Abstract
As the application of nanoparticles (NPs) and their release to the environment has increased, it is important to verify their toxicity, with a special emphasis on particle solubilization and the interaction of NP mixtures. In the current study, a model luminescent bacteria, Vibrio fischeri, was employed to test the acute toxicity of individual NPs and their binary mixtures, including metal NPs (ZnNPs, CuNPs) and metal oxide NPs (ZnONPs, CuONPs). The independent action model was used to reflect the synergistic, additive, or antagonistic interactions of binary mixtures of these NPs. The results showed that the median effective concentration (EC50) inhibited the luminescence of V. fischeri were 20.5, 4.1, 11.6, and 118.7 mg L−1 for ZnNPs, CuNPs, ZnONPs, and CuONPs, respectively, suggesting that the toxicity of these NPs to V. fischeri were as the following order: CuNPs > ZnONPs > ZnNPs > CuONPs. The combined effect of NPs were found to be antagonistic for CuNPs-ZnONPs and CuNPs-CuONPs, synergistic for CuONPs-ZnNPs, CuNPs-ZnNPs, and ZnONPs-CuONPs, and additive for ZnNPs-ZnONPs, revealing a complex pattern of possible interactions. The differences of dissolved metal ions partly accounted for the different combined toxicity of binary mixtures of NPs. The findings have important implications for better understanding the true environmental risk of NP mixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Engineered nanoparticles (NPs) are defined as particles with at least two dimensions between 1 and 100 nm (Klaine et al. 2008). Due to the unique and advanced properties, NPs have been widespread used in cosmetics, biological sensors, clinical diagnosis and treatment, environmental remediation, and other applications (Bhatt and Tripathi 2011; Musee 2011; Nyberg et al. 2008; Rantala et al. 2011). Metal-based NPs are some of the most commonly incorporated engineered NPs, such as Zn, Cu, ZnO, and CuO NPs, which are often added to commercial products (Bolyard et al. 2013; Moore et al. 2016). ZnONPs are extensively used in sunscreens, cosmetics, and bottle coatings because of its ultraviolet blocking ability and the visible transparency of nanoparticulate forms (Rousk et al. 2012). CuONPs have been used in wood preservation and antimicrobial textiles because of its antibacterial properties (Heinlaan et al. 2008). CuNPs and ZnNPs have also been widely used in catalytic and coating fields (Mortimer et al. 2010). A large amount of publications have reported that these metal-based NPs were toxic to bacteria (Xu et al. 2010), algae (Navarro et al. 2008), plants (Ma et al. 2010), and fishes (Baek and An 2011) due to particle-specific effect and/or the dissolved metal species. With increasing use, the potential release of these NPs into the environments and the subsequent impacts on ecosystem health are raising greater concern.
Most studies focused on the toxicity of a single type of NPs (Bondarenko et al. 2013; Chang et al. 2012). However, in the natural environment, a variety of NPs may coexist (Hochella et al. 2012; Sharma et al. 2015), so that the toxicity results of individual NPs may not reflect its true environmental impact (Huang et al. 2019). Due to the interactions between different NPs or differences with respect to their interaction with the biological interface, the toxicity of NPs may be substantially higher (synergistic) or lower (antagonistic) than that would be predicted simply by the sum of effects (additive) (Liu et al. 2018; Pagano et al. 2017; Ye et al. 2017). For example, Tong et al. (2015) found that ZnONPs alleviated the toxicity of TiO2NPs by reducing the contact between bacterial cells and TiO2NPs, and, in turn, TiO2NPs decreased the toxicity of ZnONPs due to their adsorption of Zn ions. Wilke et al. (2018) reported that TiO2NPs and AgNPs exhibited synergistic bacterial stress during light exposure due to their enhanced photocatalytic activity and the production of reactive oxygen species (ROS). Therefore, it is important and necessary to evaluate the combined toxicity of NPs for a better understanding of environmental risk caused by NPs. Nevertheless, the study on the joint effects of NP mixtures is very limited.
