Abstract
In this paper, heavy metal contamination in surface sediments along the Mediterranean coast of Morocco was investigated. Determining pollution degree as well as heavy metal origins were the main objectives of this investigation. For this reason, concentrations of nine heavy metals (Cd, Cu, Cr, Ni, Pb, Zn, Hg, Fe, and Mn) were analyzed at ten stations sampled during three different periods. The obtained concentrations showed significant variation between sampling periods, which was controlled by several environmental and chemical processes. According to contamination indices results using pollution load index (PLI), modified contamination degree (mCd), geoaccumulation index (Igeo), enrichment factor (EF), and potential ecological risk index (RI), sampling stations were classified between uncontaminated and strongly contaminated without detecting any intense heavy metal pollution in surface sediment. Likewise, the EF values were comprised between no enrichment and moderate to severe enrichment. According to sediment quality guidelines, the calculated M-ERM-Q indicated that heavy metal mixtures have between 9 and 49% probability for being toxic. This result revealed lowest to medium-low potential of adverse effects to biota populations. Regarding heavy metal origins, multivariate statistical investigation showed that Cd, Cu, Hg, Pb, and Zn are derived mainly from anthropogenic activities, while Fe, Mn, Cr, and Ni were derived from natural sources. Despite Cd is considered as a typical anthropogenic metal, the very low concentrations obtained in this study support the involvement of natural factor in the enrichment with this metal. Therefore, the surface sediments contamination along the Mediterranean coasts of Morocco is particularly caused by a combination of anthropogenic and natural factors. As a result, the study area can be considered as not significantly enriched by human activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal environments around the world are increasingly exposed to heavy metal pollution. This phenomenon, which mainly affects surface sediments, has emerged as a serious environmental issue due to toxicity, persistence, non-biodegradability, and bioaccumulation of heavy metals (Chapman et al. 1998; Gao and Chen 2012; Ayadi et al. 2015; El Zrelli et al. 2015; Strbac et al. 2018). The heavy metals released into aquatic environments subsequently contaminate the biota populations (Miller et al. 2000; Bastami et al. 2012; Wang et al. 2012; Pejman et al. 2015). According to many researchers, surface sediments potentially adsorb heavy metals (Chen et al. 2000; Hortellani et al. 2008; de Paula Filho et al. 2015; Frémion et al. 2016). For this reason, sediments are considered as reliable indicators used for pollutant monitoring in the environment. Besides, their geochemical compositions can be used to determine the degree and the origin of suspicious metal contamination (Suresh et al. 2012; Pejman et al. 2015).
Heavy metals are issued mainly from natural processes, such as rock alteration, and/or anthropogenic factors, such as wastewater, industry, and agricultural activities (Carman et al. 2007; Accornero et al. 2008; Alves et al. 2014; Hussain et al. 2015; Pejman et al. 2015; Frémion et al. 2016; Duodu et al. 2017; Marrugo-Negrete et al. 2017; Strbac et al. 2018). Whatever the source is, natural or anthropogenic, once released into the aquatic environment, these pollutants exhibit high affinity for sediments (Gaur et al. 2005; El Zrelli et al. 2015). In some cases, natural concentrations of heavy metals may be higher than any anthropogenic contamination (Reimann and Caritat 2005; Sakan et al. 2015; Duodu et al. 2017; Strbac et al. 2018). Therefore, it is very important to distinguish between these two sources of contamination.
