Abstract
Phytoplankton and epipelon assemblages form the main constituents, and they are producers in aquatic ecosystems, such as streams and rivers. This study was carried out between May 2008 and April 2009 to determine the impacts of polluted water on species variations, compositions, and community metrics in phytoplankton and epipelon at six stations on Ankara Stream. A total of 231 taxa were recorded during the study period, with 131 Bacillariophyta, 3 Charophyta, 41 Chlorophyta, 30 Cyanobacteria, 25 Euglenophyta, and 1 Ochrophyta. Heterogeneity of the stream stations was determined by the use of hierarchical cluster analysis (HCA). Community metrics were compared by using non-parametric tests, while canonical correspondence analysis (CCA) was used for the relationships between environmental variables and species. Variations in water quality and species composition along the stream flow revealed a significant spatial heterogeneity (p < 0.05). However, the upper stations of the stream were represented by unpolluted water quality with low nutrients and conductivity, and the mid- and downstream stations were characterized by high concentrations of ammonia (up to 60 mg L−1) and o-phosphate (up to 25 mg/L), with low concentrations of dissolved oxygen (< 1 mg L−1). The results, clearly supported by indicator taxa, showed that various domestic and industrial discharges affected the increase in pollution and the spatial heterogeneity. The findings obtained in this study will contribute to future improvements in Ankara Stream watershed studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Distribution, abundance, diversity, and composition of algae in different habitats reflect physicochemical conditions of aquatic ecosystems in general and their pollution and nutrient statuses in particular (Anene 2003). Spatial and temporal variations in algal assemblages of running waters are important tools for environmental assessment, because algae, especially diatoms, respond rapidly to varying conditions (Ács et al. 2004; Bere and Tundisi 2011). Several studies have reported spatial and temporal variations of algae (Bowes et al. 2012; Kadhim et al. 2013; Gillett et al. 2016). While temporal variations have been predominantly observed in large rivers (Tornés et al. 2014; Nardelli et al. 2016), spatial variations have been recorded to a greater degree in small rivers and streams (Dalu et al. 2015; Shulkin and Nikulina 2015).
Discharges from industrial activities and urban settlements result in high concentrations of various pollutants in the receiving waters. High concentrations of organic matter, nitrogen, and phosphorus, resulting in low concentrations or deficits of dissolved oxygen, are the most important indicators of anthropogenic eutrophication (Duong et al. 2006). Temporally and spatially varying environmental factors can influence the compositions of epipelon and phytoplankton in streams (Pan et al. 1999; Leira and Sabater 2005). On the other hand, suspended algae originate from benthic habitats in small lotic systems and are transported downstream in the water column (Stevenson et al. 1999). Therefore, monitoring of both phytoplankton and epipelon for assessment of watershed conditions in streams may provide more reliable results.
Ankara Stream flows through a multi-purpose environment, receiving wastes from industrial and urban/rural areas at different stream reaches. It is clear that temporal and spatial variations of algal communities in a stream exposed to different impacts may be more conspicuous. The objective of this study was to determine the spatial and temporal variations in phytoplankton and epipelon communities and their relationships with water quality variations and distributions in a polluted urban stream.
Material and methods
Study area and sampling stations
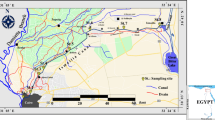
Ankara Stream is one of the major tributaries of the Sakarya River, which flows into the Black Sea. The stream originates through the confluence of different stream reaches and runs through Ankara, the capital of Turkey (Fig. 1). The stream, which is approximately 140 km in length and has a drainage basin covering 3153 km2, receives 90% of the domestic and industrial effluents of Ankara Province (Vural et al. 1997).
Station 1 is fed mainly by a small irrigation reservoir (Bayındır), with a surface area of 1 km2, and is located near the watercourse. Station 2, near Ortaköy, is affected by the village’s waste effluents. Station 3, located in the city center, is the station most affected by municipal discharges, while Station 4, at the entrance to Sincan, receives a lot of domestic sewage. Station 5 is located in an area where agricultural and industrial enterprises are concentrated and has a wastewater treatment plant adjacent to the station, while station 6 is located solely within an agricultural region.
Data collection
All samples were taken monthly at six stations on Ankara Stream (Fig. 1) between May 2008 and April 2009. For qualitative analysis, phytoplankton sampling was done with a 25-μm mesh size plankton net and 1-l PVC flasks, and the samples were preserved in formaldehyde in such a way as to have the final concentration at 4% (APHA et al. 1998). Epipelic biofilm samples were collected by pipetting 10 aliquots of streambed subsamples from 20 to 30 cm below the surface. Environmental variables were measured in situ using temperature and dissolved oxygen (DO) with YSI 55, conductivity and salinity with YSI 30, pH with Thermo Orion 210, and current speed with Global Water FP 211 model instrument, respectively. To analyze other variables (Fig. 2), water samples stored at 4 °C were transported to the State Water Works (DSI) laboratory and processed by standard methods (APHA et al. 1998).