Vibrio fischeri is a species of bioluminescent bacterium and is ubiquitously distributed in sub-tropical and temperate marine environments (Abbas et al. 2018). Bioluminescence inhibition assay is a rapid, sensitive, cost-effective, and reproducible method for acute toxicity estimation, which has been applied for the joint effects test (Wang et al. 2014). Hence, V. fischeri was chosen as the target organism for the bioluminescence inhibition assay to evaluate the acute toxicity of individual NPs and their binary mixtures. Moreover, the two classic concepts of additivity (i.e., independent action (IA) and concentration addition (CA)) were often used for mixture toxicity predictions (Huang et al. 2019; Yang Liu et al. 2016). The IA model was reportedly better for fitting the data of NP mixtures (Yang Liu et al. 2016) and accordingly was used to elucidate interactions for binary mixtures of NPs. In addition, dissolved metal ions from individual NP and their binary mixtures were determined for the elucidation of the mechanisms underlying the combined toxicity of NP mixtures. This study not only provided new data on metal and metal oxide NP mixtures’ toxic effect, but it also helped to build a better theoretical construct for the systematic evaluation of the ecological risk of NPs.
Materials and methods
Preparation of NPs and bacteria
CuNPs, ZnNPs, CuONPs, and ZnONPs with mean particle sizes of ~ 40 nm and purity greater than 99% were purchased from Sigma Aldrich (St. Louis, MO). Prior to toxicity tests, stock suspensions of NPs were prepared in ultrapure water (pH 7.0) and then sonicated (25 °C, 40 kHz and 250 W) for 1 h to break aggregates and diluted into different concentrations. V. fischeri (ATCC 49387) was obtained from Li Hop Science and Technology Biology Laboratory, and the bacterial suspension was prepared in 3% sterilized NaCl solution for use.
Toxicity tests
Four types of NPs were individually added to sterilized Erlenmeyer flasks containing V. fischeri suspension to perform the single toxicity test. The exposure concentration of NPs were showed in Table 1. The control samples were treated by an equal volume of ultrapure water replacing NP suspension. After exposure for 15 min at 25 °C, the luminescence of V. fischeri was measured using a toxicity analyzer (BHP 9514, Beijing Hamamatsu Co., Ltd., China). Luminescence inhibition, INH (%), was determined as follows:
where ITt was the luminescence of the sample at time t, and ICt was the luminescence value of the control group at time t.
Based on the decrease in luminescence, the obtained concentration relationship data were fitted using linear regression. The median effective concentration (EC50), i.e., the concentrations of NPs that inhibit 50% of the luminescence, was obtained and used as an indicator of individual toxicity. Equitoxic binary mixtures of NPs were prepared based on the EC50 values of individual NP to determine their joint effects. The toxicity test of binary mixtures was conducted as described in the single toxicity test.
Quantification of soluble metal ions
Four types of NPs were individually added to sterilized Erlenmeyer flasks containing 3% NaCl suspension. Following a 15-min incubation at 25 °C, the NP suspensions were centrifuged at 10,000g for 10 min, and the supernatant was collected for dissolved metal ions measurement using inductively coupled plasma-atomic emission spectroscopy (ICP-AES, Thermo Scientific iCAP6000 SERIES, MA, USA).
Data analysis
IA model based on the rules of “additivity” was used to predict the combined toxicity of binary mixtures of NPs. The model assumed that the two toxicants in the mixture did not react with each other and is supposed to be satisfactory for modeling effects of mixtures in which the components differ in uptake pathways or mode of action.
The mathematical expression of the IA model is as follows:
where Rmix is the total effect of binary mixtures and Ri is the effect of the individual NP.
The interactive toxicity in binary mixtures was studied by comparing the observed luminescence inhibition (INHObs) of the binary mixtures with the estimated luminescence inhibition (INHEst). Model values indicate the type of interaction between the two NPs in the mixture, namely, (1) for negative values of INHDiff (INHDiff = INHEst − INHObs), the interaction is considered to be antagonistic, implying that the observed toxicity of the mixture is lower than the sum of toxicities; (2) for positive values of INHDiff, the interaction considered to be synergistic, implying that the observed toxicity of the mixture is higher than the sum of toxicities; (3) otherwise, for values of INHDiff that were not statistically significant from 0 (95% confidence level), the interaction was considered to be merely additive.
