Abstract
Ecological assessment was performed in different thermal springs located in the Ethiopian Rift Valley area: Gergedi, Sodere, Halaba, and Gara. We assessed the habitat conditions, physicochemical characteristics, and macroinvertebrate composition at 12 sampling sites in four thermal spring systems. The physicochemical properties of the water samples varied among the sampling stations. Temperature ranged from 38.5 to 90°C among the 12 sites. Dissolved oxygen, which is essential to aquatic ecosystem normal functioning, was not detected at three sampling sites. An average of 109 macroinvertebrates were identified in three sampling campaigns. Relatively higher macroinvertebrate abundance 94 (86.2%) was registered in Gergedi than any other thermal spring sites. The macroinvertebrate abundance was 9 (8.3%) and 6 (5.5%) for the Gara and Sodere thermal springs. No macroinvertebrate communities were observed in all sites of Halaba thermal spring. From all macroinvertebrate groups, 82 (75.2%) were dipterans, 20 (18.4%) Oligochaeta, 5 (4.6%) Gastropoda, and the rest Hemipterans and Coleopterans, which comprises 2 (1.8%) individuals. Chironomidae was the dominant invertebrate taxa at all sites, and when the water temperature exceeds 42°C, it becomes the only taxon in the thermal springs. Macroinvertebrates were absent in thermal springs where the water temperature is 52°C or higher. Other diversity measures were not sensitive enough to discriminate sampling sites regardless of physicochemical variabilities except the richness and abundance. Water temperature, chloride, pH, and phosphate were identified as major determinants of macroinvertebrate richness in the Ethiopian Rift Valley region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ethiopia has thermal springs scattered in different parts of the country. They are used for different purposes. Thermal springs and thermal pools are generally used for swimming, bathing, cooking, and healing the body and soul (Mazibuko & Mwendera, 2006). However, they have been significantly impacted by different social and development activities, attracting numerous human visitors who are frequently interested in bathing in thermal springs. Various anthropogenic activities around thermal spring areas cause species reduction, extinction, and habitat loss (Holt, 2007). Thermal spring ecosystems are very susceptible to anthropogenic activities because they provide specific services and are easily altered since they are small and isolated. In Ethiopia, many people believe that water from thermal springs can relieve several diseases and is considered the cleanest and economical to treat a disease (authors’ observation). The water is thus consumed in excess and used as a thermal bath. However, the water may cause acute infectious diarrhea and chronic diarrhea, which can arise from the excess mineral concentration found in the thermal spring water (Haki & Gezmu, 2012).
Thermal gradients in aquatic ecosystems can be formed either by natural processes, such as geothermal activity, or by human activities, for instance, the release of heat wastewater from electric power–generating plants or climate change (Lamberti & Resh, 1985; Burgmer et al., 2006). Generally, thermal springs are defined as springs where water temperature lies significantly above the mean of the annual air temperature of that region (Thompson, 2003). Water becomes heated when it percolates down through rocks in the earth’s crust or volcanic areas when the water encounters thermal volcanic magma. Thermal springs are a habitat for very simple and unique species (Holt, 2007). Hence, they can be considered as unique ecosystems that harbor different aquatic biota, including macroinvertebrates. Macroinvertebrates are found in almost every type of aquatic habitat, such as thermal spring, stream, river, and other stagnant water bodies. They are an important indicator of the system’s water quality and habitat condition as they respond to anthropogenic and natural environmental variabilities. Aquatic temperature was reported among the variables that govern macroinvertebrates communities; an elevated aquatic temperature increases metabolic costs (Glazier, 2012), resulting in reduced diversity and abundance.
A study done by Lamberti and Resh (1985) indicated that thermal springs with a water temperature above 45°C did harbor macroinvertebrate communities. In contrast, macroinvertebrates were found in thermal springs with temperatures between 52 and 58°C (De Jong et al., 2005).
