Abstract
Sandy beaches are challenging ecosystems, in which biota experience extreme physical conditions. We sampled meiofauna in conjunction with environmental factors that are well-known to affect faunal associations to describe the ecological state of sandy beaches that experience natural and human-made disturbances. We applied a random stratified sampling design with monthly collections (1800 cores) at three beaches on the Alexandria, Egypt, coast during two sampling periods over 1 year from November to April and May to September. We used multivariate analyses to compare beaches for water quality, particle size, and meiofaunal assemblages. The environmental analysis explained 60% of the total variation of physical factors among beaches and grouped beaches that moderately sorted fine-grained sand and high water salinity vs. the beach with well-sorted, coarse-grain, and low salinity. Meiofaunal analyses revealed unexpected results. The abundance and temporal variation were low, and the explained proportion of natural variation by the putative environmental factors was small. The natural variation was an indicator of long-term beach ruin and oligotrophic conditions. Our results suggest that a large fraction of natural variation in beach meiofauna is stochastic or that other, non-measured, the natural forces (e.g., storm events) or human-made forces (e.g., tourism activities) are essential contributors to variation. Our best models indicate that meiofauna is more resilient to natural disturbances than to human-made stressors, and the higher the beach exposure to the synergetic effects of natural forces and anthropogenic stressors, the lower the ecological state is.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sandy beaches are physically controlled ecosystems that are challenging for different biota to withstand and flourish. They may appear to be devoid of life due to the absence of attached plants, the small-sized animals, and the high mobility of animals on exposed beaches. This physical habitat is determined by water, sand, and wind, which in turn govern the biotic distribution and community structure (Brown & McLachlan, 2010). Storms and associated erosion cause the most faunal challenges. This ecosystem contains a complex array of microscopic and macroscopic biota that interact in a trophic network of sandy beach ecosystems (McLachlan & Defeo, 2017). Anthropogenic influences including coastal engineering, pollution, and tourism development extensively affect sandy shores, and these activities vary from beach to beach, inhibit or alter the natural sand transport and budget, and cause severe erosion. However, this ecosystem varies in space and time, even without human influence. The understanding of the physical, ecological, and socio-economic factors impinge on any sandy beach, which is very important for beach assessment and management (McLachlan et al., 2013). Natural variability is an essential ecological assessment (Landres et al., 1999), and it is the key to understand the mechanisms of population regulations (Ranta et al., 1998). Schlacher et al. (2014) addressed some cases of habitat change and loss at sandy beaches based on the natural variability of fauna and flora. Others recorded that the higher the oscillation rates of natural variability, the higher the indication of ecosystem stresses is (Armenteros, 2006; Brown & McLachlan, 2010).
Benthos are sensitive indicators of natural or anthropogenic disturbances (Reiss & Kroncke, 2005), and their distributions depend on physicochemical factors and biological interactions (Montagna, 1984; Moreno et al., 2006). Meiofauna is metazoans with a patchy distribution that ranges in size between 63 and 1000 µm (Giere, 2009) and dominated by two main taxa: nematode and harpacticoida with exceptions (Coull, 1999). Meiofauna organisms are known to be sensitive indicators of environmental perturbation due to high abundance, lack of larval dispersion, small size, ubiquitous distribution, high turnover, and intimate association with sediments (Alves et al., 2013; Semprucci et al., 2015). Marine sediment bacteria act as food for macrofauna, meiofauna, and microfauna (Ha et al., 2014; Moens et al., 2013). Bacteria are vital for the breakdown of organic matter in the sediment (Danovaro, 1996), and Coliform bacteria are sewage pollution indicators in the marine environment (Abdelhamid et al., 2013).
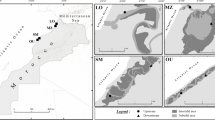
The Alexandria coast extends for about 42 km (Fig. 1; Table 1) and experiences natural and anthropogenic stressors (EEAA, 2015), beach erosion, rip currents, sea-level rise, and coastal engineering (El-Raey et al., 2015). The Egyptian Environmental Affairs Agency has declared two polluted hotspots sandy beaches: Abo-Qir Bay and El-Mex Bay at the east and the west of Alexandria coast, respectively, due to high anthropogenic influences (EEAA, 2015). Several studies have documented the deterioration of the ecological status of these two beaches (El Nemr et al., 2013; Shreadah et al., 2019). Few studies have investigated the meiofaunal abundance along the Egyptian Mediterranean coast (Mitwally, 1999; Mitwally et al., 2004), and few studies have attempted to correlate meiofauna and bacterial abundance (Jammo, 2004).
The current study aimed first to assess the responses of natural variability of meiofauna assemblages to relevant physicochemical, sedimentological factors, and microbes at three sandy beaches that experience different stressors, and second to test which factor is the best predictor of or contributor to meiofaunal variability. Finally, the study aims for the first time to use the meiofaunal variability as an environmental tool discriminating and assessing the ecological status of subtropical sandy beaches at the Alexandria Southeastern Mediterranean coast, Egypt.
Materials and methods
Study design
We collected meiofaunal sediment samples for 10 months during two sampling periods over the years 2012–2013: the mild-cold period (MC) November to April, except for March, and the mild-warm period (MW) May to September. The study design is a random stratified sampling design (Schlacher et al., 2008). We sampled four random perpendicular profiles at each beach. Each profile extended between the drift and the wrack lines and consisted of five random stations, where triplicate sediment samples were collected. Beaches designated as highly polluted high energy (HPHE), highly polluted low energy (HPLE), and clean high energy (CHE). The design has two fixed factors: period and beach. The interval distances among profiles were 150 m at HPHE and CHE and 100 m at HPLE, whereas the distances between stations were 5, 2, and 8 m apart, respectively, for HPHE, HPLE, and CHE.
The study area (Fig. 1)
Abo-Qir Bay (HPHE)
It is a shallow, semi-closed basin border by Rosetta mouth of the Nile at the northeastern and Abo-Qir headland at the southwestern (Table 1). The bay occupies an area of ~ 500 km2 with an average depth of 10–12 m. It received several land-based sources of nutrients, a high load of freshwater nutrients from the Rosetta mouth of the Nile, and Lake Edku discharge. El-Tabia pumping station is the essential source of industrial and domestic wastes. The bay also experiences hydrocarbon oil and thermal pollution due to fishing boats and Electrical Power stations. The physicochemical characteristics of the bay have received attention (Ismail et al., 2017; Khairy et al., 2012) and designated as a highly energetic dissipative sandy beach with dramatic erosion and sea-level rise (Frihy et al., 1996; Nafaa & Frihy, 1993).
El-Mex Bay (HPLE)
It has an elliptical shape that extends for about 15 km at the Western coast of Alexandria (Table 1) and a mean depth of 10 m. Lake Maruti’s discharge through the El-Mex Pump station and El-Umum drain canal are the essential sources of different industrial, agricultural, domestic, and hydrocarbon pollutants. The bay has a rocky shoreline with a narrow sandy beach, a micro-tidal estuary character, and the eddy current affect many parts (Hamdy, 2015; Shreadah et al., 2014).
North West Coast beach (CHE)
The Northwest coast beach lies at 100 km to the west of Alexandria near the touristic “Marakia” village (45 m; Table 1). This area is oligotrophic, highly energetic (Zaki et al., 2009), far away from urban development, and characterized by water clarity, extensive beachfront, and a very gentle shore slope (Frihy, 2009). The Northwest coast beaches of Alexandria classified between reflective and moderately dissipative beaches, with hazards rip currents (Frihy et al., 1996). Egyptian government built several engineering projects to create safe places for swimming (Iskander et al., 2007) that have disturbed the hydrodynamic system by creating a new pattern of sedimentation (Frihy & Deabes, 2012).
