Abstract
The study assessed the concentration and bioavailability of Pb, Cd, Zn, Cr and Fe metals in soil from rice fields in Abakaliki, Nigeria. The concentrations of these heavy metals in rice grains were also determined, with a view to assessing their human health risk on consumers. Heavy metal concentrations in soil and rice were determined using FAAS after digestion with 5:1 HNO3:HClO4 while Tessier’s sequential extraction method was used to obtain the fractionated soil digests. The mean concentrations (μg/g) of the total heavy metal ranged from 1.036 ± 1.86 (Cd) to 6900.537 ± 734.82 (Fe) in soil while that of rice grains ranged from 0.024 ± 0.07 (Cd) to 101.446 ± 75.26 (Fe).The concentrations of Fe and Cd in most of the soil samples and Pb in rice grains exceeded some Nigerian and International standards. The heavy metal sequential extractions of soil samples showed that lead and zinc were less mobile in the soil and are bioavailable for plant uptake. The estimated daily intake of the metals was below the tolerable daily intake. The hazard quotient and the total hazard index were less than one showing that at present, there is no possible adverse health effect on rice consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Monitoring of heavy metal contamination of agricultural products has intensified over the last two decades. This is as a result of their widespread release into the environment and surrounding ecosystem. The increasing rate of industrialization, urbanization, mechanized agricultural practices and quest for increased crop productivity has attributed to significant release of heavy metals in the environment. Soil is a major sink for these heavy metals when released into the environment. The indiscriminate use of agrochemicals has made agricultural lands even more prone to heavy metal contamination, and this in turn has had a huge effect on the heavy metal content of food products grown on these lands. Exposure to most of these heavy metals could pose adverse health effects, including kidney damage, abdominal pains and carcinogenic effects (WHO 1992; Tchounwou et al. 2012).
Unlike organic contaminants which are oxidized to carbon (IV) oxide by microbial action, heavy metals do not undergo microbial or chemical degradation (Kirpichtchikova et al. 2006) and the total concentration of these heavy metals in soil persist for a long time after their introduction (Adriano 2003). Most often, these heavy metals are dispersed in the various components of the soil which may determine their mobility and bioavailability (Ahumada et al. 1999). Some reports have shown that the particular behaviour of metals in the environment is determined by their specific physico-chemical forms rather than by their total concentration (Bermond 2001; Landonin 2002; Olajire et al. 2003). Sequential extraction procedures have been used to obtain information on the chemical speciation of heavy metals in the soil components. These procedures utilize a series of chemical reagents which are used to dissolve metals linked to various soil components (Ma and Uren 1998; Ahumada et al. 1999; Olajire et al. 2003; Pueyo et al. 2003). Metals that are available to plants are mostly metals which are weakly bound to various soil components.
Rice is the world’s most important staple food crop consumed by more than half of the world population (FAO 1991). It is an important food commodity for most people in sub-Saharan Africa particularly West Africa where the consumption of cereals, mainly sorghum and millet, has decreased from 61% in the early 1970s to 49% in the early 1990s while that of rice has increased from 15 to 26% over the same period (Jones et al. 1981; FAO 2001). Consumption of rice in Nigeria has risen drastically over the years. This is a development that, as welcome as it should be, has rather presented a huge economic challenge to the Nigerian Government. Importation of foreign rice has been the bane of Nigeria’s rice industry, leading to government spending excessively to ensure that rice is available to Nigerians at an affordable price. In 2016, the Nigerian Government banned the importation of foreign rice from other countries in her quest to diversify the economy by investing much in agriculture, especially in rice cultivation. The Central Bank of Nigeria (CBN) initiated a programme known as Anchor Borrower Programme (ABP), whereby billions of naira was made available as loans for rice farmers. This has intensified rice production in the various parts of Nigeria where rice is being cultivated. These areas are the northwest, north central and southeastern parts of the country.
There is a general low level of confidence amongst Nigerians on the production and processing method of locally grown rice, owing to the perceived poor agricultural practices employed by the Nigerian farmers and poor evaluation of the chemical contents. Many literatures on rice consumption have been mainly concerned with the nutritional analysis, neglecting the obvious health issues posed by many possible contaminants in the product. Considering the uncertain environmental arrangement of the Nigeria’s lithospheric and atmospheric space, that is, the poor spacing and demarcation of business areas, agricultural areas, industrial areas and residential areas in Nigeria, as well as the constituents of most agrochemicals used in rice cultivation, it becomes imperative for continued monitoring of possible contaminants in rice, grown and produced in various parts of Nigeria. Thus, the objectives of this study were to (i) determine some physiochemical parameters of the paddy soil, (ii) assess the concentrations of some heavy metals (Pb, Cd, Zn, Cr, Fe) in rice grains and soil from two rice farms, (iii) determine the mobility and bioavailability of the metals for uptake by the rice plant and the environmental risk of the metals to the soil and (iv) estimate any potential health risk arising from the consumption of rice grown in this area by the locals.
Materials and method
Sampling
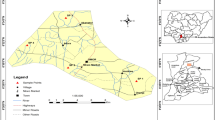
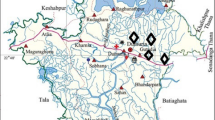
Samples (soil and grain) were collected from Ndi Nnachi Osso-Edda and Ndi Uche Osso-Edda community rice fields designated as farm A and farm B, respectively. The sampling was carried out in December 2015. Regular systematic composite grid sampling technique was used for the collection of 40 soil samples and 40 rice grain samples from farm A and farm B, making a total of 80 samples. Each farm was divided into 50 hectare sectors with a geo-reference point assigned to each grid. At the geo-reference point, top soil samples (1–10 cm) and rice grains were each taken and combined into composite samples. Two separate composite samples each for soil and rice grains were collected at each 5-ha grid, resulting in multiple geo-referenced soil tests for each farm (Fig. 1). The soil samples were collected within 0–10 cm depth using a polyethylene bag and a plastic digger and then transferred into a plastic bucket. The soil was thoroughly mixed in the plastic bucket, breaking up all cores and then filling the soil bag with about one cup of soil and discarding the extra soil. Soil samples were randomly collected from a fallow land which has not been cultivated for over 10 years within the study area to serve as control.
