Abstract
In ecological risk assessment, sum-of-toxic-unit approaches based on measured water quality factors such as trace metals are used to infer ecological impacts in the environment. However, it is uncertain whether the use of such approaches yields accurate risk predictions. To address this issue, we investigated and compared (1) water quality, including trace metals, and (2) benthic macroinvertebrate communities in a northern Japanese river receiving treated discharge from an abandoned mine and in a nearby reference river. As a sum-of-toxic-unit approach, we employed a cumulative criterion unit (CCU), namely, the sum of the ratios of the dissolved concentrations of a metal (Cu, Zn, Cd, or Pb) divided by the US Environmental Protection Agency hardness-adjusted environmental water quality criterion for that metal. Compared with the reference sites, at the metal-contaminated sites, the richness, abundance, and structure of macroinvertebrate communities were little affected, with CCUs of 1.7 to 7.4, suggesting that CCU values exceeding 1 do not always indicate marked adverse impacts on these metrics. Further study is still required to derive a more compelling conclusion on the generally applicable relationships between CCUs and ecological impacts on river invertebrates. This would lead to better ecological risk assessments based on sum-of-toxic-unit approaches.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ecological impact of trace metal contamination is a major concern in rivers worldwide (Luoma and Rainbow 2008; Iwasaki and Ormerod 2012). Mine discharges are important sources of trace metals such as Zn, Cu, Cd, and Pb. Although mine discharges are often estimated to be minor sources of overall emissions to surface waters (Naito et al. 2010; Nriagu and Pacyna 1988), elevated concentrations of metals have been frequently observed in freshwater (particularly in rivers near mining sites; (Clements et al. 2000; Hirst et al. 2002; Schmidt et al. 2012; Iwasaki et al. 2011).
In individual mines, including abandoned ones, treatment of discharges and compliance with discharge standards are typical regulatory options to prevent ecological impacts on the receiving aquatic environment. However, it is uncertain whether compliance with discharge standards leads to desirable levels of protection of aquatic ecosystems. This is because, for example, in Japan, the discharge standards for metals such Cu, Cd, and Pb were designed only to protect human health, and it is uncertain whether compliance with the nationwide discharge standard for Zn (5 mg/L for abandoned mines) protects local aquatic life. In Canada, the Metal Mining Environmental Effects Monitoring Program has been used effectively to assess the adequacy of metal mining effluent regulations (Environment Canada 2012).
Sum-of-toxic-unit approaches have been frequently used to evaluate the field impacts of metal mixtures. Typical examples are the cumulative criterion unit (CCU; Clements et al. 2000) and its modification based on the biotic ligand model (chronic criterion accumulation ratio, CCAR; Schmidt et al. 2010). The CCU is calculated as
where Mi and Ci are, respectively, the measured concentration of total or dissolved metal i and the hardness-adjusted environmental water quality criterion (EQC) for metal i (U. S. Environmental Protection Agency 2002). In this approach, a CCU value of 1.0 has been used as a threshold at which adverse ecological effects may be expected (Clements et al. 2000), but compelling evidence about whether the use of such a threshold is appropriate for predicting adverse effects occurring in the environment is still lacking. Given that predicting the ecological impacts of metal mixtures is challenging, even for laboratory experiments (Iwasaki and Gauthier 2016), to improve our understanding of this issue, it is still important to directly investigate the actual ecological impacts in rivers receiving metal mining discharges.
Here, to obtain useful insights into ecological risk assessments based on sum-of-toxic-unit approaches (in this case, CCU), we investigated the effects of treated discharge from an abandoned mine on benthic macroinvertebrates in a northern Japanese river. Benthic macroinvertebrates have different, taxon-specific sensitivities to metals (Iwasaki et al. 2018b) and have been used widely to assess the ecological impacts of chemicals, including metals, on river communities (Clements et al. 2000; Hirst et al. 2002; Schmidt et al. 2012; Iwasaki et al. 2011; Jones et al. 2019). For our investigation, we employed two study designs: (1) a comparison of water quality parameters, including metal concentrations and macroinvertebrate communities, upstream and downstream of the inflow of the treated discharge (i.e., an upstream-downstream comparison), and (2) a comparison between multiple study sites established in a river receiving mine discharge and a reference river.
