Abstract
The effects of childhood exposure to ambient air pollution and their influences on healthcare utilization and respiratory outcomes in Memphis pediatric asthma cohort are still unknown. This study seeks to (1) investigate individual-level associations between asthma and exposure measures in high asthma rate and low asthma rate areas and (2) determine factors that influence asthma at first year of a child’s life, first 2 years, first 5 years, and during their childhood. Datasets include physician-diagnosed asthma patients, on-road and individual PM2.5 emissions, and high-resolution spatiotemporal PM2.5 estimates. Spatial analytical and logistic regression models were used to analyze the effects of childhood exposure on outcomes. Increased risk was associated with African American (AA) (odds ratio (OR) = 3.09, 95% confidence interval (CI) 2.80–3.41), aged < 5 years old (OR = 1.31, 95% 1.17–1.47), public insurance (OR = 2.80, 95% CI 2.60–3.01), a 2.5-km radius from on-road emission sources (OR = 3.06, 95% CI 2.84–3.30), and a 400-m radius from individual PM2.5 sources (OR = 1.33, 95% CI 1.25–1.41) among the cohort with residence in high asthma rate areas compared to low asthma rates areas. A significant interaction was observed between race and insurance with the odds of AA being approximately five times (OR = 4.68, 95% CI 2.23–9.85), public insurance being about three times (OR = 2.65, 95% CI 1.68–4.17), and children in their first 5 years of life have more hospital visits than other age groups. Findings from this study can guide efforts to minimize emissions, manage risk, and design interventions to reduce disease burden.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing interest to better understand the effects of ambient air pollution across the life course of individuals or its aggregate effects on communities is the principle focus of previous exposure assessment studies (Hertzman 1995; Hall et al. 2002; Ben-Schlomo and Kuh 2002; Kuh et al. 2003; Vrijheid et al. 2014; Gilliland et al. 2005; Saenen et al. 2015; Kulkarni and Grigg 2008; Wild 2012; Deng et al. 2016). Current area of significant interest in epidemiological studies is on understanding the health effects of ambient air pollution and its influence on different perspectives of well-being, equity, environmental justice, and disparities related to healthcare utilization over a lifetime (Ben-Schlomo and Kuh 2002; Kuh et al. 2003; Wild 2005; Smith et al. 2015; Fecht et al. 2016). Using a life course approach, prior studies have developed spatially explicit measures of ambient air pollution, which are known to have a link to chronic pulmonary and respiratory conditions (Dockery et al. 1993; Brunekreef and Holgate 2002; Brunekreef and Sunyer 2003; Oyana et al. 2004; Pope and Dockery 2006; Swartz et al. 2004; McCreanor et al. 2007; Pope et al. 2009; Halonen et al. 2016; Veremchuk et al. 2014; Friberg et al. 2016)
Previous population health studies on short-term exposure to fine particulate matter (PM2.5) with 2.5 μm or smaller have suggested a positive association with childhood asthma (Eggleston et al. 1999; Clark et al. 1999; Gorai et al. 2015; Shankardass et al. 2015; Halonen et al. 2016). However, the evidence of associations between lifetime/course PM2.5 exposure and childhood asthma is still inconsistent/understudied (Lin et al. 2018; Kanchongkittiphon et al. 2014; Gorai et al. 2015; Shankardass et al. 2015; Halonen et al. 2016), and therefore requires further investigation (Halonen et al. 2016). In Memphis, which is known as the Asthma Capital of the USA, there is still little information and knowledge on the effects of PM2.5 on pediatric asthma. Yet the Memphis metropolitan area (MMA) pediatric asthma cohort could draw from other studies conducted in several major cities and metropolitan areas, including New York (Acosta et al. 2013; Nicholas et al. 2005; Shmool et al. 2015), Boston (Gottlieb et al. 1995; Levy et al. 2004), Atlanta (Friberg et al. 2016), Los Angeles (Whittemore and Korn 1980; Huynh et al. 2010; Camacho-Rivera et al. 2014; Berhane et al. 2016), Beijing (Zheng et al. 2002; Zhao et al. 2010; Yangzong et al. 2012), and London (Soyiri et al. 2011; Sturdy et al. 2012; Halonen et al. 2016), and use this scientific knowledge to further understand the effects of PM2.5 exposure to physician-diagnosed asthma.
Thus, we completed a recent study where it was observed the asthma burden among the MMA pediatric cohort was more prevalent in a socioeconomically deprived community living in the south, downtown, and north of Memphis than in other areas (Oyana et al. 2017). These geographical areas present children with the high rates of asthma compared to the east and other areas with high socioeconomic conditions (Oyana et al. 2017).
This study assessed PM2.5 exposure and its potential association with asthma-related emergency department (ED) visits and inpatient hospitalizations among children living in high and low asthma rate areas (reference group), measured short-term and long-term PM2.5 exposure at various stages of child development in relation to socioeconomic and environmental risk factors. We hypothesized that the geographical heterogeneity of the emergency room (ER) and hospitalization rates among this pediatric cohort could be explained by the effects of PM2.5 exposure at different stages of child development. We evaluated whether there is a link between the number of ER visits or hospitalizations and individual PM2.5 exposure during the first year of life, first 2 years of life, first 5 years of life, and during their early childhood (age < 12 years) and teenage years (age 12–18 years). This study was accomplished with two specific aims: (1) examined the temporal and spatiotemporal relationship between healthcare utilization in children with a physician-diagnosed asthma diagnosis and PM2.5 exposure and (2) examined the temporal and spatiotemporal relationship between ED visits of children with an asthma diagnosis and PM2.5 exposure.
Materials and methods
Study settings, design, and datasets
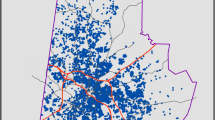
The study area consists of a pediatric population living within residential locations identified as high and low asthma rate areas. Figure 1 shows the study area and specific locations of high and low asthma rate areas, potential exposure sources, and major population centers. The MMA region consists of eight counties with a population of about 1,320,000 (US 2010 Census Bureau), and is one of the largest metropolitan areas in the southern United States. Twenty-nine percent of the population living in the MMA region is 18 years or younger. The combined asthma ED and hospitalization rate for this region is 75.9 per 1000 children, whereas the rate for ED is 56.9 per 1000 while hospitalization rate is 10.8 per 1000 (Oyana et al. 2017). This retrospective, longitudinal study covers a period of 11 years (2005–2015) and the spatial unit of analysis for studying the effects of PM2.5 exposure and healthcare utilization is at residential geocodes (individual-level measure) for all unique cases.