Water quality characteristics of Ankara Stream during the study period. Horizontal lines in the boxes indicate median values, while plus symbols indicate averages. Italic letters on the graphs indicate the results of Dunn’s test, after Kruskal–Wallis, in which levels not connected by the same letter are significantly different
Identification of algae
The collected water samples were transported to the laboratory in a cool box. One milliliter of concentrated and homogenized phytoplankton samples was transferred into a Sedgewick-Rafter counting chamber and examined with a Nikon binocular microscope at a magnification of 200×. Epipelic samples were emptied into petri dishes, covered with 22 × 22 mm lamels, and allowed for photo-taxis. The sediment samples were left to settle, and after which temporary slides were made with glycerin for counting on a Nikon binocular microscope of 600×. For the identification of diatoms, frustules were mounted on Canada Balsam/Entellan after cleaning and subjected to treatment with hot concentrated hydrochloric acid and potassium permanganate and examined on a magnification of 1000×. Reference resources were used for identification (Huber-Pestalozzi et al. 1982; Korshikov 1987; Krammer and Lange-Bertalot 1986, 1988, 1991a, b; Round et al. 1990; John et al. 2002).
Phytoplankton species counting was performed with a counting chamber and reported as organisms per milliliter (APHA et al. 1998), while benthic species were recorded as units or organisms per unit area (cm2). Phytoplankton biomass was determined through chlorophyll a (Chl-a) by using the methanol method (Youngman 1978) and a Shimadzu brand spectrophotometer. The average bio-volumes of all the cells were calculated to the geometric models or shapes that are most similar to the real shapes, and individual numbers were converted to bio-volumes (Hillebrand et al. 1999). Species richness (S) was considered to be the number of taxa present in each sample, and diversity (H′) was calculated (Shannon and Weaver 1963).
Statistics and data analysis
The normality of data on the water quality, epipelon and phytoplankton metrics, and composition was tested using Shapiro–Wilk test, and it showed that the dataset did not follow a normal distribution. Data comparison was done using Kruskal–Wallis signed-rank test (K-W). Dunn’s multiple comparison test was used to discriminate significant differences. Basic statistics were conducted using GraphPad Prism v.7 software.
Heterogeneity of sampling stations was determined using hierarchical cluster analysis (HCA). Using squared Euclidean distances as a measure of similarity, hierarchical agglomerative cluster analysis was performed on the transformed data set according to Ward’s method. The linkage distance was reported as Dlink/Dmax, which represents the quotient of the linkage distance for a particular case divided by the maximum distance; the quotient was multiplied by 100 to standardize the linkage distance represented on the y-axis. Dlink/Dmax × 100 < 50 indicates the statistical meaningfulness of the similarity of the clusters (Simeonov et al. 2003; Sinha et al. 2009; Wunderlin et al. 2001). HCA was conducted using Statistica v.8 software.
In the first step of the explanatory analysis, detrended correspondence analysis (DCA) was performed to estimate the species gradient length. In DCA with detrending via segment and Hill’s scaling, the length of the longest axis provides an estimate of the beta diversity in the data set. Since DCA showed the high gradient length of the first axis with standard deviation values > 1.2, a unimodal model was used, which indicated that most species respond unimodally to the underlying ecological gradients. Canonical correspondence analysis (CCA) by scaling the inter-species distances was performed to examine the relationship between the cell density of the dominant and frequent species in the epipelon and phytoplankton communities and environmental variables and to test the significance of the ordination axes. Monte Carlo permutations (499 iterations) specifying the first, second, and third CCA axes, respectively, were also performed to test the significance of the ordination axes. The significance of the environmental variables under CCA was tested using forward selection with a fixed p value. The data set was logarithmically transformed [log (A × y + C)] to obtain the statistically attractive properties of the normal distribution of log-normally distributed variables, where the default values of “A” and “C” are 1.0; “y” is the data value, which neatly maps the zero values again to zero; and the other values are positive. All ordination analyses were performed using CANOCO version 4.5 with no down weighting of the species data. Triplot creation, which is the plot the species and environmental variables using the sample data was selected to show the results of the CCA and the sample plots were then colored manually (ter Braak 1995; ter Braak and Verdonschot 1995; Lepš and Šmilauer 2003).
Results
Variation of water quality
Throughout the study period, temperature values were recorded from 3.89 to 25.8 °C (mean 12.20), while pH values ranged between 4.25 and 8.10 (mean 6.25). Dissolved oxygen, nitrate, and current speed values were recorded 0.17 to 10.2 mg/l (mean 3.39), 0.00 to 7.56 mg/l (mean 0.99), and 1.95 to 9.32 m/s (mean 4.75), respectively, with the highest values recorded at the upstream stations (stations 1 and 2). Suspended solids, ammonium, and o-phosphate values were recorded 0.2 to 1029 mg/l (mean 81.79), 0.00 to 63.45 mg/l (mean 27.75), and 0.02 to 25.1 mg/l (mean 5.51), respectively. The highest values of those factors were recorded at the downstream stations, while nitrite values varied between 0.00 and 4.86 mg/l (mean 0.84), with the highest value recorded at station 4 (Fig. 2).