Water quality can be described in terms of physicochemical properties and biological characteristics, such as the use of macroinvertebrate indicators (United Nations and World Health Organization, 1996). Physicochemical factors of the ecosystem are among the strongest determinants of the community structure of benthic macroinvertebrates at different locations (Buss & Borges, 2008). Moreover, aquatic organisms are sensitive in varying degrees to the physical and chemical characteristics of their aquatic environment, such as water conductivity, temperature, dissolved oxygen concentration, turbidity, pH, nutrient, and chemical composition (Mochan & Mrazik 2000; Graça et al., 2004a; Holt, 2007; Masese et al., 2009; Getachew et al., 2012; Klerk & Wepener, 2013). By measuring those parameters, it is possible to discover sites that reduce, stress, and control the aquatic life (Mochan & Maraazki, 2000). Most aquatic organisms’ diversity depends on a specific range of temperature to maintain optimal health (Brock, 1967). Hence, measuring species diversity in different habitat is a valuable tool for assessing ecosystem health (Resh, 1979). Macroinvertebrate communities and their diversity are an indicator of habitat condition, and they are highly investigated in lakes, rivers, and different marshlands in many parts of the world. Still, they are rarely used in the assessment of thermal spring water quality and habitat conditions.
Biomonitoring with macroinvertebrates among different aquatic ecosystems has advanced in Ethiopia (Ambelu, 2009; Ambelu et al., 2010; Mereta et al., 2012; Ambelu et al., 2013). However, the ecological status of thermal springs in Ethiopia, except recently assessed few thermal springs in Ethiopia’s highlands (Derso et al., 2015), or somewhere else in Africa is not investigated. Overall, studies focused on thermal springs are rare and there is no information about macroinvertebrate assemblages in the thermal springs of Ethiopian Rift Valley regions. Therefore, to determine the macroinvertebrate assemblages of the thermal springs with an emphasis on the relationship between their diversity and the physicochemical characteristics of the water, this study investigated four thermal springs in the Ethiopian Rift Valley region.
Materials and methods
Study area
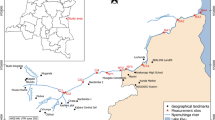
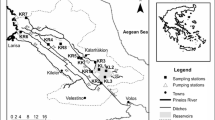
The study was conducted in thermal springs of the Ethiopian Rift Valley region (Fig. 1). The rift system is known for many thermal springs used for recreational and therapeutic purposes (Haki & Gezmu, 2012). This study assessed four thermal springs: Gergedi, Sodere, Halaba, and Gara. Most of these hot springs are considered holy waters and are believed to alleviate different Ethiopian communities’ health problems. These thermal springs are prone to various anthropogenic activities on which the most common ones are bathing, swimming, and washing clothes.
Sodere thermal spring is located in the central part of the Ethiopian rift system and 125 km to the East of Addis Ababa. This thermal spring is located at 8° 24′ 9″ North and 39° 23′ 35″ East at an altitude of 1359 m above sea level (m a.s.l.). Grasses and shrubs surround the spring. As a result, grazing activities are common. The spring is the major water source for Sodere resort, and there is a mixing of water from the swimming pool and bathing areas to downstream of the thermal spring. The thermal spring joins river Awash at about 500 m downstream from the spring’s point of discharge. Coarse sand and bedrock are the dominant substrate materials of Sodere thermal spring.
Halaba thermal spring is located in the south part of Ethiopian rift system at 317 km south of Addis Ababa. It is located at 06° 59′ 36″ North and 38° 31′ 14″ East at an altitude of 1781 m a.s.l. This spring is artesian, spraying thermally heated subsurface water into the air at 3-min intervals. Every time we visited the spring for sampling purposes, people from the surrounding area were observed taking a natural steam bath that most mentioned to get relieved from their ailment. At about 520 m downstream from the point of discharge, the water enters into a locally protected marshland. Agricultural and grazing activities are other common anthropogenic activities observed around the spring. Meanwhile, bedrock bottom, firm, and fine sand are the dominant substrate materials of Halaba thermal spring.
Gergedi thermal spring is located in the central part of the Ethiopian Rift System which is 120 km far from Addis Ababa. The spring is located at 39° 23′ 23″ East and 8° 24′ 9″ North at an altitude of 1702 m a.s.l. It is characterized by mixtures of two main substrate materials, with medium cobble-sized and gravel substrate. The spring flows for about 600 m downstream and joins river Awash. Agriculture is the most common activity near the spring area.
Gara thermal spring is located in the south part of the Ethiopian rift system, 286 km far from the south of Addis Ababa. The spring is located within 38° 01′ 14″ East and 7° 17′ 47″ North at an altitude of 1776 m a.s.l. Gara thermal spring substrate materials are dominated by firm and fine sand and little submerged vegetation. The thermal spring is highly covered with vegetation located downhill of the nearby Gara hill. The spring extends up to 500 m from the spot and enters the nearby forest, and it becomes inaccessible for macroinvertebrates sampling.
