidity, light level and prey concentration on feeding of juvenile weakfish Cynoscion regalis. Marine Ecology Progress Series, 131, 11–16.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 1–9.
Hart, R. C., & Allanson, B. R. (1976). The distribution and diel vertical migration of Pseudodiaptomus hessei (Mrázek) (Calanoida: Copepoda) in a subtropical lake in southern Africa. Freshwater Biology, 6(2), 183–198.
Hattori, H. (1989). Bimodal vertical distribution and diel migration of the copepods Metridia pacifica, M. okhotensis and Pleuromamma cutullata in the western North Pacific Ocean. Marine Biology, 103, 39–50.
Hovel, K. A., & Morgan, S. G. (1997). Planktivory as a selective force for reproductive synchrony and larval migration. Marine Ecology Progress Series, 157, 79–95.
Jacoby, C. A., & Greenwood, J. G. (1989). Emergent zooplankton in Moreton Bay, Queensland, Australia: Seasonal, lunar, and diel patterns in emergence and distribution with respect to substrata. Marine Ecology Progress Series, 51, 13–154.
Jessopp, M. J., & McAllen, R. J. (2008). Go with the flow: Tidal import and export of larvae from semi-enclosed bays. Hydrobiologia, 606, 81–92.
Karabin, A. (1985). Pelagic zooplankton (Rotaria+ Crustacea) variation in the process of lake eutrophication. II: Modifying effect of biotic agents. Ekologia Polska, 33(4), 617–644.
Kouassi, E., Pagano, M., Saint-Jean, L., Arfi, R., & Bouvy, M. (2001). Vertical migrations and feeding rhythms of Acartiaclausi and Pseudodiaptomushessei (Copepoda: Calanoida) in a tropical lagoon (Ebrié, Côte d’Ivoire). Estuary Coastal and Shelf Science, 52(6), 715–728.
Krumme, U., & Liang, T. H. (2004). Tidal-induced changes in a copepod-dominated zooplankton community in a macrotidal mangrove channel in northern Brazil. Zoological Studies, 43(2), 404–414.
Leandro, S. M., Morgado, F., Pereira, F., & Queiroga, H. (2007). Temporal changes of abundance, biomass and production of copepod community in a shallow temperate estuary (Ria de Aveiro, Portugal). Estuary Coastal and Shelf Science, 74(1–2), 215–222.
Loose, C. J., & Dawidowicz, P. (1994). Trade-offs in diel vertical migration by zooplankton: The costs of predator avoidance. Ecology, 75(8), 2255–2263.
Lopes, M. R. M., Ferragut, C., & Mattos Bicudo, C. E. D. (2009). Phytoplankton diversity and strategies in regard to physical disturbances in a shallow, oligotrophic, tropical reservoir in Southeast Brazil. Limnetica, 28(1), 159–174.
Lopez-Duarte, P. C., Christy, J. H., & Tankersley, R. A. (2011). A behavioral mechanism for dispersal in fiddler crab larvae (genus Uca) varies with adult habitat, not phylogeny. Limnology and Oceanography, 56(5), 1879–1892.
Madhupratap, M., & Rao, T. S. S. (1979). Tidal and diurnal influence on estuarine zooplankton. Indian Journal of Marine Sciences, 8, 9–11.
Margalef, R. (1958). Temporal succession and spatial heterogeneity in phytoplankton. In A. A. Buzzati-Traverso (Ed.), Perspectives in marine biology. (pp. 323–349). University of California Press.
Marques, S. C., Azeiteiro, U. M., Martinho, F., Viegas, I., & Pardal, M. A. (2009). Evaluation of estuarine mesozooplankton dynamics at a fine temporal scale: The role of seasonal, lunar and diel cycles. Journal of Plankton Research, 31(10), 1249–1263.
Menendez, M. C., Dutto, M. S., Piccolo, M. C., & Hoffmeyer, M. S. (2012). The role of the seasonal and semi-diurnal tidal cycle on mesozooplankton variability in a shallow mixed estuary (Bahía Blanca, Argentina). ICES Journal of Marine Science, 69(3), 389–398.
Menon, H. B., Sangekar, N. P., Lotliker, A. A., & Vethamony, P. (2011). Dynamics of chromophoric dissolved organic matter in Mandovi and Zuari estuaries—A study through in situ and satellite data. ISPRS Journal of Photogrammetry and Remote Sensing, 66(4), 545–552.
Micheli, F. (1999). Eutrophication, fisheries, and consumer-resource dynamics in marine pelagic ecosystems. Science, 285, 1396–1398.
Mishra, R. K., Shaw, B. P., Sahu, B. K., Mishra, S., & Senga, Y. (2009). Seasonal appearance of Chlorophyceae phytoplankton bloom by river discharge off Paradeep at Orissa Coast in the Bay of Bengal. Environmental Monitoring and Assessment, 149, 261–273.