The water temperature showed pronounced seasonal fluctuations [x2(11) = 51.3; p < 0.001], recording significantly higher temperatures (p < 0.05) during summer (June, July, and August) when compared with late autumn (November) and winter (December, January, and February). There were also temporal variations in the pH [x2(11) = 44.3; p < 0.001], with the lowest values (p < 0.05) measured in autumn (October and November) and winter (December and January) (Fig. 2).
There was a strongly decreasing trend of dissolved oxygen from upstream to downstream [x2(5) = 44.3; p < 0.001], with an increasing trend of total suspended solids [x2(5) = 41.1; p < 0.001]. Ammonium increased significantly in the midstream region [x2(5) = 30.0; p < 0.001], while o-phosphate was highest at the downstream stations [x2(5) = 21.8; p < 0.05]. The variation of current speed along the stream flow, which primarily depends on streambed morphology, did not show a statistically significant difference among the stations (p > 0.05). The flow profile of water quality affected chlorophyll a concentration, which was significantly higher in the downstream reaches compared to upstream [x2(5) = 21.2; p < 0.001].
HCA showed spatial heterogeneity of water quality parameters and created a dendrogram (Fig. 3). Although cluster 1 grouped the upstream sampling stations (1 and 2), similarity of the clusters was statistically significant at Dlink/Dmax × 100 < 50. However, cluster 2, formed by stations 3 and 4 and the downstream sampling stations (5 and 6), resulted in a statistically significant group. HCA clearly showed the differences between the upstream sampling stations and the others.
Epipelon metrics and composition
During the study period, epipelic assemblages of the stream were constituted by the following phyla: Bacillariophyta (114 taxa), Charophyta (1), Chlorophyta (6), Cyanophyta (8), and Euglenophyta (11). Epipelon cell densities and bio-volumes varied between 0.012 to 98.285 × 103 cells/cm2 and 0.073 to 833 × 106 μm3/cm2, respectively, showing an increase along the downstream. Maximum cell density and bio-volume were recorded at station 5 in July, when Nitzschia umbonata (Ehrenb.) Lange-Bert. was dominant in summer and autumn at the mid- and downstream reaches (Fig. 4).
When the dominance of N. umbonata in July was ignored, no statistically significant differences in the temporal variability of epipelon cell densities and bio-volumes (p > 0.05) were noted. However, cell densities [x2(5) = 19.5; p < 0.01] and bio-volume [x2(5) = 22.9; p < 0.001] exhibited a spatial pattern. Station 3 exhibited significantly lower densities and bio-volumes when compared with stations 1 and 5 (Fig. 4).
Species richness varied between 4 and 33 species, with a mean value of 14, recording the highest value at station 2. The highest values were recorded at the upstream stations. Species diversity varied from 0.13 to 2.66, with a mean value of 1.37, recording the highest value at station 1. There were no statistically significant differences in temporal variations of species richness and diversity (p > 0.05) (Fig. 4). While the distribution of diatom and non-diatom richness also had significant differences among the sampling stations [x2(5) = 18.6; p < 0.01 and x2(5) = 28.9; p < 0.001, respectively], species diversity did not exhibit significant differences (p > 0.05). Diatom richness was significantly higher in the upstream than the downstream, while non-diatom richness was significantly lower at the first station. Furthermore, a significant spatial variation in species diversity of non-diatom taxa was detected [x2(5) = 24.5; p < 0.001], which was significantly lower at the first station than at the downstream (p < 0.05).
Phytoplankton metrics and composition
Bacillariophyta (65 taxa), Charophyta (2), Chlorophyta (40), Cyanobacteria (28), Euglenophyta (22), and Ochrophyta (1) were identified in the phytoplankton community during the study. Phytoplankton cell densities and bio-volumes varied between 0.05 and 34.6 × 103 cells/ml and 0.08 and 52.9 × 106 μm3/ml, respectively, recording their maximums arising from Melosira varians C. Agardh dominancy in March at the first station (Fig. 5).
Although it might have appeared that there was a temporal variability in phytoplankton cell densities and bio-volumes, the K-W test did not show a statistically significant difference among the sampling months (p > 0.05). However, cell densities [x2(5) = 28.3; p < 0.001] and bio-volumes [x2(5) = 22.6; p < 0.001] exhibited a significant spatial variability, increasing longitudinally and reaching the highest values at the fifth station (Fig. 5). The variability was clear for the Bacillariophyta and Euglenophyta phyla, which were highest at the downstream stations, especially when the M. varians bloom in March was ignored at the first station. On the other hand, cell densities and bio-volumes of Chlorophyta and Cyanobacteria species were significantly higher in the midstream reach than upstream.
