Abstract
Studies on the influence of CN on Hg methylation rates in aquatic systems draining gold mining (artisanal and small-scale) communities in Africa are rare. The study assessed the influence of CN on Hg methylation in aquatic sediments of two major river systems draining artisanal and small-scale gold mining (ASGM) communities of the Prestea-Huni Valley district, Southwestern Ghana. The miners extract gold (Au) through exclusive amalgam [Hg-Au] formation or cyanidation of Au-rich Hg-contaminated tailings, or a combination of both techniques. Hg water solubility and probable mercuric compounds in sediments of Hg-contaminated CN-loaded (River Aprepre) and Hg-contaminated non-CN (River Ankobra) aquatic systems within the district were investigated. THg was determined by CV-AAS after HF/HNO3/HCl digestion. MeHg in sediments were extracted with H2SO4/KBr/CuSO4-CH2Cl2; followed by aqueous-phase propylation, preconcentration-on-Tenax, and GC-CV-AFS. River Aprepre showed 4.58–14.83 ngMeHg/g as Hg (1.4–3.7% THg as MeHg), with 241–415 ngTHg/g, and 0.05–0.21 mgCN/kg. For River Ankobra, MeHg ranged 0.24–1.21 ngMeHg/g (0.08–0.35% THg as MeHg) with 162–490 ngTHg/g dw and CN < 0.001 mg/kg. There was positive correlation (r2 = 0.5974; p < 0.01) between MeHg and CN in River Aprepre. The water-soluble fraction of Hg in sediment from both rivers was < 1% of THg. Hg in sediments from River Aprepre were generally more soluble than that from River Ankobra, indicating that Hg in sediments from River Aprepre were potentially more bioavailable for methylation. Accordingly, the presence of CN in Hg-dominated river sediments potentially influences and enhances the solubility and mobility of Hg, resulting in increased Hg methylation rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gold has been one of the mainstay of Ghana’s socio-economic development, contributing about 6% of Ghana’s gross domestic product (GDP). The gold mining sector is a major economic driving force and the biggest single contributor to government revenue, as well as leading source of export earnings. Globally, Ghana is among the top ten leading gold-producing nations. On the African continent, Ghana and South Africa are the top two gold producing nations (Ghana Chamber of Mines, 2019). Ghana’s gold output of 4.8 million ounces in 2018 surpassed South Africa’s 4.2 million ounce total for the first time (Theafricareport, 2019). Ghana’s mining industry contributes around 37% to the country’s total exports, 38.3% of total corporate tax earnings, and 27.6% of government revenue in recent years. Gold exports from Ghana constitute about 90% of total mineral exports (Ghana Chamber of Mines, 2019). Artisanal and small-scale gold mining (ASGM) has contributed significantly to Ghana’s gold production; and gold exports over decades. Gold production from ASGM accounted for 35% of total national gold production in 2019 (Ghana Chamber of Mines, 2019).
Over one million people are engaged in artisanal and small-scale gold mining (ASGM) in Ghana. Artisanal and small scale represents a significant source of employment and means of livelihood for rural and poverty-driven population in the gold mining communities. ASGM populary called ‘GALAMSEY’ (meaning ‘gather and sell’) in Ghana has been a vibrant indigenous industry for over a century (Hilson, 2002; Hilson & MsQuilken, 2014; Wilson et al., 2015). ASGM has grown extremely in recent years with the rising unemployment rate, endemic poverty, and deregulation of artisanal gold mining (Hilson and Potter, 2005). ASGM is undertaken by individuals, group of individuals, workers laid-off by large scale gold mining (LSGM) companies, or co-operatives with weak monetary base with little or no mining expertise. Reports on extensive damage to farm lands (cocoa, food crops, and rubber plantations) as well as contamination of water bodies are widespread (Mensah et al., 2015).
Southwestern Ghana, the gold mining hub in Ghana, has seen intense gold mining activities over the years. The artisanal and small-scale miners extract gold through exclusive Hg amalgamation or cyanidation of Hg-contaminated tailings, or a combination of both techniques. During mining operations, mercury released during amalgamation, water-soluble Hg-CN complexes released with cyanidation tailings along with Hg in solution are discharged into nearby rivers (Castilhos et al., 2006; Marshall et al., 2018; Seney et al., 2020). The presence of Hg(CN)2 in the aquatic environment leads to significant increase in the solubility and mobility of Hg. This makes Hg more bioavailable for methylation (Velasquez-Lopez et al., 2011; Veiga et al., 2006; Coles & Cochrane, 2006) or directly bioaccumulated (Marshall et al., 2020; Velasquez-Lopez et al., 2011). Studies conducted on Hg in aquatic environments of Southwestern Ghana due to ASGM activities have concentrated on THg in sediments and other environmental media. Few studies done on Hg speciation have focused mainly on fish. The limited work conducted on Hg speciation in sediments from gold mining communities in Ghana and Africa at large did not look at the influence of CN on Hg solubility, mobility, and subsequent impact on Hg methylation. The solubility of Hg is directly proportional to CN concentration, and the influence of CN increases mercury methylation.
The present study assessed the influence of CN on Hg methylation in sediments from two major river systems draining gold mining communities within the Prestea Huni-Valley district of Southwestern Ghana: a Hg contaminated-CN loaded (River Aprepre) and a Hg contaminated non-cyanide (River Ankobra). River Aprepre receives effluent/tailings containing Hg as well as Hg-CN complexes (from cyanidation of Hg-rich gold-tailings), whereas River Ankobra receives effluent/tailings containing predominantly Hg from ASGM operations through the exclusive use of Hg amalgamation. The study in addition evaluated the Hg water-soluble fraction of the Hg-contaminated sediments from the two rivers (resulting from extraction of Hg using water). Measurement of Hg water-soluble fraction in sediment is an important tool for the assessment of the potential biological uptake or bioavailability of Hg (Wahle & Kordel, 1997; Reis et al., 2014). The probable mercuric compounds present in the sediment from the two rivers were also identified using temperature fractionation.
Gold extraction by ASGM in Southwestern Ghana
Mercury amalgamation is the predominant gold extraction technique for ASGM (Veiga et al., 2006; Rajaee et al., 2015). The miners add metallic mercury to the impure gold dust obtained to form a mercury-gold amalgam. The ‘gold dore’ obtained is recovered through amalgam roasting in open fire to volatize Hg. ASGM have no system for Hg recovery to reduce Hg releases into the environment; resulting in the discharge of large amount of liquid Hg and gaseous elemental Hg into the environment during the process of amalgamation and roasting of the amalgam respectively (Rajaee et al., 2015). Accordingly, aquatic environments within ASGM communities have become Hg-contaminated.