Coastal sediment pollution caused by heavy metals has been the subject of several studies worldwide (Bastami et al. 2012; Gao and Chen 2012; El Zrelli et al. 2015; Ayadi et al. 2015; Pejman et al. 2015). These studies were essentially based on sediment quality, which constitutes an important information source about metallic contamination (Gao and Chen 2012; Rabaoui et al. 2015; El Zrelli et al. 2015). However, determination of heavy metal concentrations in sediments is not sufficient to establish pollution state. For this reason, numerous geochemical and quantitative approaches have been developed to assess the degree of heavy metal contamination (MacDonald et al. 2000; Abrahim and Parker 2008; Feng et al. 2011; Zhu et al. 2012; Pejman et al. 2015; Strbac et al. 2018). These quantitative methods allow determining possible origin of heavy metals and their impacts on sediment quality and biota. In addition, they provide an efficient tool, which transforms geochemical data into comprehensible information for decision-makers. The indices frequently used to assess sediment contamination with heavy metals are EF, Igeo, contamination factor (Cf), and pollution load index (PLI) (Muller 1969; Hakanson 1980; MacDonald et al. 2000; Abrahim and Parker 2008; Feng et al. 2011; Zhu et al. 2012; Pejman et al. 2015; Strbac et al. 2018). Alternative methods commonly used to estimate ecological risk of human activities include guidelines on the effect range low (ERL), effect range median (ERM), and mean ERM quotient (M-ERM-Q).
The standing touristic, economic, maritime, and ecological importance in Moroccan coasts is obvious. However, these coastal areas are exposed to extreme emissions of contaminants issued from various anthropogenic activities (Er-Raioui et al. 2009; Bloundi et al. 2009; Ben Omar et al. 2015). This critical situation has encouraged several researchers to investigate pollution status of sediments in Moroccan coastal environments (Bellucci et al. 2003; Cheggour et al. 2005; Gonzalez et al. 2007; Ruiz et al. 2006; Er-Raioui et al. 2009; Bloundi et al. 2009; Laissaoui et al. 2013; Ben Omar et al. 2015). Their main purposes were to quantify the contamination degree in sediments resulting from heavy metals and hydrocarbons. Despite these various investigations, surface sediments along Moroccan coasts are still exposed to hazardous contamination by heavy metals, and requires more attention and reflection.
The main objectives of the current study are (1) determination of heavy metal concentrations in surface sediments, (2) assessment of sediment pollution by heavy metals using plausible contamination indices, and (3) determination of heavy metals origins using multivariate statistical techniques. This study is the first to cover a large part of the north Moroccan coast during three different periods. In this way, this investigation will provide global overview about the contamination degree in this coastal region. Therefore, the obtained results should offer essential orientations and directives for monitoring heavy metal contamination in the Moroccan coast.
Material and methods
Study area overview
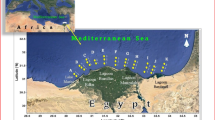
The study area belongs to the Mediterranean coast of Morocco, between Oued Moulouya in the east and Tanger in the west (Fig. 1). This zone is subdivided into three main geological domains: the internal zone, the Flysch basin, and the external zone (Durand Delga and Fontbote 1980; Didon et al. 1973).
The internal zone is essentially formed by elements issued from the Alboran plate (Andrieux et al. 1971). This domain includes three structural ensembles:
-
Sebtides: They include ultrabasic and basic materials, which are rich in metallic elements, such as Co, Cr, Fe, Mn and Ni (Saddiqui 1988; Gueddari et al. 1996; Ben Omar et al. 2015).
-
Ghomarides: Composed of metamorphic Paleozoic terrains with Hercynian structures from Silurian to Miocene age (Michard and Chalouan 1978).
-
Dorsale Calcaire: This unit is characterized by three formations (Didon et al. 1973): Triassic-Liassic limestone, Jurassic-Cretaceous limestone, and Tertiary terrigenous formations.
The Flysch basin includes detritic formations of flysch type from the lower Cretaceous age (Durand Delga and Fontbote 1980; Olivier 1984), while the external zone is represented by roof layers of different Mesozoic-Cenozoic successions.
Rivers in the study area (Fig. 1) are characterized mostly by seasonal and torrential regime. The humid period extends from December to March, with occasional peaks during November and April. During this period, significant daily flood flows are recorded. The torrential regime, due to steep slopes and precipitation time concentration, results in a high flow rate. However, during the dry season, water flows are usually low or absent.
