Abstract
The protection of wetlands is essential for sustainable development. The particular hydrology of wetlands creates conditions for the formation of hydric soils. Hydric soils are formed in oxide-reducing environments and perform important removal and re-oxidation of Fe and Mn oxides and hydroxides. They are also characterized by the formation of a superficial horizon enriched by the accumulation of organic matter. The objective of this study was to determine the concentration of essential (Co, Cu, Fe, Mn, Ni, Zn) and toxic (Al, Cd, Pb) elements in the soil solution in a wetland, surrounded by an intense agricultural activity area. The concentrations of the elements were evaluated according to the level of hydromorphy (border to the center) and the depth of the soil. The physical fractionation of these elements was also assessed (total and < 30 kDa), and the results were compared with the chemical speciation of these elements. Despite the high total concentrations of Al and Fe, the concentration of these elements was very low in the < 30 kDa fraction, which suggests that these elements are complexed with organic matter, in the form of hydroxides. Evidence of removal of Fe and Mn from the surface horizon was observed at the center of the wetland, where the highest level of hydromorphy is found. The results showed that the concentration of metals in the fraction < 30 kDa is insignificant, suggesting that the mechanisms of precipitation and/or adsorption to soil colloids play an important role in the regulation of this ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands are terrestrial soil reservoirs of carbon and water. Although providing a wide range of ecosystem services of great social and economic values, wetland degradation and loss are faster than those of other ecosystems, especially in tropics and subtropics (Finlayson et al., 2011). Wetlands cover 6% of Earth’s surface; however, the biogeochemical dynamics are still poorly understood. Insufficient information and data have a direct impact on the quality and management of these areas; therefore, it becomes difficult to stop wetland degradation and losses (Junk et al., 2013). Worldwide, land conversion to agriculture, eutrophication, and pollution are major factors that can increase pollutants inside humid areas (Chithra et al., 2015; Guo & Yang, 2016). Fine-grained components of hydric soils, as clay minerals and organic matter, play an important role on heavy metal retention, preventing their release to water due to environmental changes at the sediment–water interface (Xu et al., 2016).
In the case of metals, potential contaminants of soil and water due to their toxicity and persistence in the environment, the main processes that regulate the concentration and impact of these elements on the biota are precipitation, adsorption, complexation, and leaching (Alloway, 1995). These elements are naturally found as free hydrated ions, complexed with inorganic ligands, bound to colloids, e.g., complexed with organic matter or adsorbed to hydroxides, adsorbed to particulate material, and assimilated by the biomass (Xu et al., 2019). The total content of metal represents a complex pool of fractions that, in terms of their reactivity, are classified as inert, labile or soluble, the last two being the ones that most pose risks for contamination of water bodies or assimilation by the biota (Mora & Harrison, 1983).
In wetlands, where soil layers are periodically saturated (aquic conditions according to Soil Survey Staff, 2010), oxidation–reduction conditions are formed with important removal and re-oxidation of Fe and Mn oxides and hydroxides. The formation of a superficial horizon enriched by the accumulation of organic matter also occurs (Vepraskas & Lindbo, 2012). In hydromorphic soils, biogeochemical cycling depends on the amount of water that fills the soil pores, the water permanence time, the nature of the formed organic molecules, and the activity of the microorganisms (Castellano et al., 2012). The capacity of the soil to perform ion exchange, transport, and transformation are equally affected by the amount of solute in the soil (Hobbie et al., 2007). The chemical stress of soil is reflected by the concentrations of trace elements present in the interstitial water (Kabata-Pendias & Pendias, 2001).
The Brazilian Cerrado natural landscape has been rapidly changing to the monoculture of soybean, corn, and sugarcane for the external market. The region is considered to be the last biome to be reached by the advance of agriculture frontier, characterized by the farming based on fertilizers and pesticides. The network of wetlands, developed in between the uplands of the catchment, is surrounded by intensive agriculture, becoming susceptible to receive agrochemicals due to runoff or dissolved in water. This is of serious concern because wetlands store rainwater and slowly supply aquifers and rivers in the region (Furlan et al., 2020). Additionally, wetland floods according to seasonal rainfall represent hotspots for conservation due to the occurrence of endemic fauna and flora and the storage of organic carbon in the hydric soil, which contributes to reducing atmospheric gas emission (Mitsch et al., 2013). Despite its ecological, hydrological, and biogeochemical importance, there is a serious gap in monitoring water and soil quality whereas the Brazilian Forest Code threatens these ecosystems (Sousa et al., 2011). In the country, the fast expansion of agricultural and mining activities over the last decades caused severe metal contamination of several aquatic ecosystems (Lucca et al., 2018).
Thus, the objective of this study was to (a) determine the concentration of essential (Co, Cu, Fe, Mn, Ni, Zn) and toxic (Al, Cd, Pb) elements in the soil solution from a wetland in Cerrado (Brazilian savanna) as a function of the level of hydromorphy (border and center of the wetland) and the depth of the soil, (b) perform the physical fractionation of the elements and compare the results with the chemical speciation of them, considering the concentrations of humic substances in the area; and (c) evaluate the possibility of relating the data obtained in this study to possible impacts from the surrounding activities.