Previously regarded as a gold extraction technique solely used in large-scale gold mining, cyanidation has been introduced into ASGM operations as a supplement to mercury amalgamation. Though the miners recognize the beneficial, simple, and less tedious nature of amalgamation; its gold recovery efficiency of about 30% is considerably lower compared with alternate process like cyanidation which has about 90% efficiency (Veiga et al., 2009). Cyanide processing is not preferred among smaller miners due to the large capital investment required for gold extraction (Sousa et al., 2010; Sulaiman et al., 2007).
The artisanal and small-scale miners use exclusive amalgamation, or a combination of amalgamation and cyanidation (Velasquez-Lopez et al., 2011; Veiga et al., 2014). The miners first employ amalgamation, and subsequently, the Au-rich Hg-contaminated tailings are subjected to cyanidation to extract residual gold. The use of cyanide mostly occurs at gold processing centres and larger AGSM operators within gold mining communities (Velasquez-Lopez et al., 2011; Veiga et al., 2014). Central Processing Centres in AGSM communities are facilities where miners send their gold-bearing ores or tailings to be extracted by dedicated operators for a fee. Accordingly, the miners do not invest in costly equipment to crush, grind, concentrate, and amalgamate the gold (Veiga et al., 2014). The processing centres purchase Au-rich Hg-contaminated tailings from the miners and subsequently use cyanide to extract residual gold.
Furthermore, miners in Southwestern Ghana sell the Au-rich Hg-contaminated tailings to processing centres or larger ASGM operators within the community and other parts of Ghana for reprocessing with cyanide. The miners in addition sell their mercury-laced tailings to ASGM agents from bordering West African nations like Cote d’Ivoire and Burkina Faso (Veiga et al., 2014; Macdonald et al., 2014).
Materials and methods
The Prestea Huni-Valley District
Geographical location, geology, and drainage
The study was conducted in the Prestea-Huni Valley District (5° 58′ 0″ North, 1° 55′ 0″ West) of Southwestern Ghana (Fig. 1). The Prestea Huni Valley Municipality is a major gold mining hub in Ghana and one of the Administrative Districts in Southwestern Ghana . Lying about 300 km west of Accra (capital city of Ghana), the municipal is rich in gold and cocoa, timber, and manganese. The area covers a land area of about 1376 km2. It has four major towns namely Prestea, Huni Valley, Aboso, and Bogoso (district capital); with about 29 villages and small-towns. Notable among the small-towns are the popular gold mining towns, Damang and Dumasi. Gold mining takes place in all four major towns of the municipality.
The geology of the study area is dominated by Birimian and Tarkwaian formations (Kesse, 1985). Gold deposits from the Birimian metavolcanic and metasedimentary rocks contain more quartz lodes that consist of quartz vain type and desseminated sulphide type. The gold occurs in lens sulphide-bearing quartz veins, mainly in carbonaceous phylillite as disseminated sulphides in the metavolcanics, or as oxidised derivatives of the two types in the Birimian rocks and in conglomeritic horizons as free milling gold in the Tarkwaian (Akabzaa & Yidana, 2011). Gold deposits of the Tarkwaian are in most cases considered as paleo-placers, which is the source of ‘alluvial gold’ in Ghana (Milesi et al., 1991).
Major rivers draining the municipality are the Ankobra (which flows through Ashanti Region into the Ankobra River of Ankobra Town-Prestea), Mansi (flows from Ashanti Region into Ehyireso River and joins Ankobra), Peme (flows from Pemeso into Mansi), Opong (flows from Opong Valley into Ankobra), Huni (flows from Huni Valley into Ankobra), and River Aprepre at Dumasi.
Gold mining in Prestea Huni Valley District
The district has about 156 galamsey sites (informal artisanal mining) with over 1130 individual operations (Owusu-Nimo et al., 2018). Also, there are about 89 registered and actively operating small-scale gold mining companies (Ghana Statistical Service, 2014). The district also boasts a reasonable number of ASGM processing centres. The artisanal and small-scale gold miners in the district mine gold from three main sources: gold-bearing rock ore, tailings from large scale gold mining, and gold-rich sediment of water bodies (alluvial mining). The miners basically use exclusive Hg amalgamation for gold extraction or cyanidation of Hg-contaminated tailing, or a combination of both techniques. Mercury released (amalgamation, amalgam roasting, and amalgam squeezing) during mining operations are discharged into nearby water bodies/environment. The post-amalgamation tailings are Au-rich but Hg-contaminated. The miners sell the post-amalgamation tailings to processing centres or ASGM agents from other areas. Some miners after using the services of the processing centres leave the Au-rich Hg-contaminated tailings behind as payment.
The processing centres as well as larger ASGM who purchase the Au-rich Hg-contaminated tailings operate close to River Aprepre in Dumasi and its catchment areas. The Hg-contaminated tailings are subjected to cyanidation to extract residual gold. The net tailings contaminating Hg and Hg(CN)2 complexes are discharged finally into nearby River Aprepre, resulting in Hg-contaminated CN-loaded river sediments.
Miners along the Ankobra River apply solely amalgamation and subsequently sell the Hg-contaminated tailings to ASGM agents who convey them to processing centres several kilometres away from River Ankobra for cyanide processing. Accordingly, the section of the Ankobra River within the catchment area of the study has only Hg inputs from the mining operations.
Rivers used for the study
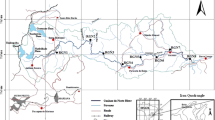
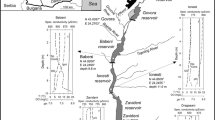
Two major rivers (River Aprepre and the Ankobra River) within the district were used for the study. River Aprepre is located at Dumasi (a village about 3 km from Bogoso, on the Bogoso-Prestea main road). Dumasi is regarded as one of the hubs of ASGM activities in the district, and Southwestern Ghana as a whole. Historically, Dumasi village has been known to be an active Artisanal and Small-Scale Gold Mining (ASGM) community. The Aprepre River which drains Dumasi flows from the eastern to the western part of the village. Tailings/effluents from artisanal/small-scale gold mining activities (both amalgamation and cyanidation of Hg-contaminated tailings) are discharged directly into River Aprepre. Thus, River Aprepre receives effluents containing CN and Hg (largely as Hg(CN)2) and considered a Hg-contaminated CN-loaded aquatic environment.
The Ankobra River (Fig. 1) which flows through Ankobra Town-Prestea is about 20 km from Dumasi township and 3.8 km from the Centre of Prestea township. The Ankobra River receives mine-impacted water (Hg-contaminated) predominantly from Artisanal and small-scale miners (alluvial and gold-bearing mineral ore mining) operating along the banks of the river and within the catchment area. A survey contacted during the study revealed that no processing centre engaged in cyanidation of Hg-contaminated tailings operates close to River Ankobra within the catchment area of Ankobra Town (samples from River Ankobra where collected from this town). This made the Ankobra River flowing through Ankobra Township a probable Hg-contaminated non-cyanide aquatic ecosystem. This accounted for the selection of Ankobra River along the Ankobra Township stretch.