Memphis pediatric asthma cohort
The Memphis pediatric asthma cohort is regionally representative of physician-diagnosed asthma patients residing in the MMA region. The cohort was obtained from a large-scale longitudinal electronic health record (EHR) database maintained by Methodist Le Bonheur Healthcare (MLH), a large not-for-profit healthcare system with about 48% overall market share in the MMA region. The cohort contains (1) unique asthma patients (n = 28,793) identified using the International Classification of Diseases Code, Ninth Revision, Clinical Modification (ICD-9-CM), diagnosis code 493.xx (Asthma and sub-classifications); (2) medical records for each patient’s ED visits and/or inpatient hospitalizations from 2005 to 2015; and (3) residential addresses for each asthma patient (≤ 18 years old at baseline and subsequent visits).
In the first published asthma report, we investigated spatiotemporal patterns of healthcare utilization among the entire pediatric asthma cohort (n = 28,793) (Oyana et al. 2017). Current study only focused on 24,005 children with asthma identified with residence in high or low asthma risk areas (Fig. 1) (Oyana et al. 2017). The pediatric cohort with residence in high asthma rate areas is highly vulnerable to asthma attacks probably due to PM2.5 exposure from potential industrial facilities and on-road mobile emission sources. We conducted an in-depth study on the effects of early life exposure. This study (15-04053-XM) was approved by the Institutional Review Boards at the University of Tennessee Health Science Center and MLH.
PM2.5 exposure data
High-resolution 1 km × 1 km satellite-derived PM2.5 dataset
This is a high-resolution spatiotemporal PM2.5 dataset that was obtained from Surface PM2.5 data downloaded from the website for the Atmospheric Composition Analysis Group, Dalhousie University, Canada (Boys et al. 2014; Van Donkelaar et al. 2015; Van Donkelaar et al. 2016), and is based on satellite observations of top-of-atmosphere reflectance, optimal estimation aerosol optical depth retrieval, and adjusted North America estimations using geographically weighted regression. They validated the surface 1 km × 1 km PM2.5 estimates for the period 2004–2014 using PM2.5 observations from the US Environmental Protection Agency (EPA) ground-level monitoring data. They established through robust validation methods that their estimates were unbiased, comparable, and representative of ground measurements of ambient air pollution. Ground-level monitoring was obtained from 108 IMPROVE sites, 128 sites under the Federal Reference Methods/Federal Equivalency Methods for the 52 largest cities with more than 1 million people, and 1125 ambient air monitoring sites.
Proximity to exposure sources (stationary and mobile) data
PM2.5 exposure measures included patient’s resident distance to on-road mobile sources and industrial facilities. The residential exposure estimates were derived using major roadways known to have high traffic-related pollution and the locations of 26 identified hotspots/top-ranked emission sources from the US Environmental Protection Agency Air Quality System.
PM2.5 measures and health outcomes
-
Specific measures of a child’s residential/maternal exposure were defined using age at the baseline. We developed eight temporally varying exposure measures that represent a child’s age of exposure to PM2.5 through the study period (first 2 years of life, first 3 years of life, first 4 years of life, first 5 years of life, first 6 years of life, first 7 years of life, first 8 years of life, and 18 years of life). Each time stamp was assigned a matching PM2.5 single value for its respective period as indicated below. Years 9 through 11 measures were excluded due to dwindling sample and reduced power.
-
Measures of PM2.5 exposure. Residential proximity to major individual PM2.5 emission sources was measured using residential geocodes within a radius of 2.5 km. Derived measures were assigned a value of 1 and those outside a value of 0. Residential proximity to major on-road mobile emission sources was measured using residential geocodes within a radius of 400 m. Derived measures were assigned a value of 1 and those outside a value of 0. A calculated childhood average PM2.5 exposures was derived from a high-resolution 1 km × 1 km satellite-derived PM2.5 dataset and all geocodes at individual-level for the cohort were assigned extracted values from the surface PM2.5 layer. Children living in areas which have more than the average PM2.5 (x̅ = 12.2 μg/m3 for the MMA region, EPA’s allowable limit is 12 μg/m3) were assigned a value of 1 and those below 0.
-
Outcome measure. Residence in high and low asthma rate areas for children with positive and negative health outcomes was determined by calculating ER and hospitalization asthma rates, and performing hotspot analysis to identify higher (negative outcomes)/lower (positive outcomes) than expected rates for the population at risk using Kulldorff’s retrospective space-time statistics, discrete Poisson models (Oyana et al. 2017).
Spatial and statistical analysis
The residential addresses of asthma patients were geocoded using a multi-step process to maximize positional accuracy in residence exposure estimates. Descriptive statistics, bivariate, and multivariate logistic regressions were used to summarize statistical distributions, characterize, and determine how PM2.5 exposure influences healthcare and respiratory outcomes.
The sample was stratified by a child’s exposure at baseline age and focus was mainly on individuals with continuous encounters in each or subsequent year over the study period.
Initial screening for all variables was conducted, including testing for multicollinearity based on a variance inflation factor (VIF) criterion of less than 3.0. Adjusted odds ratios (OR) and 95% confidence intervals (CI) were reported after adjusting for covariates. Potential associations were investigated using a binary response variable measuring outcome for children with asthma residing in high or low asthma rate areas and eight predictors (age at a time of visit, gender, race, insurance type, calculated childhood average PM2.5, proximity to major individual PM2.5 emissions sources, and proximity to major roadways/mobile emission sources). The outcome (response) variable was specified as 0/1 to either represent a child with residence in a low asthma rate area (0) or high asthma rate area (1).