Sample Pre-treatment
The soil samples were air dried for 48 h and then pulverized to fine particles using a plastic mortar and pestle. It was then sieved using a 2-mm mesh and stored, ready for digestion. The rice samples were threshed, and the husks were manually removed by hand. It was oven dried to constant weight, ground with a plastic mortar and pestle, and sieved with a 2-mm mesh, after which it was then stored, ready for digestion.
Digestion
A mass of 2 g of the pretreated sample (soil and rice grains) was each digested with 20 mL of 5:1 HNO3:HClO4 and heated with a mantle in the fume cupboard until a clear colourless digest was obtained at about 20–30 min (Allen et al. 1986; Markert 1996). The digest was transferred to the desiccator and cooled to ambient temperature. The colourless digest was then filtered and transferred to a 50-mL standard flask and then made up to mark with de-ionized water and stored in the frigid cab below 4 °C (Explosion proof frigid-cab, Model No. 078, Lab-Line Instruments Inc., USA). Digestion with a mixture of nitric acid and perchloric acid was done to determine the total concentration of each heavy metal. Heavy metal analysis was carried out using a Flame Atomic Absorption Spectrophotometer (FAAS) (Shimadzu AA-6300, Japan). Sample blanks were analysed for Pb, Cd, Cr, Zn and Fe metals by taking 20 mL of the digestion reagents through the same procedure.
Physico-chemical analysis of soil
Physico-chemical properties of the soil such as moisture content, pH, conductivity, bulk density and organic matter were determined. Moisture content was determined by oven drying a known quantity of the soil to constant weight at 105 °C. pH and conductivity were determined using Onion Star pH meter, A-211, Thermo Scientific (Japan) and Setra Conductivity Meter, DDS-11A Conductometer model (China) respectively. Bulk density was determined according to the method prescribed by ASTM (2000).Total organic matter content was determined using the hydrogen peroxide (H2O2) digestion method as proposed by Schumacher (2002).
Heavy metal sequential extraction analysis
A modified Tessier’s sequential extraction procedure (SEP) was used in this study (Tessier et al. 1979). This was used to determine the mobility and bioavailability of the metals for plant uptake. The fractions were labelled as follows; exchangeable fraction, labelled SE1, oxidisable fraction (bound to organic matter), labelled as SE2, acid soluble fraction (bound to carbonates), labelled as SE3 and finally the reducible fraction (bound to Fe/Mn oxides and hydroxides) labelled as SE4. The 5th fraction which is the residual metals was ignored owing to the fact that metals in this fraction, which are tightly bound to silicates, are hardly available for uptake by plants; thus, it constitutes no significant contaminative danger to the rice plants. Risk assessment code (RAC) which is the fraction of metal in SE1 was used to evaluate the environmental risks of heavy metals in soils. The values were interpreted in accordance with the RAC classifications (Martley et al. 2004). RAC values, (%), < 1, 1–10, 11–30, 31–50 and > 75, are associated with no risk, low, medium, high and very high risk to the environment respectively.
Sequential extraction process
SE1
About 2 g of the soil sample was placed in a centrifuge tube and shaken at room temperature with 20 mL of deionized water for 1 h. Sixteen millilitres of 1 M Mg(NO3)2 at pH 7.0 was also added and the mixture shaken for 1 h. The mixture was centrifuged (Kokusan Industrial Centrifuge, A-11, Japan), and the supernatant made up to 50 mL mark with deionized water. The supernatant was separated from the residue by filtration, labelled as SE1 and stored in the frigid cab below 4 °C prior to FAAS analysis.
SE2
Ten millilitres of 8.8 M H2O2 + 6 mL of 0.02 M HNO3 was added to the residue from SE1 and then shaken for 5 + 1 h at 98 °C. Ten millilitres of 3.5 M CH3COONH4 was added as an extracting agent. The mixture was then centrifuged, and the supernatant made up to 50 mL with deionized water. The supernatant was then filtered out, labelled as SE2 and stored in the frigid cab below 4 °C.
SE3
Twenty-five millilitres of 0.05 M Na2EDTA was added to the residue from SE2; the set up was then shaken for 6 h and centrifuged. The supernatant was made up to 50 mL mark with deionized water and then filtered out, labelled as SE3 and stored in the frigid cab below 4 °C.
SE4
An addition of 17.5 mL of 0.1 M NH2OH·HCl + 17.5 mL of 3.5 M CH3COONH4 to the residue from SE3 was made and shaken for 4 + 1 h at 98 °C. It was extracted with 10 mL of 3.5 M CH3COONH4, shaken for 1 h. The set up was centrifuged, and the supernatant made up to 50 mL mark with deionized water. It was then filtered and labelled as SE4 and then stored in the frigid cab below 4 °C.
The prepared extracts were then analysed for the heavy metals under study using the FAAS.