Sample collection and processing
All Teflons, glass, and high-purity polyethylene wares for sampling and sample processing were thoroughly acid cleaned using procedure described by Horvat et al. (1993a, b).
Sampling plan
The two water bodies were divided into three main zones (up-stream, mid-stream, and down-stream), and seven (7) sampling stations were chosen: four stations (Stations A to D) on the Aprepre River and three stations (Stations E to G) on the Ankobra River. The choice of the sampling stations was due to the bulk of human activities (mainly gold mining) happening at the banks and/or within these water bodies and their effect on the aquatic environment. Station A, the reference station, had no ASGM activities involving amalgamation; however, there was an ASGM processing centre where cyanidation of Au-rich Hg-contamination tailings are undertaken. These processing centres purchases Au-rich Hg-contaminated tailings from the ASGM and subject the tailings to cyanidation. Four (4) sediment samples were randomly collected at each Station. Additional samples were collected in suspected hotspots. A total of 57 sediment samples were collected from the two rivers, processed, and analysed. Detailed descriptions of the sampling locations are presented in Table 1.
Collection of sediment samples
Surficial river sediment samples were collected at the upper 4-cm depth with a pre-cleaned plastic shovel on board a locally manufactured canoe (Serfor-Armah et al., 2006; Beldowski et al., 2015). Samples were transferred into pre-cleaned tightly covered plastic containers and stored in polyethylene bags with hermitic seals. No preservatives were added to the samples (Serfor-Armah et al., 2006). Samples were transported to the laboratories of the Ghana Atomic Energy Commission in thermally insulated polyethylene containers for processing and shipment to Slovenia for analysis.
Processing of samples
Processing of sediment samples was done at the laboratories of the Ghana Atomic Energy Commission, Accra. Macro-organisms, organic debris, stones, and shelly fragments were gloved hand-picked from the sediment sample (Horvat et al., 2003; Tulasi et al., 2013; Reis et al., 2014). The samples were then placed in pre-cleaned plastic bowls and frozen at − 20°C. Subsequently, the frozen samples were placed on the stacks of the Thermo Scientific Super Modulyo freeze dryer and lyophilized at −40°C and 120 mbar (Adotey et al., 2011). After lyophilization, the samples were homogenized by milling and sieving at a pore size of 100 µm. Precautions taken during sample preparation were according to the methods described by Horvat et al. (2003). The lyophilized homogenate sediment samples were stored in hermitically closed polyethylene bags and kept in a freezer until shipment by courier to the Jozef Stefan Institute , Ljubljana, Slovenia.
Analysis of samples
Analysis of samples was done at the laboratories of the Department of Environmental Sciences, Jozef Stefan Institute, Ljubljana, Slovenia.
Chemicals used were of analytical grade. Standard solutions and all other solutions were prepared with ultra-pure water (resistivity, 18.2 MΩ cm) obtained from a Milli-Q purification system (Millipore Co., Bedford, MA, USA). Stock solutions of standard inorganic mercury (1 µg/mL and 5 µg/mL as Hg2+ in 5% (v/v) HNO3) were prepared from NIST SRM 3133 (Mercury Standard Solution, 10 g/mL) for calibration. Individual stock solutions of standard organomercury chloride (1 µg/mL as Hg) were prepared from methylmercury chloride (CH3HgCl, ≥ 98%) and ethylmercury chloride (C2H5HgCl, ≥ 97%) in 5% (v/v) HNO3 respectively. The organomercury compounds were purchased from Merck, Darmstadt, Germany. Mixed working standard (1 ng/mL) of MeHg and EtHg were prepared by appropriate dilution of the stock standard solutions (1 µg/mL) in 1% HNO3 daily.
Total mercury analysis
THg levels in sediment were determined according to the methods described by Horvat, et al. (1986, 1991). The method involves the oxidation of all Hg species in the sediment to Hg(II) by acid digestion, followed by reduction of oxidized Hg (ionic Hg) to Hg0 and detection by cold vapour atomic absorption spectrometry (CVAAS).
Digestion
About 200 mg of the lyophilized homogenized sediment sample was transferred into a Teflon digestion vessel. This was followed by the addition of 5 mL HNO3/HF (2:1) and 1 mL HCl; the vessel was tightly closed, and the mixture was left at room temperature for 1 h. Digestion was completed by heating the mixture overnight (720 min) on a hot plate at 100°C. The digested sample was cooled and diluted to the mark (25.8 mL) with 5% H3BO3 to neutralize excess HF.
A blank, two standard solution digests (made up 30 and 20 µL of 1 and 5 µg/mL standard Hg solution respectively) and certified reference materials (CRM): BCR-320R (Channel Sediment) and BCR-580 (Estuarine Sediment)) were analyzed together with the samples. The results obtained for twelve (12) independent replicates of the CRM were comparable to the certified values: BCR-320R (Measured: 0.79 ± 0.01 mg/kg; Certified: 0.85 ± 0.09 mg/kg); BCR-580 (Measured: 131 ± 1.43 mg/kg; Certified: 132 ± 3 mg/kg).
Reduction and detection
Detection of THg in the digest was carried out using a HG-201 Semi-automated Mercury Analyzer (Sanso Seisakusho Co., Ltd, Tokyo, Japan), after reduction (using 10% (w/v) SnCl2·2H2O in 10% HCl) (Horvat et al., 1991; Akagi, 1997; Voegborlo & Akagi, 2007). The limit of detection of the procedure was calculated on the basis of three times the standard deviation of the sample processing blank (0.05 ng). The limit of quantification (1.2 ng/g) was calculated as five times the limit of detection divided by the sample mass. The repeatability and reproducibility of the entire analytical procedure expressed as the relative standard deviation for four replicate measurements from parallel analysis was 3 to 5%.
Methyl mercury analysis
MeHg was determined according to Horvat et al. (1993a, b), Liang et al. (1994), and Bloom et al. (1997). The method involves acid leaching of MeHg from sediment, extraction into organic phase, and back extraction into aqueous phase. Derivatization by aqueous phase propylation, pre-concentration on Tenax, heat desorption, GC separation, and detection using cold vapour atomic fluorescence spectrophotometry (CVAFS). The reproducibility of the entire analytical method was 5 to 10%.
Acid leaching and extraction
About 0.2 g of homogenized sample was transferred into acid-cleaned Teflon bottles, and acid leached with 18% KBr, 5% H2SO4, and 1 M CuSO4 solution. The bottles were agitated over a shaker for 15 min at 3000 rpm. This was followed by solvent extraction of MeHgBr into CH2Cl2 (15 mL) by shaking for 15 min, back extracted into 30 mL Milli-Q water (aqueous phase) by hot plate heating at 80°C to evaporate CH2Cl2. The extract was purged for 5 min with Hg free N2 to quantitatively remove any traces of CH2Cl2, followed by MeHg measurement.