Materials and methods
Study site
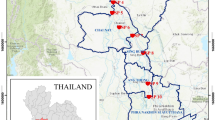
Field samplings were performed in spring (27 and 28 June) in 2017 at a total of eight sites located on the Ofuntarumanai and Tokushibetsu rivers in Hokkaido Prefecture, northern Japan (Fig. 1). An abandoned mine is located in the upstream area of the Ofuntarumanai River. The mine yielded mainly Cu, Zn, and Pb but was abandoned in 1976. Mine discharge was treated by flow through artificial wetlands and was discharged into a small tributary (stream width < 1 to 2 m) of the Ofuntarumanai River. However, we chose not to establish study sites in the tributary because the water flow there was very limited during the field sampling.
First, to assess the effects of the treated mine discharge on water quality and macroinvertebrates just downstream of the inflow into the Ofuntarumanai River, we chose two sites, S1a and S1b (i.e., a typical upstream–downstream comparison; surface–water width approximately 5 m; Fig. 1). Second, to investigate the effects at contaminated sites chosen downstream of S1b (i.e., at S2 to S4), we also selected three reference sites (R2 to R4) with respective elevations similar to those of S2 to S4. The surface–water width was approximately 10 to 20 m at these sites. The contaminated and reference sites were chosen carefully to ensure that the physicochemical factors (other than the concentrations of metals) were as similar as possible. All study sites, including S1a and S1b, were cobble-dominated, well-defined riffles.
Benthic macroinvertebrates
At each study site, we used a Surber sampler (mesh size, 0.355 mm) to collect macroinvertebrates from five stones randomly chosen from riffles (maximum diameter, 15 to 20 cm). Similar methods have been used in previous studies (Iwasaki et al. 2009, 2012, 2018a). Benthic samples were preserved in 99.5% ethanol in the field, and the macroinvertebrates remaining on a 0.5-mm sieve were generally identified to genus or species level in the laboratory. Sampling was timed to be done when the river levels decreased to normal after the end of the snowmelt runoff period and when most macroinvertebrate species were likely to be present in their aquatic larval stages, before adult emergence.
The following invertebrate community metrics for richness (number of taxa per stone) and abundance (number of individuals per stone) were used: total taxon richness; total abundance; total abundance and taxon richness of three major aquatic insect groups (Ephemeroptera (mayflies), Trichoptera (caddisflies), and Diptera); and abundance of four major families (Baetidae, Ephemerellidae, Heptageniidae, and Hydropsychidae). In addition, we complementarily analyzed the responses of 20 dominant taxa (mostly genera or species) that were defined as those that accounted for more than 5% of the total abundance at any sampled stone and that were present at more than 20% of the sampled stones (i.e., > 8 stones out of a total of 40 stones collected). Stonefly richness and abundance were not included in this analysis, because limited taxon richness (≤ 3 taxa at 80% of sampled stones) and limited numbers of individuals (≤ 10 individuals at more than half of the sampled stones) were present in our samples. For all of these invertebrate metrics, averages of the five stones at each site were calculated and used for later analyses. Macroinvertebrate abundance per stone was log-transformed (x + 1) before calculation of the site means to satisfy the assumptions (i.e., normality and homoscedasticity) of the statistical analyses used (see below).
Physicochemical parameters
We measured water temperature, dissolved oxygen (DO), and pH during the sampling by using multiparameter portable meters (AS810, AS ONE, Osaka, Japan). Electrical conductivity was also measured by using a water quality meter (PCTestr 35, Eutech Instruments, Singapore PCTestr 35, Eutech Instruments, Singapore). Water samples for analysis of dissolved metals, dissolved organic carbon (DOC), and major ions, including calcium and magnesium, were collected at the time of macroinvertebrate sampling. All the water samples were filtered (0.45-μm mesh size), and the water samples for dissolved metals analysis were acidified by adding ultrapure nitric acid and then refrigerated in the field. Copper (Cu), zinc (Zn), cadmium (Cd), and lead (Pb) concentrations were determined by using an inductively coupled plasma mass spectrometer (NexION 350; PerkinElmer Japan, Yokohama, Japan) after sample preparation according to EPA method 200.8 (U. S. Environmental Protection Agency 1994). The limits of quantification were 0.038 μg/L for Cu, 0.46 μg/L for Zn, and 0.008 μg/L for both Cd and Pb. The DOC samples were analyzed with a total organic carbon (TOC) analyzer (TOCCSN; Shimadzu, Tokyo, Japan). The concentrations of major ions (Na, K, Ca, Mg, Cl, and SO4) were analyzed with an ion chromatograph (DIONEX ICS-1100/2100; Thermo Fisher Scientific, Tokyo Japan). Water hardness was then calculated as 2.497 × [Ca] + 4.118 × [Mg].