Human health risk assessment
Non-carcinogenic health risk from rice consumption was estimated using the hazard quotient (HQ) and total hazard index (THI). HQ is the ratio of determined dose of a pollutant to a reference dose level. Estimated daily intake (EDI) of the metals analysed was estimated using the formula proposed by Manish et al. (2009):
HQs were determined following the US EPA (2015) Region I Risk-based Concentration Table defined as follows:
where Efr is the exposure frequency (350 days/year); EDtot is exposure duration (70 years); MI is rice ingestion (70 g/person/day); MC is mean metal concentration in rice (μg/g); BW is the average bodyweight of an adult (60 kg); ATn is averaging time for non-carcinogens (365 days/year × EDtot);. RfDo is the oral reference dose (mg/kg/day): Cd = 0.001, Pb = 0.004, Zn = 0.3, Fe = 0.7 and Cr(III) = 1.5 (USEPA 2000). The RfDo of Cr(III) was used to represent Cr in the study because any Cr(VI) would be reduced to Cr(III) under the acidic conditions present in the stomach (DeFlora et al. 1997).
THI was calculated to evaluate the potential risk of adverse health effects from a mixture of chemical constituent in the rice sample. Total hazard index was calculated by the summation of the HQ of the metals in the samples, thus
Data analysis
Descriptive statistics was used to present the results as mean and standard deviations. Pearson correlation was used to correlate the metal levels in the rice and soil samples from the two farms. One-way analysis of variance (ANOVA) was used to compare the levels of the heavy metals in rice samples from the two farms. All statistical analysis was performed with SPSS ver 20.0 for windows.
Quality assurance protocols
Analytical grade reagents and chemicals were used in this study. Deionized water was also used throughout the analysis. Procedural and reagent blanks were used, and a clean laboratory environment was ensured during the analysis and preparation of solutions. Glassware, plastic containers, crucibles, pestle and mortar were washed with liquid soap, rinsed with deionized water and then soaked in 10% HNO3 solution for 24 h. They were rewashed with deionized water and dried in the oven at 80 °C for 5 h before use. The Flame Atomic Absorption Spectrophotometer (Shimadzu AA-6300, Japan) used for the metal analyses was calibrated with multi-element standard solution (MESS), and the calibration standards analysed after 10 sample runs to ensure that the instrument remained calibrated. The concentrations of the metals in the MESS was determined with the FAAS and documented. Aliquot of the MESS was used to spike digested samples of soil and rice for the spiking/recovery experiment. The spiked samples were analysed for the metals under study using the FAAS. Concentrations of metals in spiked and unspiked samples were determined. The percent recovery was calculated using the following formula:
where
a = concentration of spiked sample
b = concentration of un-spiked sample
c = concentration of spike
Percentage recoveries (Tables 1 and 2) obtained range from 91.04 to 101.11% and 92.66 to 105.59% for the soil and rice samples respectively.
Results and discussions
Physico-chemical analysis
From Fig. 2, the bulk density shows a relatively constant value for the various soil samples. This shows strong similarity in the type of soil within the locality, having sourced all the samples from Edda in Afikpo south local government area of Ebonyi state. The density of the soil samples also suggests a soil type that will not allow easy penetration of materials such as water and plant roots (Dawaki et al. 2013). This soil type is ideal for rice cultivation considering the fact that rice plants requires water-logging and does not have deep root penetration (Frei and Becker 2004). The percent moisture content shows an interesting variation in the moisture content of the samples. Samples from rice farm A and control A which are quite close to each other shows a comparatively higher moisture content than samples from rice farm B and control B (also very close). However, the control samples A and B showed higher moisture content than the two rice farms. This trend suggests that rice farms A and B have been engaged with activities that take water away from the soil, thus confirming that rice plants require water for growth (Ajijola et al. 2012). However, all the soil samples showed a high percentage of moisture content, which shows a high level of water retention ability in the soil samples thus confirming the inference drawn from bulk density values considering that all the soil samples were top soils. The organic matter content slightly varied in the samples. The two control samples as expected had a slightly higher concentration of organic matter. This can be partly attributed to the non-use of the two sites for farming activities, hence no nutrient withdrawal activity, and also partly due to the presence of animal dung deposited on the sites by grazing livestock. The total carbon content follows the same trend with organic matter content. This is also quite expected considering that carbon element is an essential constituent of organic matters; thus, total organic carbon content can as well be used to estimate the quality and quantity of organic matter in a sample. The pH is fairly constant for all the samples with the exception of rice farm A which gave a slightly lower pH value. The entire pH value however suggests an acidic soil type within the locality. This soil type generally supports formation of metal complexes considering that metals reacts with mineral acids to form stable complexes (Dawaki et al. 2013). Also, Misra and Mani (1991) reported that increase in soil pH and decrease in redox potential leads to a decrease in heavy metals available to plants. There was slight variation in the conduction ability of the soil samples, with the two control samples having higher conductivity than that of the two rice farms. This also suggests that the two rice farms have been engaged in activities that takes away much minerals necessary for conduction (Mclaren et al. 2005).
Heavy metal concentrations in soil and rice grains
The heavy metal concentrations in the paddy soils of farms A and B are presented in Table 3. The mean concentrations of the heavy metals in soil from farm A were found to be in the order Fe > Zn > Cr > Pb > Cd while that of farm B were in the following order Fe > Zn > Cr > Cd > Pb. The results of the heavy metal concentrations were compared with standard acceptable limits stipulated by some local and international organizations. It was observed that some of the metals had values below limits set by the Nigerian Department of Petroleum Resources (DPR 2002), China (Anon 1995) and Canadian (CCME 2007) for agricultural soils. However, Fe concentrations in the soil samples from farm B exceeded the DPR limit. Also, cadmium levels in the soil samples from farms A and B exceeded DPR and MAC permissible limit for agricultural soil while only soil from farm B had Cd levels exceeding CCME limit. Though, iron is an essential element necessary in the production of chlorophyll, but its concentration in the soil of both farms were high. This can be attributed to a number of factors such as soil pH, aeration and compaction, organic matter and moisture content amongst others (Yoneyama et al. 2015). It has been reported that concentration of iron in the soil increases as soil pH decreases (Yoneyama et al. 2015). This could be inferred by the higher concentration of iron in paddy soil from both farms since the soils from both farms have low pH (4.57 and 4.67). Poorly aerated and compacted soil can have more iron availability especially if the soil is acidic (Yoneyama et al. 2015). Organic matter also makes iron available by combining with it thereby reducing chemical fixation and precipitation (Yoneyama et al. 2015). The reduction in precipitation results in higher concentration of iron remaining in soil. Excess water in the soil especially in acidic soil increases the availability of iron even to the point of toxicity. This could also have attributed to the higher concentration of iron in paddy soil from both farms.