Results and discussion
Toxicity of individual metal NPs
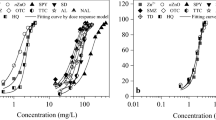
As the concentration of CuNPs increased from 0.5 to 10 mg L−1, the INH of V. fischeri increased from 11.7 to 98.2% (Fig. 1). Similarly, as the concentration of ZnNPs increased from 1 to 50 mg L−1, the INH of V. fischeri increased from 7.4 to 90.7%. The EC50 value of CuNPs was 4.1 mg L−1, and ZnNPs had an EC50 value of 20.5 mg L−1 (Table 2), suggesting that CuNPs had considerably (~ 5 fold) higher toxicity than ZnNPs for V. fischeri. Some studies have also observed that the toxic effects of CuNPs on other organisms; such as bacteria, yeast, and algae; zebra fish; and mice (Chen et al. 2006a, b; Christl and Kretzschmar 2007; Suppi et al. 2015). It has been reported that NPs can physically adsorb over the surface of bacteria and algae cells, and then induced oxidative stress and even cell membrane damage, due to their large surface area and unique physical and chemical properties (González-Pleiter et al. 2019; Sun et al. 2018; Thiagarajan et al. 2019). Moreover, some studies have reported that the toxicity of metal NPs to some organisms could be caused by the dissolved metal ions (Xia et al. 2008). The previous studies showed Cu was more toxic than Zn to Pseudomonas putida CZ1 isolated from metal-polluted soil (Chen et al. 2006a, b). Liu et al. (2016) also found that the toxicity of Cu was higher than that of Zn to lettuce seedlings regardless of the metal being in the form of a cation or NPs.
Toxicity of individual metal oxide NPs
As the concentration of CuONPs increased from 1 to 350 mg L−1, its INH towards V. fischeri increased from 10.6 to 82.2% (Fig. 2). Similarly, as the concentration of ZnONPs increased from 1 to 15 mg L−1, its INH towards V. fischeri increased from 9.9 to 76.7%. The EC50 value of CuONPs was 118.7 mg L−1, and ZnONPs had an EC50 value of 11.6 mg L−1 (Table 2), indicating that ZnONPs were more toxic to V. fischeri than CuONPs. This observation was consistent with previous studies, where ZnONPs showed higher toxicity than CuONPs to V. fischeri and other organisms (Aruoja et al. 2009; Blinova et al. 2010; Heinlaan et al. 2008). Some studies have demonstrated that the toxicity of ZnONPs and CuONPs were associated with the particle-specific effect or/and the ion dissolution (Mu and Chen 2011; Su et al. 2015). Oxidative stress resulting from the highly ROS-generated was considered to be an important toxic mechanism for these metal oxide NPs (Mu and Chen 2011; Remans et al. 2012). The excessive ROS denatured the proteins and nucleic acids, destroyed cell membrane integrity, and eventually resulted in cell inactivation (Lv et al. 2017). Su et al. (2019) reported that ZnONPs led to the higher production of ROS compared with the same amount of CuONPs. Besides, several studies have also found that ZnONPs had a higher solubility than CuONPs. For example, Adam et al. (2015) showed that ZnONPs were largely dissolved after addition to the test medium, whereas CuONPs mostly formed aggregates and only a small fraction of CuONPs was dissolved. Wang et al. (2016) also reported that the negative effect of ZnONPs was mainly due to the dissolution of zinc ions, while the toxicity of CuONPs was caused by the combined effect of copper ions dissolution and the NPs themselves. In the current study, the dissolution of NPs in the toxicity test media were determined at EC50 of NPs. The proportion of dissolved ZnONPs (15.2%) was higher than that of dissolved CuONPs (3.0%) (Table 4), which might partly explain the higher toxicity of ZnONPs.