Field and laboratory work
Field work
A handheld corer of 4.8-cm2 surface area and 11-cm length was used for sediment sample collection and a 4% formalin solution, with rose Bengal dye for sediment preservation. For microbial and sedimentological analysis, we collected two additional samples at each station that were stored in sterilized bags in the refrigerator for a maximum of 24 h for further investigation. Water temperature was measured by the mercury thermometer. The water salinity was collected using a salinity bottle, and pH was measured using HANNA HI 98,107 pH meter.
Physico-chemical and sedimentological factors
We applied the Strickland and Parsons (1972) method for salinity determination and El Wakeel and Riley (1957) and Olausson (1975) procedures for analysis and calculation of organic carbon and total organic matter, respectively. We used the Folk and Ward (1957) method for mean grain size and sediment sorting coefficient measurements.
Microbial and meiofaunal analysis
Total bacterial abundance was determined, according to Ben-David and Davidson (2014). Serial dilutions were made by aseptically removing 1 g of wet sediment into 99 ml filtered sterilized seawater, mixed vigorously, and sonicated for 2 min. Then, 1 ml from each sample was inoculated onto marine agar medium (DifcoTM, 2216), incubated at 37 °C for 24 h, and colonies were counted and expressed as colony-forming units (CFU/ml) during the mild-cold period. Coliform bacteria (CB) were inoculated on Endo Agar–less dehydrated medium (HiMedia, M1106). All colonies were counted, purified, and biochemically characterized using API 20E and API 20NE kits (BioMérieux, Marcy I´Etoile, France) according to the manufacturer’s instructions and incubated at 37 °C for 24 h during the mild-warm period. Interpretation of results was performed using the computer-aided database API-WEB™V.5.0 software (Hamdan et al., 2016).The Huys et al. (1996) technique for staining, extraction, sorting, and identification of meiofauna to higher taxonomic groupings was applied using a stereomicroscope, and abundance was expressed as individuals 10 cm−2.
Data analysis
Physico-chemical, sedimentological, and microbial data were transformed to square-root and normalized to avoid strong skewness in the distribution over samples and to overcome different measurement scales (Anderson et al., 2008). Draftsman plots were performed to test for multicollinearity among variables. The Euclidean distance-measure index was applied to build an environmental-microbial resemblance. Principal component analysis (PCA) was conducted to assess the environmental and microbial data variation between the sampling periods and among beaches. The data matrix consisted of 300 observations for each sampling period.
Taxa that had zero cells more than 25% were omitted from the meiofaunal matrix, to avoid rare-species problems, and conduct different multivariate analyses (Cunningham & Lindenmayer, 2005; Ortega Cisneros et al., 2011). Data were square-root transformed, and the simple matching measure index applied to build a meiofaunal resemblance. The PERMANOVA (Anderson, 2005) was done to test for variation in mean meiofaunal data between periods, among beaches, within profiles, stations, and their interaction and nesting effects. The pair-wise comparison analysis was performed within fixed and random factors. The PERMANOVA ran with type III of sum squares (partial); the number of permutation was 999, a reduced model of residuals permutation, and Monte-Carlo probability. To seek temporal and spatial visual discrimination based on the meiofaunal resemblance, the non-multidimensional scaling (nMDS) analysis was applied using the fixed factors as variables, and the matrix consists of 900 observations for each sampling period.
Distance-based linear models (DISTLM) analysis was applied to estimate the proportion of variation in the meiofauna community explained by the measured environmental factors (Anderson, 2003). Replicated meiofaunal data were pooled at each station to fit the factors’ matrix. We conducted the analysis based on Euclidean distance resemblance of seven factors; physicochemical, sedimentological, bacterial predictors, and a simple-matching measure resemblance of eight meiofaunal variables. Marginal and sequential tests in a linear regression model with a step-wise procedure applied between and among the fixed effects: period and beach. The Akaike (AICc) information criterion and coefficient of determination (R2) were chosen for the best model of each sequential test (Anderson et al., 2008). The permutation number for each analysis is 999. Unlike the R2 or the adjusted R2, the AICc values will not continue to increase to get better as the number of variables increases in the model, and the best model has the smallest AICc value (Anderson et al., 2008).
Distance-based redundancy analysis (dbRDA) was performed to visualize the percentage of variability in the original meiofaunal resemblance that fitted the constrained linear combination model of response criterion and predictor variables (Legendre & Anderson, 1999). The relative contribution of each environmental variable (strength and direction) in driving the variation along dbRDA axes was visualized by the vector overlays on the ordination diagram with one vector for each variable. The analysis ran between and among fixed factors: period and beach. All the multivariate analyses applied using the PRIMER 7+ software package.
Pearson correlation analysis was applied to seek for non-linear relationships between any of/and all permanent meiofaunal taxa vs. the proposed microbial food data (TB and CB). All statistical results were described based on actual p values and sample size to avoid the arbitrary rejection rate of significant vs. non-significant (Smith, 2019).
Results
Environmental factors
The mean ± SD values of the physicochemical (T◦c, PSU, pH), sedimentological factors (φ, sorting, %TOM), and microbial data (TB and CB) were tabulated in Table 1. Physicochemical and sedimentological variables had smaller mean during the mild-cold than that during the mild-warm period except for %TOM at HPHE. Draftsman analysis revealed correlation values less than the cutoff 0.95 among the physicochemical, sedimentological, and microbial variables. Therefore, the environmental matrix included all the measured variables. During the mild-cold period (Fig. 2a), the 1st and 2nd PCs accounted for ~ 60% of the total variation of the physical factors among beaches. The PC1 separated data at HPLE (right-hand side) from HPHE and CHE (left-hand side). The PC2 barely separated within HPHE, CHE, and HPLE data. During the mild-warm period (Fig. 2b), the 1st and 2nd PCs accounted for ~ 60% of variations. The PCs separated HPLE data from HPHE and CHE. The HPHE and CHE data collapsed together. All variables loaded positively on PC2.
Principal component analysis based on the Euclidean distance index of seven environmental factors during the mild-cold period (a) and the mild-warm period (b). Abbreviations: T◦c temperature, PSU salinity, pH water alkalinity, φ mean grain size, sorting sediment sorting coefficients, %TOM total organic matter, TB total bacteria, CB coliform bacteria, and beach abbreviations listed in Fig. 1
Meiofauna assemblages
Meiofaunal community structure consisted of 12 taxonomic groups. Eight taxa are permanent meiofauna; Nematode (40%), Polychaete (31.5%), Harpacticoida (18%), Turbellaria (4%), Ostracods (1.8%), Archannelida (1.5%), Foraminifera (1.2%), and Halicardia (1%). Nauplius larvae and three temporary taxa: Gastropod, Isopoda, and Bivalve, totaled 0.8%. The overall mean total meiofaunal abundance was 38 ± 67 individuals 10 cm−2 (MC; Fig. 3a), and 46 ± 108 individuals 10 cm−2 (MW; Fig. 3b). At HPLE, total abundance was ~ 3.5 and 5 times higher than the abundance at HPHE and CHE beaches, respectively. Meiofaunal abundance attained its maximum during MC (February) and ranged from 1260 ± 1001 individuals 10 cm−2 (HPLE) to 285 ± 177 individuals 10 cm−2 (CHE; Fig. 3a). The lowest abundance was found during MW (July) and ranged from 189 ± 705 individuals 10 cm−2 (HPLE) to 69 ± 53 individuals 10 cm−2 (CHE; Fig. 3b). The abundance at HPLE had a wider standard deviation range than that at HPHE and CHE. The meiofaunal abundance at HPHE and CHE beaches tracked each other with limited oscillations, and it was 1.5 times higher at HPHE beach.