Mean Cd concentrations in soils from both farms were appreciable compared to stipulated limit. This contamination could be from atmospheric fallout from industrial and urban activities, application of sewage sludge as manure and widespread use of organic and complex fertilizers (Bai et al. 2015). Recent studies have shown that application of potassium fertilizer significantly affects Cd concentration in soils (Nouri et al. 2008). Phosphate fertilizers have been reported to contain about 0.1–170 mg/kg of cadmium (Gimeno-Garcia et al. 1996). The relative abundance of metals in the paddy soil and control soil samples is shown in Fig. 3. There was 53% Cd abundance in farm B soils and 44% abundance in farm A soil samples. Soil samples from farm A showed 37% abundance of Pb while farm B was about 36%. A positive correlation was observed between the concentrations of Pb in the soil samples from the two farms. This suggests a similar source of Pb contamination in the soil from the two farms. A major highway (Afikpo-Okigwe Road) separates the two farms. Vehicular movement, exhaust emissions and tire wear emissions have been reported to have serious impact on air borne lead (Ugwu et al. 2011). Studies have shown that roadside soils usually contain greater Pb values (Akbar et al. 2006; Ano, Odoemelam and Ekwueme 2007). The Afikpo-Okigwe highway is major express road connecting Abakaliki State to Imo State both in south eastern Nigeria. It has an estimated average traffic rate of 150 vehicles per hour. Farm A soil had an abundance of Zn (48%) and 42% in farm B soil samples. The relative abundances for Cr in farm A and B soils were 41% and 50% respectively, while that of Fe in farms A and B soil samples were 33% and 59% respectively. The heavy metal concentrations in soil of this study were compared to levels reported elsewhere and in Nigeria. The mean concentration of iron in this study is lower than 43,231.29 ± 8767.30 mg/kg reported by Nasser et al. (2014) for paddy soil in Malaysia but higher than 255–379 mg/kg reported by Khairiah et al. (2009) for paddy soil in Malaysia. The concentration of lead, cadmium and zinc in both farms from this study were comparable to 4.64, 0.83 and 20.26 mg/kg reported by Ihedioha et al. (2016) for Pb, Cd and Zn in paddy soil from Enugu, Nigeria. However, lower cadmium level was obtained in this study than values reported by Satpathy et al. (2014) in paddy soil from India. Also, concentrations of cadmium (8.03 mg/kg), chromium (4.16 mg/kg) and zinc (13.89 mg/kg) reported by Payus and Talip (2014) were lower than the values obtained in this study while that of lead (8.03 mg/kg) was higher than that obtained in this study.
The concentrations of the heavy metals in rice grains from farms A and B are also presented in Table 3. The mean concentration of the heavy metals in farm A followed the order: Fe > Zn > Pb > Cr > Cd while farm B was Zn > Pb > Cr > Fe > Cd. All the detected metals in rice samples from both farms were below the WHO permissible limit for cadmium, zinc and chromium except lead which was above the limit. The result obtained in this study showed that the soil was not polluted by lead, but its concentration exceeded the threshold values in rice grain. This may be attributed to sewage water irrigation on the soil surface, atmospheric lead depositions especially from vehicular emissions, fertilizers and variety of rice being cultivated (Mar et al. 2012). Recent studies have shown that transport emissions can influence the concentration of some heavy metals in plants especially Pb concentration (Feng et al. 2012). Also, Ugwu et al. (2011) has reported a 185% Pb enrichment in cassava meal samples sundried along roadside above what was obtained in samples sun dried under ambient atmosphere. The iron concentration in the rice grain is quite lower than values obtained in the soil. This could be attributed to the fact that most of the Fe absorbed by the rice plant is used up by the rice leaves for chlorophyll formation during photosynthesis (Nnaji and Igwe 2014), thus allowing just a small fraction of the metal into the rice grain. The mean concentration of lead in this study is lower than values reported by Zhuang et al. (2009) in Dabaoshan mine in Guangdong China while the concentration of zinc and cadmium in this study was higher. Reports of Satpathy et al. (2014) have shown much lower concentration of Zn (7.2 mg/kg), Pb (1 mg/kg), Cd (0.05 mg/kg) and Cr (0.6 mg/kg) respectively than those reported in this study. Numerous studies have shown that different factors can directly influence the migration and accumulation of heavy metals in rice grains. The factors include soil type, interactions between elements, soil electrical conductivity, clay contents, nutrients, enzyme activity and other physico-chemical properties (Adams et al. 2004). Other influences include the following: type of field under study; irrigation water used and soil microbiological activities (Li et al. 2009).
A Pearson correlation analysis was done on the metal levels in the paddy soil samples (Table 4) and the rice grain samples (Table 5) from the two farms. The tables showed that there was a significant correlation in both the soil and rice samples metal levels at both 0.01 and 0.05 significance level. This suggests that the heavy metal source in the two rice fields could be from same source and is most probably from vehicular emissions and agrochemicals applied during planting. There is also the possibility of heavy metal contamination being released from the numerous artisan mining activities prevalent in the State. Zinc–lead mining sites are located some kilometres before the rice farms. These mining sites are not properly managed and regulated which results to indiscriminate mining activities all over the area. The one-way ANOVA analysis showed that there was no significant difference in the metal levels of the rice samples at a 0.05 significance level, except in the case of Fe which showed a significant difference with a p value of 0.007. The one-way ANOVA result once again confirms close similarities in the source of the heavy metal contaminants in the rice samples from the two farms.