Several urban and rural centers as well as touristic campuses can be found in the study area, which is considered as one of the most populated zone along the Moroccan coasts (Ben Omar et al. 2015; Royaume du Maroc 2014). It is also important to report that many centers within the study area do not possess any wastewater treatment system (Lahbabi and Anouar 2005; Ben Omar et al. 2015).
Sampling and chemical analysis
Surface sediments sampling was carried out at ten stations distributed along the Mediterranean coast of Morocco, between Oued Moulouya and Tanger, during three periods: June 2009, June 2012, and November 2012. The sampling stations are distributed as follows (Fig. 1): Oued Fnideq (St1), Oued Souani (St2), Oued Mghogha (St3), Oued Martil (St4), Oued Laou (St5), Oued Cabaillo (St6), Oued Selouane (St7), Bni Ansar (St8), Kariat Arkmane (St9), and Oued Moulouya (St10). The sampling locations include industrial zones and urban agglomerations. For each station, a representative composite sample of approximately 500 g was prepared by mixing three samples from different locations. The composite samples were placed in polyethylene bottles and stored at a temperature below 4 °C before being transported to the laboratory.
In the laboratory, samples were dried at 60 °C for 48 h. Afterwards, dried samples were sieved in < 2-mm sieve. To determine heavy metal concentrations (Cd, Cu, Cr, Ni, Pb, Zn, Hg, Fe, and Mn), 0.5 g of sediment was digested at 180 °C during 4 h using 4 ml of a solution composed by nitric acid (HNO3), hydrochloric acid (HCl), and fluoric acid (HF) at 5:2:1 ratio (HNO3:HCl:HF). After sample preparation, final determination of heavy metal concentrations was performed using inductively coupled plasma atomic emission spectrometry. The detection limits for each element were 0.1 μg/g for Cd; 1 μg/g for Cr, Cu, Pb, and Hg; and finally, 4 mg/g for Zn, Fe, Ni, and Mn. The standard error of each sediment samples was less than 5%.
Statistical analysis
Multivariate statistical approaches such as principal component analysis (PCA), hierarchical cluster analysis (HCA), and Pearson correlation coefficients (PCC) have been demonstrated to be appropriate for investigating the relationships between heavy metal elements. These methods, which are useful for reducing the dimensions of the observed variables, were widely used to investigate the heavy metal contamination (Jamshidi-Zanjani and Saeedi 2013; Fujita et al. 2014; Pejman et al. 2015; Li et al., 2016; Wang et al. 2016). Therefore, multivariate statistical analysis was used in this study on normalized data to establish the relationships between the different heavy metals and to identify possible sources of surface sediment contamination. The statistical analyses were performed using Excel Stat under Microsoft Office Excel.
Assessment of sediment contamination
In this study, assessment of surface sediment contamination by heavy metals was based on the following indices: PLI, modified degree of contamination (mCd), Igeo, EF, potential ecological risk index (RI), sediment quality guidelines (SQGs), and M-ERM-Q. Local geochemical background (LGB) was used to calculate contamination indices with respect to Cd, Cu, Cr, Ni, Pb, Zn, Fe, and Mn. However, to calculate contamination indices for Hg, the concentration before global industrialization given by Hakanson (1980) was chosen as geochemical background for this metal.
-
a.
Pollution load index (PLI)
The PLI provides a standard comparative average to assess the degree of contamination by defined metals (Tomlinson et al. 1980). The PLI was defined by the following equation (Eq. 1):
where \( {C}_f^i \) is the Cf (Hakanson 1980), \( {C}_s^i \) and \( {C}_n^i \) are respectively the measured concentration and the geochemical background value of defined metal. Two classes of PLI have been proposed by Tomlinson et al. (1980): if PLI > 1 there is a pollution, while if PLI ≤ 1 there is no metal pollution.
-
b.