Material and methods
Study area
The studied wetland covers an area of 426,064.80 m2 (19° 28′ 08″ S, 47° 48′ 20″ W) at 966 m of altitude (Fig. 1). The humid area is located in the high position of the sedimentary plateau, in the Upper Rio Claro Basin (west of the state of Minas Gerais). These waterlogged topographic depressions are not channelized headwaters and maintain the hydrologic connectivity with rivers throughout internal flows. The site was chosen because represents a typical wetland landscape, an isolated wetland that stores freshwater from precipitation and aquifer. The water dynamic and volume are related to rainfall seasonality and the fluctuation of shallow groundwater. In the wet season, the water level overtakes the ground surface whereas in the dry season the evapotranspiration and water infiltration empty the depression and the water level approaches 50 cm from the topsoil. The wetland exhibits a sequence of horizons with organic matter accumulated in topsoil and iron-depleted subsurface horizon indicating aquic conditions (USDA-NRCS, 2010). It is covered with grassy savanna vegetation and is surrounded by large farms with soybean and corn cultivation. On the old lateritic flat plateau shaped from sandstones of the Marilia Formation (Upper Cretaceous) (Fernandes & Coimbra, 2000), wetland systems develop in the upland position of relief, with hydrological connection with basin.
On the terrain, an extensive network of isolated wetlands can be recognized as a group of smooth circular concavities covered by grasses adapted to the soil hydromorphy (Gleysol), which contrasts with the surrounding open savanna vegetation in well-drained soils (Oxisol). Frequency and duration of saturation in the soil are related to seasonal rainwater, which also controls the level of the water table. The water table reaches the topographic surface during the summer wet months (October to March).
The tropical climate has an average annual precipitation of 1464 mm and an average annual temperature of 23 °C (Queiroz, 2012). The regional economy is based on agribusiness and the totality of the well-drained soils in the plateau is, since the 1970s, used for intensive agriculture where a crop cycle is followed by other, generally soybean and corn. The small proportion of the remaining Cerrado vegetation covers the water bodies (trees) and the center of the wetlands (grasses). Crop fields advance on wetlands from its border, drastically reducing their areas. The crops are dependent on chemical fertilizers and pesticides, both might be a source of heavy metals in the environment.
Sampling campaign and sample preservation
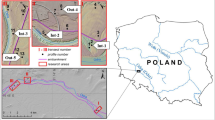
The sampling campaign was carried out in December 2018. Water samples were collected in piezometers installed at six sites along a transect (Fig. 2), from the center to the border of the wetland. The sampling points were guided by topography and soil morphology (soil color and mottles and topsoil enriched with organic matter), which responds to hydromorphy (Vepraskas, 1999). In the studied wetland, the duration and frequency of soil saturation increase from border to the center of the topographic depression. For each site, samples were collected at two depths, one at 0.60 m and the other at 2.5 m.
Sampling points P1, P2, and P4 were collected at the depths of 0.6 and 2.5 m. The sampling point P3, as it presented similar features to P2, was collected only at 2.5 m. P5 and P6 samples were collected only at the greater depth, as they were not saturated at the depth of 0.6 m during the sampling event. The water samples (500 mL) were collected into deionized-prewashed and site-water rinsed bottles and conserved in a thermal box to transportation. At each sampling site, an aliquot was collected and acidified with HNO3 (final concentration of 2%, v v−1) immediately after sampling to determine the total content of the elements, and an aliquot intended for physical fractionation was collected from the surface layer, kept at 4 °C and acidified in the laboratory, after the fractionation.
The pH and ionic strength parameters were measured in the field, during sample collection by the portable meters K39-0014P (Kasvi) and the EC was determined using a C-702 (Analion).
Reagents
All solutions used in this work were prepared with ultrapure water (resistivity 18.2 MΩ cm−1) and analytical grade reagents. For the preparation of the standard solutions and acidification of the samples, subboiling bidistilled nitric acid was used.
Before use, all materials involved in the preparation and storage of solutions, such as glassware and centrifuge tubes, as well as syringes, membrane filters, and ultracentrifugation tubes, were decontaminated with 20% HNO3 (v v−1) and washed with ultrapure water at least five times.
Fractionation and determination of elements
To determine the total content of the elements, three samples at 0.60 m and six at 2.5 m were collected from piezometers and acidified with HNO3 to the final concentration of 2% (v v−1). Aliquots of the samples collected from the 2.5 m piezometers were used to perform the physical fractionation of the elements. These aliquots were filtered with hydrophilic PFTE syringe filters (pore diameter: 45 μm) before the ultrafiltration with ultrafiltration tubes (Vivaspan 6, 30 kDa MWCO). The ultrafiltration procedure was performed, selecting the fraction < 30 kDa, to separate humic substances, which molecular weight can reach up to a few dozens of kDa (LI et al., 2004). Finally, the ultrafiltered aliquots were acidified with HNO3 to the final concentration of 2%.