Fractions and RAC of heavy metal in the soil
Sequential extraction procedure was used to fractionate the metals in the soils. This helped to provide information on the mobility, bioavailability and toxicity of metals in soils. Figure 4 shows the relative abundance of the metals in the four fractional digests of the rice farm A soil samples. The reducible fraction (SE4) has the highest abundance of Pb (34%), followed by the exchangeable fraction (SE1), 27%; oxidisable fraction (SE2), 25%; and acid soluble fraction (SE3) with 14%. The values obtained showed that about 27% of the Pb in the soil was available for uptake by the rice plant, considering that exchange of ions between the soil and plant majorly take place in the exchangeable fraction (Zhang et al. 2010; De la Rosa et al. 2003). The amount of Pb available for plant uptake suggests that there will be a relative high concentration of Pb in the rice grain sample. The relative abundance of Cd in the soil fractions showed that oxidisable fraction (SE2) gave the highest abundance at 40%, followed by acid soluble fraction (SE3), 37%; reducible fraction (SE4), 21%; and the exchangeable fraction (SE1) with 2% abundance. The low abundance of Cd metal in the exchangeable fraction of the soil samples explains the low concentration of the metal in the rice samples analysed. It has been reported that flooding paddy fields before and after heading help to reduce Cd uptake by rice. This is because in flooded condition, the soil becomes reductive and any Cd in the soil is converted to low soluble CdS while in a drained field, the soil is oxidative and CdS in the soil is converted to Cd2+ which is readily available for uptake by the rice plant (Ito and Iimura 1976).The exchangeable fraction (SE1) has the highest relative abundance of zinc (60%), followed by the acid soluble fraction (SE3) with 28%, oxidisable fraction (SE2) and reducible fraction (SE4) at 10% and 2% respectively. The high percentage of Zn in the exchangeable fraction suggests that Zn is not very mobile in the soil and thus is available for plant uptake. This may be as a result of the rice plant excreting solutions which alters the chemistry of the soil, making the metal less mobile in the soil (McLaughlin et al. 1998). A positive correlation was observed between the zinc in the exchangeable fraction and the rice grain sample. The reducible fraction (SE4) has the highest relative abundance of Cr (50%). It was followed by the oxidisable fraction (SE2) with 27% abundance; acid soluble fraction (SE3), 12%; and exchangeable fraction (SE1), 11%. The trend suggests that the metal is quite mobile in the soil having efficiently migrated mostly to the 4th fraction of the soil. The 11% abundance of the metal in the exchangeable fraction shows that most of the metal in the soil were not available for plant uptake. The acid soluble fraction (SE3) has the highest abundance of Fe in the samples at 62%, followed by the reducible fraction (SE4) at 17%, oxidisable fraction (SE2) and exchangeable fraction (SE1) with 11% and 10% respectively. The high percentage of Fe in the 3rd fraction which is also known as “bound to carbonates” shows a high affinity for the metal in the acid soluble fraction. This can be explained by the fact that this fraction contains materials which tends to immobilize the Fe metal. The predicted immobilization of Fe metal in the fraction can be attributed to carbonates as the fraction is known to contain metals that are easily and tightly bound to carbonates such as Fe, Zn, Mg and Ca. The attraction of the Fe to the acid soluble fraction ensures that a very limited amount of the metal is available at the exchangeable fraction for plant absorption. However, there might be a very high efficiency of absorption of the bio-available Fe metal by the rice plant as the metal is needed by rice leaves for photosynthesis (Nnaji and Igwe 2014). According to RAC analysis, soil from farm A showed low risk for cadmium and iron (RAC values ≤ 10%), medium risk for lead and chromium (RAC values = 11–30%) while high risk was observed for zinc(RAC values = 60%) .
Figure 5 shows the relative abundance of the metal in the four fractional digests of the rice farm B soil samples. The relative abundance of Pb in the four fractions showed that SE1 had the highest abundance of the metal with 40%, followed by SE2 (27%), SE4 (26%) and SE3 (7%). The high percentage abundance of Pb in the exchangeable fraction explains why the rice grain samples from farm B had an appreciable amount of the metal. Thus, a positive correlation was observed between the rice Pb content and the amount of Pb in the exchangeable fraction of the soil. Also, Fig. 5 showed that SE1 had 35% abundance of Cd in the soil samples. The oxidisable fraction (SE2) had 30%, SE3 had 28% while SE4 had 7%. The Cd content of the rice showed a positive correlation with that in the exchangeable fraction. The relative abundance of zinc showed the highest value of 55% for SE1. This was also the case in the farm A soil samples, thus further emphasizing the fact that the mobility of Zn in the soil samples is quite slow and thus makes zinc bioavailable for plant uptake. The zinc content of the rice grain from this farm has a positive correlation with that of the exchangeable fraction. The reducible fraction (SE4) had a 39% relative abundance for Cr, followed by SE3, SE2 and SE1 with 35%, 20% and 6% relative abundance respectively. This trend suggests a minimal Cr metal contamination in the rice grain sample. The SE3 has the highest abundance of 62% for Fe, followed by SE2, SE4 and SE1 at 25%, 10% and 3% respectively. The acid soluble fraction once again demonstrates its strong affinity for Fe metal while the exchangeable fraction still has the lowest value of the metal just as was the case in farm A. Also, a positive correlation was observed between the rice grain and the exchangeable fraction for Fe. RAC analysis showed low risk for chromium and iron (RAC values < 10%) while high risk was observed for zinc, lead and cadmium (RAC values > 30%).