CCU values were calculated on the basis of the measured concentrations of dissolved metals and the US EPA hardness-adjusted EQCs (U. S. Environmental Protection Agency 2002; see the Introduction for more details). Hardness-adjusted EQCs for Cu, Zn, Cd, and Pb were calculated at a water hardness of 10 mg/L, which was based on the observed range of water hardness (Table 1). We did not take water quality parameters other than water hardness (e.g., pH and DOC) into account in calculating the EQCs, because these values varied little among study sites (Table 1; see Results section for more details), and at the time of writing US EPA EQCs based on biotic ligand models (BLMs), which consider the influences of such water quality parameters on metal bioavailability and toxicity, were available only for copper (U. S. Environmental Protection Agency 2007).
Surface–water width at the studied riffles was measured onsite and was averaged if benthic samples were collected at multiple riffles within individual sites. Maximum current velocity and depth were determined on the basis of measurements taken at 10 to 20 locations in the studied riffles; current velocity was measured at 60% of water depth by using an electromagnetic velocity meter (VR-301; KENEK, Tokyo, Japan). Before invertebrate collection, water depth and current velocity (at 60% depth) were also measured above the upper surface of each stone sampled. Stone size was calculated as the product of maximum diameter and maximum circumference; it differed little among study sites (Table 2). No clear relationships between stone size and macroinvertebrate metrics were observed (data not shown; as was also observed by Iwasaki et al. 2009, 2012). Therefore, we did not standardize all macroinvertebrate metrics to unit area. The catchment area of each study site was estimated by using a digital elevation model (50-m grid; Geographical Survey Institute of Japan, www.gsi.go.jp/ENGLISH/index.html) and the hydrologic tools in ArcGIS 10.2 for desktop (Esri Japan, Tokyo, Japan).
Statistical analysis
All statistical analyses were done with R version 3.4.4 (R Core Team 2018). A significance level (α) of 0.05 was used.
For the upstream–downstream comparison, differences in the mean values of macroinvertebrate metrics between S1a and S1b were analyzed by using a two-sample two-sided Welch’s t-test (Welch 1947). To compare macroinvertebrate metrics between the downstream-contaminated sites (S2 to S4) and the corresponding reference sites (R2 to R4), we first calculated the 90% confidence interval of each metric on the basis of the standard deviation of the site means for the three reference sites; we regarded this as the range for the reference sites (hereafter called the reference range). We determined whether the 90% confidence intervals of the site means at the contaminated sites overlapped with the reference range, and we operationally interpreted overlap with the reference range to be a noncritical observed effect. Although each reference site was carefully selected to match the elevation and other physicochemical parameters of its paired-contaminated site, it would not have been accurate to compare the macroinvertebrate metrics of each pair by assuming that each reference site was perfectly matched to the corresponding contaminated site. Therefore, we chose to use reference ranges to evaluate the effects of mine discharge on the river receiving that drainage.
In addition, nonmetric multidimensional scaling (NMDS) based on Bray-Curtis dissimilarities (Manly and Navarro Alberto 2016) was performed to compare the structures of macroinvertebrate communities among the study sites. Sites S1a and S1b were included in this analysis to complementarily visualize the overall patterns of the community structures of the macroinvertebrates sampled. Statistical differences in community structure between the three contaminated sites and three reference sites, as well as between S1a and S1b, were evaluated by using permutational multivariate analysis of variance (PERMANOVA; Anderson 2001). The NMDA and PERMANOVA analyses were performed with the “metaMDS” and “adonis” functions, respectively, of the R package vegan version 2.5–3. Data used for Tables and Figs. 2–4 are available in Electronic Supplementary Material (ESM).
Results
Upstream–downstream comparison
No substantial changes in any of the measured physicochemical parameters, including concentrations of metals, were observed between S1a and S1b (Tables 1 and 2; see Table A1 in ESM for major ions, including calcium and magnesium), although we had expected to see higher concentrations of metals at S1b, the site immediately downstream of the treated water inflow. The dissolved concentrations of metals at S1a and S1b were 1.1 to 4.6 times the US EPA EQCs at a water hardness of 10 mg/L (see Table 1; U. S. Environmental Protection Agency 2002); these exceedances were more evident for Cd and Pb than for Cu and Zn. Also, the metal concentrations at S1a and S1b were approximately 7 to 100 times the mean concentrations at R2 to R4 (Table 1).