The determination of the elements was performed by inductively coupled plasma–mass spectrometry (ICP-MS, Thermo Scientific, model X-Series 2). 6Li and Ge were used as online internal standards to correct possible spectral interferences.
Characterization of samples
The determination of organic carbon in the samples was performed by the TOCV carbon analyzer (SHIMADZU, Japan). Carbonates were eliminated by addition of HCl 2.5% (v v−1).
The pH and electrical conductivity were measured by K39-0014P-Kasvi and Analion C-702, respectively, previously calibrated with the solutions provided by the manufacturers.
Statistical analysis
The concentrations of the elements were expressed by the mean and standard deviation, calculated for the three runs of each sample obtained during the quantification. To perform the statistical treatments, values below the limit of quantification were assumed to be \(\frac{LQ}{2}\). The normality of the data was assessed using the Shapiro–Wilk test. The comparison between two normal variables at the same sampling point was assessed using the paired Student’s t test (α = 0.05). The comparison between two or more variables with non-normal distribution was performed using the Kruskal–Wallis test (α = 0.05) and, when applicable, the results were further compared using the Dunn post hoc test. Multivariate analysis of grouping by similarity (dendrogram) was also performed to compare the sampling points according to the concentrations of the elements, adopting the attributes Euclidean distance and complete method for calculating similarity. The evaluation of similarity patterns between the elements was performed using Pearson’s correlation matrix (normal data). All statistical tests were performed with the aid of the public domain Software R Studio.
Speciation model
Visual MINTEQ geochemical modeling software, version 3.1 was used (Gustafsson, 2012). The modeling was performed by using the data from the program’s database. The input parameters were pH, ionic strength (I), dissolved organic carbon (DOC) concentration and the total contents determined in the 2.5 m piezometer of the Al3+, Fe2+, Mn2+, Co2+, Ni2+, Cu+, Zn2+, Cd2+, and Pb2+ ions. The oxidation states attributed to the elements were based on Eh–pH diagrams (Takeno, 2005, LLNL database for pH = 6.5 and Eh = − 0.2 to 0.2 mV).
To calculate element complexation with organic matter, the dissolved organic carbon concentrations in the samples were input, according to the model NICA (Non-Ideal Competitive Adsorption)-Donnan. The NICA-Donnan model combines non-ideal competitive adsorption isotherms, used to describe the bonding of ions to a heterogeneous surface, to the Donnan electrostatic model, used to describe the electrostatic interactions between ions and humic substances (Milne et al., 2003).
Since fulvic acids have a lower molecular weight than humic acids (Beckett et al., 1987), water-extractable organic matter tends to have a higher concentration of fulvic acids. Furthermore, the stability constant between humic and pure fulvic acids with metals shows that metal–humic acids complexes are less stable when compared to metal-fulvic counterparts (Kabata-Pendias & Pendias, 2001). Thus, as pre-established in the model used by the Visual Minteq software, 100% of the DOC concentration was assumed to be fulvic acid.
The values of the input parameters are shown in Table 1 of the Supplementary Material.
Results and discussion
Distribution of elements concentration
Table 1 shows the concentrations of the elements at the six sampling points. P1 was the innermost point, at the center of the wetland, and P6 the outermost point, at the border of the wetland. For the elements, Fe, Mn, Co, Ni, Zn, Cd, and Pb, a tendency to increase the concentration towards the border was observed. The concentrations of these elements in P6 ranged from 4 to 20 times higher than the median.
The lowest concentrations were found for Co, Cd, Pb, and Ni. These results are expected, since Pb and Ni are considered to be poorly mobile elements, as they are bonded to silicates. Additionally, Cd occurs strongly retained to organic matter in wetlands. Co is not found in high concentrations in soil interstitial water due to high adsorption to Fe and Mn oxides and clay minerals (Kabata-Pendias & Pendias, 2001).
The concentration of inorganic elements in the upper layer of the soil is the result of processes involved in soil formation and anthropic activities, mainly agriculture. Elements such as Cd and Pb are one of the main trace elements found in soils of agricultural plantations with a long history of fertilizer use (Jiao et al., 2012). Thus, given the natural vulnerability that the studied wetland presents due to the landform conditions and the characteristics of the surroundings of the wetlands in the study area, contamination by metals is possible (Rosolen et al., 2015). In the present study, the highest levels of metals were found at the point closest to the agricultural cultivation area.
Table 2 shows a comparison of the metal concentrations determined in this study and the values reported for the soil solution in the literature.
The total content of the elements Mn, Co, Ni, Cu, Zn, Cd, and Pb in the soil solutions were within the range reported from different parts of the globe. The concentration range of the elements in the soil solution varied by up to four orders of magnitude, depending on the element. The results obtained in the present study are close to the values reported for the organic soil and the results obtained for the wetland, with notably lower levels. The higher similarity of the results is possibly related to the similarity of the type of soil and sampling method for organic soil, and to the type of soil, sampling method, landform characteristics, climate, and geology for the wetland.
