Abstract
The centrifuge-less dispersive liquid-liquid microextraction (DLLME) technique was used to separate selenium species in aqueous samples. According to the salting-out effect, a simple approach was used to eliminate the centrifugation step. The optimization of the independent variables was performed using chemometric methods. Under optimal conditions, this methodology was statistically validated. The linearity was between 20 and 300 μg L−1. The limit of detection and quantification were calculated 3.4 μg L−1 and 10.4 μg L−1, respectively. The values of reproducibility and repeatability were determined ≤ 9.5% and ≤ 6.4, respectively. The possibility of the method was successfully assessed by analyzing the analytes in real samples clarified satisfactory recoveries (98.1–101.4% for Se (IV) and 98.4–101.5% for Se (VI)).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The DLLME technique has been applied since the end of 2006. It was the consequence of researchers’ attempt to find a way to reduce certain problems encountered during sample preparation. Dispersive liquid-liquid microextraction (DLLME) technique has been applied since the end of 2006. It was the consequence of researchers’ attempt to find a way to reduce certain problems encountered during sample preparation. The DLLME is an extraction technique developed within the last decade that involves the dispersion of fine droplets of extraction solvent in an aqueous sample. Partitioning of analytes into the extraction phase is instantaneous due to the very high collective surface area of the droplets. Hence, high enrichment factors and very low solvent consumption were achieved in comparison with conventional extraction techniques (Quigley et al. 2016). This method also aimed to overcome the incompatibility with the twelve principles of green analytical chemistry (Lichtfouse 2005). The advantages of this method have led to its widespread use in preparing the sample and preconcentrations of the organic and inorganic analytes in various matrices. Low solvent consumption, high speed, and simplicity are considered as some advantages of the DLLME method. However, this method has long been criticized for its disadvantages such as the need for halogenated solvents, the requirement of a second dispersive solvent, and difficulties in collecting extraction solvent following the extraction process. Furthermore, this method requires centrifugation. Attempts to overcome the aforementioned disadvantages resulted in introducing modified methods and/or new techniques. In this vein, the use of ionic liquids (Rykowska et al. 2018), supramolecular solvent (Zong et al. 2018), and switchable solvent (Timofeeva et al. 2017) to replace toxic halogenated solvents have made these methods more environmentally friendly. In addition, dispersion using air (Nejad et al. 2017), vortex (Abdallah and Ahmed 2019), and ultrasound (Panhwar et al. 2017; Tuzen et al. 2018) assistance instead of dispersive solvent could remove the second solvent, resulting in more compliance with green chemistry principles. Moreover, using dissolved gas flotation (Seidi 2017) and magnetic ionic liquid (Fiorentini et al. 2018; Wei et al. 2019) as well as salt addition (Farahani 2019) have led to independence from centrifugation. It should also be noted that using novel tools and replacing traditional solvents with ones with lower density compared with water and having a melting point approaching (or near) the surrounding temperature makes it possible to collect the extraction solvent following the extraction (Chen et al. 2015; Jain and Verma 2011). In this study, some strategies have been developed to overcome the shortcomings of classical DLLME for the determination of selenium. In this way, the disperser solvent was replaced by vortex agitation, 1-undecanol was used as a more sustainable solvent instead of toxic organic solvents, and the salting-out effect was employed for omitting the centrifugation step. Selenium is a necessary trace mineral that is important for many bodily processes. Selenium has two inorganic forms in aqueous samples: Se (VI) and Se (IV), which have higher toxicity compared with selenium’s organic forms (LeBlanc et al. 2018; Tan et al. 2016). Therefore, the development of analytical methods based on the principles of green chemistry is very crucial for monitoring selenium in dietary sources.