Exposure to PM2.5, child health outcomes, age, race, and insurance type in the main effect and interactive models were examined using type 3 analysis of effects at a conservative p ≤ 0.10 level. If significant, post hoc comparisons between levels of interactions were assessed at a significance level (p ≤ 0.05). The main effect and interactive models were constructed using PROC LOGISTIC or PROC GLIMMIX procedures in SAS/Stat 13.1 in version 9.4 (SAS Institute Inc., Cary, NC, USA).
All the data processing, mapping, and analyses were conducted using ArcGIS 10.2.2 (ESRI Inc., Redlands, CA, USA), SaTScan (developed jointly by Kulldorff M., Boston, Massachusetts and Information Management Services, Inc., Silver Spring, MD, USA), and SpaceStat 4.0.21 (ClusterSeer, a BioMedware Inc., Ann Arbor, MI). The data and knowledge obtained from all the above spatial and statistical analyses were used to spatially and temporally explain pediatric asthma disease risk, potential trends, and study the effects of PM2.5 exposure on asthma ED visits and hospitalizations in this cohort.
Results
Characteristics of asthma patients living in high and low asthma rate areas
In the pediatric asthma cohort, 53.5% (12,830/24,005) and 46.5% (11,175/24,005) of the children with asthma had their residence in high and low asthma rate areas, respectively. The asthma cohort sample distribution is stratified by high and low asthma rate areas (Table 1 and Fig. 2). The majority of samples with continuous visits to ED and hospitalizations mainly occurred during the early childhood period (Table 1). The sample distribution further shows 61% and 51% of children with asthma aged less than 5 years had their residence in high asthma rate and low asthma rate areas, respectively. Nearly 5.3% children in the 12–18 age group live in a high asthma rate area compared to 8.4% in a low asthma rate area. Sixty percent of the cohort living in either high asthma rate or low asthma rate areas was male. African American children constituted slightly over 82.2%; specifically, 90.1% of them lived in high asthma rate areas and 73.1% low asthma rate.
Cohort characteristics and respective statistical results for the study population are presented in Table 2. Three quarters of the cohort were on public (Medicaid/TennCare) insurance; and this number went up to 85.3% for those in the high asthma rate area compared to only 63.5% in the low asthma rate area. Nearly 32% of the children living in a low asthma rate area were on private insurance; this is three times more than the high asthma rate area (11.5%). Figure 2 shows that one-third of children with asthma in high asthma rate areas lived in close proximity to a major individual PM2.5 sources. Three quarters of the children with asthma in high asthma rate areas lived within a 400-m radius from on-road mobile emission sources. In the low asthma rate area, only 11.2% had residential proximity to a major individual PM2.5 source while 67.6% lived within a 400-m radius from on-road mobile emission sources.
The rest of the section is structured around three major themes that address the specific aims of this paper: (1) the effects of PM2.5 exposure and other risk factors on pediatric asthma over an 11-year study period; (2) the temporal effects of PM2.5 residence exposure on pediatric asthma in high and low asthma rate areas; and (3) type 3 analysis of effects of PM2.5 residence exposure on pediatric asthma over time.
The effects of PM2.5 exposure and other risk factors on pediatric asthma over an 11-year study period
Children younger than 5 years and living in high asthma rate areas were at a higher risk (OR = 1.79, 95% CI 1.61–1.98) of asthma encounter compared to those in the other age groups (5–8 years, OR = 1.46, 95% CI 1.30–1.63; 9–11 years, OR = 1.38, 95% CI 1.22–1.56) (Table 2). Male children living in high asthma rate areas were 8% (OR = 0.92, 95% CI 0.88–0.97) less likely to have asthma encounters than female children. African American children living in high asthma rate areas were approximately five times (OR = 4.57, 95% CI 4.18–5.01) more likely to have asthma encounters than whites as suggested by the main effects model. Children on public insurance and living in the high asthma rate areas were approximately four times (OR = 3.77, 95% CI 3.52–4.04) more likely to have asthma encounters than those on private insurance. Children living in high asthma rate areas and within 400-m radius from major roadways were 38% (OR = 1.38, 95% CI 1.31–1.46) more likely to have asthma encounters than those living beyond 400-m radius to major roadways/mobile emission sources. Children living in high asthma rate areas and within a 2.5-km radius of individual PM2.5 emission sources were approximately three times (OR = 3.24, 95% CI 3.02–3.48) more likely to have asthma encounters than those living beyond a 2.5-km radius of individual PM2.5 emission sources.
In the overall model, children aged 11 years or younger were at a higher risk for ED asthma visits and hospitalizations compared to those aged 12 years or older (Table 2). But, children aged 11 years old or younger, especially those less than 5 years old, were at a higher risk (31% more likely (OR = 1.31, 95% CI 1.17–1.47)) than those in the 5–8 and 9–11 age groups. African American children were three times (OR = 3.09, 95% CI 2.80–3.41) more likely to have ED asthma visits and hospitalizations compared to white children. While the overall main effects model showed that children within the “Other” category (Hispanic, Asian/Pacific Islander, etc.) were 58% more likely to have ED asthma visits and hospitalizations than whites, the interaction of age and race revealed that children less than 5 years under the “Other” category were two times (OR = 1.96, 95% CI 1.01–3.78) more likely to have ED asthma visits and hospitalizations compared to those in the 12–18 age group.
Even though gender had no effect (OR = 0.95, 95% CI 0.89–1.00) over the 11-year study period among children living in high asthma rate areas, we found that female children were 5% less likely than male children to have asthma encounters. The most influential (and consistently observed) risk factors accounting for the high number of ED asthma visits and hospitalizations were being an African American child with residence in high asthma rate areas, on public insurance, and living either within a 2.5-km radius from individual PM2.5 sources or 400-m radius from major roadways. However, after adjusting for covariates, the odds of asthma from effects of PM2.5 exposure in high asthma rate areas compared to low asthma rate areas was less than 18% with the true population effect between 16 and 20%.