The results found for Fe and Al in the present study exceed the maximum limits reported in a survey performed by Kabata-Pendias and Pendias (2001), for soils in different parts of the world. Possibly, the higher concentration herein found is due to most of the previously reported studies being concentrated in regions of temperate climate, whereas the conditions of formation of tropical soils favor the higher concentration of Fe and Al elements. The concentrations of these elements in the Triângulo Mineiro wetland were even higher and this difference was even greater when considered that the concentrations reported by Ribeiro et al. (2019) correspond to those of dissolved metals (< 0.45 µm) and collected in a more superficial layer (0–0.10 m). The concentrations reported for Mn were also higher than those obtained in the present study. A fundamental process that characterizes soils periodically saturated by water is the removal of Fe and Mn compounds from the soil matrix, released by dissolution and reduction induced by saturation and anaerobic conditions (Vepraskas & Lindbo, 2012).
In the present study, Zn concentrations were higher than those reported for Triângulo Mineiro wetland, possibly due to the presence of Zn in the particulate fraction in the results obtained in the present study.
Comparison of concentrations as a function of depth
Table 3 shows the extractable water concentrations of the elements at two depths
For Al, a significant difference (p = 0.0244) was observed in the concentrations at the two studied depths (0.60 and 2.5 m). For the other elements, there was no significant difference (Ni: p = 0.5602, Cu: p = 0.2589, Zn: p = 0.5992) in concentrations as a function of depth.
In a recent study carried out at the same sampling points (data not shown), an increase in the stability constant between total humic substances (HS) and Al or Cu as a function of depth was observed. As the concentration of water extractable organic matter (WEOM) decreased with depth, it was suggested that WEOM was adsorbed to the surface of minerals and oxides in the soil. Therefore, the lower concentrations of Al for samples collected at 2.5 m suggest that, in greater depths, the complex of this element and HS is found immobilized by the mineral fraction of the soil. The iron-depleted matrices in the soils of the wetland (gleysol) are enriched in kaolinitic clay minerals and aluminum hydroxides (gibbsite) formed after a long and continuous process of chemical weathering and the action of humic substances is not effective in mineral disequilibrium. The high content of extracted Al is linked to the chemical–mineralogical composition of soil developed in that wetlands, which is enriched of Al-hydroxides (gibbsite) and low-activities clay (kaolinite) resulted from long and intensive tropical chemical weathering.
In P1, Zn concentration at the 0.60 m piezometer was three times higher, when compared to the 2.5 m one. Zn is easily adsorbed to minerals and organic compounds in the soil and, therefore, in most soil types, its accumulation is observed in the surface horizons (Kabata-Pendias & Pendias, 2001). However, the same pattern was not observed for points P2 and P4, and a higher concentration in the 2.5 m piezometer was observed for P2. In both P2 and P4, the mineralization of the organic matter considerably decreased with the depth increase (Table 4), following an opposite trend of that found at P1, suggesting that Zn concentration is related to organic carbon mineralization processes, possibly as Zn being required in microbial activities (Esther et al., 2014).
Ni concentration did not vary according to depth, except for P1. The Ni concentration is stable along with the soil profile; therefore, increases in the superficial horizon are indications of contamination (Malavolta, 1994).
Although it was not possible to observe a difference in the concentrations as a function of depth for Fe (p = 0.3941) and Mn (p = 0.57) when comparing the three sampling points, for P1, the two elements presented a similar pattern of variation. Table 3 shows an inversion in the sampling point (P1) concentration pattern when compared to the other sampling points, being lower at 0.60 m than at 2.5 m piezometer for both elements (Mn: p = 0.00478 and Fe: p = 0.003102). This inversion may be related to an advanced process of Fe removal from the soil, wherein the seasonal fluctuation of the water table results in the leaching of Fe from the superficial horizon to greater depths (Rosolen et al., 2015). As P1 is the most central among the sampling points, it is subject to a more expressive effect of this dynamic.
To a lesser extent, the same process may have occurred for Mn, given the geochemical similarity between these elements (Kabata-Pendias & Pendias, 2001). Reiss and Chifflard (2015) evaluated the effect of dissolved organic carbon on the concentration of extractable Mn in water as a function of depth in a wetland in Germany, characterized by a lithological discontinuity. For the lowest sampling point in the terrain, the concentration of Mn increased with depth, being significantly higher at the greatest depth studied (1.10 m). This was attributed to the upward transport of the subsoil element (gleysol) through the interstitial water of the soil.