Data collection
A total of 36 triplicate samples were collected from the 12 study sites in three sampling campaigns. Samples were taken at 2-month intervals during the dry season. The study sites were selected a priori based on the criteria of accessibility, geographical distribution, and existing variations of natural and anthropogenic activities. The collected data were categorized into three parts: (a) physicochemical data, (b) macroinvertebrate data, and (c) physical habitat (physiographic and visual rabid bioassessment protocol (RBP)) data (e.g., water depth, water width, spring bed type, vegetation cover).
Physicochemical and habitat data
Physicochemical parameters like temperature, electrical conductivity, pH, and dissolved oxygen concentration were measured onsite at each sampling location using standard HACH® Multi meter hand-held probe (model HQ40D). Water samples collected from all sampling sites were analyzed for fluoride, chloride, sulfate, phosphate, and nitrate in the laboratory according to standard methods (American Public Health Association et al., 2005).
The water width, water depth, and flow velocity were assessed according to Ambelu (2009). The riparian vegetation, sinuosity, spring bank status, and embeddedness were estimated using the US-EPA habitat assessment protocol (Barbour et al., 1999). Human impact assessment was made following the Maine Department of Environmental Protection method (MDEP, 2008).
Macroinvertebrate data
Macroinvertebrates were collected using the kick-sampling technique, consisting of a D-frame net having a mesh size of 300-µm diameter (Ambelu et al., 2010). Kick sampling was performed along a 10-m stretch of the spring for 5 min, including all the microhabitats within the sampling reach (IBN, 1984). During sampling, the riverbed (spring bed) was thoroughly disturbed by kicking with the feet to dislodge the macroinvertebrates from the substrate. All substrates in the sampling reach were thoroughly checked to capture organisms. Within the 5 min of kick sampling, all areas of pool, riffle, edge, and center were sampled. After sampling, macroinvertebrates were sorted alive onsite and preserved in 70% ethanol. The benthic macroinvertebrates were identified up to their family level at the Jimma University Department of Environmental Health Sciences & Technology laboratory unit using standard systematic keys (McCafferty, 1983; Bouchard, 2004; Oscoz et al., 2011). Another expert cross-checked the identification process to maintain the quality of the data set.
Statistical analysis
Physicochemical and macroinvertebrate data were log (x+1) and chi-square transformed for statistical analyses to meet the assumption of normality. Spearman’s rank-order correlation test was performed between the macroinvertebrate indices and physicochemical parameters.
Different diversity and biotic indices were calculated to identify the sensitive index suitable to characterize anthropogenic alteration of thermal springs in the Rift Valley region. The Simpson diversity index (1−D), Shannon diversity index (H′), Shannon evenness (J′), and abundance were calculated (Magurran, 2013). Margalef index of richness (d) and family-level taxa richness (in terms of the number of families) were also calculated (Gamito, 2010). Among biotic indices, Hilsenhoff family-level biotic index (FBI) (Hilsenhoff, 1988), biological monitoring working party (BMWP) score (Bartram & Balance, 1996), and South African scoring system (SASS) (Dallas, 2007) were computed.
Hierarchical cluster analysis using Wards algorithms was performed in PAST software version 2. Multiple regression analysis was conducted using Statistica (STATSOFT, 2007) to identify variables affecting macroinvertebrate communities’ diversity. A stepwise forward selection method was performed to select the best environmental predictors for family-level richness. The Statistica automatically calculated the standard errors for the regression coefficients, and it uses traditional multiple leaner regression method based on error mean square and variance covariance matrix of the predictors.
Results
Physicochemical characteristics
Physicochemical characteristics of the springs varied widely among sampling sites (Table 1). Minimum water temperature was recorded at Gergedi3, 38.5°C, while the maximum was at Halaba1, which was 90°C. Dissolved oxygen ranged from 0 to 6.28 mg/L. The highest electrical conductivity (EC) was measured at Sodere1, which is 3060 µS/cm. Relatively, water samples from Geregedi had the lowest EC value, which is about 933 µS/cm. The pH ranged from 5.0 to 8.5. However, most of the thermal springs had pH value in the acidic range.
Based on the habitat assessment, all sampling sites had marginal habitat conditions, except for Halaba2 and Halaba3 sites with poor habitat. All sampling reaches in the thermal springs are under the moderate human impact. The habitat condition and human impact scores corresponding to each sampling site are given in Table 2.