Modified contamination degree (mCd)
The contamination degree (Cd) allows global assessment of sediment pollution using concentrations of seven specific heavy metals and the organic pollutant PCB (Hakanson 1980). This approach requires Cf calculation for each pollutant as presented in Eq. 2. However, Cf calculation requires a minimum of five sediment samples to obtain the mean concentration, which is subsequently compared to background value. Then, Cd is defined as the sum of Cf for the eight pollutant species according to the following formula (Eq. 3):
To avoid such limitations, Abrahim and Parker (2008) proposed the Modified mCd. This index was defined as the sum of all contamination factors divided by the number of analyzed pollutants (n), as shown in the following equation (Eq. 4):
The pollution classification, using mCd proposed by Abrahim and Parker (2008), is given as follows:
mCd < 1.5: nil to very low degree of contamination
1.5 < mCd < 2: low degree of contamination
2 < mCd <4: moderate degree of contamination
4 < mCd < 8: high degree of contamination
8 < mCd < 16: very high degree of contamination
16 < mCd < 32: extremely high degree of contamination
mCd > 32: ultra-high degree of contamination
-
c.
Geoaccumulation index (Igeo)
The Igeo assesses sediment contamination by metals with respect to geochemical background. It is calculated using the equation defined as follows (Muller 1969):
where Ci and Bi are respectively heavy metal (i) concentration and geochemical background. The 1.5 factor is used to minimize possible effect in background value variations, which can be attributed to lithological changes in sediment. Muller (1969) distinguishes seven classes of contamination based on the Igeo:
-
Igeo > 5: extremely contaminated
-
4 < Igeo ≤ 5: strongly to extremely contaminated
-
3 < Igeo ≤ 4: strongly contaminated
-
2 < Igeo ≤ 3: moderately to strongly contaminated
-
1 < Igeo ≤ 2: moderately contaminated
-
0 < Igeo ≤ 1: uncontaminated to moderately contaminated
-
Igeo ≤ 0: uncontaminated
-
d.
Enrichment factor (EF)
The EF is used to determine the degree of anthropogenic pollution through heavy metals. This factor is based on the normalization of examined metals with respect to a reference element. EF is calculated using the Eq. 6 (Buatmenard and Chesselet 1979; Ergin et al. 1991):
where Ci and Cref are respectively examined metal and reference element concentrations, Bi and Bref are respectively examined metal and reference element backgrounds.
In EF method, Fe, Sc, Al, Mn, and Li are usually used as reference elements (Quevauviller et al. 1989; Jamshidi-Zanjani and Saeedi 2013; Abrahim and Parker 2008; Wang et al. 2018). In this study, iron (Fe) was chosen as reference element for geochemical normalization. This metal is characterized by high natural concentration and, therefore, cannot be enriched significantly due to anthropogenic sources (Alexander et al. 1993; Niencheski et al. 1994; Abrahim and Parker 2008; Wang et al. 2018). Moreover, Fe is considered as major adsorbent and quasi-conservative tracer of natural metallic phases in river and coastal sediments (Schiff and Weisberg 1999; de Paula Filho et al. 2015). Yet, the calculated EF values will be interpreted using the classification reported by Sakan et al. (2009):
-
EF > 50: extremely severe enrichment
-
25 ≤ EF < 50: very severe enrichment
-
10 ≤ EF < 25: severe enrichment
-
5 ≤ EF < 10: moderate to severe enrichment
-
3 ≤ EF < 5: moderate enrichment
-
1 ≤ EF < 3: minor enrichment
-
EF < 1: no enrichment
-
e.
Potential ecological risk index (RI)
The RI is used to assess characteristics and environmental behaviors of heavy metal contamination in coastal sediments (Hakanson 1980). This index reflects the susceptibility of biological populations to toxic substances and illustrates the potential ecological risk caused by contamination levels (Bastami et al. 2015). The RI is calculated using the following equation:
where \( {E}_r^i \) is the individual potential ecological risk, \( {T}_r^i \) is the toxic response factor for a given heavy metal; Ni = 5, Hg = 40, Cd = 30, Cu = Pb = 5, Cr = 2, and Zn = 1 (Hakanson 1980; Zhu et al. 2013), and \( {C}_f^i \) is the contamination factor. The following classification was adopted according to RI values (Hakanson 1980):
-
RI ≥ 600: very high ecological risk
-
300 ≤ RI < 600: considerable ecological risk
-
150 ≤ RI < 300: moderate ecological risk
-
RI < 150: low ecological risk
-
f.