As was the case with the water quality measurements, no significant differences in all 12 invertebrate metrics, with the exception of mayfly (Ephemeroptera) richness, were observed between S1a and S1b (Fig. 2). A significant increase in mayfly richness—one of the most metal-sensitive metrics (Iwasaki et al. 2012; Clements et al. 2000)—was detected at S1b. However, the quantitative difference in mean mayfly richness between the two sites was not large (1.1 taxa), and the mean mayfly abundances at these sites were below ten individuals per stone. In the case of the dominant taxa (Fig. A1 in ESM), significant decreases in the abundances of Agathon sp. (Blephariceridae) and Simulium sp. (Simuliidae) and a significant increase in the abundance of Polypedilum sp. (Chironomidae) were detected at the downstream site, although the abundances of these insects were also limited (site means were generally below ten individuals per stone; Fig. A1).
Comparison between downstream-contaminated sites and reference sites
The dissolved concentrations of Cu, Zn, Cd, and Pb at the three downstream-contaminated sites (S2 to S4) were approximately 3 to 50 times those at the three reference sites (Table 1). As expected, sites farther upstream in the river receiving the mine discharge were more heavily contaminated than sites farther downstream, as shown by the metal concentrations and the CCU values (S2, 7.4; S3, 3.4; S4, 1.7: Table 1). The concentrations of metals at the most downstream site (S4) were either marginally or much lower than the US EPA EQCs, but the CCU value was higher than 1. Although the pH and water temperature were marginally lower at the contaminated sites than at the reference sites, other water quality measurements (e.g., DO) were comparable (Table 1). Comparison of physical parameters revealed that the estimated catchment areas in the reference areas were generally larger (Table 2: particularly when comparing S2 vs. R2, S3 vs. R3, and S4 vs. R4), but there was little difference in the other measurements between the downstream-contaminated sites and the three reference sites.
The fact that the 90% confidence intervals of almost all the macroinvertebrate metrics calculated at S2 to S4, with the exception of caddisfly (Trichoptera) abundance and the abundance of hydropsychiid caddisflies, overlapped with the reference ranges (i.e. the 90% confidence intervals calculated from the site means of the three reference sites; Fig. 3) indicated that there were noncritical effects. Even the mean values of most of the macroinvertebrate metrics at S2 to S4 fell within the corresponding reference ranges (Fig. 3). Similar longitudinal changes in several metrics (e.g., increased mayfly (Ephemeroptera) richness and abundance at the more downstream sites) were evident between the two rivers; in such cases, macroinvertebrate richness and abundance were comparable between sites with similar elevations (i.e., S2 vs. R2, S3 vs. R3 and S4 vs. R4).
In contrast, the 90% confidence intervals of caddisfly abundance at S2 and of the abundance of hydropsychiid caddisflies at S2 and S3 did not overlap with the corresponding reference ranges (Fig. 3). Except in the case of one stone collected at S2 where > 20 individuals of limnephilid caddisflies (Neophylax japonicus) were present, 89% of the caddisflies counted at S2 were hydropsychiid caddisflies, as were 38% of those counted at S3. Compared with the mean abundance at the reference sites, a 56% reduction in the abundance of hydropsychiid caddisflies was observed at S2 and an 80% reduction at S3 (see also the results for Hydropsyche orientalis in Fig. A2 in ESM).
Similar results were obtained for the dominant taxa (Fig. A2). Noncritical effects on the abundances of the 20 dominant taxa at the contaminated sites were found, with the exception of a few examples, namely, H. orientalis at S2 and S3, Epeorus curvatulus at S2, and Antocha sp. at S2. Interestingly, relatively high abundances of Baetis thermicus, which is often present at heavily metal-contaminated sites (e.g., Iwasaki et al. 2012), were observed at S2 and S3 compared with those at the reference sites.
Nonmetric multidimensional scaling
Results of the NMDS ordination and PERMANOVA showed that the community structures of macroinvertebrates did not differ significantly between S2–S4 and R2–R4 (Fig. 4; PERMANOVA, F = 1.4, P = 0.17). The values of NMDS 1 increased at the more downstream sites in both rivers, as was observed with the longitudinal changes in some of the macroinvertebrate metrics (see Fig. 3). A difference in the structures of the invertebrate communities between S1a and S1b was detected (PERMANOVA, F = 2.7, P = 0.022).