Phytoplankton species richness varied from 6 to 37 species, with a mean value of 15, and the highest values recorded were those at the downstream stations. Species diversity varied between 0.17 and 2.76, with a mean value of 1.69, with the highest value at station 5 (Fig. 5). Statistically significant temporal and spatial variabilities for both species richness and species diversity metrics were not recorded (p > 0.05).
Spatial and temporal variations of species
Nitzschia palea (Kütz.) W.Sm., N. umbonata, and Ulnaria ulna (Nitzsch) Compère were the only species present in more than 50% of all epipelon samples, while M. varians, Nitzschia fonticola (Grunow) Grunow, N. palea, U. ulna, and Leibleinia epiphytica (Hieron.) Compère were recorded as the most frequent species in all phytoplankton samples (Fig. 6). Although the relative abundances of those species exhibited variability in both epipelon and phytoplankton at the sampling stations, no statistically significant seasonality patterns were determined (p > 0.05). M. varians had a higher occurrence frequency in phytoplankton (53%) than in epipelon (32%) and constituted the majority of the phytoplankton community of the upstream reach in March with bloom. N. fonticola was mainly distributed in the midstream and downstream reaches in summer and autumn in both phytoplankton (48%) and epipelon (24%). Its relative abundance in phytoplankton at those stations was significantly different [x2(5) = 15.6; p < 0.01]. N. palea was the second most abundant species in both epipelon (57%) and phytoplankton (67%). It reached high relative abundance in both communities in spring and summer. Its temporal change in phytoplankton was significantly different (p < 0.05), while its spatial and temporal variabilities in epipelon were not statistically significant (p > 0.05). N. umbonata was the most frequent species (58%) and was extremely dominant in the downstream epipelon in summer and autumn. Spatial variability in relative abundance of N. umbonata in epipelon was highly significant [x2(5) = 21.6; p < 0.001] by dominance in the downstream reach when compared with upstream. Another frequent taxon in both epipelon (58%) and phytoplankton (48%) was U. ulna. Contrary to other frequent diatom species, U. ulna exhibited a dominant trend in the upstream and midstream reaches (Fig. 6), with significantly higher relative abundance in epipelon [x2(5) = 20.5; p < 0.01] and phytoplankton [x2(5) = 18.2; p < 0.01].
Leibleinia epiphytica, the most abundant taxon (82%) in phytoplankton, had a rare occurrence of 1% in epipelon, and a clear spatial variability in phytoplankton was observed [x2(5) = 21.1; p < 0.001] due to the higher relative abundances of L. epiphytica in phytoplankton observed in the midstream in comparison with the upstream stations (p < 0.01).
Euglena spp. were recorded as dominant/sub-dominant in epipelon in all sampling stations, except for the first, between autumn and spring (Fig. 6). On the other hand, spatial variation of Euglena sp. in epipelon at the sampling stations was significantly different [x2(5) = 19.7; p < 0.01] in that it was of higher relative abundance at station 5 (p < 0.01). Also, spatial variation of Schizothrix sp. in phytoplankton was highly significant [x2(5) = 0.2; p < 0.001] in that the relative abundance in station 2 was significantly different from the other sampling stations (p < 0.001).
CCA indicated 61.3% of cumulative variance in the data, comprising the density of dominant species in epipelon and water quality with the first four axis eigenvalues greater than two (Table 1). CCA clearly displayed temporal and spatial variations of the species and water quality, supporting the results of univariate tests. The first axis (axis 1), representing 21.7% of the variance, was positively loaded with the variations of C. placentula var. euglypta (Ehrenberg) Grunow, Fragilariforma virescens (Ralfs) D. M. Williams & Round, and Fragilaria acus (Kütz.) Lange-Bert. at station 1. The second axis (axis 2), explaining 16.4% of total variance, was mainly loaded with the variations in Planothidium lanceolatum (Bréb. ex Kütz.) Lange-Bert., Amphora libyca Ehrenb., Craticula halophila (Grunow) D. G. Mann, Halamphora veneta (Kützing) Levkov, Navicula trivialis Lange-Bertalot, and Nitzschia linearis W. Sm. at station 2. Dissolved oxygen, the highest in the upstream reach, was also positively loaded on axes 1 and 2. On the other hand, other water quality parameters, which increased from the upstream to the downstream stations, were located on the negative sides of axes 1 and 2, together with the dominant species of the midstream and downstream sampling stations (Fig. 7a).