Morgan, C. A., Cordell, J. R., & Simenstad, C. A. (1997). Sink or swim? Copepod population maintenance in the Columbia River estuarine turbidity-maxima region. Marine Biology, 129, 309–317.
Murali, R. M., Dhiman, R., Choudhary, R., Seelam, J. K., Ilangovan, D., & Vethamony, P. (2015). Decadal shoreline assessment using remote sensing along the central Odisha coast, India. Environmental Earth Sciences, 74, 7201–7213.
Naik, S., Mishra, R. K., Panda, U. S., Mishra, P., & Panigrahy, R. C. (2020). Phytoplankton community response to environmental changes in Mahanadi Estuary and its adjoining coastal waters of Bay of Bengal: A multivariate and remote sensing approach. Remote Sensing in Earth Systems Sciences, 3(1), 110–122.
Nandy, T., & Mandal, S. (2020). Unravelling the spatio-temporal variation of zooplankton community from the river Matla in the Sundarbans Estuarine System. India. Oceanologia, 62(3), 326–346.
Oishi, K., & Saigusa, M. (1997). Nighttime emergence patterns of planktonic and benthic crustaceans in a shallow subtidal environment. Journal of Oceanography, 53, 611–621.
Panda, U. C., Sundaray, S. K., Rath, P., Nayak, B. B., & Bhatta, D. (2006). Application of factor and cluster analysis for characterization of river and estuarine water systems–a case study: Mahanadi River (India). Journal of Hydrology, 331(3–4), 434–445.
Paradeep Fishing Harbour (2020). http://www.msfhparadeep.com/. Accessed on 13 Dec 2020.
Paula, J., Bartilotti, C., Dray, T., Macia, A., & Queiroga, H. (2004). Patterns of temporal occurrence of brachyuran crab larvae at Saco mangrove creek, Inhaca Island (South Mozambique): Implications for flux and recruitment. Journal of Plankton Research, 26(10), 1163–1174.
Pielou, E. C. (1966). The measurement of diversity in different types of biological collections. Journal of Theoretical Biology, 13, 131–144.
Raj, S., Jee, P. K., & Panda, C. R. (2013). Textural and heavy metal distribution in sediments of Mahanadi estuary, East coast of India. Indian Journal of Geo-Marine Sciences, 42(3), 370–374.
Rao, A. D., Dash, S., & Babu, S. V. (2004). Numerical study of the circulation and sediment transport in the Mahanadi Estuary. Natural Hazards, 32, 219–237.
Raymont, J. E. G. (1983). Plankton and productivity in the oceans. 2nd edition, Volume 2-Zooplankton. Pergamon Press, Oxford.
Robertson, A. I., Dixon, P., & Daniel, P. A. (1988). Zooplankton dynamics in mangrove and other nearshore habitats in tropical Australia. Marine Ecology Progress Series, 43, 139–150.
Samantray, P., Mishra, B. K., Panda, C. R., & Rout, S. P. (2009). Assessment of water quality index in Mahanadi and Atharabanki Rivers and Taldanda Canal in Paradip area. India. Journal of Human Ecology, 26(3), 153–161.
Shang, X., Wang, G., & Li, S. (2008). Resisting flow–laboratory study of rheotaxis of the estuarine copepod Pseudodiaptomus annandalei. Marine and Freshwater Behaviour Physiology, 41(2), 109–124.
Shannon, C. E., & Weaver, W. (1963). The mathematical theory of communication. University of Illinois Press.
Sivasankar, R., Ezhilarasan, P., Kumar, P. S., Naidu, S. A., Rao, G. D., Kanuri, V. V., Rao, V. R., & Ramu, K. (2018). Loricate ciliates as an indicator of eutrophication status in the estuarine and coastal waters. Marine Pollution Bulletin, 129(1), 207–211.
Srichandan, S., Baliarsingh, S. K., Prakash, S., Lotliker, A. A., Parida, C., & Sahu, K. C. (2019). Seasonal dynamics of phytoplankton in response to environmental variables in contrasting coastal ecosystems. Environmental Science and Pollution Research, 26, 12025–12041
Srichandan, S., Tarafdar, L., Muduli, P. R., & Rastogi, G. (2021). Spatiotemporal patterns and impact of a cyclone on the zooplankton community structure in a brackish coastal lagoon. Regional Studies in Marine Science, 101743.
Steinberg, D. K., & Landry, M. R. (2017). Zooplankton and the ocean carbon cycle. Annual Review of Marine Science, 9, 413–444.
Sundaray, S. K., Nayak, B. B., & Bhatta, D. (2009). Environmental studies on river water quality with reference to suitability for agricultural purposes: Mahanadi river estuarine system, India—A case study. Environmental Monitoring and Assessment, 155, 227–243.