Toxicity of binary mixtures of NPs
In the natural environment, various NPs are likely to be found together and thus it is important to evaluate the combined toxicity of NPs mixtures. Binary mixtures of NPs were each added at their EC50 values, and the toxicity of six mixtures to V. fischeri was tested, based upon the combinations of four NPs: CuNPs-ZnONPs; CuNPs-ZnNPs; CuONPs-ZnONPs; CuNPs-CuONPs; ZnNPs-CuONPs and ZnNPs-ZnONPs (Table 3). According to the IA model formula, INHEst = Rmix = 50% + 50% − 50% * 50% = 75%. The INHObs of the bioluminescence of V. fischeri for the combinations: ZnONPs-CuONPs, CuNPs-ZnNPs, and ZnNPs-CuONPs were 98.47%, 79.21%, and 98.34%, respectively, which reflected their synergistic interactions. The INHObs of the CuNPs-ZnONPs and CuNPs-CuONPs combinations to the bioluminescence of V. fischeri were 62.61% and 65.01%, respectively, and thus the combined toxicity of these particles was antagonistic. The INHObs of ZnNPs-ZnONPs was 74.84%, implying the additive toxicity of ZnNPs and ZnONPs.
The mixed NPs were likely to interact with each other, and thus affected their toxicity. For example, the presence of HemNPs alleviated the toxicity of AgNPs by surface adsorption of Ag ions (Huang et al. 2019). Wilke et al. (2018) showed synergistic toxicity of AgNPs and TiO2NPs due to the enhanced photocatalytic activity and cellular production of ROS. In the present study, the binary mixtures of ZnONPs-CuONPs, CuNPs-ZnNPs, and ZnNPs-CuONPs exhibited synergistic toxicity to V. fischeri. These binary NPs could be observed to release different metal irons, and it can be speculated that Zn and Cu uptake by V. fischerii did not occur competitively. Furthermore, the concentrations of dissolved ions in these mixture suspensions were found to be higher than those in the individual NPs suspensions (Table 4). The observation suggested that the coexistence of these binary NPs were able to induce the dissolution of ions, which partially accounted for the synergistic effect of these binary NPs to V. fischerii. ZnNPs-ZnONPs, CuNPs-CuONPs combinations released the same metal ions (Zn2+ or Cu2+). According to the point competition theory, when a metal ion occupied a binding site on the cell surface, the binding opportunity of ions of the same property was lowered (Fulladosa et al. 2005). The antagonistic effect between CuNPs and CuONPs might be the result of the receptors for Cu uptake being saturated. The combined effects of ZnNPs and ZnONPs were found to be additive, indicating that there is no obvious interaction between ZnNPs and ZnONPs and the receptors for Zn uptake might be not saturated. For Zn/ZnO, Cu/CuO combinations that released the same metal ions (Zn2+ or Cu2+), it was not able to show synergistic effects due to the capability of bacterial cells to take in metals (Zn or Cu) was limited. Additionally, an antagonistic effects was observed for CuNPs-ZnONPs mixtures, which was consistent with the research of Liu et al. (2016), where the antagonistic effect for ZnONPs on the toxicity of CuNPs might be attributed to “interactions” between dissolved Cu and dissolved Zn, particulate Cu and dissolved Zn, particulate Zn and dissolved Zn, and particulate Zn and dissolved Cu. Besides, NPs had a high adsorption capacity due to their high surface-area-to-volume ratio (Liu et al. 2005), and thus the dissolved irons were likely to be attracted to other NPs. In this study, we observed the lower concentrations of Cu ions in the mixture suspensions of CuNPs-ZnONPs compared to that in the individual CuNP suspensions (Table 4), which might be partly responsible for the antagonistic effects of CuNPs-ZnONP mixtures. In summary, the coexistence of different NPs may exhibit different interactions, including synergistic, additive, or antagonistic interactions. Further study was needed to reveal the mechanisms underlying the combined toxicity of these NPs for a better understanding of their true environmental impact.