Canonical correspondence analysis plots of dominant and/or frequent species in (a) epipelon and (b) phytoplankton communities. Axes represent linear combinations of environmental gradients; the compositions of these axes are shown by the black arrows. Green “X” marks represent the species. Other colored symbols represent sites and sampling months [station 1, circle; station 2, up-triangle; station 3, diamond; station 4, star; station 5, down-triangle; station 6, square; red, summer (June, July, August); yellow, autumn (September, October, November); gray, winter (December, January, February); blue, spring (March, April, May)] (Afor, Asterionella formosa; Agra, Aulacoseira granulata; Plan, Planothidium lanceolatum; Alyb, Amphora libyca; Asta, Astasia sp.; Ccyc, Cyclotella cretica var. cyclopuncta (H. Hakansson & J. R. Carter) R. Schmidt; Chal, Craticula halophila; Kirc, Kirchneriella irregularis; Coce, Pantocsekiella ocellata; Cpla, Cocconeis placentula var. euglypta; Dten, Diatoma tenuis; Eugl, Euglena sp.; Facu, Fragilaria acus; Fcro, Fragilaria crotonensis; Fvir, Fragilariforma virescens; Hven, Halamphora veneta; Lepi, Leibleinia epiphytica; Mvar, Melosira varians; Nfon, Nitzschia fonticola; Nlin, Nitzschia linearis; Npal, Nitzschia palea; Ntri, Navicula trivialis; Numb, Nitzschia umbonata; Osci, Oscillatoria sp.; Pdig, Phormidium diguetii; Schi, Schizothrix sp.; Ulna, Ulnaria ulna)
For phytoplankton, CCA showed 70.5% of variance in relation to dominant species and water quality (Table 1). The first axis (axis 1), explaining 27.4% of the cumulative variance, was positively loaded with the variance of Schizothrix sp. at station 2. Dissolved oxygen was located on the positive sides of axes 1 and 2, while the other water quality parameters, increasing from upstream to downstream stations, negatively loaded these axes (Fig. 7b).
Discussion
Statistically, significant differences among the sampling stations for many parameters, except for temperature and pH, displayed spatial heterogeneity, revealing industrial and rural impacts along the Ankara Stream. While low-conductivity values, dissolved anion and cation contents, and concentrations of inorganic nitrogen and o-phosphate increased in the downstream direction, important decreases in concentrations of nitrate nitrogen and dissolved oxygen were recorded (Fig. 2). The same situation was recorded in many rivers (Duong et al. 2006; Bowes et al. 2012; Kıvrak and Uygun 2012). Also, it was reported that chemical oxygen demand and the content of ammonium compounds of dissolved nitrogen are the most definite indicators of total anthropogenic impacts (Shulkin and Nikulina 2015). The responses of the algal groups and species to those impacts were clearly observed along the stream flow. The upstream stations in Ankara Stream had a lower level of pollution, and HCA clearly demonstrated dissimilarities between the upstream and other stations.
The dominant phytoplankton species in the upstream reach were clearly related to water quality. Furthermore, the phytoplankton community of the upper courses of the stream was influenced by tail water of the Bayındır Reservoir, as shown in some studies (Sullivan et al. 2001). Phytoplankton density and bio-volume at the first station were significantly lower than those farther downstream when the M. varians bloom of March was not considered (Fig. 5). In addition, Achnanthidium minutissimum (Kütz.) Czarn., Pantocsekiella ocellata (Pantocsek) K. T. Kiss & Ács, and Kirchneriella irregularis (G.M.Sm.) Korshikov were dominant at the first station (Fig. 6). CCA clearly discriminated against P. ocellata and K. irregularis as characteristic species of the first station, in that Cyclotella and Kirchneriella species were reported as highly abundant phytoplankton taxa of Bayındır Reservoir (Atıcı et al. 2005). While Cyclotella species were widely reported as dominant in oligotrophic lakes (Van Dam et al. 1994), K. irregularis was found in clear, meso-eutrophic lakes (Reynolds et al. 2002). In epipelon, C. placentula var. euglypta, F. virescens, F. acus, and Navicula radiosa Kütz. were the dominant species at the first station as shown by CCA (Fig. 7). While P. lanceolatum, A. libyca, C. halophila, H. veneta, N. trivialis, N. linearis, and U. ulna were recorded as dominant in the epipelon community at the second station, which had more domestic sewage and agricultural inputs, the dominance of Schizothrix spp. was mostly observed in plankton in late summer and autumn. Also, while P. lanceolatum and N. trivialis were distinguished among the characteristic species of epipelon of the station by CCA, U. ulna is also known to be dominant in urban-polluted rivers and to be highly pollution-tolerant (Soininen 2002; Ács et al. 2004; Bere and Tundisi 2011). Schizothrix species are considered to be characteristic species of stream assemblages (Radloff et al. 2010). Differences in species richness between the two upstream stations were conspicuous.
The physical destabilization done to the riverbed may have been the reason for the sudden fall in the density and bio-volume of cells in epipelon at the third station (Fig. 4). Also, there was an increase in organic loading due to domestic and animal wastes, which caused a serious rise in ammonium values (11.3–44.0 mg/l). At the fourth station, wastes from yeast and sugar factories in the densely populated settlements led to high turbidity and ammonium concentration, unlike when sharp drops were recorded in nitrate and DO values. The sudden fall in the number of algal species, as well as bio-volume, was a response to this result especially in the epipelon of the midstream reach. A. minutissimum, M. varians, N. umbonata, N. palea, U. ulna, Phormidium diguetii (Gomont) Anagn and Komárek, and Euglena sp. were species of relative importance (Fig. 6). Relatively conspicuous in the midstream phytoplankton were Fragilaria crotonensis Kitton, Nitzschia fonticola (Grunow) Grunow, N. palea, and L. epiphytica. N. fonticola and N. palea were reported to be able to use organic nitrogen (Licursi et al. 2016). High sewage loading followed by anaerobic digestion effects led to the growth of appropriate anoxic medium-dominant taxa (e.g., L. epiphytica, Oscillatoria sp., and Euglena spp.), which were also reported in other studies (Duong et al. 2006; Kadhim et al. 2013).