Archannelida was omitted from the meiofaunal analysis due to high number of zero cells. PERMANOVA results revealed that there was no significant variation among meiofaunal communities between the mild-cold and mild-warm periods (Table 2; Pseudo-F1, 1200 = 1.34, Pperm = 0.211, U-Perm = 999, PMC = 0.306). Among beaches, variation in meiofaunal communities was significant (Pseudo-F2, 1200 = 13.36, Pperm = 0.001, U-perm = 999, PMC = 0.001). The variability in the meiofaunal assemblages was significant within month nested in period (Pseudo-F8, 1200 = 5.93, Pperm = 0.001, U-perm = 999, PMC = 0.001) and stations nested in profile and beach (Pseudo-F48, 1200 = 3.83, Pperm = 0.001, U-perm = 999, PMC = 0.001). The interaction effect between the nested factors (month (period) ∗ profile (beach)) revealed higher significant variability in meiofaunal assemblages than the interaction effect between the nested factors (month (period) ∗ (station (profile (beach)), (Pseudo-F72, 1200 = 2.60 and F 384, 1200 = 2.04, respectively, at Pperm = 0.001, U-perm = 999, PMC = 0.001). The smallest variability was detected for the interaction between month nested in period and beach (Pseudo-F16, 1200 = 1.08, Pperm = 0.008, U-perm = 998, PMC = 0.015).
Results of pair-wise tests based on meiofaunal communities, revealed small significant variations between MC and MW periods (Table 3; t-test = 1.73, Pperm = 0.008, U-perm = 996, PMC = 0.075). Among beaches, the highest significant variation was detected between HPHE and HPLE, then between HPLE and CHE and after then between HPHE and CHE (t-test = 3.74, 3.67, and 3.46 at Pperm = 0.001, U-perm = 999, 998, and 997, PMC = 0.001, 0.001, and 0.003 respectively). The within-month variability was higher during the mild-cold than that during the mild-warm period (Fig. 3; Appendix 1). The pair-wise analysis within different levels of the fixed factors and their interactions revealed scattered results shown at Appendices 1–4 at actual probability values. The nMDS analysis did not visualize clear groupings between periods and among beaches. Therefore, the results were not shown here.
The DISTLM model during the mild-cold period, marginal test, revealed that six environmental factors, each alone, had a significant contribution to meiofaunal communities at p = 0.001: φ, sorting, pH, T◦C, PSU, and TB (Table 4). However, each factor explained a low proportion of meiofaunal community variation, and salinity explained the highest contribution (9.5%). The sequential analysis showed the best model based on the lowest AICc (5499.4) value contained six factors and explained 15% of the cumulative variation in meiofaunal communities. During the mild-warm period, each variable out of four environmental factors contributed significantly to meiofaunal assemblages, but with a low proportion of variation: %TOM, φ, sorting, and PSU at p-value (0.001), and grain size explained the highest proportion (3.5%). The best model consisted of the combination of six physicochemical and sedimentological factors and explained 9% of the cumulative variation at the lowest AICc value (4389.2). However, DISTLM models capture a little of explained variation that fitted the linear regression with R2 equals to 0.151 and 0.089 at significant p = 0.020 and 0.010 during the mild-cold and warm periods, respectively.
Results of dbRDAs analysis revealed that the 1st and 2nd dbRDA plots were responsible for 75.3% and 19.8% of the fitted variation and 11.3% and 3% of the total variation, respectively, during MC (Fig. 4a). The 1st dbRDA correlated negatively with the PSU (−0.75) and sorting (−0.50) data. The 2nd dbRDA correlated positively and negatively with TB (0.63) and pH (−0.67), respectively. The dbRDA plots visualized two groupings: the HPLE grouping loaded on the right-hand side, whereas the HPHE and CHE grouping loaded on the left-hand side. During the mild-warm period, the 1st and 2nd dbRDAs were responsible for 69.9% and 27.4% of the fitted variation, respectively (Fig. 4b), and 6.1% and 2.5% of total variations, respectively. The 1st dbRDA had a positive correlation with sorting (0.60), φ (0.45), and PSU (0.42) data, whereas the 2nd dbRDA correlated positively with φ (0.5), T◦C (0.44), and %TOM (0.43) data. The dbRDA plots visualized two groupings of data. The first grouping consists of HPHE, CHE, and some HPLE data loaded on the right-hand side, whereas the second grouping consists of the rest of HPLE data on the left-hand side.
The dbRDA plots, a constrained model based on the resemblance of simple matching index of 8 criterion data; Nematodes, Polychaete, Foraminifera Harpacticoida, Turbellaria, Halicardia, Ostracods, total meiofauna, and Euclidean distance resemblance of 7 predictors’ data between (a) the mild cold and (b) the mild-warm periods. A list of abbreviations is in Figs. 1 and 2
The DISTLM analysis among beaches, marginal tests, revealed that one or two factors explained a small amount of the variation in meiofaunal assemblages at each beach during the mild-cold period; sorting at HPHE, TB at HPLE, sorting, and pH at CHE (Table 5) at p = 0.04, 0.03, 0.03, 0.02 respectively. The sequential analysis based on the lowest AICc selection revealed that the best model consisted of sorting, T◦C, and TB at HPHE; TB and sorting at HPLE; and pH, sorting, and φ at CHE that explained 6.0% (R2 = 0.059), 6.5% (R2 = 0.065), and 10% (R2 = 0.098) of the total variation in meiofaunal assemblages, respectively. During the mild-warm period, marginal test (Table 6), %TOM, was the only factor that explained significant contribution to the community, but with a small proportion of variation at HPLE (p = 0.03) and CHE (p = 0.01). At HPLE, the best model based on the lowest AICc value consisted of the combination of %TOM and φ that explained a small proportion of variations, 6.5% (R2 = 0.065) in the meiofaunal assemblages, whereas the best model at CHE consisted of five combined factors and explained 16% (R2 = 0.16) of the total variation in the meiofaunal community. The sequential analysis at HPHE revealed that the combination of the measured environmental factors did not contribute significantly to meiofaunal assemblages (Table 6).
Pearson’s correlation (Table 7) revealed that during the MC, total bacterial counts correlated significantly with total meiofaunal abundance and the abundance of different meiofaunal taxa, and the R values for all significant correlations ranged between ~ 0.2 and ~ 0.4, except for Ostracods and Archannelida. During the mild-warm period, Harpacticoida and Turbellaria abundance had a weak significant positive and negative correlations with the coliform count at p = 0.001 and 0.016, respectively.
Discussion
In an attempt to assess the ecological states of three sandy beaches that experience natural disturbances and anthropogenic stressors at the Alexandria coast, Egypt (Nafaa & Frihy, 1993), we studied the natural variability of the meiofaunal organisms as an ecological assessment tool (Balsamo et al., 2012; Costa et al., 2016). Our study addressed the most well-known environmental factors affecting meiofaunal distribution (Giere, 2009), and contributor to the variability. The study revealed unexpected results, low abundances, lack of temporal variation, and a low contribution of environmental and microbial factors to meiofaunal variability. However, there was evidence that the measured factors controlled meiofaunal natural variability, the highest contribution of salinity, and sand particles to meiofaunal variability during the cold and warm periods, respectively. Despite the low proportion of environmental contribution, our results indicated that natural variability at each beach is a case study, stand-alone indicating beach-stress level. A thorough discussion will go through points that explain our findings.