Macroinvertebrate composition and environmental condition
A total of 109 macroinvertebrates were identified from the thermal springs. Collected macroinvertebrates belonged to five orders and six families. Dipterans were the most dominant macroinvertebrate communities, constituting 82 individuals (75.2%), followed by Oligochaeta (18.4%). The Gastropoda were represented by five individuals (4.6%). Hemipteran and Coleopteran groups were the least among the total macroinvertebrate communities that constituted only 0.92%. The highest abundance of macroinvertebrate communities recorded at Gergedi thermal spring (94[86.2%]) compared with Gara (9 [8.3%]) and Sodere (6[5.5%]). On the contrary, macroinvertebrates were absent at all sites of Halaba and Sodere1.
Among the macroinvertebrate families, chironomids were the most abundant dipteran group, which were about 81 (74.3%) individuals, followed by Oligochaeta with 20 (18.4%) and Physidae 5 (4.6%) individuals. Other macroinvertebrate groups such as Dytisidae, Coroxidae, and Tipulidae represented 1 (0.92%) individuals. Chironomids were recorded in all sampling sites except at Halaba and Sodere sites, where the water temperature is elevated. The other very common macroinvertebrate group in this study was Oligochaeta, found in four different sampling sites (Gergedi1–3 and Gara2).
The macroinvertebrate distribution at each site was largely dictated by water temperature (Fig. 2). However, the macroinvertebrate diversity at the two sites (Gergedi 2 and 3) which have relatively lower water temperature (<40.5°C; Table 1), was mainly affected by anthropogenic activities such as minimal vegetation covers where only two taxa colonized them. Only one thermal spring site (Gergedi 1) had the maximum number of taxa. The number of taxa (richness) and the number of individuals (abundance) fall below 3 and 15, respectively, at sites where the temperature exceeds 40.5°C. Chironomidae dominates sampling sites with a water temperature greater than 42°C.
We calculated different diversity indices for each site and Gergedi1 scored the highest (1.375) Shannon index. On the contrary, Sodere2, Gara1, and Gara3 had zero value of this index. Diversity and biotic indices were not calculated for Sodere1 and Halaba1–3 sites as there were no macroinvertebrate communities. A higher Simpson index of diversity (1.0) was calculated for Sodere2, followed by Gergedi1 (0.758) and Gara2 (0.667). Relatively, Geregedi1 had the highest biotic index value than all sampling sites. Most study sites (Sodere2, Gergedi2, 3, Gara1, 2, and 3) have a similar FBI score of 8 (Table 3). Comparatively, Geregdi sampling sites had the highest value for most biotic indices, while Gara thermal spring had the lowest. Spearman correlation test indicated that temperature was negatively correlated with all indices, while dissolved oxygen and habitat condition score (HCS) were positively correlated (Table 4).
Among the significant multiple regression predictors (p-value < 0.05), temperature and phosphate showed a negative correlation, while pH, chloride, and altitude have a positive relationship to taxa richness. In addition, EC showed negative correlation with macroinvertebrates abundance (r = −0.70, p < 0.05) and richness (r = −0.63, p < 0.05) (Table 5). The predicted and observed values of the regression model showed a strong significant correlation (R2 = 0.936, p-value < 0.00109), and only a few data points deviated from the 95% confidence interval (Fig. 3).
Temperature, pH, chloride, phosphate, and altitude were selected as the best environmental predictors for family-level richness
Diversity and biotic indices were not calculated for Sodere1 and Halaba1-3 sites as there were no macroinvertebrate communities.
Discussion
This study has highlighted the ecological status of thermal springs in Ethiopia’s Rift Valley region which could help to indicate the major ecological threats in such unique ecosystems. The response of macroinvertebrate communities reflected the different environmental variabilities in the Rift Valley thermal springs. Our study found that macroinvertebrates in the Rift Valley thermal springs could live in water with a ≤ 51.9°C temperature. This is in disagreement with Lamberti and Resh (1985) findings and Herbst and Sada (2001). They have concluded that macroinvertebrates cannot survive in a water body with a temperature of greater than 45°C. However, it is in agreement with the findings of De Jong et al. (2005) and Glazier (2012), which indicated macroinvertebrates in a water body with elevated temperature even above 52°C. The parallel study done in Ethiopia’s highland thermal springs (Derso et al., 2015) also indicated that macroinvertebrate communities could exist having water temperature up to 52°C.