Compositionally similar certified reference material, BCR-580 (Estuarine Sediment), and reference material, IAEA 433 (Marine Sediment), were analyzed together with the samples. The results obtained for twelve (12) independent determinations were in agreement with the certified and recommended values respectively: BCR-580 (Measured: 75.7 ± 2.5 µg/kg; Certified: 75 ± 4 µg/kg) and IAEA 433 (measured value: 0.17 ± 0.008 µg/kg; recommended value: 0.17 ± 0.07 µg/kg).
Determination of Hg water-soluble fraction from sediment (solubility test)
The total mercury content of the Hg water-soluble fraction in the sediment samples was determined after water-leaching of the soluble Hg, filtration, and acid digestion of the filtrate.
Extraction of Hg water-soluble fraction
Lyophilized homogenized river sediment samples were respectively shaken with 100 mL Milli-Q water and rain water in an end-over-end mechanical shaker for 24 and 48 h (Rodrigues et al., 2010) followed by centrifugation at 3200 rpm. The supernatant was filtered through a 0.2-µm pore size nylon filter. THg content of the Hg water-soluble fraction in the filtrate was determined by the USEPA Method 1631E (Mercury in Water by Oxidation, Purge and Trap, and CVAFS).
Identification of mercuric compounds in river sediment by thermal fractionation
Mercuric compounds in river sediment was established by thermal fractionation using a Jozef Stefan Institute ‘in-house’ built Hg detection device (Sadlar et al., 2015). The instrument is an RA-915+ Hg Analyzer with a PYRO-915+ pyrolysis unit used for detection of Hg. It is developed for direct determination of total Hg by combustion of samples and detection by cold vapour atomic absorption spectrometry (CV AAS) with Zeeman background correction (Sholupov et al., 2004). Detailed description of the apparatus and the experimental procedure can be found in Sadlar et al. (2015).
The results for the mercuric compounds detected in the sediments are depicted as Hg thermo-desorption curves (TDC); the curve is a plot of Hg released (ng/m3) versus temperature (°C) (Fig. 5). The mercuric compounds were characterized by the temperature range at which their thermal-release commences, through the peak maximum, to the point where the curve returns to baseline.
Total, free, and WAD cyanide determination
Total, free, and WAD (weak acid dissociation) cyanide in the lyophilized sediment samples were determined by Skalar segmented flow auto-analyzer (Skalar Method 295) after samples have been digested and solubilized. The method was adopted from the ‘Standard Methods for the Examination of Water and Waste Water, 23rd Edition (2005), Methods 4500-CN’.
Free cyanide was analysed using Colorimetric method. WAD cyanide was determined using Colorimetric method with distillation step, while total cyanide was analysed using colorimetric method with coupled distillation and UV digestion.
For total cyanide determination, cyanide in solution was released from all cyanide complexes through UV digestion and distillation. The cyanide was converted to cyanogen chloride by reaction with chloramine-T, which subsequently reacts with isonicotinic and barbituric acid to form a blue-coloured complex. The intensity of this complex is concentration-dependent and measured at 600 nm.
Free cyanide and WAD cyanide were determined the same way as total cyanide; however, the UV lamb was switched-off for free cyanide, and instead ZnSO4 solution was added to precipitate any iron cyanide present. In the determination of WAD cyanide, the UV digestion lamb was switched off and the samples were deionized.
Ten (10) sediment samples (six from River Aprepre and four from Ankobra River) were randomly selected for cyanide determination. The same samples were used for organic carbon determination as well as test for Hg solubility in water.
Organic carbon determination
The dry combustion method using Leco CS844 Carbon/Sulphur Determinator was used in the estimation of the total organic carbon content in the lyophilized sediments (Santi et al., 2006).
Statistical analysis
To explore the relationship between the parameters (THg, MeHg, OC, CN, and solubility) determined with respect to the sampling sites, hierarchical cluster analysis was used. Hierarchy cluster analysis (HCA) was performed on mean centred data set using Wards linkage with squared Euclidean distance between data for similarity groupings of sampling sites with respect to parameters. The results obtained are reported as dendrograms in Fig. 4.
Result and discussion
Total and methyl mercury distribution in sediment
The concentrations of THg and MeHg in all surface sediment samples from the Aprepre and Ankobra Rivers (Stations B, C, D, E, F, and G) varied from 11 to 624 ngTHg/g (mean range: 147 to 316 ng/g); and 0.07 to 14.8 ngMeHg/g (mean range: 0.11 to 3.45) respectively (Fig. 2). THg concentration in sediment from the Reference Station (Station A) ranges from 31 to 80 ng/g with a mean of 50.8 ng/g. Station A, the reference station, chosen upstream River Aprepre had no ASGM activities involving amalgamation, however, there was an ASGM processing centre (~ 500 m) where cyanidation of Au-rich Hg-contamination tailings is undertaken. This processing centre purchases Au-rich Hg-contaminated tailings from the ASGM and subject the tailings to cyanidation.
High THg levels of 624 ng/g, 432 ng/g, and 490 ng/g were found in Stations B, D, and G, respectively. About 75% and 90% of THg levels in sediment from sampling stations B and D respectively of the Aprepre River exceeded the USEPA recommended levels of 0.2 ug/g THg in aquatic sediments; and average background levels of 50.8 ng/g. The elevated levels of THg in sediment from these 3 stations were as a result of their closeness (short distance) to ASGM operation sites, the likely source of Hg contamination in the area. THg in sediments from Stations C, E, and F were generally below the USEPA recommended guideline value of 0.2 µg/g; except one sampling location in Station C with THg level (220 ng/g) slightly above the recommended guide. The observed levels may be attributed to the relatively long distances of Stations C, E and F from the source of Hg input.
To establish that levels of THg is not the sole factor influencing levels of MeHg, the relationship between THg and MeHg was assessed using Pearson’s correlation, performed with SPSS 16. The results showed a positive correlation (r = 0.359, p > 0.001) between THg and MeHg from Rivers Aprepre and Ankobra. This suggests that there may be influencing contributions from other factors like CN, OC and solubility; in agreement with observations by Kelly et al. (1995).
Statistical analysis (THg: p > 0.001; MeHg: p < 0.01) using one-way ANOVA by SPSS confirmed that there is no significant difference in the means of THg and MeHg levels between the Aprepre and Ankobra Rivers. However, there is significant difference in THg and MeHg levels between the two water bodies. Hence, the positive correlation (r2 = 0.359; p > 0.001) between THg and MeHg in all the sediment samples from both Rivers is an evidence that mercury methylation factors may influence methylation differently between the two water bodies. Additionally, different methylation parameters may exist between the Aprepre and Ankobra Rivers.