Materials and method
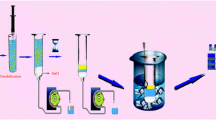
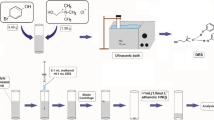
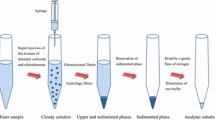
The chemicals Na2SeO3, Na2SeO4, 1-dodecanol, 1-undecanol, 1-decanol, n-hexadecane, and 3,3′-diaminobenzidine hydrochloride (DAB) were obtained from Sigma-Aldrich company at the highest grade of purity (99%). Appropriate amounts of Na2SeO3 and Na2SeO4 were dissolved in a 100-mL volumetric flask, and the resulting solution was diluted with distilled water to obtain stock standard solutions of Se (IV and VI, 1000 μg mL−1). The resulting solutions were then kept in a refrigerator at 4 °C and away from light. HCl (37%) and NaCl (≥ 99.0%) were purchased from Merck Company. Injectable Se solution as a veterinary pharmaceutical was prepared from Razak Drug Co (Alborz, Iran). The working solutions and the DAB solution were made every day. Double-beam spectrophotometry (PerkinElmer Lambda 25 with 350 μL quarts microcell) was employed to read the absorbance. Metrohm 780 pH Meter and the FINE PCR vortex were utilized to read the pH of the solution and accelerate the dispersion of the cloudy solution. The experiments were carried out with the optimal values of the independent variables. In the first step, a 5 mL selenium (IV) solution (0.2 mg L−1) was transferred to a test tube and 0.6 mL of a DAB solution (0.005 mol L−1) was subsequently added. A hydrochloric acid solution was then added to the solution to adjust the pH to 2–2.5. The sample was put in a 100 °C water bath for approximately 5 min, and then, the pH was adjusted. After adding the extraction solvent, the sample was placed in a vortex mixer. Next, a filter paper was used, and 2.5 g of sodium chloride salt was added. The vortex solution was passed through a salt column, and organic and aqueous phases were separated. After the separation, the organic phase was then placed in an ice cooling bath and the sample was loaded to read the absorbance at 434 nm. To measure Se (VI), it was converted to Se (IV) with hydrochloric acid (Mostafavi et al. 2019).
Results and discussion
Solvent extraction is considered as a very important variable affecting the extraction efficiency. In the present study, 1-dodecanol, 1-undecanol, 1-decanol, and n-hexadecane were used as the extracting solvent. As Table S1 indicates, the extraction efficiency with 1-undecanol is higher, leading to its selection as the extracting solvent.
Multivariate optimization analysis based on PBD and CCD was performed to screen and identify the significant factors, respectively. First, PBD was used to screen the important variables like extracting solvent volume (A) and vortex time (B), vortex intensity (C), complexing agent concentration (D), pH value (E), and the sample flow rate (F).
Altogether, twenty-three experiments were performed randomly with three replications. Replication helps obtain a better estimate of experimental error. The details of the experimental design are presented in Table 1.
The significance of the model was assessed through analysis of variance. As shown in Table 2, the results indicate the significant effects of the extracting solvent volume (A), vortex time (B), and the complexing agent concentration (D) on extraction efficiency (p < 0.05). However, no significant impact was seen on the efficiency of the extraction. Moreover, the adverse effect of the complexing agent concentration (D) is shown on a normal curve plot (Fig. 1).
As the results of the screening design indicate, the extracting solvent volume (A) and vortex time (B) were utilized at low, central, and high levels under CCD in 13 randomly evaluated experiments. Table 3 shows the results of CCD experiments. The results presented in Table 4 demonstrate the significance of the regression (p < 0.05) and non-significance (p > 0.05) of the lack of fit (LOF). In line with this, Eq. (1) was used as the basis to formulate a second-order polynomial model:
In the above formula, Y denotes the dependent variable, β0 denotes the intercept term, A and B denote the independent variables, β1 and β2 are the linear coefficients, the quadratic coefficients are denoted by β11 and β22, while the cross-product coefficient of the opposite is denoted by β12. Two primary effects (β1 and β2), two curvature effects (β11 and β22), and one two-factor interactions (β12) were derived from this relationship.