Tackx, M. L., De Pauw, N., Van Mieghem, R., Azémar, F., Hannouti, A., Van Damme, S., Fiers, F., Daro, N., & Meire, P. (2004). Zooplankton in the Schelde estuary, Belgium and The Netherlands. Spatial and temporal patterns. Journal of Plankton Research, 26(2), 133–141.
Thakur, R. K., Jindal, R., Singh, U. B., & Ahluwalia, A. S. (2013). Plankton diversity and water quality assessment of three freshwater lakes of Mandi (Himachal Pradesh, India) with special reference to planktonic indicators. Environmental Monitoring and Assessment, 185(10), 8355–8373.
Venkataramana, V., Sarma, V. V. S. S., & Reddy, A. M. (2017). River discharge as a major driving force on spatial and temporal variations in zooplankton biomass and community structure in the Godavari estuary India. Environmental Monitoring and Assessment, 189(9), 474.
Villate, F. (1997). Tidal influence on zonation and occurrence of resident and temporary zooplankton in a shallow system (estuary of Mundaka, Bay of Biscay). Scientia Marina, 61(2), 173–188.
Vineetha, G., Jyothibabu, R., Madhu, N. V., Kusum, K. K., Sooria, P. M., Shivaprasad, A., Reny, P. D., & Deepak, M. P. (2015). Tidal influence on the diel vertical migration pattern of zooplankton in a Tropical Monsoonal Estuary. Wetlands, 35, 597–610.
Walter, T. C. (1987). Review of the taxonomy and distribution of the demersal copepod genus Pseudodiaptomus (Calanoida: Pseudodiaptomidae) from southern Indo-West Pacific waters. Australian Journal of Marine and Freshwater Research, 38(3), 363–396.
Williamson, C. E., Fischer, J. M., Bollens, S. M., Overholt, E. P., & Breckenridge, J. K. (2011). Toward a more comprehensive theory of zooplankton diel vertical migration: Integrating ultraviolet radiation and water transparency into the biotic paradigm. Limnology and Oceanography, 6(5), 1603–1623.
Winder, M., & Jassby, A. D. (2011). Shifts in zooplankton community structure: Implications for food web processes in the upper San Francisco Estuary. Estuaries and Coasts, 34, 675–690.
Wooldridge, T. H. (1999). Estuarine zooplankton community structure and dynamics. In B. R. Allanson & D. Baird (Eds.), Estuaries of South Africa. (pp. 141–166). Cambridge University Press.
World Register of Marine Species (2021). Retrieved April 27, 2021, from http://www.marinespecies.org/
Youn, S. H., & Choi, J. K. (2008). Distribution pattern of zooplankton in the Han River estuary with respect to tidal cycle. Ocean Science Journal, 43(3), 135–146.
Acknowledgements
Authors are thankful to Director, Indian National Centre for Ocean Information Services (INCOIS), Ministry of Earth Sciences, Hyderabad, India, for the encouragement. One of the authors (RR) is thankful to Director, National Remote Sensing Centre (NRSC), for his constant support. Authors express their deep gratitude to Dr. Paula Reis of Université du Québec à Montréal (UQAM), Canada, for her help in editing the manuscript. This is INCOIS contribution no. 415.
Funding
This study has been undertaken as a part of the project entitled “Coastal Monitoring” under the umbrella of “Ocean Services, Modelling, Application, Resources and Technology (O-SMART)” scheme, sanctioned by the Indian Ministry of Earth Sciences (MoES) vide Administrative Order no. MoES/36/OOIS/CM/2019 dated 07–05-2019. A part of the funding was received from Indian Space Research Organisation (ISRO)-National Carbon Project—Coastal Carbon Dynamics (NCP-CCD).
Author information
Authors and Affiliations
Contributions
AAL conceived and conceptualized the study; SS, SKB, AAL, BKS, and RR collected the data; SS and SKB carried out laboratory analysis; SS, SKB, AAL, BKS, and RR interpreted data; SS prepared the first draft of manuscript with critical input of SKB and AAL; SS and SKB prepared the graphical illustrations with substantial input of AAL and RR; TMB, RR, and BKS reviewed, edited, and revised the manuscript; TMB supervised the entire study; AAL, RR, and TMB acquired funding resource. SS and SKB revised the manuscript as per the reviewer’s comments with critical input from AAL. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• First report of tidal zooplankton dynamics in contrasting salinity regimes
• Catalogued 92 zooplankton taxa belonging to 13 groups indicating higher diversity
• Higher zooplankton abundance during high tide and night irrespective of salinity regimes
• Prominent variability of zooplankton abundance at diurnal scale in the lower estuary
• Prevalence of limnetic taxa in the lower estuary during low tide
Rights and permissions
About this article
Cite this article
Srichandan, S., Baliarsingh, S.K., Lotliker, A.A. et al. Unravelling tidal effect on zooplankton community structure in a tropical estuary. Environ Monit Assess 193, 362 (2021). https://doi.org/10.1007/s10661-021-09112-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09112-z