The temporal effects of PM2.5 residence exposure on pediatric asthma in high and low asthma rate areas
The OR data was plotted against three PM2.5 exposure predictors using forest plots in Fig. 3. Association estimates stratified by age at the baseline (on the x-axis) represent the eight stages of a child’s development, thus indicating potential impacts from PM2.5 exposure over time. Individual PM2.5 sources and PM2.5 emissions from major roads were found to be statistically significant over time with the exception of PM2.5 exposure for ED asthma visits (first 2 years through the first 5 years). However, the association between asthma and PM2.5 exposure was significant for all inpatient hospitalizations from the first 2 years of life through the first 8 years of life. Exposure effects over time among the inpatient group were statistically associated with 1.4 (OR ranges from 1.35 to 1.39) times the odds of asthma in high asthma rate areas compared to low asthma rate areas. Exposure effects over time among the ED visits and inpatient hospitalization groups were also statistically associated (OR ranges from 2.62 to 3.92) times the odds of asthma in the pediatric population living near individual PM2.5 sources in comparison to those in low asthma rate areas. Children residing within a 2.5-km radius of individual PM2.5 sources were about three times (OR ranges from 2.62 to 3.04) more likely to have ED visits and approximately four times (OR ranges from 3.61 to 3.92) more likely to have inpatient hospitalizations.
Estimated associations between measures of asthma trajectories and PM2.5 exposure and the odds of having ED asthma visit and inpatient hospitalization (IP) after stratifying for child’s age at the baseline. Panel (a) odds from childhood PM2.5 exposure, (b) odds from individual PM2.5 sources exposure, and (c) odds from major on-road PM2.5 emission sources exposure. Adjusted OR and 95% CI were calculated for all individual-level categorical predictors, adjusted for eight predictors, and statistically significant at p < 0.05
We observed the greatest risk of 3.9 odds (OR = 3.92 (3.21–4.80)) of having an asthma attack from effects of individual PM2.5 sources was among children from high asthma rate areas that required inpatient hospitalization followed by a 49% odd among children with asthma living near major roadways. Children with asthma living within a 400-m radius from the major roadways (55 to 71% more likely), PM2.5 exposure (35 to 39% more likely), and PM2.5 first 5 years of life (19 to 21% more likely) are more likely to have an asthma attack requiring ED visit than those living in low asthma rate areas. The data suggests that PM2.5 exposure is an influential risk factor and drives healthcare utilization and outcomes in infants than in older children.
Type 3 analysis of the effects of PM2.5 residence exposure on pediatric asthma over time
Results showing associations between predictors of health outcomes and the odds of having asthma among unique pediatric asthma cases stratified by continuous encounters over a study period are presented in Table 3. Table 4 shows type 3 analysis of effects to further explain variations in health outcomes from high and low asthma rate areas. Type 3 analysis of effects showed a significant (p = 0.085) interaction between age and race in the overall study population when evaluated at a conservative p level of ≤ 0.10. White children less than 5 years old were significantly different than those in the other age strata (5–8 years, 9–11 years, and 12–18 years); they had more asthma-related encounters, i.e., 77% more likely to have ED asthma visits and hospitalizations compared to those in the 12–18 age group. An analysis of African American data showed that children in the 12–18 age group were significantly different than those in the other age strata (< 5 years, 5–8 years, and 9–11 years). African American children in the other age group were only 20–23% more likely to have ED asthma visits and hospitalizations compared to children in the 12–18 age group.
Further examination of interactions between age and race showed statistically significant results in years 1 and 5, and age and insurance type in year 4. In year 1, children with asthma aged and younger than 5 years were 18% (OR = 1.18, 95% CI 1.03–1.36) more likely to have additional ED visits and hospitalizations than those in the 12–18 age group. Children in the 5–8 and 9–11 age groups and living in the high asthma rate areas were no different from those younger than 5 years among the continuous asthma encounters. From year 1 to year 6, children with continuous asthma encounters were predominately African American residing in high asthma rate areas.
They were notable interactions between age and race (p = 0.1054) (Table 4). White children aged below 5 years were in the range from 43 to 51% (< 5 years vs. 5–8 years, OR = 1.96, 95% CI 1.01–3.78; and < 5 years old vs. 12–18 years, OR = 1.43, 95% CI 1.08–1.90) more likely to have an asthma encounter than those in the 5–8 and 12–18 age groups. Children who were less than 5 years and 9–11 years old and from “other” racial category by age (Hispanic, Asian/Pacific Islander, etc.) were found to be about 2.34 to 2.68 times more likely to have ED asthma visits and hospitalizations.
Children in the 9–11 age group with asthma encounters for four consecutive years (up to year 4) were three times (OR = 2.95, 95% CI 1.23–7.09) more likely than those in the 12–18 age group to have continuous ED asthma visits and hospitalizations. Children on public insurance (Medicaid/TennCare) and living in high asthma rate areas with continuous encounters up to year 6 were two times (OR = 1.97, 95% CI 1.45–2.68) more likely to have ED asthma visits and hospitalization compared to those on private insurance. We found a significant interaction between age and insurance type (p = 0.0775) after controlling for race. Further in-depth analysis of this interaction revealed that children on private insurance and younger than 5 years old were 51% (OR = 0.49, 95% CI 0.27–0.89) less likely to have ED asthma visits and hospitalizations than those in the 5–8 age group, whereas strikingly those on public insurance and aged below 12 years were almost three times more likely to have ED asthma visits and hospitalizations than those in the 12–18 age group (Table 4). Children younger than 5 years old and on “other insurance care” were approximately five times (OR = 4.81, 95% CI 1.12–20.54) more likely than those in the 5–8 age group to have more continuous ED asthma visits and hospitalizations.
The main effects model for patients with continuous ED asthma visits and hospitalizations for five consecutive years showed that children younger than 5 years old were equally more likely than those in the other age groups (5–8 years, 9–11 years, and 12–18 years) to have continuous ED asthma visits and hospitalizations (Table 4). African American children were about five times (OR = 4.68, 95% CI 2.23–9.85) and children on public insurance were about three times (OR = 2.65, 95% CI 1.68–4.17) to be in the group with hospital visits over the first 5 years of life. We found a significant interaction between age and race (p < 0.0001) after controlling for insurance. The interaction revealed that children on other insurance care and < 9 years were at a higher risk of more ED asthma visits and hospitalizations than those in the 9–11 age group.