Discussion
Our results from the comparison of contaminated and reference sites in a northern Japanese river demonstrated that the richness, abundance, and structure of macroinvertebrate communities were affected little at metal-contaminated sites, where CCU values were as high as 7.4. Although our study sites were limited in number, these results are generally consistent with the conclusions of Iwasaki et al. (2011, 2012), who have conducted field surveys in other regions of Japan. For example, Iwasaki et al. (2011) have estimated the “safe” concentrations of Zn (70 to 115 μg/L) for multiple richness metrics of river macroinvertebrates, and their study sites with those Zn concentrations had CCU values of 9.0 to 15.0. However, Clements et al. (2000) have demonstrated significant reductions in several metrics, including mayfly richness and abundance, at sites with CCU values of 2.0 to 10.0 in Colorado mountain rivers. Also, Schmidt et al. (2010) have shown some adverse changes in benthic communities at a CCAR value of less than 1 when US EPA EQC values were corrected on the basis of BLM predictions. In general, macroinvertebrate communities in rivers receiving mine discharges may be affected by metals other than the typically measured ones, e.g., Cu, Zn, Cd, and Pb. Failure to measure the concentrations of the metals that are actually affecting macroinvertebrates leads to the underestimation of CCUs and then to the overestimation of the ecological impacts of metals on the basis of CCUs (Iwasaki et al. 2018b). Further study is still required to derive more compelling conclusions on the general applicability of the relationship between CCUs and effects on river macroinvertebrate richness and abundance.
Although relatively large reductions were observed in caddisfly abundances (mainly hydropsychiid caddisflies) at some of the contaminated sites, hydropsychiid caddisflies are usually not the most sensitive family among aquatic insect families: their sensitivity is often reported to be moderate to low (Iwasaki et al. 2018b; Clements et al. 2000). Similar results (i.e., reduced abundance at S2 and S3) were observed only for Antocha sp. (Tipulidae; Fig. A2), which are sessile on the river bottom as well as net-spinning hydropsychiid caddisflies. Therefore, the reduced abundances of these sessile invertebrates might have been due to lower stability of the gravel beds at S2 and S3 rather than to the metal contamination itself.
The dissolved metal concentrations reported here were obtained from single occasion grab samples during the field survey (June, 2017). From multiple grab samples collected at S2 (seven samples in July–October, 2016) and S4 (four samples in July–December, 2016), the mean respective concentrations (± standard errors) of total Zn were 37 (± 3) and < 10 (NA) μg/L (Hokkaido Prefecture 2017). The mean concentration of Zn at S2 was close to our measurement (29 μg/L). The total concentrations of other metals (Cu, Cd, and Pb) at these sites were below the limits of quantification (0.01, 0.01, and 0.005 μg/L, respectively; Hokkaido Prefecture 2017), and these results do not contradict results of our original measurements. Although the available measured data are limited, these comparisons suggest that our measurements of dissolved metal concentrations are less likely to be overestimates of the annual mean concentrations of the metals.
In contrast to our initial expectation, the dissolved concentrations of metals differed little between the sites upstream and immediately downstream of the mine discharge inflow (i.e., S1a and S1b). Consistent results were obtained in further water samplings conducted in July 2018 and September 2018 (Iwasaki et al., in preparation). Although we need to accumulate monitoring data on metal concentrations and knowledge about their sources, an important implication of our findings is that the contribution of treated mine discharge to the overall loads of metals in the particular river studied may be limited.
Our results also suggested that there were no marked changes in the abundance and richness of macroinvertebrate communities between the upstream and downstream sites (i.e., S1a and S1b), although reduced abundances of a few dominant taxa were observed at the downstream site. The values of several community metrics, including mayfly abundance and richness, were lower at these sites than at the three downstream reference sites (R2 to R4; Figs. 2 and 3); moreover, although qualitative, the results of NMDS supported the hypothesis that the macroinvertebrate communities at S1a and S1b differed from those at R2 to R4 (Fig. 4). However, because we did not collect benthic macroinvertebrate samples at reference sites relevant to S1a and S1b, further study is needed to evaluate whether such differences were associated with the metal pollution or with natural physical parameters associated with the evaluation.