The fifth station was affected by industrial and waste treatment plants along with agricultural inputs, while the sixth station was mostly affected by agricultural inputs. N. umbonata, often found in the outflows of treatment systems (Krammer and Lange-Bertalot 1988), supported the bloom at the fifth station. N. palea, F. crotonensis, Aulacoseira granulata (Ehrenberg) Simonsen, Asterionella formosa Hassall, Oocystis marssonii Lemm., Chlamydomonas sp., L. epiphytica, and Euglena species (especially at station 5), all in the downstream phytoplankton, were conspicuous (Fig. 6). N. palea, N. umbonata, U. ulna, F. crotonensis, A. granulata, Diatoma tenuis C. Agardh, P. diguetii, Oscillatoria sancta Kütz. ex Gomont, Oscillatoria tenuis C. Agardh ex Gomont, and Euglena spp. were in the epipelon and in harmony with organic pollutants. A. granulata, N. palea, and N. umbonata were recorded as substantial biomass at the downstream stations of Ankara Stream, and those species are defined as resilient taxa to increasing pollution gradient and nutrients (Licursi and Gómez 2002; Duong et al. 2006). In the downstream phytoplankton, contrary to the epipelon, both species richness and diversity were observed to be high. Soluble inorganic nitrogen compounds are usually important determinants of algal growth and community composition in streams (Ting et al. 2013). Throughout the downstream, low oxygen and high ammonium, with increasing o-phosphate concentration, may have affected the growth of algae in different ways. More C. placentula var. euglypta and M. varians were recorded at stations with low ammonium and o-phosphate in the upstream portion of the study, while there were more N. palea and N. umbonata at stations with high ammonium and o-phosphate in the mid-to-downstream sections, as reported by Licursi and Gómez (2002). In addition, the increase in chlorophyll a throughout the downstream reach, especially at the fifth station, supported that situation. Even if it is not the only factor, o-phosphate concentration is known to have a limiting effect on chlorophyll a (Bowes et al. 2012). Generally, an increase in o-phosphate is positively correlated with an increase in algal biomass (Stevenson et al. 2012).
In lotic systems, a trend from low toward high organic pollution levels forms a gradient that plays a more important role according to environmental conditions, climate, vegetation, and geographic factors (Duong et al. 2006; Bere and Tundisi 2011). It was also reported that there are more spatial and temporal variations when small rivers and streams are compared with large rivers because of their higher velocities (Gerald et al. 2006). Longitudinal increases in nutrients and changes in community compositions, along with increases in biomass, were observed among different species in the stream. Several studies determined the link between rising nutrient concentrations and algal biomass increases (Licursi et al. 2016; Varol and Şen 2018), very often by changing their species composition or diversity, varying from species richness to monotonous communities (Ács et al. 2004).
Conclusion
In this study, phytoplanktonic and epipelic assemblages were found to be strongly associated with spatial gradients in nutrient concentrations. However, statistically significant differences in many parameters among stations displayed spatial heterogeneity, indicating industrial and rural impacts along the streamflow (Fig. 2). In the research confirmed by FTIR analyses, it was reported that differences in environmental conditions can cause changes in the compositions of filamentous algal species due to different responses (Çelekli et al. 2016). Under the same climatic and geographic conditions, if a lotic system is uniform, that is, affected only by agricultural or urbanization activities, spatial heterogeneity is seen to be rare, but, in environmental conditions, such as in Ankara, spatial heterogeneity is pushed to the forefront with very different influences. Ankara Stream, in terms of metrics and composition of algae with physical–chemical parameters, showed significant evidence for understanding the differences. It is recommended that further studies be conducted on the stream under discussion to enlighten this subject.
References
Ács, É., Szabó, K., Tóth, B., & Kiss, K. T. (2004). Investigation of benthic algal communities, especially diatoms of some Hungarian streams in connection with reference conditions of the water framework directives. Acta Botanica Hungarica, 46, 255–278.
Anene, A. (2003). Techniques in hydrobiology. In E. Onyeike & J. O. Osuji (Eds.), Research techniques in biological and chemical sciences (pp. 174–189). Owerri: Springfield Publishers Ltd.
APHA, AWWA, & WEF. (1998). Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association.
Atıcı, T., Obalı, O., & Calışkan, H. (2005). Control of water pollution and phytoplanktonic algal flora in Bayındır Dam reservoir (Ankara). E.U. Journal of Fisheries & Aquatic Sciences, 22, 79–82.