With the exception of year 7, the odds of asthma from effects of PM2.5 exposure in high asthma rate areas compared to low asthma rate areas was less than 55% with the true population effect between 31 and 71% for year 8, and less than 70% with the true population effect between 40 and 85% for year 9. The other two exposure measures (PM2.5 from major roadways up to year 4 and individual PM2.5 sources up to year 6) were positively associated with increased risk of asthma. It is notable, however, after six continuous asthma encounters (i.e., up to year 6), the effects of exposure measures were insignificant (excluding a significant year 8 with a wide CI).
An in-depth assessment of temporal patterns in adjusted ORs for three exposure predictors ((a) PM2.5 exposure, (b) 400-m radius to major roadways, and (c) individual PM2.5 emission sources) was performed by plotting respective statistics in Fig. 4. Adjusted ORs are on the y-axis and plotted against each of the three predictors over time. On the x-axis, time represents the year in which patients had an ED visit or inpatient hospitalization. This time also depicts the number of ED visits or hospitalizations for each pediatric asthma patient with continuous encounters in each or subsequent year over the study period. The following key insights are evident in this figure: there is a steady increase in ORs in years 1 through 5 in two exposure predictors (400-m radius to major roadways and individual PM2.5 sources); and there is a decrease in ORs in childhood exposure predictor in years 1 through 3, although the trend from years 3 through 6 has an upward trajectory. However, the trend for years 7 through 9 is quite unstable and hard to determine probably due to sample size variation. For example, the reference group had to be changed from 12–18 to 9–11 age group for comparisons in years 7 and 8. We identified a decrease in ORs for individual PM2.5 exposure predictor from years 7 through 9, while the trend in years 7 through 9 has a downward trajectory. PM2.5 exposure was linked to childhood asthma (p value = 0.02), which is evident in the increased linear trend over time.
Discussion
The main findings in this study are the following: (1) a steady increase in odds ratio in the initial period/first years of child’s life was observed suggesting the increasing impacts of PM2.5 exposure. (2) PM2.5 exposure is an influential risk factor and drives healthcare utilization and outcomes in early childhood. (3) Age, insurance, race, on-road, and individual emissions acted independently during the early childhood period. Potential exposure impacts differed by age, race/ethnicity, and insurance. (4) A far greater asthma disease burden was observed among African American children living in high asthma rate areas from PM2.5 exposure than those living in low asthma rate areas over the 11-year study period. These study findings are consistent with previous studies and confirm our study hypotheses (Oyana et al. 2004; Oyana et al. 2017; Shmool et al. 2015; Huynh et al. 2010; Camacho-Rivera et al. 2014; Berhane et al. 2016; Sturdy et al. 2012; Halonen et al. 2016).
A steady increase in odds ratio in the initial period/first years of child’s life was observed suggesting the increasing impacts of PM2.5 exposure over the study period. The increase in OR is especially notable among infants living in large urban centers, which have high concentrations of industrial facilities and major roadways. The infants are at a greater risk of adverse health outcomes over their childhood than those living in farther away. Previous studies show that the early age of a child is a critical period for the development and maturation of the biological and non-biological systems, especially the lungs, immune system, and brain (National Research Council 2015; Gogtay et al. 2004; Lenroot and Giedd 2006; Tong and Colditz 2004; Ritz et al. 2006; Ritz and Wilhelm 2008; Durston 2008; Giedd et al. 2009). A child’s development continues until when they are about 6 years old (National Research Council 2015). Thus, negative health effects from PM2.5 exposure at an early age (first years of a child’s life) may have far everlasting impacts over a child’s biological, mental, and physical development (National Research Council 2015; Tong and Colditz 2004; Schmidt 2007; Ritz and Wilhelm 2008; Spenrath et al. 2011).
PM2.5 exposure is an influential risk factor and drives healthcare utilization and outcomes in early childhood up to age 6 years, thus indicating potential cumulative effects of PM2.5 emissions. Data on child/ maternal exposure risk at the baseline age could potentially offer fundamental clues on potential harmful health impacts. Environmental influences are further compounded by other socioeconomic risk factors for asthma, such as low socioeconomic status of the family and their heavy reliance on public insurance. However, caution is required when interpreting this finding because urban sources of PM2.5 are composed of different chemicals, and likely have no protective effect on the microbiome.
Furthermore, our data shows a significant loss of private insurance and gain in public insurance beneficiaries during the period. This is a critical health disparity finding since it uncovers a widening racial/ethnic gap in the quality of healthcare received based on insurance type. According to the CDC, childhood asthma prevalence rates of 20–40% were reported in low-resourced, minority communities. Yet asthma accounts to about $56 billion dollars each year in preventable hospital visits. In this study, as the number of consecutive years in asthma encounters increased, we observed an increase in the number of patients on public insurance in comparison to preceding years. Private to public insurance shift may be attributable to the growing changes in the US healthcare ecosystem. For example, asthma was considered as a pre-existing condition and eligible for Medicaid coverage under the 2010 Affordable Care Act (ACA) passed by the Congress. Unfortunately, the chronicity of the patients who were on private insurance prior to 2010 may have increased over time due to the lack of appropriate and timely care, high co-payments, and medication costs.
Prior research has shown that increase in share of medication costs was significantly associated with a decreased adherence to medications (Weiss et al. 2000; Barnett and Nurmagambetov 2011; Sullivan and Ghushchyan 2015; Mao et al. 2017). The annual cost for asthma medications was estimated at $4900 per person, and as the co-payments double, adherence to medications reduced by 32% since 2010 (Weiss et al. 2000; Barnett and Nurmagambetov 2011; Eaddy et al. 2012; Sullivan and Ghushchyan 2015; Mao et al. 2017. Future studies are required to investigate why patients on the private insurance opt out of the preventive programs and why they end up on public insurance.
Age, insurance, race, on-road, and individual acted independently during the early childhood period. Potential exposure impacts on children with asthma also differed by age, race/ethnicity, and insurance. The effects of ambient air emissions with a continuous over reliance on public insurance among African American children in this pediatric asthma cohort may be significantly impacting the use of medications and medication management. In our type 3 analysis of effects, there were no significant interactions between the age groups and any of the insurance type, although a significant interaction was observed between race and insurance type.