Effect of cyanide on methylmercury levels
The levels of THg, MeHg, percentage (%) THg as MeHg, organic carbon, and cyanide levels obtained in the ten (10) randomly selected sediment samples (sampling points S1 to S6; and SA to SD from the Aprepre and Ankobra rivers respectively) are presented in Table 2. Sampling points S1 to S6 represent samples taken from Stations D of the Aprepre River (known to receive effluent from ASGM activities (through application of both amalgamation, and cyanidation of Hg-contaminated tailings)). SA to SD are samples taken from Station G of the Ankobra River (close to ASGM operation site (exclusively amalgamation), but several kilometres away from processing sites engaged in cyanidation of Hg-contaminated tailing). MeHg levels in sediment from River Aprepre (S1 to S6) vary between 4.58 and 14.83 ng/g (1.41 to 3.65% of THg as MeHg; with 241 to 415 ng/g dry mass of THg). MeHg from the Ankobra River (which is kilometres away from processing sites engaged in cyanidation of Hg-contaminated tailing (SA to SD)) ranged from 0.24 to 1.21 ng/g (0.08 to 0.35% of THg as MeHg; with THg levels ranging from 162 to 490 ng/g).
Free cyanide, weak acid dissociation (WAD) cyanide, and total cyanide (TCN), levels in sediment from the Aprepre River varies from 0.05 to 0.21, 1.05 to 3.90, and 4.76 to 13.9 mgCN/kg sediment respectively. About 60% of measured free cyanide (ranging from 0.11 to 0.21 mg/kg) were above the USEPA (1977) sediment standard of 0.1 mg/kg and within 0.1 to 0.25 mg/kg USEPA (1977) sediment standard for moderate pollution. Hence, sediment from the Aprepre River is contaminated with cyanide. This may be due to the continuous increasing number of processing sites engaged in cyanidation of Au-rich Hg-contaminated tailings. Free, WAD, and TCN levels were all below the instrumental detection limit of 0.001 mg/kg in the Ankobra River. The insignificant levels of cyanide in River Ankobra may be attributed to the absence of processing sites engaged in cyanidation of Hg-contaminated tailings around the catchment area of River Ankobra where the study was undertaken.
It was therefore observed that samples from the Aprepre River with high cyanide levels (compared with the Ankobra River) show higher MeHg levels, as well as high %THg as MeHg. This could possibly be attributed to the high formation or presence of Hg(CN)2 making Hg more soluble and bioavailable (Marshall et al., 2020) for methylation (Velasquez-Lopez et al., 2011; Veiga et al., 2006; Coles & Cochrane, 2006). Guimaraes et al. (2011) reported low MeHg in free cyanide contaminated streams. This is contrary to what was found for this study, probably due to relatively low free cyanide (about an average of 6.3% of WAD), with about 40% of free cyanide (in samples) below the USEPA (1977) guideline value.
MeHg levels in the river sediments showed a positive relationship with free, WAD, and total cyanide (Free-CN: r2 = 0.5974, p < 0.01; WAD: r2 = 0.632, p < 0.05 (Fig. 3); TCN: r2 = 0.788, p < 0.0 1) Also, the percentage of THg as MeHg shows a positive relationship with all the cyanide species (Free-CN: r2 = 0.773, p < 0.01; WAD: r2 = 0.749, p < 0.05; TCN: r2 = 0.777, p < 0.01) in all the selected sediment samples from both rivers.
MeHg correlated positively (r2 = 0.8803; p < 0.001; n = 10; Fig. 3) with organic carbon in all the ten (10) randomly selected sediment samples from the Aprepre and the Ankobra rivers. The Aprepre River sediments are characterized by relatively higher organic carbon content (0.9 to 1.73%) compared with the Ankobra River (0.21 to 0.48%). Several studies have also reported a corresponding increase in MeHg levels in sediment with increasing organic carbon (Warner et al., 2005; Chavon et al., 2011). Sediment from the Aprepre River (midstream to downstream) recorded significantly higher MeHg levels with the highest %THg as MeHg of 3.7% compared with 0.35% for the Ankobra River (Table 2).
Normally in sediment, only about 1 to 1.5% of THg content occurs as MeHg, and even much lower (typically < 0.5%) in marine and estuarine sediment (Gobeil & Cossa, 1993; Kelly et al., 1995; Ullrich et al., 2001).
Though increase in both cyanide and organic carbon correlated positively with MeHg levels, organic carbon is not the main factor for the higher MeHg level in the Aprepre River. This is because mean organic carbon level of 0.99% in sediment from the Reference Station shows no difference from that obtained in Stations B and D of the River Aprepre. Thus, the organic carbon levels at the reference station and Station B and D were comparable. However, the Reference Station reported the highest cyanide level of 0.49 mg/kg which is higher than that reported for Station B and D by a factor of about 2. Mean MeHg levels for the Reference Station varies from 0.23 to 1.83 ng/g; with 0.74 to 3.98% of THg occurring as MeHg. Hence, the high percentage of THg occurring as MeHg shows Hg methylation is highest at the Reference Station due to high levels of cyanide. Also normalizing OC with MeHg (MeHg-OC) and correlating with FCN, WAD, and TCN shows a positive correlation of r2 = 0.6227, p < 0.05; r2 = 0.747, p > 0.01; and r2 = 0.798, p < 0.01 respectively. Hence, the presence of cyanide is a major factor for the high MeHg levels in the Aprepre River.
Mercury water-solubility fraction in sediment
To ascertain the potential biological uptake for Hg methylation, the Hg water-soluble fractions in sediment from both Rivers were assessed. The solubility test was performed using Milli-Q water and Rain water (Rodrigues et al., 2010; Reis et al., 2014). Twenty four (24) hours of extraction was selected for the test. This is because, beyond 24 to 48 h of extraction, the amount of leachable Hg was the same. The results obtained for the Hg water-soluble fraction in sediment samples from Rivers Aprepre and Ankobra are presented in Table 2 (expressed in absolute values as well as in percentage of total content). The Milli-Q Hg water-soluble fraction in the sediment ranged from 0.16 to 1.67 ng/g; for rain water extraction, the fraction of Hg soluble in rain water ranged from 0.60 to 2.4 ng/g.
A critical assessment of the percentage of Hg water-soluble leached using Milli-Q and Rain water (Table 2) shows that in general, solubility of Hg was slightly higher in rain water than Milli-Q water. On a whole, the percentage of the water soluble fraction of Hg in the samples was below 1% (that is 0.09–0.6%). Milli-Q water is usually used in various schemes designed to extract water soluble fractions from soils and sediment. In this study, rain water (obtained locally) was also used as an extractant alongside Milli-Q water, because it reflects more realistically natural conditions. In the case of river sediments, the influence of rain water is most important after precipitation events and consequent elevated water levels of the river that in turn can effect mobility and availability of Hg.