The formation of soluble complexes with organic ligands is often related to the high mobility of the element in the soil profile and its bioavailability (Kabata-Pendias & Pendias, 2001). Water level fluctuations due to wet-dry cycles and DOC concentration in wetlands affect biogeochemical processes in wetlands (Chow et al., 2013). The C:N ratio is indicative of SOM mineralization. The contents of organic carbon are higher in topsoil at 0–10 cm in all soil profiles (Table 4). In this depth, carbon contents decrease from P1 to P5, closely related to the topography, which determines the permanence of water in soil. The profile P6 is different, possibly influenced by agriculture, which is strongly dependent on farm inputs. Overall, C contents diminish from topsoil to deeper horizons in all profiles because litter deposited on the soil surface is an active process under anaerobic conditions (Everett, 1983). Comparing soil profiles and horizon depth, the C:N ratio diminish at 0–20 cm in the profiles P2, P3, and P4 and at 0–50 cm in P1, indicating mineralization of SOM in soil surface due to aerobic conditions during the dry season. C:N ratio increases in deeper soil horizons probably because the high level of the water table along the year creates the anaerobic conditions through the redox reactions by microorganisms (Vepraskas, 2004). In P5, the C:N ratio diminishes towards soil depth, indicating increased mineralization in soil depth, and in P6, except at 0–10 cm, the ratio is higher and stable. The transfer of C and N through soil is complex; however, the results indicate that microorganisms of the wetland soil metabolize carbon, and DOC could be produced at an independent reaction by microbes utilizing SOC as C source, which enhances the forming metal-DOC complexes (Christensen et al., 1996; Liu et al., 2017).
It is possible that microsites (hotspots) with high biological activity are present within the soil profile. These spots contain organic residues, such as dead roots or leaves, which are actively biodegraded by microorganisms (Parkin, 1987). The action of microorganisms consumes dissolved oxygen, and in the absence of oxygen for the degradation of organic matter, the two main electron acceptors will be Fe(III) and Mn(IV), which will then be reduced to their more soluble forms Fe(II) and Mn(II). This process could be related to the fact that the removal of Fe was observed only in the most central point of the wetland.
Relationship between the elements
Figure 3 shows a cluster analysis by the similarity of the sampling points as a function of the total content of the elements at the 2.5 m piezometer. The similarity between the points is inversely proportional to the distance between them.
It can be seen from the figure that P6, at the border of the wetland, is separated from the others. The cluster analysis suggests that the points P1 and P2, located inside the wetland, present a similar pattern for the concentration of the elements. This may indicate similar mechanisms influencing the dynamics of the distribution of the elements. In addition, the concentration of the elements on the border and at the innermost points results from different processes.
Given this result, a Pearson correlation matrix (Table 5) was performed to compare the total contents of the elements in the study area and evaluate the affinity between the elements, which could indicate origin by similar processes.
In this table, there is a high correlation between Fe, Mn, Co, Ni, Zn, Cd, and Pb and between Al and Cu. However, from the results presented in Table 1, it is observed that only Al and Cu concentrations were not higher at P6 compared to the other sampling points, which explains the low correlation between Al and Cu and the other elements. In the well-drained soil (profile P6), Cu2+ and Al3+ are the usual stable forms of Cu and Al, and the complexes or chelate formation with organic matter play an important role in element movement from soil (Matos et al., 2001).
Fractionation and speciation
Comparisons of the concentration of the elements between the fractions < 30 kDa and total are herein presented (Figs 4, 5). The interpretation of the results was carried out using ionic speciation data from the geochemical modeling available in the Visual Minteq software. The dissolved organic matter (DOM) concentrations of the samples were input to the modeling. Refer to Supplementary Material for additional details about the modeling (input and output data).
Despite the high total concentrations of Fe and Al found in the samples, values above the limit of detection (LOD) were not found for any of the sampling points for the fraction < 30 kDa (Fig. 4). Similar results have been widely reported in the literature. Pédrot et al. (2008) found more than 80 and 90% Fe and Al, respectively, in the fraction > 30 kDa. Also, the DOM distribution was uniform throughout the studied fractions; therefore, the distribution of Fe and Al did not follow the same pattern as the DOM. In the ionic speciation of Al under the conditions of the present study (Fig. 4), most of the Al occurred as AlOHx. AlOHx tend to form large size polymers and the model predicted most of the AlOHx formed had positive charges, which have great affinity for the negative functional groups of humic substances (Gérard et al., 2001). Therefore, the data from the speciation model and the results from the fractionation of the element corroborate the interactions described in the literature. Similar results were reported by Barbiero et al. (2016), where the absence of Fe and Al in the fraction > 30 kDA was attributed to the rapid formation of large (oxy-)hydroxides of Al and Fe and complexation with organic ligands.
Fe (Fig. 4) occurred in the samples predominantly as a free ion; therefore, its presence was expected in the fraction <30 kDa. However, results similar to those observed in this study, i.e., higher concentrations in the fraction >30 kDa, have been previously reported for Fe in a wetland. These results were related to the presence of Fe oxides, which had already been reported for the same size range at the wetland in the area of study. Also, Fe was found to be present in large colloids and almost completely removed from the soil solution in ultrafiltration procedures (Pourret et al., 2007).