Human health risk assessment
Two factors (estimated daily intake and hazard quotient) were used to determine human risk assessment of these heavy metals through rice consumption. Table 6 presents the estimated daily intake (EDI) and hazard quotient (HQ) of these heavy metals. Tolerable and recommended daily intake values established by some international organization like WHO and Joint FAO/WHO Expert Committee Food Additive (JECFA) were used to determine exposure to contaminants as well as related health risk by comparing with the estimated daily intake of heavy metals obtained in this study. It was observed that the EDIs of the metals in rice from farm B were higher than those obtained in farm A except in Fe where EDI in rice from farm A was higher. Also, the EDIs of Cd, Zn, Cr and Fe in rice from farm A were 3, 0.6, 0.4 and 15% of the safe values while farm B had 14, 0.6, 0.5 and 4% as EDI values for Cd, Zn, Cr and Fe respectively. However, EDIs of Pb in rice from farm A and B were 56 and 78% of the safe value. These values are appreciable compared to other EDIs of the other metals. Much lower EDI of Pb (0.37 μg/kg bw/day) has been reported in rice obtained in Zhejiang, China (Huang et al. 2013). However, Ihedioha et al. (2016) reported EDI of lead and cadmium to be 129% and 154% higher than their safe values respectively. The low EDI obtained for the metals in this study is an indication that there will be no adverse health effect from the heavy metal ingestion through consumption of rice grown in the region. This is confirmed from the results of the hazard quotient and total hazard index obtained for rice from the two farms. The hazard quotient of adult from rice consumption from farm A was in the following order: Pb > Fe > Cd > Zn > Cr while farm B was Pb > Cd > Fe > Zn > Cr. Amongst the toxic heavy metals, ingestion of Pb had the highest potential for adverse effect while Cr ingestion had the minimal potential. None of the HQ of these metals was above 1, and thus, consumption of rice from this region cannot lead to any probable health risk to the consumers. The total hazard index for rice consumption from farm A is 0.693 with relative contributions as follows: Pb (69.84%), Cd (3.89%), Zn (2.89%), Cr (0.08%) and Fe (23.37%). A higher THI (0.852) was obtained for rice consumption from farm B with relative contributions as follows: Pb (79.23%), Cd (12.91%), Zn (2.46%), Cr (0.08%) and Fe (5.28%). Ihedioha et al. (2016) has reported THI of 3.028 in rice consumption from Ada rice fields in Enugu, Nigeria. In their work, Cd and Pb contributed about 40% and 37% of the THI respectively. The THIs obtained in rice consumption from farms A and B were lower than unity which still indicates that consumers of rice from that region are not exposed to any probable health risk. Though this assessment was to measure intake of heavy metals through rice consumptions, human beings can still be exposed to heavy metals through other food such as consumption of contaminated vegetables, water and fish or pathways like inhalation and dermal contact (Chary et al. 2008).
Conclusion
This study showed that most of the heavy metals in both soil and rice were below the permissible limit except for Fe, Pb and Cd in some cases. Probable sources of these metals have been attributed to use of agrochemicals, vehicular emissions and artisan mining around the study area. Metal uptake from the soil to the plant is quite viable and primarily occurs in the exchangeable fraction of the soil. Zinc and lead were less mobile in the soil and thus more available for plant uptake. Heavy metal contamination of rice grain within the study area was minimal. The total hazard risk was less than unity, and therefore, consumption of rice from the study area does not pose any immediate threat to consumers. However, it must be noted that with continuous industrial spread and activities and the use of heavy metal–laden chemicals for farming, the potential for heavy metal contamination of the soil and, subsequently, rice grown on the soil will increase.
References
Adams, M., Zhao, F. J., McGrath, S. P., Nicholson, F. A., & Chambers, B. J. (2004). Predicting cadmium concentration in wheat and barley grain using soil properties. Journal of Environmental Quality, 33, 532–541.
Adriano, D. C. (2003). Trace elements in terrestrial environments: bio-geochemistry, bio availability and risks of metals (2nd ed.). New York City: Springer.
Ahumada, I., Mendoza, J., Navarrete, E., & Ascar, L. (1999). Sequential extraction of heavy metals in soils irrigated with waste water. Communications in Soil Science and Plant Analysis, 30, 1507–1519.
Ajijola, S., Usman, J. M., Egbetokun, O. A., Akoun, J., & Osalusi, C. S. (2012). Appraisal of rice production in Nigeria: a case study of north central states of Nigeria. International Journal of Stored Product and Post-Harvest Research, 3(9), 133–136.
Akbar, F. A., Hale, W. H. G., Headley, A. D., & Athar, M. (2006). Heavy metal contamination of roadside soils in Northern England. Soil and Water Research, 1(4), 158–163.
Allen, S. E., Grimshaw, H. M., & Rowland, A. P. (1986). Chemical analysis. In P. D. Moore & S. B. Chapman (Eds.), Methods in plant ecology (pp. 285–344). London: Blackwell Scientific.
Ano, A. O., Odoemelam, S. A., & Ekwueme, P. O. (2007). Lead and cadmium levels in soils and cassava (Mannihot esculanta crantz) along Enugu-Port Harcourt expressway in Nigeria. Electronic Journal of Environment, Agriculture and Food Chemistry, 6(5), 2024–2031.
Anon. (1995). Maximum allowable concentrations in the China Environmental Quality Standards for Soil (GB 15618–1995, grade II for the agriculture land).
ASTM (2000). Standard test methods for moisture, ash, and organic matter of peat and other organic soils. Method D 2974–00. American Society for Testing and Materials. West Conshohocken, PA.
Bai, L. Y., Zeng, X. B., Su, S. M., Duan, R., Wang, Y. N., & Gao, X. (2015). Heavy metal accumulation and source analysis in green house soils of Wuwei District, Gansu Province, China. Environmental Science and Pollution Research, 22, 5359–5369.