Understanding of natural variability is essential to provide reference points as a basis not only for evaluating ecosystem management (Swanson et al., 1994) but also for distinguishing between disturbed and undisturbed ecosystems (Power, 1999). Investigating meiofaunal natural variability herein revealed some points that could indicate the ecosystem disturbances. The number of recorded higher meiofaunal taxa (12) agreed with other subtropical studies (Kotwicki et al., 2005), but sensitive taxa in many coastal areas, according to Zeppilli et al. (2015), were absent herein (Gastrotrichs, Tardigrade), or had a low contribution; Ostracods. However, a total number of permanent taxa (8) assigned our sandy beaches within the lower limit of sufficient environmental quality score according to Danovaro's classification (Gambi & Dappiano, 2004; Pusceddu et al., 2007). The overall mean meiofaunal abundance was smaller than the minimal values worldwide with few exceptions (Baldrighi et al., 2019; Gheskiere et al., 2002; Moreno et al., 2006). It was, also, smaller than earlier studies (Mitwally, 1999; Mitwally et al., 2004), whereas the abundance decreased from the overall mean 1581 ± 606 individuals 10 cm−2 during 1996 to overall mean values 38 ± 67 and 46 ± 108 individuals 10 cm−2 during 2012–2013, suggesting long-term beach ruin probably due to the coastal engineering that started at the end of the last century. Some studies documented a significant decrease in macrofauna and meiofaunal abundances, and their community structures due to coastal engineering (Hamdy & Ibrahim, 2019) or in the post dredging sites (Szymelfenig et al., 2006). To conclude, that the low meiofaunal abundance was an indicator of human-made activities. Moreover, the low meiofauna herein could also indicate the low productivity along beaches. Coull and Fleeger (1977) commented that low meiofaunal density is an indicator of low area productivity. The oligotrophic conditions dominate the eastern Mediterranean (Danovaro et al., 1999), southeastern Mediterranean (Dowidar, 1984), and the northwest coast of Alexandria (Zaki et al., 2009), suggesting that meiofaunal natural variability could be a good indicator of the low productivity.
Lack of temporal variations and a low contribution of environmental factors to meiofaunal variability, in the current study, suggested that meiofaunal assemblages could have a stochastic distribution. The fundamental causes of a stochastic pattern are the rate of organisms birth, death, immigration, and emigration that occur at random (Gansfort et al., 2020). Despite the well-known patchy distribution of spatial meiofaunal abundance (Giere, 2009; Higgins & Thiel, 1988), some studies commented that meiofaunal distribution tends to have a stochastic pattern over time and space (Mitwally & Abada, 2008; Traunspurger & Majdi, 2017). In the current study, three tolerant taxa having different dispersal abilities Nematodes, Polychaete, and Harpacticoida were dominating our sandy beaches, and they could be the reason behind the increase in the role of local stochastic distribution. Dorgham et al. (2014) documented the dispersion patterns of macrofaunal polychaete along the Alexandrian coast that could impact the temporal meiofaunal distribution. At the same time, high energy could disturb meiofaunal abundance causing the passive migration into the water column or deeper inside the sediment, reducing the numbers, masking the responses to the environmental factors, and increasing the chances of stochastic distribution. Erosion causes a reduction in meiofaunal abundance and richness (Giere, 2009; Semprucci et al., 2011), and meiofauna at the exposed beaches can reach deep in sediment to avoid the effects of currents and wave action (Rodrı́guez, 2003).
Despite the lack of temporal variations, the within-month variability during the mild-cold period was high (Table 2; Appendix 1), suggesting that the temporal pattern was due to, perhaps, density-independent events; winter storms. Seasonal changes in natural forces drastically affect the temporal fluctuations of meiofaunal density (Riera et al., 2011; Sevastou et al., 2011; Sun et al., 2014), and winter storms effect could last for 2 weeks for meiofaunal recovery (Grémare et al., 2003). Groupings of meiofaunal data (Figs. 2a and 4a) resembled the physical data groupings, suggesting the importance of water quality factors as meiofaunal drivers. Salinity and pH explained about 12.5% of the total meiofaunal variation during the mild-cold period (Table 4), suggesting that they act as a proxy for other natural events, e.g., winter storms. Rain decreases water salinity over the Mediterranean Sea (Milner et al., 2012), salinity gradients affect nematode abundances (Adao et al., 2009), and the pH explained most of the benthic biomass variability (First & Hollibaugh, 2010). A low water salinity value with a high standard deviation (Table 1) indicated that natural disturbances, winter storms, and heavy rain could be a key factor affecting the meiofaunal community. Moreover, the frequent intrusion of freshwater from the River Nile at the HPHE and HPLE could impact the faunal assemblages.
During the mild-warm period, despite the overall low contribution of environmental factors, sand particles alone explained a significant proportion, 3.5% (Table 4) to the total meiofaunal variation suggesting the importance of grain size in affecting meiofaunal distribution but other non-quantified factors, e.g., touristic activities, could homogenize sediment, minimize the within-month variability (Appendix 1), and mask the particle contribution. Many studies documented that despite the socio-economic profits of tourism, the rapid development of these activities resulted in beach disturbance, that characterized by low organic matter, low meiofaunal abundances, and species diversity (Defeo et al., 2009; Gheskiere et al., 2005; Nordstrom, 2004; Sun et al., 2014). Besides, touristic activities have proved as the essential source of fecal pollution (Korajkic et al., 2018; Torres-Bejarano et al., 2016). Studies along the Alexandrian coast classified beaches from very clean to highly polluted (El-Shenawy & El-Shenawy, 2009) based on coliform bacteria. However, the detected numbers were less than the Egyptian guide standards (500 CFU/100 ml) for recreational waters (George, 2009). Most of the unexplained variation in the meiofaunal community herein could be due to the effect of some non-quantified factors, e.g., winter storms and human-made stress, or because of the stochastic distribution. Alves et al. (2013) concluded that anthropogenic effluents caused a lack of meiofaunal temporal variations. Ostracods community analysis showed a large proportion of unexplained variation due to its highly stochastic variation (Gansfort et al., 2020). The proportion of variation in the meiofaunal community was higher during the mild-cold period (15%) than that during the mild-warm period (9%), suggesting that meiofaunal assemblages are more resilient to sources of natural disturbances than to anthropogenic stressors.
Among beaches, the contribution of environmental factors to the total variation of meiofaunal communities stated different ecological states, despite the overall low proportion of the explained variation, suggesting that each beach should be considered a unique case study for management decision-makers. Sediment sorting and total bacteria had a significant contribution to meiofaunal communities at the two highly polluted beaches (Table 5), indicating the importance of the sorting coefficient as an indicator of the wave action effect on sediment composition, which in turn drive meiofaunal community. The sediment sorting coefficient is a fundamental factor reflecting the hydrodynamic severity on grain particles, reshaping, influences sediment characteristics, and affect meiofaunal communities (Maria et al., 2013; Santos et al., 2019; Urban-Malinga et al., 2004). The significant contribution of total bacteria to the total meiofaunal (Table 5) is an indication of the biodegradation levels, and meiofauna could stimulate bacteria for biodegradation rather than for the feeding process. The contribution of total bacteria to the total meiofaunal variation was higher at a low energy beach (3.7%) than that at the high energy (2.4%), indicating that wave action affects meiofaunal-bacterial relationships. Meiofauna should have a high abundance to affect the microbial structure (Montagna, 1984), graze on 3% of total bacterial production (Pascal et al., 2008), and both communities responded to the environmental variability in the same way (Papageorgiou et al., 2007). High energy causes a large scale of pollution dispersion (Defeo et al., 2009), and low energy induces a high retention rate of pollutants and reduces the biodegradation rate (Lee & Levy, 1991; Lo et al., 2018). To conclude, the synergetic effects of energy strength, pollution, and stochastic distribution at the highly polluted beaches could be the main reasons behind the low prediction of meiofaunal drivers during the cold period (6% and 6.5%).