There is a far greater burden of asthma disease on African American children living in high asthma rate areas from PM2.5 exposure than those living in low asthma rate areas over the study period. African American children on public insurance were more likely (p = 0.09) to have asthma-related hospital visits compared to white children on public insurance. Other minority share a similar burden like African American children. We found children in the “Other” (which includes Hispanic, Asian, American and Pacific Islander, etc.) racial category were 60% (OR 1.58, 95% CI 1.36–1.82) more likely than white children to have pediatric asthma visits to a healthcare facility. Indeed, white children fared way better than African American or other minority children in obtaining in early and/or timely care because of having private insurance, thus had a reduced asthma burden with low hospital visits.
On further decomposition of the interaction between age and race, we observed that African American children younger than 5 years old were significantly (p = 0.03) less likely to have encounters compared to those in the 12–18 age group. This age-related comparison gives us key insights into the impact of delayed care for asthma-related conditions among African American children. Delayed healthcare in early childhood period among this group could be the reason behind increased hospital visits in older children. This finding highlights age-related disparity and calls for more focused and appropriate asthma care coordination for young African Americans. Further investigation of clinical data elements such as body mass index and chronic comorbid conditions is required (Boulet and Boulay 2011). Robust data on how metabolic and homeostatic systems are adjusted in early years of life has potential to inform personalized medicine, therapeutic interventions, and asthma diagnostics/management.
Conclusions and implications
There is strong evidence of associations between exposure to PM2.5 and increased pediatric asthma risk in the MMA region. Racial disparities in healthcare utilization and outcomes due to PM2.5 exposure are far starker in this Memphis pediatric asthma cohort than originally reported. This study highlights a widening disparity gap in this pediatric asthma cohort through numerous analytical strategies stratified by residence (high vs. low asthma rate areas), age (exposure during early childhood period vs. teenage age period), race (African American vs. white, white vs. other), and insurance (public vs. private). Upon a detailed and critical examination of all these factors, we observed that the size of racial and age disparities in childhood asthma varies geographically in the MMA region, and these spatial variations have significant cost implications on asthma care and management.
References
Acosta, J. C., Banito, A., Wuestefeld, T., Georgilis, A., Janich, P., Morton, J. P., et al. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology, 15(8), 978–990. https://doi.org/10.1038/ncb2784.
Barnett, S. B. L., & Nurmagambetov, T. A. (2011). Costs of asthma in the United States: 2002-2007. Journal of Allergy and Clinical Immunology, 127(1), 145–152.
Ben-Schlomo, Y., & Kuh, D. (2002). A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology, 31, 285–293.
Berhane, K., Chang, C., McConnell, R., Gauderman, W. J., Avol, E., Rapapport, E., Urman, R., Lurmann, F., & Gilliland, F. (2016). Association of changes in air quality with bronchitis symptoms in children in California, 1993-2012. JAMA., 315(14), 1491–1501. https://doi.org/10.1001/jama.2016.3444.
Boulet, L. P., & Boulay, M. E. (2011). Asthma-related comorbidities. Expert Review of Respiratory Medicine, 5(3), 377–393.
Boys, B. L., Martin, R. V., van Donkelaar, A., MacDonell, R., Hsu, N. C., Cooper, M. J., Yantosca, R. M., Lu, Z., Streets, D. G., Zhang, Q., & Wang, S. (2014). Fifteen-year global time series of satellite-derived fine particulate matter. Environmental Science & Technology, 48(19), 11109–11118. https://doi.org/10.1021/es502113p.
Brunekreef, B., & Holgate, S. T. (2002). Air pollution and health. Lancet, 360, 1233–1242.
Brunekreef, B., & Sunyer, J. (2003). Asthma, rhinitis and air pollution: is traffic to blame? [Comment]. The European Respiratory Journal, 21(6), 913–915.
Camacho-Rivera, M., Kawachi, I., Bennett, G. G., & Subramanian, S. V. (2014). Perceptions of neighborhood safety and asthma among children and adolescents in Los Angeles: a multilevel analysis. PLoS One, 9(1), e87524. https://doi.org/10.1371/journal.pone.0087524.
Clark, N. M., Brown, R. W., Parker, E., Robins, T. G., Remick, D. G. J., Philbert, M. A., Keeler, G. J., & Israel, B. A. (1999). Childhood asthma. Environmental Health Perspectives, 107(Suppl 3), 421–429.
Deng, Q., Lu, C., Yu, Y., Li, Y., Sundell, J., & Norbäck, D. (2016). Early life exposure to traffic-related air pollution and allergic rhinitis in preschool children. Respiratory Medicine, 121, 67–73.
Dockery, D. W., Pope, C. A. I. I. I., Xu, X., Spengler, J. D., Ware, J. H., Fay, M. E., et al. (1993). An association between air pollution and mortality in six U.S. cities. The New England Journal of Medicine, 329(24), 1753–1759.
Durston, S. (2008). Converging methods in studying attention-deficit/hyperactivity disorder: what can we learn from neuroimaging and genetics? Development and Psychopathology, 20(4), 1133–1143.
Eaddy, M. T., Cook, C. L., O’Day, K., Burch, S. P., & Cantrell, C. R. (2012). How patient cost-sharing trends affect adherence and outcomes: a literature review. Pharmacy and Therapeutics, 37(1), 45–55.
Eggleston, P. A., Buckley, T. J., Breysse, P. N., Wills-Karp, M., Kleeberger, S. R., & Jaakkola, J. J. (1999). The environment and asthma in U.S. inner cities. Environmental Health Perspectives, 107(Suppl 3), 439–450.
Fecht, D., Hansell, A. L., Morley, D., Dajnak, D., Vienneau, D., Beevers, S., Toledano, M. B., Kelly, F. J., Anderson, H. R., & Gulliver, J. (2016). Spatial and temporal associations of road traffic noise and air pollution in London: Implications for epidemiological studies. Environment International, 88, 235–242. https://doi.org/10.1016/j.envint.2015.12.001.
Friberg, M. D., Zhai, X., Holmes, H. A., Chang, H. H., Strickland, M. J., Sarnat, S. E., Tolbert, P. E., Russell, A. G., & Mulholland, J. A. (2016). Method for Fusing Observational Data and Chemical Transport Model Simulations To Estimate Spatiotemporally Resolved Ambient Air Pollution. Environmental Science & Technology, 50(7), 3695–3705. https://doi.org/10.1021/acs.est.5b05134.