Bermond, A. (2001). Limits of sequential extraction procedures reexamined with emphasis on the role of H+ ion reactivity. Analytica Chimica Acta, 445, 79–88.
Canadian Council of Ministers of Environment. (2007). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health. Available at http://www.ccme.ca/publications/ceqg_rcqe.html?category_id=125. Accessed 28 Dec 2016.
Chary, N. S., Kamala, C. T., & Raj, D. S. (2008). Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicology and Environmental Safety, 69(3), 513–524.
Dawaki, U. M., Dikko, A. U., Noma, S. S., & Aliyu, U. (2013). Heavy metals and physicochemical properties of soils in Kano urban agricultural land. Journal of Basic Applied Science, 21(3), 239–246.
De la Rosa, G., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2003). Utilization of ICP/OES for the determination of trace metals binding to different humic fractions. Journal of Hazardous Material, 97, 207–218.
DeFlora, S., Camoirano, A., Bagnasco, M., Bennicelli, C., Corbett, G. E., & Kerger, B. D. (1997). Estimates of the chromium (VI) reducing capacity in human body compartments as a mechanism for attenuating its potential toxicity and carcinogenicity. Carcinogenesis, 18, 531–537.
Department of Petroleum Resources DPR (2002). Environmental guidelines and standards for soil in Nigeria (revised edition). Department of Petroleum Resources, Nigeria: Ministry of Petroleum and Natural Resources.
Feng, J. F., Zhao, J., Bian, X. M., Zhang, W. (2012). Spatial distribution and controlling factors of heavy metal contents in paddy soil and crop grains of rice wheat cropping system along highway in East China. Environmental Geochemistry and Health, 34, 605–614.
Food and Agricultural Organization of the United Nations (FAO) (2001). Statistical databases. Available online at: http://faostat.fao.org. Accessed 15 Nov 2016.
Food and Agricultural Organization. (1991). African fisheries and the environment. Journal of Food and Agriculture Organization of the United Nations Regional Office for Africa, 9, 2–8.
Frei, M., & Becker, K. (2004). Agro-biodiversity in subsistence-oriented farming systems in a Philippine upland region: nutritional considerations, biodiversity and conservation. Journal of the Institute of Animal Production, Tropics and Subtropics, 13, 1591–1610.
Gimeno-Garcia, E., Andreuand, V., & Boluda, R. (1996). Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environmental Pollution, 92, 19–25.
Huang, Z., Pan, X., Wu, P., Han, J., & Chen, Q. (2013). Health risk assessment of heavy metals in rice to the population in Zheijang, China. PLoS One, 8(9), e75007. https://doi.org/10.1371/journal.pone.0075007.
Ihedioha, J. N., Ujam, O. T., Nwuche, C. O., Ekere, N. R., & Chime, C. C. (2016). Assessment of heavy metal contamination of rice grains (Oryza sativa) and soil from Ada field, Enugu, Nigeria: estimating the human health risk. Human and Ecological Risk Assessment- an International Journal, 22(8), 1665–1667.
Ito, H., & Iimura, K. (1976). The absorption and translocation of cadmium in rice plants and its influence on their growth, in comparison with zinc: studies on heavy metal pollution of soils (part 1). Bulletin of the Hokuriku National Agricultural Experiment Station, 19, 71–139.
JECFA (1982). Evaluation of certain food additives and contaminants, twenty-sixth report of the joint FAO/WHO expert committee on food additives. World Health Organization, Technical Report Series 683.
JECFA (1983). Evaluation of the Joint FAO/WHO Expert Committee on Food Additive: iron. Available online at: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=2859. Accessed 15 Apr 2018.
Joint FAO/WHO Expert Committee on Food Additives (JECFA) (2010). Evaluation of certain food additives and contaminants: summary and conclusion on the seventy-third meeting, Geneva.
Jones, L. H. P., Jarvis, S. C., Green, D. J., & Hayes, M. H. B. (1981). The fate of heavy metals in the chemistry of soil processes. New York: John Wiley & Sons publishers.
Khairiah, J., Habibah, H. J., Anizan, I., Maimon, A., Aminah, A., & Ismail, B. S. (2009). Content of heavy metals in soil collected from selected paddy cultivation areas in Kedah and Perlis, Malaysia. Journal of Applied Sciences Research, 5(12), 2179–2188.
Kirpichtchikova, T. A., Manceau, A., Spadini, L., Panfili, F., Marcus, M. A., & Jacquet, T. (2006). Speciation and solubility of heavy metals in contaminated soil using X-ray microfluorescence, EXAFS spectroscopy, chemical extraction, and thermodynamic modeling. Journal of Geochimica et Cosmochimica Acta, 70(9), 2163–2190.
Landonin, D. V. (2002). Heavy metal compounds in soils: problems and methods of study. Eurasian Soil Science, 35(6), 605–613.
Li, P. J., Wang, X., Allinson, G., Li, X., & Xiong, X. (2009). Risk assessment of heavy metals in soil previously irrigated with industrial wastewater in Shengyang, China. Journal of Hazardous Material, 161, 516–521.
Ma, Y. B., & Uren, N. C. (1998). Transformation of heavy metals added to soil: application of a new sequential extraction procedure. Geoderma, 84, 157–168.
Manish, A., Weuve, J., Schwartz, J., & Wright, R. O. (2009). Association of environmental cadmium exposure with periodontal disease in U.S. adults. Environmental Health Perspective, 117(5), 739–744.
Mar, S. S., Okazaki, M., & Motobayashi, T. (2012). The influence of phosphate fertilizer application levels and cultivars on cadmium uptake by Komatsuna (Brassica rapa. L.var. perviridis). Soil Science, Plant and Nutrition, 58, 492–502.