Bere, T., & Tundisi, J. G. (2011). Diatom-based water quality assessment in streams influence by urban pollution: effects of natural and two selected artificial substrates, São Carlos-SP, Brazil. Brazilian Journal of Aquatic Sciences and Technology, 15, 54–63.
Bowes, M. J., Gozzard, E., Johnson, A. C., Scarlett, P. M., Roberts, C., Read, D. S., Armstrong, L. K., Harman, S. A., & Wickham, H. D. (2012). Spatial and temporal changes in chlorophyll-a concentrations in the River Thames basin, UK: are phosphorus concentrations beginning to limit phytoplankton biomass? Science of the Total Environment, 426, 45–55.
Çelekli, A., Aslanargun, H., Soysal, Ç., Gültekin, E., & Bozkurt, H. (2016). Biochemical responses of filamentous algae in different aquatic ecosystems in South East Turkey and associated water quality parameters. Ecotoxicology and Environmental Safety, 133, 403–412.
Dalu, T., Bere, T., Richoux, N. B., & Froneman, P. W. (2015). Assessment of the spatial and temporal variations in periphyton communities along a small temperate river system: a multimetric and stable isotope analysis approach. South African Journal of Botany, 100, 203–212.
Duong, T. T., Coste, M., Feurtet-Mazel, A., Dang, D. K., Gold, C., Park, Y. S., & Boudou, A. (2006). Impact of urban pollution from the Hanoi Area on benthic diatom communities collected from the Red, Nhue and Tolich Rivers (Vietnam). Hydrobiologia, 563, 201–216.
Gerald, V. S., Michael, M. E., Ketterer, E., & Johansen, J. R. (2006). Ecology and assessment of the benthic diatom communities of four Lake Erie estuaries using Lange-Bertalot tolerance values. Hydrobiologia, 561, 239–249.
Gillett, N. D., Pan, Y., Asarian, E. J., & Kann, J. (2016). Spatial and temporal variability of river periphyton below a hypereutrophic lake and a series of dams. Science of the Total Environment, 541, 1382–1392.
Hillebrand, H., Dürselen, C. D., Kirschtel, D., Pollingher, U., & Zohary, T. (1999). Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology, 35, 403–424.
Huber-Pestalozzi, P., Huber-Pestalozzi, G., Förster, K., & Med, G. (1982). Das Phytoplankton des SuBwassers: section 8 pt 1, Conjugatophyceae Zygnematales und Desmidiales (excl. Zygnemataceae). Stuttgart: E Schweizerbart'sche Verlagsbuchhandlung.
John, D. M., Whitton, B. A., & Brook, A. J. (2002). The freshwater algal flora of the British Isles: an identification guide to freshwater and terrestrial algae (Vol. 1). Cambridge: Cambridge University Press.
Kadhim, N. F., Al-Amari, M. J., & Hassan, F. M. (2013). The spatial and temporal distribution of epipelic algae and related environmental factors in Neel stream, Babil province. International Journal of Aquatic Science, 4, 23–32.
Kıvrak, E., & Uygun, A. (2012). The structure and diversity of the epipelic diatom community in a heavily polluted stream (the Akarçay, Turkey) and their relationship with environmental variables. Journal of Freshwater Ecology, 27(3), 443–457.
Korshikov, O. B. A. (1987). The freshwater algae of the Ukrainian SSR. Dehra Dun: Bishen Singh Mahendra Pal Singh and Koeltz Scientific Books.
Krammer, K., & Lange-Bertalot, H. (1986). Bacillariophyceae. 1. Teil: Naviculaceae. In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süßwasserflora von Mitteleuropa, Band 2/1. Stuttgart: Gustav Fischer Verlag, Jena.
Krammer, K., & Lange-Bertalot, H. (1988). Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süsswasserflora von Mitteleuropa, Band 2/2. Stuttgart: Gustav Fischer Verlag, Jena.
Krammer, K., & Lange-Bertalot, H. (1991a). Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süsswasserflora von Mitteleuropa, Band 2/3. Stuttgart: Gustav Fischer Verlag, Jena.
Krammer, K., & Lange-Bertalot, H. (1991b). Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis Teil 1–4. In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süsswasserflora von Mitteleuropa, Band 2/4. Stuttgart: Gustav Fischer Verlag, Jena.
Leira, M., & Sabater, S. (2005). Diatom assemblages distribution in Catalan rivers, NE Spain, in relation to chemical and physiographical factors. Water Research, 39, 73–82.
Lepš, J., & Šmilauer, P. (2003). Multivariate analysis of ecological data using CANOCO. Cambridge: Cambridge University Press.
Licursi, M., & Gómez, N. (2002). Benthic diatoms and some environmental conditions in three lowland streams. Annales de Limnologie - International Journal of Limnology, 38, 109–118.
Licursi, M., Gómez, N., & Sabater, S. (2016). Effects of nutrient enrichment on epipelic diatom assemblages in a nutrient-rich lowland stream, Pampa Region, Argentina. Hydrobiologia, 766, 135–150. https://doi.org/10.1007/s10750-015-2450-7.