The lack of significant contribution or the weak contribution of environmental factors to total meiofaunal variations (Table 6) could be a proxy for the beach ruin due probably to the touristic activities that synergistically added more stressor effect at the polluted beaches (Table 6). Itoh et al. (2011) considered a lack of significant contribution of environmental factors to meiofaunal variation as a proxy for important non- quantified forces in their study, and beaches experience high recreational levels are designated moderately and highly disturbed areas (Pereira et al., 2017). The higher the proportion of the environmental contribution to the total meiofaunal variation at the mild-cold period than that at the mild-warm periods at the highly polluted beaches suggested that the meiofaunal community was more resilient to natural forces act on sediment structure high energy during the winter than to human-made disturbance during summer tourism activities.
The clean high energy beach, CHE, stated a different ecological state, where it occupied the lowest meiofaunal abundances (Fig. 3) and the highest proportion of environmental contribution to the total meiofaunal variation (Tables 5 and 6). In contrast to the polluted beaches, this proportion was higher during the mild-warm period than during the mild-cold period (16% vs. 10%), indicating that the lower the human-made activities, the higher the response of meiofaunal assemblages to their environmental factors during the warm period. This beach could be under the antagonistic effects of different natural and human-made forces. The CHE is non-urbanization and had a low rate of touristic activities that could explain the higher the environmental contribution to the meiofaunal communities, whereas wave energy, rip currents (Nafaa & Frihy, 1993), and the dominance of oligotrophic conditions (Zaki et al., 2009) affect meiofaunal assemblages, concluding that touristic activities and coastal engineering harm meiofaunal communities more than the natural disturbances.
Our best model results indicate that the low contribution of environmental factors to the meiofaunal community was a beach assessment tool. The beach, Abo-Qir Bay, under the synergetic effect of natural disturbances and many land-based sources of nutrients, industrial, and domestic wastes at HPHE, revealed the worse prediction. The beach EL-Mex Bay that experiences mainly anthropogenic discharges: industrial, agricultural, sewage, and hydrocarbon pollution at HPLE, had a low estimation, and the beach North West Coast that experiences natural disturbances and limited anthropogenic stressors, such as coastal engineering at CHE, revealed the most tolerable prediction. However, many studies commented that it is difficult to differentiate between the effect of the natural disturbance from the anthropogenic stressors (Schratzberger et al., 2009; Semprucci et al., 2011), and many indirect factors complicate the relationships between the faunal assemblages and their driving forces (Tolhurst et al., 2010). Our low meiofaunal abundances and their environmental contribution suggested that the more the beach exposure to different sources of anthropogenic stressors, the higher the beach ruins are. The beach state became worse when the natural disturbances integrated synergistically with human-made activities. We suggest for management decision-makers to consider each beach a unique case study and manipulate alone, putting into consideration human-made activities the mechanical engineering, pollution, tourism activities, besides the natural forces winter storms, high energy, and rip currents.
The weak and a lack of correlation between bacterial communities and different meiofaunal taxa (Table 7) suggested the idea of the alternative food meiofauna grazed bacteria when algal abundance and biomass are very low (Pascal et al., 2009). Studies documented strong meiofaunal-algal correlations (Evrard et al., 2012; Mitwally et al., 2004). The dynamical character of our sandy beaches and the high levels of sediment toxicity at the polluted beaches are other causal factors that could mask the meiofaunal-bacterial interaction as many studies (Maria et al., 2016; Montagna et al., , 1987, 1989; Urban-Malinga et al., 2004) suggested. Our study does not prove the importance of bacteria as a meiofaunal food source. However, the low counts of coliform bacteria (Table 1) evidenced that Alexandrian sandy beaches are free of pathogenic diseases, and we recommended further studies for a better understanding of the meiofaunal-bacterial relationship.
Conclusions
The current work is the first study that has investigated the environmental drivers of meiofaunal natural variability at the southeastern Mediterranean coast, Alexandria, Egypt, in an attempt to assess the ecological status of three challenging sandy beach ecosystems. Low meiofaunal abundance indicates that natural variability is a good indicator of long-term beach ruin and oligotrophic conditions. Lack of temporal variation indicates that the meiofauna could have a stochastic distribution. The significant contribution of water salinity and sand particles to the total meiofaunal variations, despite their low proportion, indicate their role as proxies for winter storms and touristic activities. The synergetic effect of stochastic variation, natural disturbances, and human-made disturbances controlled the meiofaunal natural variability and masked the contribution of the well-known environmental factors to the total meiofaunal variation. The ecological assessment of sandy beaches ranged from very bad, bad, and to some extent, tolerable according to the contribution of the well-known environmental factors to the meiofaunal natural variability.
References
Abdelhamid, M. A., El-Barbary, M. I., & El-Deweny, M. E. M. (2013). Bacteriological status of ashtoum El-Gamil protected area. Egyptian Journal of Aquatic Biology and Fisheries, 17, 11–23.
Abo-Taleb, H. A., El Raey, M., Abou Zaid, M. M., Ezz, S. A., & Aziz, N. A. (2015). Study of the physico-chemical conditions and evaluation of the changes in eutrophication-related problems in El- Mex Bay. African Journal of Environmental Science and Technology, 9, 354-364.
Adao, H., Alves, A. S., Patrıacio, J., Neto, J. M., Costa, M. J., & Marques, J. C. (2009). Spatial distribution of subtidal Nematoda communities along the salinity gradient in southern European estuaries. Acta Oecologica, 3(5), 287–300.
Alves, A. S., Adão, H., Ferrero, T. J., Marques, J. C., Costa, M. J., & Patrício, J. (2013). Benthic meiofauna as indicator of ecological changes in estuarine ecosystems: The use of nematodes in ecological quality assessment. Ecological Indicators, 24, 462–475.
Anderson, M. J. (2003). DISTLM forward Distance-based multivariate analysis for a linear model using forward selection A computer program, Department of Statistics University of Auckland, pp. 1–10.
Anderson, M. J. (2005). PERMANOVA, Permutational multivariate analysis of variance (pp. 1–23). University of Auckland, New Zealand.
Anderson, M. J., Gorley, R. N., & Clarke, K. R. (2008). PERMANOVA+for PRIMER: Guide to software and statistical methods (pp. 1–214). Plymouth, Uk.
Armenteros, M., Marti´N, I., Williams, J. P., Creagh, B., Gonza´Lez-Sanso´ N, G., & Capetillo, N. (2006). Spatial and temporal variations of meiofaunal communities from the Western Sector of the Gulf of Batabano. Cuba. I. Mangrove Systems. Estuaries and Coasts 29, 124-132.
Baldrighi, E., Semprucci, F., Franzo, A., Cvitkovic, I., Bogner, D., Despalatovic, M., Berto, D., Formalewicz, M. M., Scarpato, A., Frapiccini, E., Marini, M., & Grego, M. (2019). Meiofaunal communities in four Adriatic ports: Baseline data for risk assessment in ballast water management. Marine Pollution Bulletin, 147, 171–184.