Pédrot et al. (2008) classified Mn as “truly dissolved” and present in the interstitial water of the soil at the studied wetland entirely as a free ion. From the comparison with these results, the difference between the total content and <30 kDa in the present study may be related to the presence of the element in the particulate fraction. According to the speciation of Mn in equilibrium conditions, the element occurs predominantly as a free ion and complexed by fulvic acids, with the fraction complexed with fulvic acids more expressive at P3 (9.5%), P5 (5.5%), and P6 (4%). When comparing the data for the fraction <30 kDa and the speciation model, the percentage of Mn2+ in the samples showed a positive correlation with the percentage of Mn in the fraction <30 kDa (R2 = 0.46). For all samples, the concentration of MnOHx found in the model was low, and organic matter and free ion seem to be the main factors for the presence of Mn in <30 kDa.
Free ion fraction was the only occurring species for Cu+ and the dominant one for Zn2+ and Ni2+(Fig. 5). Thus, it is expected that the concentration of the elements in the fraction <30 kDa will be very close to the total content of these elements. However, not only is the concentration in the fraction <30 kDa lower than expected for Cu, Zn, and Ni at P6, but very similar variations were observed in the fractionation of the elements. This similarity indicates that the same mechanism may be influencing the fractionation dynamics, mainly for Ni and Zn. Except for Al, Cd, and Pb, all elements showed a concentration above the limit of quantification (LOQ) in the fraction <30 kDa at P6. For Pb and Cd, the estimated fraction complexed with fulvic acids is quite expressive, which may be the reason for the absence of these elements in the fraction <30 kDa.
The occurrence of Co and Fe in the fraction <30 kDa was only observed in P6 and the percentage in relation to the total content was 59.3 and 1.3%, respectively. For Cu, Mn, Ni, and Zn, the percentages in the fraction <30 kDa in relation to the total content in P6 were 41.8, 77.8, 60.7, and 50.5%, respectively. For Ni and Zn, the occurrence in the fraction <30 kDa in P6 was lower than in the other points (medians 81.5% and 57.2%, respectively). The occurrence in the fraction <30 kDa for P6 was higher for Mn (median = 61.2%) and equal for Cu (median = 41.8%).
Thus, despite the significant increase in the total content of the elements (except Al and Cu) at P6 in relation to the other sampling points, the data for fraction < 30 kDa indicate that, except for Mn, the colloidal and particulate fractions are the most expressive at the border of the wetland.
Conclusion
Despite the high concentrations of Al and Fe in the study area, the concentration of these elements was very low in the fraction <30 kDa, which suggests that these elements are complexed with organic matter in the form of hydroxides. Evidence of removal of Fe and Mn from the superficial horizon was observed at the innermost point of the wetland, where a higher degree of decomposition of organic matter was found.
The grouping of the sampling points by the similarity of the total content of the elements suggests that the points located in the transition segment of the wetland have a similar pattern, which may indicate that similar mechanisms influence the dynamics of the distribution of the elements. Also, it indicates that the concentration of the elements at the border and the innermost point result from different processes from those located at the other points. The transition segment is subject to a greater fluctuation of the oxidation–reduction conditions as it is the segment that most rapidly loses the flood water through the lateral flow towards the interior of the wetland.
The comparison of the concentration in < 30 kDa fraction at the border and the other points, the concentrations of the elements as a function of depth and the comparison with the elements soil solution concentration from the literature suggest that the area is not currently contaminated. Also, the metal leached from the surrounding areas to the wetland can be precipitated and/or adsorbed to the colloidal fraction. The presence of elements in the fraction < 30 kDa, which is more likely to generate environmental damage to the biota is currently very small. However, wetlands tend to accumulate potentially toxic contaminants in the soil, increasing its enrichment factor and ecological risk (Gao et al., 2014); therefore, long-term monitoring of the studied are is necessary to prevent worsening of possible contaminations.
The comparison of data obtained from different depths, fractionation, speciation modeling, and interaction between the elements allowed to interpret the distribution of the fractions of the elements, as well as the mechanisms that control the mobility of these elements between the soil profiles. However, in this study, the data obtained in speciation modeling were consistent for some elements (e.g., Al), but for others, the interpretation needed to be carried out with caution (e.g., Cu and Zn). Finally, the present study provides data on the concentration and fractionation of metals in wetlands of the Cerrado, which are still scarce in the literature.
Data Availability
The authors declare that the data supporting the findings of this study are available within the article and the Supplementary Material of this paper.
References
Alloway, B. J. (1995). Heavy metals in soils (2nd ed.). London: Blackie Academic & Professional.
Barbiero, L., Berger, G., Rezende Filho, A. T., et al. (2016). Organic Control of Dioctahedral and Trioctahedral Clay Formation in an Alkaline Soil System in the Pantanal Wetland of Nhecolândia, Brazil. PLoS One, 11, 1–23.
Beckett, R., Jue, Z., & Giddings, J. C. (1987). Determination of molecular weight distributions of fulvic and humic acids using flow field-flow fractionation. Environmental Science and Technology, 21, 289–295.