In addition, due to the presence of various minerals and dissolved gases (e.g., CO2), rain water can be slightly acidic, which might contribute to better extraction efficiency of Hg compared with Milli-Q water. This approach was used due to the absence of clean river water matrix that had no particulates. In addition, in situ river water was probably already enriched with Hg.
Positive correlation (r2 = 0.3067; p < 0.05; n = 10) was observed between MeHg levels and water soluble mercury content. Also, the percentage of THg as MeHg shows a positive relationship with water-soluble mercury content (r2 = 0.2079; p < 0.01; n = 10). The highest Hg water-soluble fraction obtained in this study was observed in sediments from the Aprepre River (0.67–1.67 ng/g with Milli-Q, and 1.04–2.4 ng/g for rain-water respectively) with corresponding high MeHg levels. Therefore, Hg would be potentially bioavailable in sediment from Aprepre River than the Ankobra River. This is in agreement with a similar observations by Wahle and Kordel (1997) and Reis et al. (2014).
Statistical analysis
Hierarchical cluster analysis
The dendrograms obtained using variables (THg, MeHg, CN, OC, and solubility) and using sampling sites show two main clusters at Euclidean distances of 5 and 15 respectively: Cluster 1 in Fig. 4a consisting of MeHg, CN, OC, and solubility and Cluster 2 consisting only of THg. The grouping of solubility, MeHg, CN, and OC in Cluster 1 can be attributed to the influence of CN, OC, and Hg solubility on MeHg contents. Also, MeHg together with CN shows that this system is contaminated with both Hg and CN. Cluster 1 in Fig. 4b consists of sampling sites S1, S2, S3, S4, S5, S6 (Aprepre River), and SA (Ankobra River) which receives effluent containing both CN and Hg.
The existence of only Hg in Cluster 2 of Fig. 4a shows that there is no CN contamination in this system and could be attributed to River Ankobra consisting of sampling sites SB, SC, and SD.
Interestingly, sampling site SA in the Ankobra River clustered with the sampling sites in the River Aprepre. This could be as a result of the mobility of CN from River Aprepre into Ankobra River (through River Mansi, a tributary of Ankobra River). There is the likelihood that traces of cyanide may be present at sampling site SA due to its closeness to River Aprepre before the CN finally degrade completely as it transports to sampling sites SB, SC, and SD. Cyanide was below detection limit (Table 2) in the samples from the Ankobra River, as a result of degradation in the presence of sunlight as it travel down the stream.
Thermal fractionation of mercury compounds in sediment
The possible Hg compounds present in the sediment samples from the two Rivers were determined using temperature fractionation. The results for thermal release of Hg compounds from sediment samples are presented in thermo-desorption curves in Fig. 5. The Hg compounds and the corresponding temperatures obtained from this study have been discussed in relation to the study by Rumayor et al. (2013) on pure mercury compounds and their temperatures (Table 3). Generally, the thermo-desorption curves obtained in this study (Fig. 5) were comparable/similar to the thermo-desorption curves obtained in the study by Rumayor et al. (2013) that characterized mercury compounds using thermal desorption.
The thermograms for all the samples generally shows two clear distinguishable peaks (Fig. 5). The first peak on each thermogram (Fig. 5a–d), released at 200–360°C, with maximum temperature at 270°C for Fig. 5a–b and 220°C for Fig. 5c–d. These suggest the presence of complexes of Hg with one or a combination of the following: humic material, HgCl2, and Hg(CN)2 with reference to thermal desorption temperature by Rumayor et al. (2013) (Table 3). The second peak for each thermogram (Fig. 5a–d) was released at 420–600°C, with a maximum peak height at 510°C which is most likely to contain Hg2SO4 (Rumayor et al., 2013) (Table 3). It was observed that the first peaks on thermograms from sediment samples from Hg-contaminated cyanide-loaded areas (Fig. 5a–b) were distinct with a maximum peak height temperature of 270°C. This is comparable with the maximum peak height of 267°C reported for Hg(CN)2 by Rumayor et al. (2013) (Table 3). Hence, the composition of the first peaks (Fig. 5a–b) is likely to contain a high proportion of Hg(CN)2. The first peak on the thermograms for sediment from River Ankobra occurs at 220°C. This maximum temperature does not correspond to the temperature for Hg(CN)2 reported by Rumayor et al. (2013). This is a confirmation of the absence of cyanide in the Ankobra River.
Conclusion
The study established high levels of MeHg in the Hg contaminated cyanide-loaded aquatic environment (River Aprepre) compared with low levels of MeHg in River Ankobra (Hg-contaminated non-cyanide aquatic environment). Primarily due to the use of cyanide (in addition to the use of Hg) by ASGM to extract gold from Au-rich Hg-contaminated tailings at processing centres within the catchment area of River Aprepre.
Hg in sediments from River Aprepre were generally more soluble than that from River Ankobra; indicating that Hg in sediments from River Aprepre were more bioavailable for methylation. Accordingly, the presence of CN in Hg-contaminated river sediments quantitatively influences and enhances the solubility and mobility of Hg; resulting in high rates of Hg methylation. Thermal fractionation of sediment confirmed Hg(CN)2 as the probable mercuric compound present in sediments of River Aprepre.
References
Adotey, D. K., Stibilj, V., Serfor-Armah, Y., Nyarko, B. J. B., & Jacimovic, R. (2011). Dietary supply of selenium for adolescents in three residential care orphanages in southern Ghana. Science of the Total Environment, 410, 72–79.
Akabzaa, T. M., & Yidana, S. M. (2011). Evaluation of sources and Options for possible clean-up of anthropogenic mercury contamination in the Ankobra River basin in Southwestern Ghana. Journal of Environmental Protection, 2, 1295–1302.
Akagi, H. (1997). Analytical methods for evaluating human exposure to mercury due to gold mining. Proceedings of the International workshop on health and environmental effects of mercury due mining operations. Manila, pp. 131 – 141. Available at: http://www.nimd.go.jp/kenkyu/docs/march_mercury_analysis_manual(e).pdf
Beldowski J., Miotk M., & Pempkowiak, J. (2015). Methylation index as means of quantification of the compliance of sedimentary mercury to be methylated. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-4716-y
Bloom, N. S., Colman, J. A., & Barber, L. (1997). Artifact formation of methylmercury during aqueous distillation and alternative techniques for the extraction of methyl mercury from environmental samples. Fresenius Journal of Analytical Chemistry, 358, 372.
Castilhos, Z. C., Rodrigues-Filho, S., Rodrigues, A. P. C., Villas-Bôas, R. C., Siegel, S., Veiga, M. M., & Beinhoff, C. (2006). Mercury contamination in fish from gold mining areas in Indonesia and human health risk assessment. Science of the Total Environment, 368, 320–325.
Chavon, R. W., Leaner, J. J., Somerset, V. S., & Nel, J. M. (2011). Mercury concentrations at a historically mercury-contaminated site in KwaZulu-Natal (South Africa). Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-011-0458-8.