Balsamo, M., Semprucci, F., Frontalini, F., & Coccioni, R. (2012). Meiofauna as a tool for marine ecosystem biomonitoring. Marine Ecosystems, 4, 77–104.
Ben-David, A., & Davidson, C. E. (2014). Estimation method for serial dilution experiments. Journal of Microbiological Methods, 107, 214–221.
Brown, A., & McLachlan, A. (2010). The ecology of sandy shores. Elsevier.
Costa, A., Valenca, A., & Santos, P. (2016). Is meiofauna community structure in Artificial Substrate Units a good tool to assess anthropogenic impact in estuaries? Marine Pollution Bulletin, 110, 354–361.
Coull, B. (1999). Role of meiofauna in estuarine soft-bottom habitats. Australian Journal of Ecology, 24, 327–343.
Coull, B., & Fleeger, J. W. (1977). Long-term temporal variation and community dynamics of meiobenthic copepods. Ecology, 58, 1136–1143.
Cunningham, R. B., & Lindenmayer, D. B. (2005). Modeling count data of rare species: Some statistical issues. Ecology, 86, 1135–1142.
Danovaro, R. (1996). Detritus-bacteria-Meiofauna interactions in a seagrass bed (Posidonia oceanica) of the NW Mediterranean. Marine Biology, 127(121), 113.
Danovaro, R., Dinet, A., Duineveld, G., & Tselepides, A. (1999). Benthic response to particulate fluxes in different trophic environments: A comparison between the Gulf of Lions-Catalan Sea (western-Mediterranean) and the Cretan Sea (eastern-Mediterranean). Progress in Oceanography, 44, 287–312.
Defeo, O., McLachlan, A., Schoeman, D. S., Schlacher, T. A., Dugan, J., Jones, A., Lastra, M., & Scapini, F. (2009). Threats to sandy beach ecosystems: A review. Estuarine, Coastal and Shelf Science, 81, 1–12.
Dorgham, M. M., Hamdy, R., El Rashidy, H. H., Atta, M. M., & Musco, L. (2014). Distribution patterns of shallow water polychaetes (Annelida) along the Alexandria coast, Egypt (Eastern Mediterranean). Mediterranean Marine Science, 15, 635.
Dowidar, N. M. (1984). Phytoplankton biomass and primary productivity of the South-Eastern Mediterranean (pp. 983–1000). Faculty of Science, Alexandria University.
EEAA. (2015). Alexandria Coastal Zone Management Project (ACZMP). Ministry of Environmental Affairs & Egyptian Environmental Affairs Agency (pp. 1–15). SFG1484V2.
El-Raey, M. F., Nasr, S., & Hendy, D. (2015). Integrating knowledge to assess coastal vulnerability to sea-level rise. In El- Mex Bay (Ed.), Egypt: The Application of the DIVA Tool. International Journal of Marine Science.
El-Shenawy, M. A., & El-Shenawy, M. (2009). Enterohaemorrhagic Escherichia coli O157 in coastal environment of Alexandria. Egypt. Microbial Ecology in Health and Disease, 17, 103–106.
El Nemr, A., El-Sadaawy, M. M., Khaled, A., & Draz, S. O. (2013). Aliphatic and polycyclic aromatic hydrocarbons in the surface sediments of the Mediterranean: Assessment and source recognition of petroleum hydrocarbons. Environmental Monitoring and Assessment, 185, 4571–4589.
El Wakeel, S., & Riley, J. (1957). The Determination of organic carbon in marine muds. ICES Journal of Marine Science, 22, 180–183.
Evrard, V., Huettel, M., Cook, P. L. M., Soetaert, K., Heip, C. H. R., & Middelburg, J. J. (2012). Importance of phytodetritus and microphytobenthos for heterotrophs in a shallow subtidal sandy sediment. Marine Ecology Progress Series, 455, 13–31.
First, M. R., & Hollibaugh, J. T. (2010). Environmental factors shaping microbial community structure in salt marsh sediments. Marine Ecology Progress Series, 399, 15–26.
Folk, R. L., & Ward, W. C. (1957). Brazos River bar [Texas]; A study in the significance of grain size parameters. Journal of Sedimentary Research, 27, 3–26.
Frihy, O. E. (2009). Morphodynamic implications for shoreline management of the western-Mediterranean sector of Egypt. Environmental Geology, 58, 1177–1189.
Frihy, O. E., & Deabes, E. (2012). Erosion chain reaction at El Alamein Resorts on the western Mediterranean coast of Egypt. Coastal Engineering, 69, 12–18.
Frihy, O. E., Dewidar, K. M., & El Raey, M. M. (1996). Evaluation of coastal problems at Alexandria, Egypt. Ocean & Coastal Management, 30, 281-295.
Gambi, M. C., & Dappiano, M. (2004). Mediterranean marine benthos: A manual of methods for its sampling and sudy. Società Italiana di Biologia Marina.
Gansfort, B., Fontaneto, D., & Zhai, M. (2020). Meiofauna as a model to test paradigms of ecological metacommunity theory. Hydrobiologia, 847, 2645–2663.
George, E. M. (2009). Egypt State of Environment Report. Egyptian Environmental Affaris Agency www.eeaa.gov.egeport. pp. 1–356.
Gheskiere, T., Hoste, E., Kotwicki, L., Degraer, S., Vanaverbeke, J., & Vincx, M. (2002). The sandy beach meiofauna and free-living nematodes from De Panne (Belgium). Bulletin de l’institut Royal des sciences Naturelles De Belgique, 72, 43–49.
Gheskiere, T., Vincx, M., Weslawski, J. M., Scapini, F., & Degraer, S. (2005). Meiofauna as descriptor of tourism-induced changes at sandy beaches. Marine Environment Research, 60, 245–265.
Giere, O. (2009). Meiobenthology: The microscopic motile fauna of aquatic sediments (2nd ed., pp. 1–537). Springer-Verlag Berlin Heidelberg.
Grémare, A., Amouroux, J.-M., Cauwet, G., Charles, F., Courties, C., De Bovée, F., Dinet, A., Devenon, J. L., De Madron, X. D., Ferre, B., Fraunie, P., Joux, F., Lantoine, F., Lebaron, P., Naudin, J.-J., Palanques, A., Pujo-Pay, M., & Zudaire, L. (2003). The effects of a strong winter storm on physical and biological variables at a shelf site in the Mediterranean. Oceanologica Acta, 26, 407–419.
Ha, S. Y., Min, W.-K., Kim, D.-S., & Shin, K.-H. (2014). Trophic importance of meiofauna to polychaetes in a seagrass (Zostera marina) bed as traced by stable isotopes. Journal of the Marine Biological Association of the United Kingdom, 94, 121–127.
Hamdan, A. M., El-Sayed, A. F., & Mahmoud, M. M. (2016). Effects of a novel marine probiotic, Lactobacillus plantarum AH 78, on growth performance and immune response of Nile tilapia (Oreochromis niloticus). Journal of Applied Microbiology, 120, 1061–1073.
Hamdy, R., & Ibrahim, H. (2019). Recent changes in polychaete community along the Alexandria coast, Egy. Egyptian Journal of Aquatic Biology & Fisheries, 23, 1–12.
Higgins, R. P., & Thiel, H. (1988). Introduction to the study of meiofauna. Smithsonian Institution Press.
Huys, R., Gee, J. M., Moore, C., & Hamond, R. (1996). Marine and brackish water harpacticoid copepods Part I. Synopses of the British Fauna (New Series) Book. In Kermack, D. M., Barnes, R. S .K. & Crothers (Eds.) (pp.1–352). London.