The surface response method (RSM) was used to evaluate the results of the experiments, and multiple regression analysis was used to test whether a certain empirical relationship exists between the response (Y) and the independent variables, which is expressed by Eq. (2):
The quantitative measurement coefficient (R2) accounted for the fit quality of the polynomial equation. In this respect, the value of R2 as a measure of variance was around 0.9613. Figure 2 shows the three-dimensional curve of the model and the developed RSM by the model. As can be seen, the adsorption amount increases by enhancing the extracting solvent volume up to about 150 μL, which has a negative effect on the adsorption. However, increasing the vortex time to about 7 min resulted in increasing adsorption capacity. Hence, the optimum test conditions are the extracting solvent volume of 147 μL and a vortex time of 7 min as well as high vortex intensity, complexing agent concentration of 0.005 mol L−1, an injection flow rate of 0.4 mL min−1, and pH of 7.
Method validation is an important technique in analytical chemistry to guarantee the quality, reliability, and comparability of the results. In the present study, the method validation was conducted based on “The Guidelines and Rules of the International Conference on Harmonization (ICH).” Linearity range, calibration model, detection and quantification limit, and precision and accuracy were considered as the parameters for evaluating the validity of the method. The best calibration curve was constructed with ten standard concentrations of Se (IV) ranging from 5 to 500 μg L−1 (n = 3). Each concentration was examined in the optimum condition of the variables in three replicate experiments. Additionally, to determine the linearity of the calibration curve, an analysis of variance test was examined. Consequently, an F test was used to calculate the ratio of the LOF variance to squared pure errors. It is notable that the proportion of Fcritical to Fexperimental was more than 1.0. The results obtained for the F test and the high value of the coefficient of determination (R2 = 0.9613) of the analyte supports that the linear function model is statistically significant. Furthermore, linearity in the range of 20–300 μg L−1 for the Se (IV) sample was computed. The common parameters of the limit of quantitation (LOQ) and limits of detection (LOD) were used to analyze the sensitivity (IUPAC 2002; Faraji and Helalizadeh 2017). The LOD and LOQ of the analyte were estimated at 3.4 μg L−1 and 10.4 μg L−1, respectively (see Electronic Supplementary Material, Table S2).
Based on definitions provided by ICH, accuracy is known as the proximity between the obtained value and the actual or accepted value although accuracy is frequently utilized to evaluate the systematic error. In the present study, accuracy was examined using the recovery tests. In other words, it was assessed by obtaining the recovery value of each concentration level by dividing the mean concentration difference from the three replications and the actual or accepted value. Finally, a t test at a 95% confidence level was run. The empirical t statistic was calculated based on the following equation:
where \( {\overline{R}}_{\mathrm{exp}} \), N, and SR represent the mean relative recoveries, the number of samples, and the standard deviation of recoveries, respectively. In this evaluation, the null hypothesis (H0) equals \( {\overline{R}}_{\mathrm{exp}} \) = 100% while hypothesis one (H1) equals \( {\overline{R}}_{\mathrm{exp}} \) ≠ 100%.
Reproducibility was used to evaluate the accuracy, and the results were reported as the percentage of relative standard deviation. Additionally, the evaluation of the reproducibility was repeated five times for three concentrations in optimal conditions under the same testing conditions. As for reproducibility, 27 experiments were conducted under optimal conditions in three different days by two different testers. Data on the 3 days were used to analyze the variance, and the reported results had a 95% level of confidence. Furthermore, the values of reproducibility and repeatability were determined at ≤ 9.5% and ≤ 6.4%, respectively.