Giedd, J. N., Lalonde, F. M., Celano, M. J., White, S. L., Wallace, G. L., Lee, N. R., & Lenroot, R. K. (2009). Anatomical brain magnetic resonance imaging of typically developing children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 48(5), 465–470.
Gilliland, F., Avol, E., Kinney, P., Jerrett, M., Dvonch, T., Lurmann, F., et al. (2005). Air Pollution Exposure Assessment for Epidemiologic Studies of Pregnant Women and Children: Lessons Learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environmental Health Perspectives, 113(10), 1447–1454.
Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., Nugent, T. F., 3rd, Herman, D. H., Clasen, L. S., Toga, A. W., Rapoport, J. L., & Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101(21), 8174–8179.
Gorai, A. K., Kanchan, Upadhyay, A., Tuluri, F., Goyal, P., & Tchounwou, P. B. (2015). An innovative approach for determination of air quality health index. The Science of the Total Environment, 533, 495–505. https://doi.org/10.1016/j.scitotenv.2015.06.133.
Gottlieb, D. J., O’Connor, G. T., & Beiser, A. S. (1995). Poverty, race, and medication use are correlates of asthma hospitalization rates: a small area analysis in Boston. Chest., 108(1), 28–35. https://doi.org/10.1378/chest.108.1.28.
Hall, A. J., Yee, L. J., & Thomas, S. L. (2002). Life course epidemiology and infectious diseases. International Journal of Epidemiology, 31, 300–301.
Halonen, J. I., Blangiardo, M., Toledano, M. B., Fecht, D., Gulliver, J., Anderson, H. R., Beevers, S. D., Dajnak, D., Kelly, F. J., & Tonne, C. (2016). Long-term exposure to traffic pollution and hospital admissions in London. Environmental Pollution, 208(Pt A), 48–57. https://doi.org/10.1016/j.envpol.2015.09.051.
Hertzman, C. (1995). The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences, 896, 85–95.
Huynh, P., Salam, M. T., Morphew, T., Kwong, K. Y. C., & Scott, L. (2010). Residential proximity to freeways is associated with uncontrolled asthma in inner-city Hispanic children and adolescents. The Journal of Allergy, 2010, Article ID 157249, 7. https://doi.org/10.1155/2010/157249.
Kanchongkittiphon, W., Gaffin, J. M., & Phipatanakul, W. (2014 Jun). The indoor environment and inner-city childhood asthma. Asian Pacific Journal of Allergy and Immunology, 32(2), 103–110.
Kuh, D., Ben-Schlomo, Y., Lynch, J., Hallqvist, J., & Power, C. (2003). Life course epidemiology. Journal of Epidemiology and Community Health, 57, 778–783.
Kulkarni, N., & Grigg, J. (2008). Effect of air pollution on children. Paediatrics and Child Health, 18, 238–243.
Lenroot, R., & Giedd, J. N. (2006). Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews, 30(6), 718–729.
Levy, J. I., Welker-Hood, L. K., Clougherty, J. E., Dodson, R. E., Steinbach, S., & Hynes, H. P. (2004). Lung function, asthma symptoms, and quality of life for children in public housing in Boston: a case-series analysis. Environmental Health, 3, 13. https://doi.org/10.1186/1476-069X-3-13.
Lin, Y., Zou, J., Yang, W., & Li, C. Q. (2018). A Review of Recent Advances in Research on PM2.5 in China. International Journal of Environmental Research and Public Health, 15(3), 438. https://doi.org/10.3390/ijerph15030438.
Mao, J., Xu, X., McPheeters, J. T., Davis, J. R., & Tran, T. N. (2017). Patient characteristics and factors associated with persistent high costs in asthma. Journal of Allergy and Clinical Immunology, 139(2), AB58.
McCreanor, J., Cullinan, P., Nieuwenhuijsen, M. J., Stewart-Evans, J., Malliarou, E., Jarup, L., Harrington, R., Svartengren, M., Han, I. K., Ohman-Strickland, P., Chung, K. F., & Zhang, J. (2007). Respiratory effects of exposure to diesel traffic in persons with asthma. The New England Journal of Medicine, 357, 2348–2358. https://doi.org/10.1056/NEJMoa071535.
National Research Council 2015. (2015). Committee on the science of children birth to age 8: deepening and broadening the foundation for success; board on children, youth, and families; Institute of Medicine; Allen LR, Kelly BB, editors. Washington (DC): National Academies Press (US); 23.
Nicholas, S. W., Jean-Louis, B., Ortiz, B., Northridge, M., Shoemaker, K., Vaughan, R., Rome, M., Canada, G., & Hutchinson, V. (2005 February). Addressing the childhood asthma crisis in Harlem: the Harlem Children’s zone asthma initiative. American Journal of Public Health, 95(2), 245–249. https://doi.org/10.2105/AJPH.2004.042705.
Oyana, T. J., Rogerson, P., & Lwebuga-Mukasa, J. S. (2004). Geographic clustering of adult asthma hospitalization and residential exposure to pollution sites in Buffalo neighborhoods at a U.S.–Canada border crossing point. American Journal of Public Health, 94(7), 1250–1257.
Oyana, T. J., Podila, P., Wesley, J. M., Lomnicki, S. M., & Cormier, S. A. (2017). Spatiotemporal patterns of childhood asthma hospitalization and utilization in Memphis Metropolitan Area from 2005 to 2015. Journal of Asthma, 54, 842–855. https://doi.org/10.1080/02770903.2016.1277537.
Pope, C. A., III, & Dockery, D. W. (2006). Health effects of fine particulate air pollution: lines that connect. Journal of the Air & Waste Management Association (1995), 56, 709–742.
Pope, C. A., Burnett, R. T., Krewski, D., Jerrett, M., Shi, Y., Calle, E. E., & Thun, M. J. (2009). Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke. Circulation, 120, 941–948.
Ritz, B., & Wilhelm, M. (2008). Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic & Clinical Pharmacology & Toxicology, 102, 182–190.