Conclusions
The acute toxicity of four typical metal and metal oxide NPs (ZnNPs, CuNPs, ZnONPs, and CuONPs) and their binary mixtures to V. fischeri were determined. The results showed that acute toxicity decreased in the order CuNPs > ZnONPs > ZnNPs > CuONPs. Furthermore, synergistic effects were found for the mixtures CuONPs-ZnONPs, CuONPs-ZnNPs, and CuNPs-ZnNPs, whereas antagonistic effects were observed for the mixtures CuONPs-CuNPs and CuNPs-ZnONPs, and additive effect for the mixtures ZnNPs-ZnONPs. The combined toxicity of the binary mixture of NPs was associated with the situation of metal ions dissolution. This study suggested that the co-existence of different NPs should be taken into account in assessing the realistic environmental risks of NPs.
References
Abbas, M., Adil, M., Ehtisham-ul-Haque, S., Munir, B., Yameen, M., Ghaffar, A., Shar, G. A., Asif Tahir, M., & Iqbal, M. (2018). Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Science of the Total Environment, 626, 1295–1309.
Adam, N., Vergauwen, L., Blust, R., & Knapen, D. (2015). Gene transcription patterns and energy reserves in Daphnia magna show no nanoparticle specific toxicity when exposed to ZnO and CuO nanoparticles. Environmental Research, 138, 82–92.
Aruoja, V., Dubourguier, H. C., Kasemets, K., & Kahru, A. (2009). Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Science of the Total Environment, 407, 1461–1468.
Baek, Y. W., & An, Y. J. (2011). Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Science of the Total Environment, 409, 1603–1608.
Bhatt, I., & Tripathi, B. N. (2011). Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere, 82, 308–317.
Blinova, I., Ivask, A., Heinlaan, M., Mortimer, M., & Kahru, A. (2010). Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environmental Pollution, 158, 41–47.
Bolyard, S. C., Reinhart, D. R., & Santra, S. (2013). Behavior of engineered nanoparticles in landfill leachate. Environmental Science & Technology, 47, 8114–8122.
Bondarenko, O., Juganson, K., Ivask, A., Kasemets, K., Mortimer, M., & Kahru, A. (2013). Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Archives of Toxicology, 87, 1181–1200.
Chang, Y. N., Zhang, M., Xia, L., Zhang, J., & Xing, G. (2012). The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials, 5, 2850–2871.
Chen, Z., Meng, H., Xing, G., Chen, C., Zhao, Y., Jia, G., Wang, T., Yuan, H., Ye, C., Zhao, F., Chai, Z., Zhu, C., Fang, X., Ma, B., & Wan, L. (2006a). Acute toxicological effects of copper nanoparticles in vivo. Toxicology Letters, 163, 109–120.
Chen, X. C., Shi, J. Y., Chen, Y. X., Xu, X. H., Xu, S. Y., & Wang, Y. P. (2006b). Tolerance and biosorption of copper and zinc by Pseudomonas putida CZ1 isolated from metal-polluted soil. Canadian Journal of Microbiology, 52, 308–316.
Christl, I., & Kretzschmar, R. (2007). C-1s NEXAFS spectroscopy reveals chemical fractionation of humic acid by cation-induced coagulation. Environmental Science and Technology, 41, 1915–1920.
Fulladosa, E., Murat, J. C., & Villaescusa, I. (2005). Study on the toxicity of binary equitoxic mixtures of metals using the luminescent bacteria Vibrio fischeri as a biological target. Chemosphere, 58, 551–557.
González-Pleiter, M., Tamayo-Belda, M., Pulido-Reyes, G., Amariei, G., Leganés, F., Rosal, R., & Fernández-Piñas, F. (2019). Secondary nanoplastics released from a biodegradable microplastic severely impact freshwater environments. Environmental Science: Nano, 6, 1382–1392.
Heinlaan, M., Ivask, A., Blinova, I., Dubourguier, H. C., & Kahru, A. (2008). Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere, 71, 1308–1316.
Hochella, M. F., Aruguete, D., Kim, B., & Madden, A. S. (2012). Naturally occurring inorganic nanoparticles: general assessment and a global budget for one of earth’s last unexplored major geochemical components. In Nature’s nanostructures, Taylor & Francis Group: New York, 1, 1–31.