To determine how specific the approach was, various quantities of other ions were added to the sample of selenium standard solution. The assessment of analyte recovery was performed for every extrinsic ion and determination of the equivalent error was done post-extraction under ideal conditions (Table S3). In addition, the method tolerance was reported as acceptable in the presence of intrusive ions up to 5%. As the results showed, no buffer, as the source of different impurities and perturbations, was required during testing since the DAB ligand was stable in the strong acidic medium. Moreover, the results show that the complexing agent reacts with no alkaline or alkaline earth elements. Therefore, it has the potential to be successfully tested in natural samples and aquatic environments with high salt content. Furthermore, actual samples with content of river water, tap water, and the drug were analyzed to establish how precise the approach was. The recovery experiments were performed by the addition of known amounts of the standard solution (Se IV and VI) to tap and river water samples in concentrations of 30.0, 40.0, and 50 μg L−1. The injectable solution drug sample was also spiked by a standard solution of Se (IV) in concentrations of 25.0, 35.0, and 45.0 μg L−1. Accordingly, for each concentration level, the accuracy was reported as percent recovery by the assay of the known added amount of the analyte in the sample or as the difference between the mean and the accepted true value together with the confidence intervals. Table 5 shows the recovery results of each sample after adding a certain amount of Se (IV and VI) standard at three concentration levels. Finally, a t test at a 95% level was used to evaluate the results. Simply, by the subtraction of the total content of Se from the values of Se (IV) in Table 5, the Se (VI) amount was determined.
Conclusion
This study put forth a novel approach for selenium ion preconcentration and measurement in aqueous samples and assessed that approach against strategies devised by earlier research (Table 6). The findings indicated that this method is cost-effective, convenient, fast, and more environmentally friendly. To that end, the study did not employ any halogenated solvent and minimal solvent extraction was undertaken in the context of the technique of floating drop solidification. This was done so that the standard technique of dispersive liquid-liquid extraction conformed to the green chemistry tenets. In addition, the dispersive solvent was removed, and the separation procedure was carried out without centrifugation. Future studies should take more essential steps to embrace the principles of green chemistry.
References
Abdallah, O. I., & Ahmed, N. S. (2019). Development of a vortex-assisted dispersive liquid-liquid microextraction (VA-DLLME) and LC-MS/MS procedure for simultaneous determination of fipronil and its metabolite fipronil sulfone in tomato fruits. Food Analytical Methods, 12(10), 2314–2325.
Chen, S., Zhu, S., & Lu, D. (2015). Solidified floating organic drop microextraction for speciation of selenium and its distribution in selenium-rich tea leaves and tea infusion by electrothermal vapourisation inductively coupled plasma mass spectrometry. Food Chemistry, 169, 156–161.
Dadfarnia, S., Shabani, A. M. H., & Nozohor, M. (2014). Dispersive liquid-liquid microextraction-solidified floating organic drop combined with spectrophotometry for the speciation and determination of ultratrace amounts of selenium. Journal of the Brazilian Chemical Society, 25(2), 229–237.
Farahani, H. (2019). Screening of parabens in natural water by salting-out based centrifugeless dispersive liquid-liquid microextraction combined with HPLC-UV. Acta Chimica Slovenica, 66(3), 576–583.
Faraji, H., & Helalizadeh, M. (2017). Lead quantification in urine samples of athletes by coupling DLLME with UV–Vis spectrophotometry. Biological Trace Element Research, 176, 258–269.
Fiorentini, E. F., Escudero, L. B., & Wuilloud, R. G. (2018). Magnetic ionic liquid-based dispersive liquid-liquid microextraction technique for preconcentration and ultra-trace determination of Cd in honey. Analytical and Bioanalytical Chemistry, 410(19), 4715–4723.
IUPAC. (2002). Harmonized guidelines for single-laboratory validation of method of analyses (IUPAC technical report). Pure and Applied Chemistry, 74, 835.
Jain, A., & Verma, K. K. (2011). Recent advances in applications of single-drop microextraction: a review. Analytica Chimica Acta, 706(1), 37–65.
LeBlanc, K. L., Kumkrong, P., Mercier, P. H., & Mester, Z. (2018). Selenium analysis in waters. Part 2: Speciation methods. Science of the Total Environment, 640, 1635–1651.
Lichtfouse, E., Schwarzbauer, J., Robert, D. (2005). Environmental chemistry: green chemistry and pollutants in ecosystems. Springer-Verlag, Berlin, Heidelberg. https://doi.org/10.1007/b137751.