The Ethiopian Rift Valley region’s thermal springs have no sensitive taxa group such as Ephemeroptera, Plecoptera, and Trichoptera (EPT); instead, they were dominated by the dipterans and coleopteran groups. Similar findings were reported from a study done on the geothermal streams of New Zealand (Duggan et al., 2007). Research done in the Eastern Amhara region of Ethiopia’s thermal springs also indicates that dipterans were the most dominant macroinvertebrate communities (Derso et al., 2015). In our study, Chironomidae was found to be living in all sampling sites except Sodere1 and 2 and all sites in Halaba thermal spring where the environmental condition is harsher than the rest of the sites, which is most likely due to the elevated water temperature. This indicates that even tolerant macroinvertebrate taxa such as Chironomidae could be difficult to find in the aquatic environment where there are harsh environmental stressors such as a water temperature that exceeds 51°C. In a similar study, Chironomidae were absent in thermal springs with water temperature above 41°C (James, 1985). Hence, water temperature is a vital environmental parameter that determines the macroinvertebrate richness in the thermal spring discharges of the Rift Valley region of Ethiopia. It limits the diversity of aquatic species due to high temperature, deteriorated water quality, and loss of energy due to increased metabolic demand (Glazier, 2012).
Dissolved oxygen was not detected at three sampling sites (Sodere1 and Halaba1 and 2), which may be most probably due to the elevated water temperature (>55°C) and high EC (>1400 µS/cm) that could enhance the liberations of oxygen (Bunn & Davies, 1992; Homma & Tsukahara, 2008).
The highest EC (3060 µS/cm) was recorded at the first sampling sites of Sodere, which might be due to the elevated water temperature that increases the solubility of minerals from the surrounding earth material (Haki & Gezmu, 2012) and the presence of active magma around the springs. In addition to the water temperature, elevated EC value in the present study might be one of the major stressors to macroinvertebrate communities (Heishman & Mcluky, 2011). Further, Heishman and Mcluky (2011) showed a strong positive correlation between very high EC and the absence of aquatic macroinvertebrate community. Meanwhile, the highest discharge (1.38–1.74 m3/s) were recorded at Gergedi sampling sites (Table 1). These sampling sites were responsible for the 86.2% abundance of macroinvertebrate communities in the present study, which indicates the significant role of discharges in the macroinvertebrate assemblages in the thermal springs. This was further confirmed by the significant and positive correlations between discharge and different indices (Table 4).
Biotic indices such as FBI, BMWP, SASS, and ASPT could not discriminate the sites. Only one site was categorized differently by FBI and BMWP; otherwise, all sites were classified as similar. This might be due to the absence of diverse invertebrate organisms found at each site. This suggests that monitoring the ecological status using macroinvertebrate in a harsher environment (like thermal springs in the Rift Valley region) may not be appropriate. However, genus- or species-level biotic indices might be appropriate and should be tested in future studies. In a study conducted in a third-order stream, O'Leary et al. (2004) reported the association between the genus-level and family-level metrics of the same macroinvertebrate samples. In the comparisons, the genus-level taxonomy analysis showed more tolerant of pollution than the family-level analysis. In addition, the index of biological integrity (IBI) was more sensitive for the change in metrics at the genus level compared with the family level of taxonomical analysis.
In the present study, Simpson and Shannon diversity indices were not significantly linked with the water quality parameters such as the water temperature and dissolved oxygen. A Shannon diversity index below 1 could indicates the unsuitability of the habitat for biota, resulting in less diversity. Generally, values greater than 3 may indicate the suitability of the macroinvertebrate habitat and enables them to be divers. Based on this criterion, only the first sampling site of Gergedi exceeds 1.5 of Shannon diversity index value. All remaining sites were below 1.0 (Table 6).
Overall, the diversity indices like Shannon and Simpson indices may not be appropriate due to depauperate macroinvertebrate communities (De Jong et al., 2005). Among the water quality variables, a higher concentration (0.89 mg/L) of phosphate was recorded at Gara3, which is above the guideline (0.05 mg/L) set by Australian and New Zealand for fresh water quality (ANZECC, 2000). Except the Gergedi1 sampling site, the rest sites had a phosphate concentration above the guideline value. The elevated phosphate concentration in the study area might be related to agricultural activities mainly from fertilizer usage on farmlands near the thermal spring. In addition, the usage of detergents and soap for bathing and washing clothes in the thermal spring could also be the reason for elevated phosphate levels. Further, the higher phosphate concentration indicates the presence of high anthropogenic inputs to the springs because naturally phosphate concentration should not exceed 0.05 mg/L.