Bergkvist, B., Folkeson, L., Kleja, D. (1989). Fluxes of Cu, Zn, Pb, Cd, Cr, and Ni in temperate forest ecosystems. Water Air and Soil Pollution, 47, 217–286.
Castellano, M.J., Lewis, D.B., Andrews, D.M., Mcdaniel, M.D. (2012). Coupling biogeochemistry and hydropedology to advance carbon and nitrogen cycling science. In: H. Lin (Ed.), Hydropedology (pp.711–735). USA: Elsevier.
Chithra, S. V., Harindranathan Nair, M. V., Amarnath, A., & Anjana, N. S. (2015). Impacts of impervious surfaces on the environment. International Journal of Engineering Science Invention, 4, 27–31.
Chow, A. T., Dai, J., Conner, W. H., Hitchcock, D. R., & Wang, J. J. (2013). Dissolved organic matter and nutrient dynamics of a coastal freshwater forested wetland in Winyah Bay, South Carolina. Biogeochemistry, 112, 571–587.
Christensen, J. B., Jensen, D. L., & Christensen, T. H. (1996). Effect of dissolved organic carbon on the ability of cadmium, nickel and zinc in leachate polluted groundwater. Water Research, 30, 3037–3049.
de Lucca, G. S., Barros, F. A. P., Oliveira, J. V., Magro, J. D., & Lucas, E. M. (2018). The role of environmental factors in the composition of anuran species in several ponds under the influence of coal mining in southern Brazil. Wetlands Ecology and Management, 26, 285–297.
Esther, O. J., Guo, C., Tian, X., Li, H., & Zhou, Y. (2014). The Effects of Three Mineral Nitrogen Sources and Zinc on Maize and Wheat Straw Decomposition and Soil Organic Carbon. Journal of Integrative Agriculture, 13(12), 2768–2777.
Everett, K. R. (1983). Histosols. In: Wilding, L.P., Smeck, N.E., Hall, G.F. (Eds.), Pedogenesis andSoil Taxonomy, II. The Soil Orders. Elsevier, Amsterdam.
Fernandes, L. A., Coimbra, A. M. (2000). Revisão estratigráfica da parte oriental da Bacia Bauru (Neocretáceo). Revista brasileira de Geociências, 30(4), 717-728 (in Portuguese).
Finlayson, C.M., Davidson, N., Pritchard, D., Milton, G. R., and MacKay, H. (2011). The ramsar convention and ecosystem-based approaches to the wise use and sustainable development of wetlands. J. Int. Wildlife Law Policy, 14, 176-198.
Furlan, L. M., Rosolen, V., Salles, J., Moreira, C. A., Ferreira, E., Bueno, G. T., et al. (2020). Natural superficial water storage and aquifer recharge assessment in Brazilian savanna wetland using unmanned aerial vehicle and geophysical survey. Journal of Unmanned Vehicle Systems, 8, 224–244.
Gao, C., Lin, Q., Bao, K., et al. (2014). Historical variation and recent ecological risk of heavy metals in wetland sediments along Wusuli River, Northeast China. Environment and Earth Science, 72, 4345–4355.
Gérard, F., Boudot, J. P., & Ranger, J. (2001). Consideration on the occurrence of the Al 13 polycation in natural soil solutions and surface waters. Applied Geochemistry, 16, 513–529.
Guo, Y., Yang, S. (2016) Heavy metal enrichment in the Changjiang (Yangtze River) catchment and on the inner shelf of the East China Sea over the last 150 years. The Science of the Total Environment, 543 (Part A), 105–115.
Gustafsson, J. P. (2012) Visual MINTEQ 3.0 user guide. Department of Land and Water Recources Engineering, 1, 1–73.
Hobbie, S.E., Ogdahl, M., Chorover, J., Chadwick, O.A., Oleksyn, J., Zytkowiak, R., Reich, P.B. (2007). Tree species effects on soil organic matter dynamics: the role of soil cation composition. Ecosystems, 10, 999–1018.
Jiao, W., Chen, W., Chang, A. C., & Page, A. L. (2012). Environmental risks of trace elements associated with long-term phosphate fertilizers applications: A review. Environmental Pollution, 168, 44–53.
Junk, W. J., Piedade, M. T. F., Lourival, R., et al. (2013). Brazilian wetlands: their definition, delineation, and classification for research, sustainable management, and protection. Aquatic Conservation: Marine and Freshwater Ecosystems, 24(1), 5–22.
Kabata-Pendias, A., Pendias, H. (2001) The Anthropocene - Water Pollution. In: Kabata-Pendias, A. (ed) Trace Elements in Soils and Plants Trace Elements in Soils and Plants. 3rd ed. Boca Raton: CRC Press.
Li, L., Zhao, Z., Huang, W., et al. (2004). Characterization of humic acids fractionated by ultrafiltration. Organic Geochemistry, 35, 1025–1037.
Liu, X., Ruecker, A., Song, B., Xing, J., Conner, W. H., & Chow, A. T. (2017). Effects of salinity and wet-dry treatments on C and N dynamics in coastal-forested wetland soils: Implications of sea-level rise. Soil Biology & Biochemistry, 112, 56–67.