Nardelli, M., Bueno, N., Ludwig, T., & Guimarães, A. (2016). Structure and dynamics of the planktonic diatom community in the Iguassu River, Paraná State, Brazil. Brazilian Journal of Biology, 76, 374–386.
Pan, Y., Stevenson, R. J., Hill, B. H., Kaufmann, P. R. & Herlihy, A. T. (1999). Spatial patterns and ecological determinants of benthic algal assemblages in mid-Atlantic streams, USA. Journal of Phycology, 35, 460-468.
Radloff, P. L., Contreras, C., Whisenant, A., & Bronson, J. M. (2010). Nutrient effects in Small Brazos basin streams final report. WQTS-2010-02. Austin, Texas: Water Quality Program, Texas Parks and Wildlife Department.
Reynolds, C. S., Huszar, V., Kruk, C., Naselli-Flores, L., & Melo, S. (2002). Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research, 24, 417–428.
Round, F. E., Crawford, R. M., & Mann, D. G. (1990). The diatoms. Biology, morphology of the genera. Cambridge: Cambridge University Press.
Shannon, C. E., & Weaver, W. (1963). The mathematical theory of communication. Urbana: University of Illinois Press.
Shulkin, V. M., & Nikulina, T. V. (2015). Comprehensive assessment of river-water quality in Primorskii Krai, Russian Federation, with respect to chemical characteristics and composition of periphyton algae. Inland Water Biology, 8, 15–24.
Simeonov, V., Stratis, J. A., Samara, C., Zachariadis, G., Voutsa, D., Anthemidis, A., Sofoniou, M., & Kouimtzis, T. (2003). Assessment of the surface water quality in Northern Greece. Water Research, 37, 4119–4124.
Sinha, S., Basant, A., Malik, A., & Singh, K. P. (2009). Multivariate modeling of chromium-induced oxidative stress and biochemical changes in plants of Pistia stratiotes L. Ecotoxicology, 18, 555–566.
Soininen, J. (2002). Responses of epilithic diatom communities to environmental gradients in some Finnish Rivers. International Review of Hydrobiology, 87, 11–24.
Stevenson, R. J., Bennett, B. J., Jordan, D. N., & French, R. D. (2012). Phosphorus regulates stream injury by filamentous green algae, DO, and pH with thresholds in responses. Hydrobiologia, 695, 25–42. https://doi.org/10.1007/s10750-012-1118-9.
Stevenson, R. J. & Y. Pan (1999). Assessing environmental conditions in rivers and streams with diatoms. In Stoermer, E. F. & J. P. Smol (eds), The diatoms: applications for the environmental and earth sciences. Cambridge University Press, Cambridge: 11-40.
Sullivan, B. E., Prahl, F. G., Small, L. F., & Covert, P. A. (2001). Seasonality of phytoplankton production in the Columbia River: a natural or anthropogenic pattern? Geochimica et Cosmochimica Acta, 65, 1125–1139.
ter Braak, C. J. F. (1995). Ordination. In R. H. G. Jongman, C. J. F. ter Braak, & O. F. R. van Tongeren (Eds.), Data analysis in community and landscape ecology (pp. 91–173). Cambridge: Cambridge University Press.
ter Braak, C. F., & Verdonschot, P. M. (1995). Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sciences, 57, 255–289.
Ting, C., Stephen, Y. P., & Yebu, L. (2013). Nutrient recovery from wastewater streams by microalgae: status and prospects. Renewable and Sustainable Energy Reviews, 19, 360–369.
Tornés, E., Pérez, M. C., Durán, C., & Sabater, S. (2014). Reservoirs override seasonal variability of phytoplankton communities in a regulated Mediterranean river. Science of the Total Environment, 475, 225–233.
Van Dam, H., Mertens, A., & Sinkeldam, J. (1994). A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Netherlands Journal of Aquatic Ecology, 28, 117–133.
Varol, M., & Şen, B. (2018). Abiotic factors controlling the seasonal and spatial patterns of phytoplankton community in the Tigris River, Turkey. River Research and Applications Wiley, 34, 13–23.
Vural, N., Duygu, Y., Kumbur, H. (1997). Monitoring of Anionic Surfactants in Ankara Stream. Revista Internacional de Contaminación Ambiental, 13(1), 47-50.
Wunderlin, D. A., Diaz, M. P., Ame, M. V., Pesce, S. F., Hued, A. C., & Bistoni, M. A. (2001). Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquia river basin (Cordoba-Argentina). Water Research, 35, 2881–2894.
Youngman, R.E. (1978). Measurement of chlorophyll. WRC Technical Report TR82, UK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özer, T., Açıkgöz Erkaya, İ., Koçer, M.A.T. et al. Spatial and temporal variations in composition of algae assemblages with environmental variables in an urban stream (Ankara, Turkey). Environ Monit Assess 191, 387 (2019). https://doi.org/10.1007/s10661-019-7527-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7527-8