Martinis, E. M., Escudero, L. B., Berton, P., Monasterio, R. P., Filippini, M. F., & Wuilloud, R. G. (2011). Determination of inorganic selenium species in water and garlic samples with on-line ionic liquid dispersive microextraction and electrothermal atomic absorption spectrometry. Talanta, 85(4), 2182–2188.
Mostafavi, B., Feizbakhsh, A., Konoz, E., & Faraji, H. (2019). Hydrophobic deep eutectic solvent based on centrifugation-free dispersive liquid–liquid microextraction for speciation of selenium in aqueous samples: One step closer to green analytical chemistry. Microchemical Journal, 148, 582–590.
Nejad, M. G., Faraji, H., & Moghimi, A. (2017). Monitoring Pb in aqueous samples by using low density solvent on air-assisted dispersive liquid–liquid microextraction coupled with UV–Vis spectrophotometry. Bulletin of Environmental Contamination and Toxicology, 98(4), 546–555.
Panhwar, A. H., Tuzen, M., & Kazi, T. G. (2017). Ultrasonic assisted dispersive liquid-liquid microextraction method based on deep eutectic solvent for speciation, preconcentration and determination of selenium species (IV) and (VI) in water and food samples. Talanta, 175, 352–358.
Quigley, A., Cummins, W., & Connolly, D. (2016). Dispersive liquid-liquid microextraction in the analysis of milk and dairy products: A review. Journal of Chemistry, 2016, 1–12.
Rykowska, I., Ziemblińska, J., & Nowak, I. (2018). Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. Journal of Molecular Liquids, 259, 319–339.
Seidi, S. (2017). Dissolved carbon dioxide flotation-assisted in-syringe dispersive liquid–liquid microextraction coupled with microsampling flame atomic absorption spectrometry for selective determination of palladium in water samples. Journal of the Iranian Chemical Society, 14(6), 1159–1167.
Shabani, A. M. H., Dadfarnia, S., & Nozohor, M. (2013). Indirect spectrophotometric determination of ultra trace amounts of selenium based on dispersive liquid–liquid microextraction–solidified floating organic drop. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 116, 1–5.
Tan, L. C., Nancharaiah, Y. V., van Hullebusch, E. D., & Lens, P. N. (2016). Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnology Advances., 34, 886–907.
Timofeeva, I., Timofeev, S., Moskvin, L., & Bulatov, A. (2017). A dispersive liquid-liquid microextraction using a switchable polarity dispersive solvent. Automated HPLC-FLD determination of ofloxacin in chicken meat. Analytica Chimica Acta, 949, 35–42.
Tuzen, M., Uluozlu, O. D., Mendil, D., Soylak, M., Machado, L. O., dos Santos, W. N., et al. (2018). A simple, rapid and green ultrasound assisted and ionic liquid dispersive microextraction procedure for the determination of tin in foods employing ETAAS. Food Chemistry, 245, 380–384.
Wei, J., Zhang, X., Hou, Z., & Lu, X. (2019). High-quality total RNA extraction from Magnolia sieboldii K. Koch seeds: A comparative evaluation. Journal of Forestry Research, 30(1), 371–379.
Zhang, Y., Duan, J., He, M., Chen, B., & Hu, B. (2013). Dispersive liquid liquid microextraction combined with electrothermal vaporization inductively coupled plasma mass spectrometry for the speciation of inorganic selenium in environmental water samples. Talanta, 115, 730–736.
Zong, Y., Chen, J., Hou, J., Deng, W., Liao, X., & Xiao, Y. (2018). Hexafluoroisopropanol-alkyl carboxylic acid high-density supramolecular solvent based dispersive liquid-liquid microextraction of steroid sex hormones in human urine. Journal of Chromatography A, 1580, 12–21.
Funding
This study was supported by grants from the Islamic Azad University, Central Tehran branch.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Mostafavi, B., Feizbakhsh, A., Konoz, E. et al. Salting-out strategy for speciation of selenium in aqueous samples using centrifuge-less dispersive liquid-liquid microextraction. Environ Monit Assess 192, 662 (2020). https://doi.org/10.1007/s10661-020-08609-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08609-3