Here, we used river macroinvertebrates as an indicator group to infer the ecological impacts on a northern Japanese river receiving mine discharge. However, because the utility of a given indicator group in predicting the responses (e.g., species richness) of other taxonomic groups in aquatic ecosystems is generally low (Heino 2010), investigating the responses of other groups such as periphyton and fish in the study river, as well as performing macroinvertebrate sampling in different seasons (Iwasaki et al. 2011), would likely provide a more robust assessment of the overall ecosystem protection and add useful information for management of the mine discharge.
Finally, together with the previous findings (Iwasaki et al. 2011, 2012), our results suggest that CCU values exceeding 1 do not always indicate marked adverse impacts on the richness and abundance of macroinvertebrate communities. Because the dissolved concentrations of more than one metal were higher than, or close to, the values of the USEPA EQCs at downstream-contaminated sites, a similar implication is applicable when we consider the exceedances of individual metal EQCs. This result thus supports the general prediction that environmental quality benchmarks are generally conservative (Chapman 2018). However, sum-of-toxic-unit approaches can be based on other environmental benchmarks (e.g., environmental water quality standards established in other countries and those that fully consider the bioavailability of trace metals) in evaluating ecological risks. Therefore, although the ranges of these benchmarks for some metals may be similar in different countries (see, e.g., Iwasaki and Ormerod 2012)—and, as an example, the sum-of-toxic-unit values calculated on the basis of UK and Australian benchmarks were similar to the CCU values (see Table A2 in ESM)—the applicability of our conclusion to the ecological interpretation of CCU and sum-of-toxic-unit values exceeding 1 needs further investigation. International harmonization of water quality guideline derivations has been discussed (Merrington et al. 2014), so further testing of such a conclusion may become easier in the not-so-distant future.
Conclusions
The results of our field survey in a northern Japanese river receiving treated mine discharge suggest that CCU values exceeding 1 do not always indicate marked adverse impacts on the richness and abundance of macroinvertebrate communities. Further study is, however, required to derive the generally applicable relationships between CCU and sum-of-toxic-unit values and ecological impacts on river invertebrates. Accumulation of this information would be valuable for better inferring and managing ecological impacts on the basis of sum-of-toxic-unit approaches.
References
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecology, 26(1), 32–46. https://doi.org/10.1046/j.1442-9993.2001.01070.x.
Chapman, P. M. (2018). Environmental quality benchmarks—the good, the bad, and the ugly. Environmental Science and Pollution Research, 25(4), 3043–3046. https://doi.org/10.1007/s11356-016-7924-2.
Clements, W. H., Carlisle, D. M., Lazorchak, J. M., & Johnson, P. C. (2000). Heavy metals structure benthic communities in Colorado mountain streams. Ecological Applications, 10(2), 626–638.
Environment Canada. (2012). Metal mining technical guidance for environmental effects monitoring. Gatineau: Environment Canada.
Heino, J. (2010). Are indicator groups and cross-taxon congruence useful for predicting biodiversity in aquatic ecosystems? Ecological Indicators, 10(2), 112–117. https://doi.org/10.1016/j.ecolind.2009.04.013.
Hirst, H., Jüttner, I., & Ormerod, S. J. (2002). Comparing the responses of diatoms and macroinvertebrates to metals in upland streams of Wales and Cornwall. Freshwater Biology, 47(9), 1752–1765.
Hokkaido Prefecture (2017). Report on work, survey, and analysis for preventing abandoned mine pollution in the fiscal year of 2016.
Iwasaki, Y., & Gauthier, P. (2016). Concentration addition and response addition to analyze mixture toxicity: Is it worth testing? Environmental Toxicology and Chemistry, 35(3), 526–527. https://doi.org/10.1002/etc.3263.
Iwasaki, Y., Kagaya, T., & Matsuda, H. (2018a). Comparing macroinvertebrate assemblages at organic-contaminated river sites with different zinc concentrations: Metal-sensitive taxa may already be absent. Environmental Pollution, 241, 272–278. https://doi.org/10.1016/j.envpol.2018.05.041.
Iwasaki, Y., Kagaya, T., Miyamoto, K., & Matsuda, H. (2009). Effects of heavy metals on riverine benthic macroinvertebrate assemblages with reference to potential food availability for drift-feeding fishes. Environmental Toxicology and Chemistry, 28(2), 354–363.
Iwasaki, Y., Kagaya, T., Miyamoto, K., & Matsuda, H. (2012). Responses of riverine macroinvertebrates to zinc in natural streams: Implications for the Japanese water quality standard. Water, Air, and Soil Pollution, 223(1), 145–158.