Iskander, M. M., Frihy, O. E., El Ansary, A. E., El Mooty, M. M., & Nagy, H. M. (2007). Beach impacts of shore-parallel breakwaters backing offshore submerged ridges, Western Mediterranean Coast of Egypt. Journal of Environmental Management, 85, 1109–1119.
Ismail, M. M., El Zokm, G. M., & El-Sayed, A. A. M. (2017). Variation in biochemical constituents and master elements in common seaweeds from Alexandria Coast, Egypt, with special reference to their antioxidant activity and potential food uses: prospective equations. Environmental Monitoring and Assessment, 189, 648.
Itoh, M., Kawamura, K., Kitahashi, T., Kojima, S., Katagiri, H., & Shimanaga, M. (2011). Bathymetric patterns of meiofaunal abundance and biomass associated with the Kuril and Ryukyu trenches, western North Pacific Ocean. Deep Sea Research Part I: Oceanographic Research Papers, 58, 86–97.
Jammo, K. M. T. (2004). Biodegredation of organic matter in the marine environment of Alexandria (Eastern Harbor) (pp. 1–303). Ph D Alexandria University.
Khairy, M. A., Kolb, M., Mostafa, A. R., El-Fiky, A., & Bahadir, M. (2012). Risk posed by chlorinated organic compounds in Abu Qir Bay, East Alexandria. Egypt. Environ Sci Pollut Res Int, 19, 794–811.
Korajkic, A., McMinn, B. R., & Harwood, V. J. (2018). Relationships between microbial indicators and pathogens in recreational water settings. International Journal of Environmental Research and Public Health 15.
Kotwicki, L., Szymelfenig, M., De Troch, M., Urban-Malinga, B., & Weslawski, J. M. (2005). Latitudinal biodiversity patterns of meiofauna from sandy littoral beaches. Biodiversity and Conservation, 14, 461–471.
Landres, P. B., Morgan, P., & Swanson, F. J. (1999). Overview of the use of natural variability concepts in managing ecological systems. Ecological Applications, 9, 1179–1188.
Lee, K., & Levy, E. M. (1991). Bioremediation: Waxy crude oils stranded on low-energy shorelines. International Oil spill conference Proceeding, 1991, 541–547.
Legendre, P., & Anderson, M. J. (1999). Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs, 69, 1–24.
Lo, H. S., Xu, X., Wong, C. Y., & Cheung, S. G. (2018). Comparisons of microplastic pollution between mudflats and sandy beaches in Hong Kong. Environmental Pollution, 236, 208–217.
Maria, T. F., Paiva, P., Vanreusel, A., & Esteves, A. M. (2013). The relationship between sandy beach nematodes and environmental characteristics in two Brazilian sandy beaches (Guanabara Bay, Rio de Janeiro). Annals of the Brazilian Academy of Sciences, 85, 257–270.
Maria, T. F., Vanaverbeke, J., Vanreusel, A., & Esteves, A. M. (2016). Sandy beaches: State of the art of nematode ecology. Anais da Academia Brasileira de Ciências, 88, 1635–1653.
McLachlan, A., & Defeo, O. (2017). The ecology of sandy shores. Academic Press.
McLachlan, A., Defeo, O., Jaramillo, E., & Short, A. D. (2013). Sandy beach conservation and recreation: Guidelines for optimising management strategies for multi-purpose use. Ocean & Coastal Management, 71, 256–268.
Milner, A. M., Collier, R. E. L., Roucoux, K. H., Müller, U. C., Pross, J., Kalaitzidis, S., Christanis, K., & Tzedakis, P. C. (2012). Enhanced seasonality of precipitation in the Mediterranean during the early part of the Last Interglacial. Geology, 40, 919–922.
Mitwally, H. (1999). Ecological and systematic studies of the interstitial fauna and benthic diatoms in the sandy beaches of Alexandria. Ph. D thesis, Faculty of Science, Alexandria University, 324.
Mitwally, H., & Abada, A. E. (2008). Spatial variability of meiofauna and macrofauna in a Mediterranean protected area, Burullus Lake. Egypt. Meiofauna Marina, 16, 185–200.
Mitwally, H., Montagna, P., Halim, Y., Khalil, A., Dorgham, M., & Atta, M. (2004). Egyptian sandy beach meiofauna and benthic diatoms. Rapp Comm Int Mer Medit, 37, 537.
Moens, T., Braeckman, U., Derycke, S., Fonseca, G., Gallucci, F., Gingold, R., Guilini, K., Ingels, J., Leduc, D., Vanaverbeke, J., Van Colen, C., Vanreusel, A., & Vincx, M. (2013). Ecology of free-living marine nematodes. Handbook of Zoology.
Montagna, P. A. (1984). In situ measurement of meiobenthic grazing rates on sediment bacteria and edaphic diatoms. Marine Ecology - Progress Series, 18, 119–130.
Montagna, P. A., Bauer, J. E., Hardin, D., & B, R., . (1989). Vertical distribution of microbial and meiofaunal populations in sediments of a natural coastal hydrocarbon seep. Journal of Marine Research, 47, 657–680.
Montagna, P. A., Bauer, J. E., Toal, J., Hardin, D., & Spies, R. B. (1987). Temporal variability and the relationship between benthic meiofaunal and microbial populations of a natural coastal petroleum seep. Journal of Marine Research, 45, 761–789.
Moreno, M., Ferrero, T. J., Granelli, V., Marin, V., Albertelli, G., & Fabiano, M. (2006). Across shore variability and trophodynamic features of meiofauna in a microtidal beach of the NW Mediterranean. Estuarine, Coastal and Shelf Science, 66, 357–367.
Nafaa, M. G., & Frihy, O. E. (1993). Beach and nearshore features along the dissipative coastline of the nile delta. Egypt Journal of Coastal Research, 9, 423.
Nordstrom, K. F. (2004). Beaches and dunes of developed coasts. Cambridge University Press.
Olausson, E. (1975). Methods for the chemical analysis of sediments. FAO Fisheries Technical Papers (FAO).
Ortega Cisneros, K., Smit, A. J., Laudien, J., & Schoeman, D. S. (2011). Complex, dynamic combination of physical, chemical and nutritional variables controls spatio-temporal variation of sandy beach community structure. PLoS One, 6, e23724.
Papageorgiou, N., Moreno, M., Marin, V., Baiardo, S., Arvanitidis, C., Fabiano, M., & Eleftheriou, A. (2007). Interrelationships of bacteria, meiofauna and macrofauna in a Mediterranean sedimentary beach (Maremma Park, NW Italy). Helgoland Marine Research, 61, 31–42.
Pascal, P.-Y., Dupuy, C., Mallet, C., Richard, P., & Niquil, N. (2008). Bacterivory by benthic organisms in sediment: Quantification using15N-enriched bacteria. Journal of Experimental Marine Biology and Ecology, 355, 18–26.
Pascal, P.-Y., Dupuy, C., Richard, P., Mallet, C., du Chatelet, E. A., & Niquilb, N. (2009). Seasonal variation in consumption of benthic bacteria by meio- and macrofauna in an intertidal mudflat. Limnology and Oceanography, 54, 1048–1059.
Pereira, T. J., Gingold, R., Villegas, A. D. M., & Rocha-Olivares, A. (2017). Patterns of spatial variation of meiofauna in sandy beaches of northwestern Mexico with contrasting levels of disturbance. Thalassas: An International Journal of Marine Sciences, 34, 53–63.