Coles, A. C., & Cochrane, K. (2006). Mercury cyanide contamination of groundwater from gold mining and prospects for removal. Sea to Sky Geotechnigue. 1118-1122.
Ghana chamber of mines. (2019). Annual report. https://ghanachamberofmines.org/wp-content/uploads/2020/05/2019-Annual-Report_Complete.pdf on 12 August, 2020.
Ghana Statistical Service. (2014). District analytical report Prestea Huni-Valley District. Population and Housing Census-Year 2010.
Guimaraes, J. R. D., Betancourt, O., Miranda, M. R., Barriga, R., Cueva, E., & Betancourt, S. (2011). Long-range effect of cyanide on mercury methylation in gold mining area in southern Ecuador. Science of the Total Environment, 409, 5026–5033.
Gobeil, C., & Cossa, D. (1993). Mercury in sediments and sediment pore water in the Laurentian Trough. Canadian Journal of Fisheries and Aquatic Sciences, 50, 1794.
Hilson, G. M. (2002). Harvesting mineral riches: 1000 years of gold mining in Ghana. Resources Policy, 28, 13–26.
Hilson, G., & McQuilken, J. (2014). Four decades of support for artisanal and small-scale mining in sub-Saharan Africa: A critical review. Extractive Industries and Society, 1, 104–118.
Hilson, G., & Potter, C. (2005). Structural adjustment and subsistence industry: Artisanal gold mining in Ghana. Development and Change, 36, 103–131.
Horvat, M., Boom, N. S., & Liang, L. (1993a). Comparison of distillation with other current isolation methods for the determination of methylmercury compounds in low level environmental samples, Part I. Sediment Analytical Chemistry Acta, 281, 135–152.
Horvat, M., Liang, L., & Boom, N. S. (1993b). Comparison of distillation with other currency isolation methods for the determination of methylmercury compounds in low level environmental samples, Part II. Water Analytical Chemistry Acta, 282, 153–168.
Horvat, M., Lupsina, V., & Pihlar, B. (1991). Determination of total mercury in coal fly ash by gold amalgamation cold vapour atomic absorption spectrometry. Analytical Chemistry Acta, 24, 71–79.
Horvat, M., Nolde, N., Fajon, V., Jereb, V., Logar, M., Sonja, L., et al. (2003). Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou. China Science of the Total Environment, 304, 231–244.
Horvat, M., Zvonari, T., & Stegnar, P. (1986). Optimization of a wet digestion method for the determination of mercury in blood by cold vapour absorption spectrometry (CV AAS). Vestnik Slovenskega Kemijskega Drustva, 33(4), 475–486.
Kelly, C. A., Rudd, J. W. M., Louis, V. L., & Heyes, A. (1995). Is total mercury concentration a good predictor of methyl mercury concentration in aquatic systems. Water, Air, and Soil Pollution, 80, 715–724.
Kesse, G. O. (1985). The mineral and rocks resources of Ghana (pp. 20–55). Rotterdam: A.A. Balkerma.
Liang, L., Horvat, M., & Bloom, N. S. (1994). An improved method for speciation of mercury by aqueous phase ethylation, room temperature precollection, GC separation and CV AFS detection. Talanta, 41, 371–379.
Macdonald, K. F., Lund, M. A., Blanchette, M. L., & Mccullough, C. D. (2014). Regulation of Artisanal Small Scale Gold Mining (ASGM) in Ghana and Indonesia as currently implemented fails to adequately protect aquatic ecosystems. An Interdisciplinary Response to Mine Water Challenges - Sui, Sun & Wang (eds). China University of Mining and Technology Press, Xuzhou, ISBN 978–7–5646–2437–8.
Marshall B.G., Veiga M. M., Kaplan R. J., Miserendino R.A., Schudel G., Bergquist B.A., Guimarães J.R.D., Sobral L.G.S., & Gonzalez-Mueller C. (2018). Evidence of transboundary mercury and other pollutants in the Puyango-Tumbes River Basin, Ecuador− Peru. Environmental Science: Processes Impacts, 20(4): 632−641.
Marshall, B. G., Veiga, M. M., Silva, H. A. M., & Guimaraes, J. R. D. (2020). Cyanide Contamination of the Puyango-Tumbes River caused by artisanal gold mining in Portovelo-Zaruma. Ecuador: Current Environmental Health Report. https://doi.org/10.1007/s40572-020-00276-3
Mensah K.A., Mahiri1 I.O., Owusu O., Mireku O.D., Wireko I., Kissi E.A (2015). Environmental impacts of mining: a study of mining communities in Ghana. Applied Ecology and Environmental, 3(3): 81-94.
Milesi, J. P., Ledru, P., Ankrah, P., Johan, V., Marcoux, E., & Vinchon, C. (1991). The metallogenic relationship between Biriman and Tarkwaian gold deposits in Ghana. Mineral Deposita, 26, 228–238.
Owusu-Nimo, F., Mantey, J., Nyarko, K. B., Appiah-Effah, E., & Aubynn, A. (2018). Spatial distribution patterns of illegal artisanal small scale gold mining (Galamsey) operations in Ghana: A focus on the Western Region. Heliyon, 4(2), e00534. https://doi.org/10.1016/j.heliyon.2018.e00534
Rajaee, M., Obiri, S., Green, A., Long, R., Cobbina, S. J., Nartey, V., ... & Basu, N. (2015). Integrated assessment of artisanal and small-scale gold mining in Ghana—Part 2 : Natural Sciences Review, 12, 8971–9011.
Reis, A. T., Lopes, C. B., & Davidson., Duarte A.C., Pereira E. (2014). Extraction of mercury water-soluble fraction from soil: An optimization study. Geodema, 213, 255–260.
Rodrigues, S. M., Henriques, B., Coimbra, J., Ferreira da Silva, E., Pereira, M. E., & Duarte, A. C. (2010). Water-soluble fraction of mercury, arsenic and other potentially toxic elements in highly contaminated sediments and soils. Chemosphere, 78, 1301–1312.
Rumayor M., Diaz-somoano M., Lopez-Anton M. A., Martinez-Tarazona M. R., (2013). Mercury compounds characterization by thermal desorption. Talanta, 114, 318–322.
Sadlar, M., Pavlin, M., Jacimovic, R., Stergarsek, A., Frkal, P., & Horvat, M. (2015). Temperature Fractionation (TF) of Hg compounds in Gypsum from wet flue gas desulfurization system of the coal fires thermal power plant. American Journal of Analytical Chemistry, 6, 939–956.