Moreover, the present study showed a very high concentration of fluoride in all sampling points. The maximum concentration (22.2 mg/L) was found at Halaba1, and the minimum (4.03 mg/L) at Soder3; both of them are above the guideline value of 1.5 mg/L set by (WHO, 2008). The fluoride content of most sampling sites could have health risks, including skeletal and dental fluorosis, to those who had prolonged exposure to such level of fluoride (Kloos & Haimanot, 1999). A possible reason for higher fluoride of the studied site might be associated with soil texture, underground water source, and direct contact of water with the nearby rock (Kundu et al., 2001). However, a relationship between fluoride concentration gradient and macroinvertebrates assemblage was not observed.
In general, the thermal spring in the Rift Valley area could be grouped into three based on the water quality and macroinvertebrates diversity. Hierarchical cluster analyses made using physicochemical, and macroinvertebrate communities (Fig. 4) of the different sampling sites showed that sites were grouped into three major clusters. The first cluster consists of the three sites from Gergedi, which is characterized by relatively low water temperature (<42°C), better water quality, and invertebrates richness and abundance. In comparison, the second cluster consisted of four sites (Sodere1 and Halaba 1–3) characterized by elevated temperature, which most probably has liberated the DO followed by the absence of macroinvertebrate communities. The third cluster consists of five sites (Sodere2, Sodere3, Gara1, Gara2, and Gara3) with water temperature ranging from 42 to 45°C, characterized by low DO level and low macroinvertebrate abundance. In all three groups, temperature is the major stressor that affects water quality and macroinvertebrate diversity in the region. In fact, Graça et al. (2004b) found that water temperature was one of the most determinant factors in determining the richness and abundance of macroinvertebrates.
Dendrogram of sampling sites based on physicochemical and macroinvertebrate communities (Ward clustering method using Euclidian distance). Full names of abbreviated sampling sites are given in Table 2
Conclusion
Macroinvertebrates that can withstand elevated temperature such as Chironomidae were the most abundant invertebrate group found in the thermal springs with elevated water temperature reaching nearly 52°C. Biotic indices (BMWP, ASPT, and FBI) were relatively better in discriminating sampling sites than the diversity measures. Among diversity indices, richness and abundance were relatively sensitive to differentiate the sites having varying water quality. Macroinvertebrates with lowest taxonomic level (genus and species) could be more convenient to be used as a biomonitoring tool for ecosystems under harsh environmental conditions as sometimes, one family could be found. Therefore, to elucidate more on the ecology of studied thermal springs, studies based on lowest taxonomic resolutions should be promoted. Overall, this study provides important insight into the relevance of macroinvertebrate communities as a water quality monitoring tool in thermal springs and suggests further investigations on this unique ecosystem.
References
Ambelu, A. (2009). Biological monitoring based on macroinvertebrates for decision support of water management in Ethiopia. Ghent University
Ambelu, A., Lock, K., & Goethals, P. (2010). Comparison of modelling techniques to predict macroinvertebrate community composition in rivers of Ethiopia. Ecol Inform, 5, 147–152. https://doi.org/10.1016/j.ecoinf.2009.12.004.
Ambelu, A., Lock, K., & Goethals, P. L. M. (2013). Hydrological and anthropogenic influence in the Gilgel Gibe I reservoir (Ethiopia) on macroinvertebrate assemblages. Lake Reserv Manag, 29, 143–150. https://doi.org/10.1080/10402381.2013.806971.
American Public Health Association, American Water Works Association, Water Environment Federation. (2005). Standard methods for the examination of water & wastewater. Washington, D.C.: American Public Health Association.
ANZECC (Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand) (2000) National Water Quality Management Strategy, Document 4—Australian and New Zealand Guidelines for Fresh and Marine Water Quality. ANZECC, Canberra, Australia. http://www.environment.gov.au/water/publications/quality/nwqms-guidelines-4-vol3.html. Accessed 10 July 2018.html. Accessed 10 July 2018
Bartram, J., & Ballance, R. (1996). Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programmes. CRC Press.