Malavolta, E. (1994). Fertilizantes e seu impacto ambiental: micronutrientes e metais pesados, mitos, mistificação e fatos. São Paulo: Produquimica.
Matos, A. T., Fontes, M. P. F., Costa, L. M., & Martinez, M. A. (2001). Mobility of heavy metals as related to soil chemical and mineralogical characteristics of Brazilian soils. Environmental Pollution, 111, 429–435.
Milne, C. J., Kinniburgh, D. G., Riemsdijk, W. H., & Tipping, E. (2003). Generic NICA−Donnan Model Parameters for Metal-Ion Binding by Humic Substances. Environmental Science & Technology, 37, 958–971.
Mitsch, W. J., Bernal, B., Nahlik, A. M., Mander, U., Zhang, L., Anderson, C. J., et al. (2013). Wetlands, carbon, and climate change. Landscape Ecology, 28, 583–597.
Mora, S. J., & Harrison, R. M. (1983). The use of physical separation techniques in trace metal speciation studies. Water Research, 17, 723–733.
Parkin, T.B. (1987). Soil microsites as a source of denitrification variability. Soil Science Society of American Journal, 51, 1194–1199.
Pédrot, M., Dia, A., Davranche, M., et al. (2008). Insights into colloid-mediated trace element release at the soil/water interface. Journal of Colloid and Interf Science, 325, 187–197.
Pourret, O., Dia, A., Davranche, M., et al. (2007). Organo-colloidal control on major- and trace-element partitioning in shallow groundwaters: Confronting ultrafiltration and modelling. Applied Geochemistry, 22, 1568–1582. (special issue).
Queiroz, A. T. (2012). Análise e avaliação da demanda e da disponibilidade hídrica nos alto e médio curso do Rio Uberabinha e o abastecimento público em Uberlândia (MG). 137 f. Dissertation (Master). Instituto de Geografia, UFU, Uberlândia (in Portuguese).
Reiss, M., & Chifflard, P. (2015). Depth function of manganese (Mn) concentration in soil solutions: Hydropedological translocation of trace elements in stratified soils. European Journal of Soil Science, 4, 169.
Ribeiro, B. T., Nascimento, D. C., Curi, N. et al. (2019). Assessment of trace element contents in soils and water from cerrado wetlands, triângulo mineiro region. Revista Brasileira de Ciencia do Solo, 43, 1–17.
Rosolen, V. S., De-Campos, A. B., Govone, J. S., & Rocha, C. (2015). Contamination of wetland soils and floodplain sediments from agricultural activities in the Cerrado Biome (State of Minas Gerais, Brazil). CATENA, 128, 203–210.
Soil Survey Staff (2010). Keys to Taxonomy, USDA-NRCS, 338.
Sousa, P. T., Jr., Piedade, M. T. F., & Candotti, E. (2011). Brazil’s forest code puts wetlands at risk. Nature, 478, 458.
Takeno, N. (2005) Atlas of Eh-pH diagrams Intercomparison of thermodynamic databases. National Institute of Advanced Industrial Science and Technology Tokyo.
USDA-NRCS, (2010). Field indicators of hydric soils in the United States, Version 7.0. In: Vasilas, L.M., Hurt, G.W., Noble, C.V. (Eds.). USDA, NRCS in Cooperation with the National Technical Committee for Hydric Soils, Fort Worth, TX.
Vepraskas, M. J. (1999) Redoximorphic features for identifying aquic conditions. Tech. Bull., no 301. NC Agric. Research Serv., Raleigh, NC.
Vepraskas, M. J. (2004). Redoximorphic Features for Identifying Aquic Conditions. Tech. Bull. 301. NC Agric. Res. Serv. Raleigh, NC.
Vepraskas, M.J., Lindbo, D.L. (2012). Redoximorphic features as related to soil hydrology and hydric soils. In: H. Lin (Ed.), Hydropedology. (pp. 143–172). USA: Elsevier.
Xu, J., Zhuang, Q., Fu, Y., Huang, Y., Sun, Z., & Liu, Z. (2019). Spatial distribution, pollution levels, and source identification of heavy metals in wetlands of Suzhou Industrial Park, China. Wetlands Ecology and Management, 27, 743–758.
Xu, X., Cao, Z., Zhang, Z., Li, R., & Hu, B. (2016). Spatial distribution and pollution assessment of heavy metals in the surface sediments of the Bohai and Yellow Seas. Marine Pollution Bulletin, 110, 596–602.
Acknowledgements
The authors gratefully acknowledge Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Processo nº 2017/14168-1) for financially supporting this study
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Processo nº 2017/14168-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luko-Sulato, K., Rosa, V.A., Furlan, L.M. et al. Concentration of essential and toxic elements as a function of the depth of the soil and the presence of fulvic acids in a wetland in Cerrado, Brazil. Environ Monit Assess 193, 157 (2021). https://doi.org/10.1007/s10661-021-08945-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-08945-y