Iwasaki, Y., Kagaya, T., Miyamoto, K., Matsuda, H., & Sakakibara, M. (2011). Effect of zinc on diversity of riverine benthic macroinvertebrates: Estimation of safe concentrations from field data. Environmental Toxicology and Chemistry, 30(10), 2237–2243.
Iwasaki, Y., & Ormerod, S. J. (2012). Estimating safe concentrations of trace metals from inter-continental field data on river macroinvertebrates. Environmental Pollution, 166, 182–186.
Iwasaki, Y., Schmidt, T. S., & Clements, W. H. (2018b). Quantifying differences in responses of aquatic insects to trace metal exposure in field studies and short-term stream mesocosm experiments. Environmental Science & Technology, 52(7), 4378–4384. https://doi.org/10.1021/acs.est.7b06628.
Jones, J. I., Murphy, J. F., Collins, A. L., Spencer, K. L., Rainbow, P. S., Arnold, A., Pretty, J. L., Moorhouse, A. M. L., Aguilera, V., Edwards, P., Parsonage, F., Potter, H., & Whitehouse, P. (2019). The impact of metal-rich sediments derived from mining on freshwater stream life. Reviews of Environmental Contamination and Toxicology, 247, 1–79. https://doi.org/10.1007/398_2018_21.
Luoma, S. N., & Rainbow, P. S. (2008). Metal contamination in aquatic environments. Cambridge: Cambridge University Press.
Manly, B. F. J., & Navarro Alberto, J. A. (2016). Multivariate statistical methods: A primer (4th ed.). Boca Raton: CRC press.
Merrington, G., An, Y.-J., Grist, E. P. M., Jeong, S.-W., Rattikansukha, C., Roe, S., et al. (2014). Water quality guidelines for chemicals: Learning lessons to deliver meaningful environmental metrics. Environmental Science and Pollution Research, 21(1), 6–16. https://doi.org/10.1007/s11356-013-1732-8.
Naito, W., Kamo, M., Tsushima, K., & Iwasaki, Y. (2010). Exposure and risk assessment of zinc in Japanese surface waters. Science of the Total Environment, 408(20), 4271–4284.
Nriagu, J. O., & Pacyna, J. M. (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace-metals. Nature, 333(6169), 134–139.
R Core Team (2018). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Schmidt, T. S., Clements, W. H., Mitchell, K. A., Church, S. E., Wanty, R. B., Fey, D. L., Verplanck, P. L., & San Juan, C. A. (2010). Development of a new toxic-unit model for the bioassessment of metals in streams. Environmental Toxicology and Chemistry, 29(11), 2432–2442.
Schmidt, T. S., Clements, W. H., Wanty, R. B., Verplanck, P. L., Church, S. E., San Juan, C. A., Fey, D. L., Rockwell, B. W., DeWitt, E., & Klein, T. L. (2012). Geologic processes influence the effects of mining on aquatic ecosystems. Ecological Applications, 22(3), 870–879.
U. S. Environmental Protection Agency (1994). Method 200.8: Determination of trace elements in waters and wastes by inductively coupled plasma-mass spectrometry, Revison 5.4. Cincinnati, OH.
U. S. Environmental Protection Agency (2002). National Recommended Water Quality Criteria: EPA822-R-02-047. Washington.
U. S. Environmental Protection Agency (2007). Aquatic life ambient freshwater quality criteria—Copper 2007 revision, EPA-822-F-07-001. Washington
Welch, B. L. (1947). The generalization of student's' problem when several different population variances are involved. Biometrika, 34(1/2), 28–35.
Acknowledgments
We are grateful to Susumu Norota and Kazuto Ohmori of Hokkaido Research Organization for their help in study site selection, and Shosaku Kashiwada and Daiki Kitamura of Toyo University for their help in metal analysis. The paper does not necessarily reflect the policies or views of any government agencies. Useful comments by anonymous reviewers are greatly appreciated.
Funding
Preparation of this manuscript was supported partly by the Environment Research and Technology Development Fund (5RF-1801) of the Environmental Restoration and Conservation Agency of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iwasaki, Y., Fujisawa, M., Ogino, T. et al. Does a sum of toxic units exceeding 1 imply adverse impacts on macroinvertebrate assemblages? A field study in a northern Japanese river receiving treated mine discharge. Environ Monit Assess 192, 83 (2020). https://doi.org/10.1007/s10661-019-8047-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-8047-2