Huang, B., Wei, Z. B., Yang, L. Y., Pan, K., & Miao, A. J. (2019). Combined toxicity of silver nanoparticles with hematite or plastic nanoparticles toward two freshwater algae. Environmental Science and Technology, 53, 3871–3879.
Klaine, S. J., Alvarez, P. J. J., Batley, G. E., Fernandes, T. F., Handy, R. D., Lyon, D. Y., Mahendra, S., McLaughlin, M. J., & Lead, J. R. (2008). Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environmental Toxicology and Chemistry, 27, 1825–1851.
Liu, Y., Liang, P., Guo, L., & Lu, H. B. (2005). Study on the adsorption behavior of heavy metal ions on nanometer TiO2 supported on silica gel. Acta Chimica Sinica, 63, 312–316.
Liu, Y., Baas, J., Peijnenburg, W. J. G. M., & Vijver, M. G. (2016). Evaluating the combined toxicity of Cu and ZnO nanoparticles: utility of the concept of additivity and a nested experimental design. Environmental Science and Technology, 50, 5328–5337.
Liu, Y., Wang, S., Wang, Z., Ye, N., Fang, H., & Wang, D. (2018). TiO2, SiO2 and ZrO2 nanoparticles synergistically provoke cellular oxidative damage in freshwater microalgae. Nanomaterials, 8, 95–99.
Lv, Y., Niu, Z., Chen, Y., & Hu, Y. (2017). Bacterial effects and interfacial inactivation mechanism of nZVI/Pd on Pseudomonas putida strain. Water Research, 115, 297–308.
Ma, X., Geiser-Lee, J., Deng, Y., & Kolmakov, A. (2010). Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Science of the Total Environment, 408, 3053–3061.
Moore, J. D., Stegemeier, J. P., Bibby, K., Marinakos, S. M., Lowry, G. V., & Gregory, K. B. (2016). Impacts of pristine and transformed Ag and Cu engineered nanomaterials on surficial sediment microbial communities appear short-lived. Environmental Science and Technology, 50, 2641–2651.
Mortimer, M., Kasemets, K., & Kahru, A. (2010). Toxicity of ZnO and CuO nanoparticles to ciliated protozoa Tetrahymena thermophila. Toxicology., 269, 182–189.
Mu, H., & Chen, Y. (2011). Long-term effect of ZnO nanoparticles on waste activated sludge anaerobic digestion. Water Research, 45, 5612–5620.
Musee, N. (2011). Nanowastes and the environment: Potential new waste management paradigm. Environment International, 37, 112–128.
Navarro, E., Baun, A., Behra, R., Hartmann, N. B., Filser, J., Miao, A. J., Quigg, A., Santschi, P. H., & Sigg, L. (2008). Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology, 17, 372–386.
Nyberg, L., Turco, R. F., & Nies, L. (2008). Assessing the impact of nanomaterials on anaerobic microbial communities. Environmental Science and Technology, 42, 1938–1943.
Pagano, L., Pasquali, F., Majumdar, S., De La Torre-Roche, R., Zuverza-Mena, N., Villani, M., et al. (2017). Exposure of: Cucurbita pepo to binary combinations of engineered nanomaterials: physiological and molecular response. Environmental Science: Nano, 4, 1579–1590.
Rantala, A., Utriainen, M., Kaushik, N., Virta, M., Välimaa, A. L., & Karp, M. (2011). Luminescent bacteria-based sensing method for methylmercury specific determination. Analytical and Bioanalytical Chemistry, 400, 1041–1049.
Remans, T., Thijs, S., Truyens, S., Weyens, N., Schellingen, K., Keunen, E., Gielen, H., Cuypers, A., & Vangronsveld, J. (2012). Understanding the development of roots exposed to contaminants and the potential of plant-associated bacteria for optimization of growth. Annals of Botany, 110, 239–252.
Rousk, J., Ackermann, K., Curling, S. F., & Jones, D. L. (2012). Comparative toxicity of nanoparticulate CuO and ZnO to soil bacterial communities. PLoS One, 7, e34197.