Barbour, M. T., Gerritsen, J., Snyder, B. D., & Stribling, J. B. (1999). Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish (2nd ed.). Washington, D.C.: United States Environmental Protection Agency.
Bouchard, J. R. W. (2004). Guide to aquatic invertebrates of the Upper Midwest. University Of Minnesota
Brock, T. D. (1967). Life at high temperatures: Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science, 158, 1012–1019. https://doi.org/10.1126/science.158.3804.1012.
Bunn, S. E., & Davies, P. M. (1992). Community structure of macroinvertebrates fauna and water quality of saline river system in South west Australia. Hdrobiologia, 148, 143–160.
Burgmer, T., Hillebrand, H., & Pfenninger, M. (2006). Effects of climate-driven temperature changes on the diversity of freshwater macroinvertebrates. Oecologia, 151, 93–103. https://doi.org/10.1007/s00442-006-0542-9.
Buss, D. F., & Borges, E. L. (2008). Application of rapid bioassessment protocols (RBP) for benthic macroinvertebrates in Brazil: Comparison between sampling techniques and mesh sizes. Neotrop Entomol, 37, 288–295. https://doi.org/10.1590/S1519-566X2008000300007.
Dallas, H. F. (2007). River health programme: South African scoring system (SASS) data interpretation guidelines. Report produced for the Department of Water Affairs and Forestry (Resource Quality Services) and the Institute of Natural Resources.
De Jong, G. D., Canton, S. P., & Chadwick, J. W. (2005). Macroinvertebrates occurring in Sunbeam Hot Springs, an absolutely hot spring in Idaho, USA. J Freshw Ecol, 20, 611–613. https://doi.org/10.1080/02705060.2005.9664779.
Derso, S., Beyene, A., Melaku, G., & Ambelu, A. (2015). Ecological status of hot springs in Eastern Amhara Region: macroinvertebrates diversity. Am Sci Res J Eng Technol Sci ASRJETS, 14, 1–22.
Duggan, I. C., Boothroyd, I. K., & Speirs, D. A. (2007). Factors affecting the distribution of stream macroinvertebrates in geothermal areas: Taupo Volcanic Zone, New Zealand. Hydrobiologia, 592, 235–247.
Hilsenhoff, W. L. (1988). Rapid field assessment of organic pollution with a family-level biotic index. Journal of the North American benthological society, 7(1), 65–68.
Gamito, S. (2010). Caution is needed when applying Margalef diversity index. Ecological Indicators, 10(2), 550–551.
Getachew, M., Ambelu, A., Tiku, S., et al. (2012). Ecological assessment of Cheffa Wetland in the Borkena Valley, northeast Ethiopia: Macroinvertebrate and bird communities. Ecol Indic, 15, 63–71. https://doi.org/10.1016/j.ecolind.2011.09.011.
Glazier, G. D. S. (2012). Temperature affect food - chain length and macroinvertebrate species richness in spring ecosystems. Freshw Sci, 31, 575–585.
Graça, M. A. S., Pinto, P., Cortes, R., et al. (2004). Factors affecting macroinvertebrate richness and diversity in Portuguese streams: A two-scale analysis. Int Rev Hydrobiol, 89, 151–164. https://doi.org/10.1002/iroh.200310705.
Haki, G. D., & Gezmu, T. B. (2012). Physico-chemical properties of waters from some Ethiopian hot springs and the risk to the health of the community. Greener J Phys Sci, 2, 138–140.
Heishman, A., & Mcluky, R. G. (2011). Use of conductivity to define compliance with state narrative water quality standard. West Verginia: Jacken Kelly PLC.
Herbst, D. B., & Sada, D. W. (2001). Macroinvertebrates and environmental characteristics of Owens Valley Springs. California: Desert reserch institiute.
Holt, R. F. (2007). Special elements of biodiversity in British Columbia. British Colombia
Homma, A., & Tsukahara, H. (2008). Chemical characteristics of hot spring water and geological environment in the northernmost area of the Itoigawa Shizuoka Tectonic Line. Bull Earth Res Inst, 83, 217–225.
IBN (1984). Biological water quality: determination of the biotic index based on aquatic macroinvertebrates, NBN T92-402.
James, M. (1985). Change in the faunal composition of two thermal streams near Taupa, Newzeland. J Mar Fresh Water Res, 10, 439–443.
de Klerk, A. R., & Wepener, V. (2013). Macroinvertebrate assemblage changes as an indicator of water quality of perennial endorheic reed pans on the Mpumalanga Highveld, South Africa. J Environ Prot, 04, 10–21. https://doi.org/10.4236/jep.2013.47A002.