Power, D. M. (1999). Recovery in aquatic ecosystems: An overview of knowledge and needs. Journal of Aquatic Ecosystem Stress and Recovery, 6, 253–257.
Pusceddu, A., Gambi, C., Manini, E., & Danovaro, R. (2007). Trophic state, ecosystem efficiency and biodiversity of transitional aquatic ecosystems: Analysis of environmental quality based on different benthic indicators. Chemistry and Ecology, 23, 505–515.
Ranta, E., Kaitala, V., & Lundberg, P. (1998). Population variability in space and time: the dynamics of synchronous population fluctuations. Oikos, 83, 376–382.
Reiss, H., & Kroncke, I. (2005). Seasonal variability of benthic indices: An approach to test the applicability of different indices for ecosystem quality assessment. Marine Pollution Bulletin, 50, 1490–1499.
Riera, R., Núñez, J., Carmen Brito, M. D., & Tuya, F. (2011). Temporal variability of a s ubtropical intertidal meiofaunal ass emblage: Contrasting effects at the species and assemblage-level. Vie et milieu - Life and Environment, 61, 129–137.
Rodrı́guez, J.G., Lastra, M., López, J., . (2003). Meiofauna distribution along a gradient of sandy beaches in northern Spain. Estuarine, Coastal and Shelf Science, 58, 63–69.
Santos, G. H. C., Cardoso, R. S., & Maria, T. F. (2019). Bioindicators or sediment relationships: Evaluating ecological responses from sandy beach nematodes. Estuarine, Coastal and Shelf Science, 224, 217–227.
Schlacher, T. A., Jones, A. R., Dugan, J. E., Weston, M. A., Harris, L., Schoeman, D. S., Hubbard, D. M., Scapini, F., Nel, R., & Lastra, M. (2014). Open-coast sandy beaches and coastal dunes. Coastal Conservation, 19, 37–92.
Schlacher, T. A., Schoeman, D. S., Dugan, J., Lastra, M., Jones, A., Scapini, F., & McLachlan, A. (2008). Sandy beach ecosystems: Key features, sampling issues, management challenges and climate change impact. Marine Ecology. ISSN, 0173–9565(29), 70–90.
Schratzberger, M., Lampadariou, N., Somerfield, P. J., Vandepitte, L., & Vanden Berghe, E. (2009). The impact of seabed disturbance on nematode communities: Linking field and laboratory observations. Marine Biology, 156, 709–724.
Semprucci, F., Colantoni, P., Sbrocca, C., Baldelli, G., Rocchi, M., & Balsamo, M. (2011). Meiofauna in sandy back-reef platforms differently exposed to the monsoons in the Maldives (Indian Ocean). Journal of Marine Systems, 87, 208–215.
Semprucci, F., Frontalini, F., Sbrocca, C., du Chatelet, E. A., Bout-Roumazeilles, V., Coccioni, R., & Balsamo, M. (2015). Meiobenthos and free-living nematodes as tools for biomonitoring environments affected by riverine impact. Environment Monitoring and Assessment, 187, 187–251.
Sevastou, K., Lampadariou, N., & Eleftheriou, A. (2011). Meiobenthic diversity in space and time: The case of harpacticoid copepods in two Mediterranean microtidal sandy beaches. Journal of Sea Research, 66, 205–214.
Shreadah, A. M., Abdel-Mohsen M, El-Sayed, A., Mohamed A, A., & Hamam A. R. H. (2019). Evaluation of Different Anthropogenic Effluents Impacts on the Water Quality Using Principal Component Analysis: A Case Study of Abu-Qir Bay-Alexandria-Egypt. International Journal of Environmental Monitoring and Analysis, 7, 56.
Shreadah, M., Masoud, M., Khattab, A., & El Zokm, G. (2014). Impacts of different drains on the seawater quality of El-Mex bay (Alexandria, Egypt). Journal of Ecology and The Natural Environment, 6, 287–303.
Smith, E. P. (2019). Ending reliance on statistical significance will improve environmental inference and communication. Estuaries and Coasts, 43, 1–6.
Strickland, J. D. H., & Parsons, T. R. (1972). A practical handbook of seawater analysis. The Journal of the Fisheries Research Board of Canada Ottawa, Ont'ario (pp. 310) Canada.
Sun, X., Zhou, H., Hua, E., Xu, S., Cong, B., & Zhang, Z. (2014). Meiofauna and its sedimentary environment as an integrated indication of anthropogenic disturbance to sandy beach ecosystems. Marine Pollution Bulletin, 88, 260–267.
Swanson, F. J., Jones, J. A., Wallin, D. O., & Cissel, J. H. (1994). Natural variability-implications for ecosystem management. ecosystem management: Principles and applications. Jensen and Bourgeron (editors), II, 80–90.
Szymelfenig, M., Kotwicki, L., & Graca, B. (2006). Benthic re-colonization in post-dredging pits in the Puck Bay (Southern Baltic Sea). Estuarine, Coastal and Shelf Science, 68, 489–498.
Tolhurst, T. J., Defew, E. C., & Dye, A. (2010). Lack of correlation between surface macrofauna, meiofauna, erosion threshold and biogeochemical properties of sediments within an intertidal mudflat and mangrove forest. Hydrobiologia, 652, 1–13.
Torres-Bejarano, F., González-Márquez, L. C., Díaz-Solano, B., Torregroza-Espinosa, A. C., & Cantero-Rodelo, R. (2016). Effects of beach tourists on bathing water and sand quality at Puerto Velero, Colombia. Environment, Development and Sustainability, 20, 255–269.
Traunspurger, W., & Majdi, N. (2017). Meiofauna. Methods in stream. Ecology, 3, 273–295.
Urban-Malinga, B., Kotwicki, L., Gheskiere, T. A., Jankowska, K., Opalinski, K., & Malinga, M. (2004). Composition and distribution of meiofauna, including nematode genera, in two contrasting Arctic beaches. Polar Biology 27.
Zaki, H., Goma, R., Tadros, A., & Mahmoud, M. (2009). Environmental parameters of Alexnadria Inshore North western Coastall Area, Alexandria. World Applied science, 7, 715–725.
Zeppilli, D., Sarrazin, J., Leduc, D., Arbizu, P. M., Fontaneto, D., Fontanier, C., Gooday, A. J., Kristensen, R. M., Ivanenko, V. N., Sørensen, M. V., Vanreusel, A., Thébault, J., Mea, M., Allio, N., Andro, T., Arvigo, A., Castrec, J., Danielo, M., Foulon, V., … Fernandes, D. (2015). Is the meiofauna a good indicator for climate change and anthropogenic impacts? Marine Biodiversity, 45, 505–535.
Acknowledgements
The authors deeply thank Mr. A.S. Qamara for collecting and sorting samples. The first author is grateful to Prof Dr. John W Fleeger, Louisiana State University, USA, for reading the manuscript and for his valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights for review
Meiofaunal Natural variability is a good indicator of beach ruin.
Stochastic distribution, winter storms, and touristic activities are the fundamental causes of the large fraction of the unexplained variation in meiofaunal communities.
The beach that experiences the synergetic effect of natural forces and human-made activities has a worse ecological state.
Human-made disturbances; mechanical engineering, pollution, tourism activities, and the natural forces; winter storms, high energy, and rip currents are essential contributors for management decision-makers.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mitwally, H.M., Hamdan, A.M. Environmental drivers of meiofaunal natural variability, Egypt, Southeastern Mediterranean. Environ Monit Assess 193, 185 (2021). https://doi.org/10.1007/s10661-021-08927-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-08927-0