Sharma, V. K., Filip, J., Zboril, R., & Varma, R. S. (2015). Natural inorganic nanoparticles-formation, fate, and toxicity in the environment. Chemical Society Reviews, 44, 8410–8423.
Su, Y., Zheng, X., Chen, Y., Li, M., & Liu, K. (2015). Alteration of intracellular protein expressions as a key mechanism of the deterioration of bacterial denitrification caused by copper oxide nanoparticles. Scientific Reports, 5, 15824.
Su, Y., Wu, D., Xia, H., Zhang, C., Shi, J., Wilkinson, K. J., & Xie, B. (2019). Metallic nanoparticles induced antibiotic resistance genes attenuation of leachate culturable microbiota: The combined roles of growth inhibition, ion dissolution and oxidative stress. Environment International, 128, 407–416.
Sun, X., Chen, B., Li, Q., Liu, N., Xia, B., Zhu, L., & Qu, K. (2018). Toxicities of polystyrene nano- and microplastics toward marine bacterium Halomonas alkaliphila. Science of the Total Environment, 642, 1378–1385.
Suppi, S., Kasemets, K., Ivask, A., Künnis-Beres, K., Sihtmäe, M., Kurvet, I., Aruoja, V., & Kahru, A. (2015). A novel method for comparison of biocidal properties of nanomaterials to bacteria, yeasts and algae. Journal of Hazardous Materials, 286, 75–84.
Thiagarajan, V., Iswarya, V. P., Julian, A., Seenivasan, R., Chandrasekaran, N., & Mukherjee, A. (2019). Influence of differently functionalized polystyrene microplastics on the toxic effects of P25 TiO2 NPs towards marine algae Chlorella sp. Aquatic Toxicology, 207, 208–216.
Tong, T., Wilke, C. M., Wu, J., Binh, C. T. T., Kelly, J. J., Gaillard, J. F., & Gray, K. A. (2015). Combined toxicity of nano-ZnO and nano-TiO2: from single- to multinanomaterial systems. Environmental Science and Technology, 49, 8113–8123.
Wang, D., Gao, Y., Lin, Z., Yao, Z., & Zhang, W. (2014). The joint effects on Photobacterium phosphoreum of metal oxide nanoparticles and their most likely coexisting chemicals in the environment. Aquatic Toxicology, 154, 200–206.
Wang, D., Lin, Z., Wang, T., Yao, Z., Qin, M., Zheng, S., & Lu, W. (2016). Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both? Journal of Hazardous Materials, 308, 328–334.
Wilke, C. M., Wunderlich, B., Gaillard, J. F., & Gray, K. A. (2018). Synergistic bacterial stress results from exposure to nano-Ag and nano-TiO2 mixtures under light in environmental media. Environmental Science and Technology, 52, 3185–3194.
Xia, T., Kovochich, M., Liong, M., Mädler, L., Gilbert, B., Shi, H., Yeh, J. I., Zink, J. I., & Nel, A. E. (2008). Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano, 2(10), 2121–2134.
Xu, M., Fujita, D., Kajiwara, S., Minowa, T., Li, X., Takemura, T., Iwai, H., & Hanagata, N. (2010). Contribution of physicochemical characteristics of nano-oxides to cytotoxicity. Biomaterials, 31, 8022–8031.
Ye, N., Wang, Z., Fang, H., Wang, S., & Zhang, F. (2017). Combined ecotoxicity of binary zinc oxide and copper oxide nanoparticles to Scenedesmus obliquus. Journal of Environmental Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering, 52, 555–560.
Funding
This work was financially supported by the National Key Research and Development Program of China (2018YFC1901000), the National Natural Science Foundation of China (21577038, 41807462), China Postdoctoral Science Foundation (2017 M611504), and State Key Laboratory of Pollution Control and Resource Reuse Foundation (PCRRF17001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, H., Shi, J., Su, Y. et al. Acute toxicity evaluation of nanoparticles mixtures using luminescent bacteria. Environ Monit Assess 192, 484 (2020). https://doi.org/10.1007/s10661-020-08444-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08444-6