Ritz, B., Wilhelm, M., & Zhao, Y. (2006). Air pollution and infant death in Southern California, 1989–2000. Pediatrics, 118, 493–502.
Saenen, E., Horemans, N., Vanhoudt, N., Vandenhove, H., Biermans, G., Van Hees, M., et al. (2015). Induction of Oxidative Stress and Antioxidative Mechanisms in Arabidopsis thaliana after Uranium Exposure at pH 7.5. International Journal of Molecular Sciences, 16(6), 12405–12423. https://doi.org/10.3390/ijms160612405.
Schmidt, C. W. (2007). Environmental connections: a deeper look into mental illness. Environmental Health Perspectives, 115(8), A404–A410.
Shankardass, K., Jerrett, M., Dell, S. D., Foty, R., & Stieb, D. (2015). Spatial analysis of exposure to traffic-related air pollution at birth and childhood atopic asthma in Toronto, Ontario. Health & Place, 34, 287–295. https://doi.org/10.1016/j.healthplace.2015.06.001.
Shmool, J. L., Bobb, J. F., Ito, K., Elston, B., Savitz, D. A., Ross, Z., et al. (2015). Area-level socioeconomic deprivation, nitrogen dioxide exposure, and term birth weight in New York City. Environmental Research, 142, 624–632. https://doi.org/10.1016/j.envres.2015.08.019.
Smith, A. D., Heron, J., Mishra, G., Gilthorpe, M. S., Ben-Shlomo, Y., & Tilling, K. (2015). Model selection of the effect of binary exposures over the life course. Epidemiology, 26, 719–726.
Soyiri, I. N., Reidpath, D. D., & Sarran, C. (2011). Asthma length of stay in hospitals in London 2001–2006: demographic, diagnostic and temporal factors. PLoS One, 6(11), e27184. https://doi.org/10.1371/journal.pone.0027184.
Spenrath, M. A., Clarke, M. E., & Kutcher, S. (2011). The science of brain and biological development: implications for mental health research, practice and policy. Clarke M, Ghali L, eds. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 20(4), 298–304.
Sturdy, P., Bremner, S., Harper, G., Mayhew, L., Eldridge, S., Eversley, J., Sheikh, A., Hunter, S., Boomla, K., Feder, G., Prescott, K., & Griffiths, C. (2012). Impact of asthma on educational attainment in a socioeconomically deprived population: a study linking health, education and social care datasets. PLoS One, 7(11), e43977. https://doi.org/10.1371/journal.pone.0043977.
Sullivan, P. W., & Ghushchyan, V. (2015). The burden of asthma in the United States: updated nationally representative estimates of the cost of asthma. Journal of Allergy and Clinical Immunology, 135(2), AB48.
Swartz, L., Callahan, K., Butz, A., Rand, C., Kanchanaraksa, S., Diette, G., et al. (2004). Partnering with an inner city community to conduct an environmental randomized clinical trial in asthma. Environmental Research, 95(2), 156–165.
Tong, S., & Colditz, P. (2004). Air pollution and sudden infant death syndrome: a literature review. Pediatric and Perinatal Epidemiology, 18, 327–335.
Van Donkelaar, A., Martin, R. V., Spurr, R. J. D., & Burnett, R. T. (2015). High-resolution satellite-derived PM2.5 from optimal estimation and geographically weighted regression over North America. Environmental Science and Technology, 49, 10482–10491.
Van Donkelaar, A., Martin, R. V., Brauer, M., Hsu, N. C., Kahn, R. A., Levy, R. C., Lyapustin, A., Sayer, A. M., & Winker, D. M. (2016). Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environmental Science & Technology, 50(7), 3762–3772. https://doi.org/10.1021/acs.est.5b05833.
Veremchuk, L., Cherpack, N., Gvozdenko, T., & Volkova, M. (2014). Methodology for the assessment of the impact of the atmospheric air pollution on the formation of the levels of overall morbidity rate of bronchial asthma. Gigiena i Sanitariia, 94, 119–122.
Vrijheid, M., Slama, R., Robinson, O., Chatzi, L., Coen, M., van den Hazel, P., et al. (2014). The human early-life exposome (HELIX): project rationale and design. Environmental Health Perspectives, 122(6), 535–544. https://doi.org/10.1289/ehp.1307204.
Weiss, K. B., Sullivan, S. D., & Lyttle, C. S. (2000). Trends in the cost of illness for asthma in the United States, 1985–1994. Journal of Allergy and Clinical Immunology, 106(3), 493–499.
Wild, C. P. (2012). The exposome: from concept to utility. International Journal of Epidemiology, 41(1), 24–32.
Whittemore, A. S., & Korn, E. L. (1980). Asthma and air pollution in the Los Angeles area. American Journal of Public Health, 70(7), 687–696. https://doi.org/10.2105/AJPH.70.7.687.
Yangzong, Y., Shi, Z., Nafstad, P., Håheim, L. L., Luobu, O., & Bjertness, E. (2012). The prevalence of childhood asthma in China: a systematic review. BMC Public Health, 12, 860. https://doi.org/10.1186/1471-2458-12-860.
Zhao, J., Bai, J., Shen, K., Xiang, L., Huang, S., Chen, A., Huang, Y., Wang, J., & Ye, R. (2010). Self-reported prevalence of childhood allergic diseases in three cities of China: a multicenter study. BMC Public Health, 10(1), 551–557.
Zheng, T., Niu, S., Lu, B., Fan, X., Sun, F., Wang, J., Zhang, Y., Zhang, B., Owens, P., Hao, L., Li, Y., & Leaderer, B. (2002). Childhood asthma in Beijing, China: a population-based case-control study. American Journal of Epidemiology, 156(10), 977–983. https://doi.org/10.1093/aje/kwf127.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Geospatial Technology in Environmental Health Applications
Rights and permissions
About this article
Cite this article
Oyana, T.J., Podila, P. & Relyea, G.E. Effects of childhood exposure to PM2.5 in a Memphis pediatric asthma cohort. Environ Monit Assess 191 (Suppl 2), 330 (2019). https://doi.org/10.1007/s10661-019-7419-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7419-y