Santi C., Certini G., & D’Acqui P. L. (2006). Direct determination of organic carbon by dry combustion in soils with carbonates. Communications in soil Science and Plant Analysis, 37: 1–2, 155–162. https://doi.org/10.1080/00103620500403531
Seney, C. S., Bridges, C. C., Aljic, S., Moore, M. E., Orr, S. E., Barnes, M. C., et al. (2020). Reaction of cyanide with Hg-contaminated gold mining tailings produces soluble mercuric cyanide complexes. Journal of Chemical Research in Toxicology. https://doi.org/10.1021/acs.chemrestox.0c00211
Serfor-Armah, Y., Nyarko, B. J. B., Adotey, D. K., Dampare, S. B., & Adomako, D. (2006). Levels of arsenic and antimony in water and sediment from Prestea, a gold mining town in Ghana and its environ. Water, Air, and Soil Pollution, 175, 181–192.
Sholupov, S., Pogarev, S., Ryzhov, V., Mashyanov, N., & Stroganov, A. (2004). Zeeman atomic absorption spectrometer RA-915+ for direct determination of mercury in air and complex matrix samples. Fuel Processing Technology, 85, 473–485. https://doi.org/10.1016/j.fuproc.2003.11.003
Sousa, R. N., Veiga, M. M., Klein, B., Telmer, K., Gunson, A. J., & Bernaudat, L. (2010). Strategies for reducing the environmental impact of reprocessing mercury-contaminated tailings in the artisanal and small-scale gold mining sector: Insights from Tapajos River Basin. Brazil. Journal of Cleaner Production, 18(16–17), 1757–1766.
Sulaiman, R., Baker, R., Susilorini, B., Telmer, K., & Spiegel, S. J. (2007). Indonesia country report: Removal of barriers to introduction of cleaner artisanal gold mining and extractin technologies (G. M. Project, Trans.), pp. 56: UNIDO.
Theafricareport. (2019): https://www.theafricareport.com/in-depth/the-africa-reports-guide-to-2019retrieved on 23 August, 2020.
Tulasi, D., Adotey, D., Affum, A., Carboo, D., & Serfor-Armah, Y. (2013). Speciation of As (III) and As (V) in water and sediment using reverse-phase ion-pair high performance liquid chromatography- Neutron activation analysis (HPLC-NAA). Envionmental Monitoring and Assessment, 185, 7979–7991.
Ullrich, S. M., Tanton, T. W., & Abdrashitova, S. A. (2001). Mercury in the environment: A review of the factors affecting methylation. Critical Reviews in Environment Science and Technology, 31, 241–293.
USEPA. (1977). Guidelines for pollution classification of Great Lakes harbor sediments. Region V: US Environmental Protection Agency.
Veiga, M. M., Angeloci, G., Hitch, M., & Vel´asquez- L´opez P. C. (2014). Processing centers in artisanal gold mining. Journal of Cleaner Production, 64, 535–544.
Veiga, M. M., Maxson, P. A., & Hylander, L. D. (2006). Origin and consumption of mercury in small-scale gold mining. Journal of Cleaner Production, 14, 436–447.
Veiga, M. M., Nunes, D., Klein, B., Shandro, J. A., Velasquez, P. C., & Sousa, R. N. (2009). Replacing mercury use in artisanal gold mining: Preliminary tests of millleaching. Journal of Cleaner Production, 17, 1373–1381.
Velasquez-Lopez, P. C., Viega, M. M., Klein, B., Shandro, J. A., & Hall, K. (2011). Cyanidation of mercury-rich tailings in artisanal and small-scale gold mining: Identifying strategies to manage environmental risks in Ecuador. Journal of Cleaner Production, 19(9–10), 1125–1133.
Voegborlo, R. B., & Akagi, H. (2007). Determination of mercury in fish by cold vapour atomic absorption spectrometry using an automatic mercury analyser. Food Chemistry, 100, 853–858.
Wahle, U., & Kordel, W. (1997). Development of analytical methods for the assessment of ecotoxicological relevant soil contamination. Part A. Development and improvement of soil extraction methods for the determination of the bioavailable parts of contaminant. Chemosphere, 35, 223–237.
Warner, K. A., Bonzongo, J. J., Roden, E. E., Ward, G. M., Green, A. C., Chaubey, I., et al. (2005). Effect of watershed parameters on mercury distribution in different environmental compartments in the Mobile Alabama River Basin, USA. Science of the Total Environment, 347, 187–207.
Wilson, M. L., Renne, E., Roncoli, C., Agyei Baffour, P., & Tenkorang, E. Y. (2015). Integrated assessment of artisanal and small-scale gold mining in Ghana—Part 3: Social sciences and economics. International Journal of Environmental Research Public Health, 12(7), 8133–8156.
Acknowledgements
The authors are grateful to the Ghana Atomic Energy Commission for logistical support.
Funding
Funding for this research was provided by the International Centre for Theoretical Physics/International Atomic Energy Agency (ICTP/IAEA) Sandwich Training Educational Programme (STEP) for Delali Tulasi (through the IAEA Technical Cooperation Project INT0089). Additional funding from the Jozef Stefan Institute, Slovenia, through the American Roentgen Ray Society (ARRS) Programme P-0143 for Delali Tulasi is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Dr. Delali Tulasi (Principal Investigator): Involved in the Conception, Design and Formulation of the Research Study; Sampling team, Main analyst (Formal Analysis of Samples); Drafting of Manuscript, Review and Editing; Funding Acquisition; Visualization; and Validation. Ms. Vesna Fajon: Formal Analysis of samples (Total and Methylmercury Determination); Validation; and Software. Dr. Joze Kotnik: Formal Analysis of samples (Thermal fractionation of mercuric compounds in sediment); Interpretation of Results; and Software. Dr Yaroslav Shlyapnikov: Data Curation and Data Analysis. Dr. Dennis K. Adotey: Research design and formulation, Samples Collection; Sample Preparation; Manuscript Preparation, Review and Editing; and Funding acquisition. Prof. Yaw Serfor-Armah: Research Design and Formulation; Manuscript Preparation and Editing; Funding Acquisition; and Project Administration. Prof. Milena Horvat: Conceptualization, Design, Formulation and Methodology of Research; Coordination of entire Research Study, Preparation of Manuscript, Review and Editing; Funding; Resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The environmental influence of CN on Hg solubility, mobility, and bioavailability and subsequent impact on Hg methylation rates in river sediments have scarcely been published.
• The presence of Hg, CN, and Hg(CN)2 in river sediments draining artisanal and small-scale gold mining communities have significant impact on Hg methylation.
• The presence of CN in Hg-contaminated river sediments influences/enhances Hg solubility, mobility, bioavailability, and subsequent methylation.
• Hg-contaminated CN-loaded river sediments have high methylation rates due to high Hg solubility, mobility, and bioavailability.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tulasi, D., Fajon, V., Kotnik, J. et al. Mercury methylation in cyanide influenced river sediments: A comparative study in Southwestern Ghana. Environ Monit Assess 193, 180 (2021). https://doi.org/10.1007/s10661-021-08920-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-08920-7