Kloos, H., & Haimanot, R. T. (1999). Distribution of fluoride and fluorosis in Ethiopia and prospects for control. Trop Med Int Health TM IH, 4, 355–364.
Kundu, N., Panigrahi, M., Tripathy, S., et al. (2001). Geochemical appraisal of fluoride contamination of groundwater in the Nayagarh District of Orissa, India. Environ Geol, 41, 451–460. https://doi.org/10.1007/s002540100414.
Lamberti, G. A., & Resh, V. H. (1985). Distribution of benthic algae and macroinvertebrates along a thermal stream gradient. Hydrobiologia, 128, 13–21. https://doi.org/10.1007/BF00008935.
Maine Department of Environmental Protection (MDEP) (2008) Quality Assurance Project Plan for Biological Monitoring of Maine’s Rivers, Streams, and Freshwater Wetlands. Environmental Protection Documents. 98. https://digitalmaine.com/dep_docs/98
Magurran, A. E. (2013). Measuring biological diversity. John Wiley & Sons.
Masese, F., Muchiri, M., & Raburu, P. (2009). Macroinvertebrate assemblages as biological indicators of water quality in the Moiben River, Kenya. Afr J Aquat Sci, 34, 15–26. https://doi.org/10.2989/AJAS.2009.34.1.2.727.
Mazibuko, P. M., Mwendera, E. J. (2006). An assessment of the impacts of hot spring usage on water Quality in Swaziland.
McCafferty, W. P. (1983). Aquatic entomology: the fishermen’s and ecologists’ illustrated guide to insects and their relatives. Boston: Jones and Bartlett.
Mereta, S. T., Boets, P., Ambelu Bayih, A., et al. (2012). Analysis of environmental factors determining the abundance and diversity of macroinvertebrate taxa in natural wetlands of Southwest Ethiopia. Ecol Inform, 7, 52–61. https://doi.org/10.1016/j.ecoinf.2011.11.005.
Mochan, D. G., & Mrazik, S. (2000). A summary of chemistry, temperature. Oregon Department of Environmental Quality, Laboratory Division, Biomonitoring Section, Oregon: habitat and macroinvertebrate data from the Southeast Oregon ambient monitoring sites.
O’Leary, N., Vawter, A. T., Wagenet, L. P., & Pfeffer, M. (2004). Assessing water quality using two taxonomic levels of benthic macroinvertebrate analysis: Implications for volunteer monitors. Journal of Freshwater Ecology, 19(4), 581–586. https://doi.org/10.1080/02705060.2004.9664738.
Oscoz, J., Galicia, D., & Miranda, R. (2011). Identification guide of freshwater macroinvertebrates of Spain. New York: Springer, Dordrecht.
Resh, V. H. (1979). Sampling variability and life history features: basic considerations in the design of aquatic insect studies. Journal of the Fisheries Board of Canada, 36(3), 290–311. https://doi.org/10.1139/f79-047.
STATSOFT, (2007) Inc., Tulsa, OK.: STATISTICA, Version 8. AStA 91, 339–341. https://doi.org/10.1007/s10182-007-0038-x
Thompson, C. (2003). Tonopah: It’s water under the bush", the Arizona Republic 1-12-03, p. B12.
United Nations, World Health Organization (1996) Water quality monitoring: A practical guide to the design and implementation of freshwater quality studies and monitoring programmes, 1st ed. E & FN Spon, London ; New York
WHO (2008) Guidelines for drinking-water quality - Volume 1: Recommendations, 1st edn. WHO, Geneva
Acknowledgements
We are grateful to Jimma University and Arba Minch University for sponsoring this study. We are also thankful to the Water and Sewerage Office for permitting us to undertake ecological assessment in the Rift Valley region’s thermal springs. We would like to extend our thanks to Sisay Feyira, Wegayehu Erdachew, Wondemagegn Charente, and Anteneh Jenbere for their help in laboratory and fieldwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bekele, T.G., Ambelu, A., Chegen, R.G. et al. Relevance of macroinvertebrate communities as a water quality monitoring tool in ecosystems under harsh environmental conditions in the Rift Valley region. Environ Monit Assess 193, 138 (2021). https://doi.org/10.1007/s10661-021-08923-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-08923-4