Markert, B. (1996). Instrumental element and multi-element analysis of plant samples—methods and applications. Chichester: Wiley and sons.
Martley, E., Gulson, B., Louie, H., Wu, M., & Di, P. (2004). Metal partitioning in soil profiles in the vicinity of an industrial complex, New South Wales, Australia. Geochemistry: Exploration, Environment, Analysis, 4, 171–179.
McLaren, R. G., Clucas, L. M., & Taylor, M. D. (2005). Leaching of macronutrients and metals from undisturbed soils treated with metal-spiked sewage sludge: distribution of residual metals. Australian Journal of Soil Research, 43(2), 159–170.
McLaughlin, M. J., Smolders, E., & Merckx, R. (1998). Soil-root interface: physicochemical processes. In P. M. Hunag (Ed.), Soil chemistry and ecosystem health (pp. 233–277). Madison: Soil Science Society of America.
Misra, S. G., & Mani, D. (1991). Soil pollution. Punjabi Bagh: Ashish Publishing House.
Nasser, A., Khairiah, J., Talib, M. L., Ismail, B. S., & Anizan, I. (2014). Accumulation and translocation of heavy metals in soil & paddy plant samples collected from rice fields irrigated with recycled and non-recycled water in MADA Kedah, Malaysia. International Journal of ChemTech Research, 6(4), 2347–2356.
National Research Council, Food and Nutrition Board. (1989). Recommended dietary allowances (10th edit ed.). Washington, DC: National Academy Press.
Nnaji, J. C., & Igwe, O. U. (2014). Fractionation of heavy metals in soil samples from rice fields in New Bussa, Nigeria. International Journal of ChemTech Research, 6(14), 5544–5553.
Nouri, J., Mahvi, A. H., Jahed, G. R., & Babaei, A. A. (2008). Regional distribution pattern of groundwater heavy metals resulting from agricultural activities. Environmental Geology, 55, 1337–1343.
Olajire, A. A., Ayodele, E. T., Oyedirdan, G. O., & Oluyemi, E. A. (2003). Levels and speciation of heavy metals in soils of industrial southern Nigeria. Environmental Pollution, 113, 135–144.
Payus, C., & Talip, A. F. A. (2014). Assessment of heavy metal accumulation in paddy rice (Oryza sativa). African Journal of Agricultural Research, 9(41), 3082–3090.
Pueyo, M., Sastre, J., Hernandez, E., Vidal, M., Lopez-san-chez, J. F., & Rauret, G. (2003). Heavy metals in the environment: prediction of trace element mobility in contaminated soils by sequential extraction. Journal of Environmental Quality, 32, 2054–2066.
Satpathy, D., Reddy, M. V., & Dhal, S. P. (2014). Risk assessment of heavy metal contamination in paddy soil, plants and grains (Oryza sativa L) at the east coast of India. Biomedical Research International, 2014, 11. https://doi.org/10.1155/2014/545473.
Schumacher, A. B. (2002). Methods for the determination of total organic carbon (TOC) in soils and sediments. United States Environmental Protection Agency, Environmental Sciences Division National Exposure Research Laboratory, Las Vegas, NV 89193-3478.
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metal toxicity and the environment. Experientia Supplementum., 101, 133–164.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Journal of Analytical Chemistry, 51(7), 844–851.
Ugwu, J. N., Okoye, C. O. B., & Ibeto, C. N. (2011). Impacts of vehicle emissions and ambient atmospheric deposition in Nigeria on the Pb, Cd, and Ni content of fermented cassava flour processed by sun drying. Human and Ecological Risk Assessment: An International Journal, 17(2), 478–488.
US EPA (U. S. Environmental Protection Agency). (2000). Risk based concentration table. Washington DC: United States Environmental Protection Agency.
US EPA (2015). Estimating risk from contaminants contained in agricultural fertilizers. Draft Report, Office of Solid Waste, US Environmental Protection Agency. http://www.epa.gov/epawaste/index.htm. Accessed 17 December 2016.
WHO (World Health Organization) (1992). Cadmium. Environmental health criteria, Vol. 134. WHO, Geneva.
WHO. (1993). Evaluation of certain foods additives and contaminants. Forty-first report of the Joint FAO/WHO Expert Committee on Foods Additives (JECFA) (p. 53). Geneva, Technical Report Series 837: WHO.
WHO (1996). Permissible limits of heavy metals in soil and plants, (Geneva: World Health Organization), Switzerland.
Yoneyama, T., Ishikawa, S., & Fujimaki, S. (2015). Route and regulation of zinc, cadmium and iron transport in rice plants (Oryza sativa L.) during vegetative growth and grain filling: metal transporters, metal speciation, grain Cd reduction and Zn and Fe biofortification. International Journal of Molecular Science, 16, 19111–19129.
Zhang, M. K., Liu, Z. Y., & Wang, H. (2010). Use of single extraction methods to predict bioavailability of heavy metals in polluted soils to rice. Communications in Soil Science and Plant Analysis, 41(7), 820–831.
Zhuang, P., McBride, M. B., Xia, H., Li, N., & Li, Z. (2009). Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Science of Total Environment, 407(5), 1551–1561.
Acknowledgements
The authors wish to acknowledge Mr. Orji who assisted us with the local rice farmers. We also wish to thank Mr. Okoh, Mr. Kartay, Mr. Daniel Ogbebor and Miss Chioma Nweke of the Port-Harcourt Refining Company Water and Utility Lab for their invaluable help with the laboratory procedures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ihedioha, J.N., Ogili, E.O., Ekere, N.R. et al. Risk assessment of heavy metal contamination of paddy soil and rice (Oryza sativa) from Abakaliki, Nigeria. Environ Monit Assess 191, 350 (2019). https://doi.org/10.1007/s10661-019-7491-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7491-3