Sediment quality guidelines (SQGs) and mean ERM quotient (M-ERM-Q)
Sediment quality guidelines (SQG) were developed by Long et al. (1995) (Table 1). These guidelines are useful to assess ecological risks of contaminated sediments and, therefore, provide tolerable pollutant concentrations in order to protect organisms living in or near the sediment.
Chemical concentrations with adverse biological effects were called the ERL and the ERM (Long et al. 1995; McCready et al. 2006; Pejman et al. 2015). These two guideline values define three ranges of chemical concentrations (Long et al. 1995; McCready et al. 2006; Christophoridis et al. 2009):
-
≥ ERM: undesirable effects are frequent
-
≥ ERL and < ERM: undesirable effects are occasional
-
< ERL: undesirable effects are rare
The M-ERM-Q is used to identify areas of potential hazard in relation to sediment quality (Violintzis et al. 2009; Pejman et al. 2015). It is calculated using the following equation (Long and MacDonald 1998; Long et al. 2000):
where Ci, ERMi and n are respectively the concentration, ERM values and number of metal i. Four classes were defined using M-ERM-Q (Long et al. 2000):
-
M-ERM-Q > 1.5: 76% of toxic probability
-
0.51 ≤ M-ERM-Q < 1.5: 49% of toxic probability
-
0.11 ≤ M-ERM-Q < 0.5: 21% of toxic probability risk
-
M-ERM-Q < 0.1: 9% of toxic probability
Results
Heavy metal concentrations
The statistical summary of the mean concentrations measured over the three periods is presented in Table 2. With a widely varying range, heavy metal concentrations of different elements were comprised respectively between a minimum and a maximum as follows: Cd, < DL, 1.2 μg/g; Cu, 0.1–25 μg/g; Cr, < DL, 500 μg/g; Ni, < DL, 40 μg/g; Pb, 2.4–75.8 μg/g; Zn, 7.8–480 μg/g; Hg, < DL, 0.8 μg/g, Fe, 3.4–174.9 mg/g, and Mn, 113–1980 μg/g. These concentrations were compared to four geochemical backgrounds; average values of shale, continental crust (Turekian and Wedepohl 1961; Taylor 1964), concentration before global industrialization (Hakanson 1980), and LGB (Table 1, Fig. 2).
When Cu contents are below different geochemical background values, Cd concentrations exceed these limits in St2, St4, and St7. Whereas, Cr concentrations are above the three geochemical backgrounds in St1, St2, St3, and St4.
For Ni, all concentrations are below the standard geochemical backgrounds, except for LGB, where they exceeded it in St1, St2, St3, St4, and in St10. Excepting St1, St5, St8, and St9 for Pb and St8, St9 for Zn, all sampling site concentrations exceeded the three geochemical background values.
The Hg contents are higher than different geochemical backgrounds in St2, St3, St4, St6, St8, and St10. Whereas, Fe values are above different geochemical backgrounds in most sampling sites excluding St8. However, all Mn concentrations are inferior to these standards except St3, St4, St5, St6, and St10, which are superior.
To summarize for the three sampling periods, the percentage of stations that exceed LGB is 90% for Fe; 50% for Ni, Pb, Zn, and Mn; 40% for Cr and Hg; 10% for Cd; and 0% for Cu. Whereas, the percentage of stations that exceed Shale values is 50% for Pb and Fe, 40% for Zn and Hg, 30% for Cr, 20% for Cd and Mn, and 0% for Cu and Ni. Finally, the percentage of stations that exceed the continental crust value is 80% for Zn, 70% for Hg, 60% for Pb, 30% for Cd and Cr, and 0% for Cu and Ni.
The comparison of heavy metal concentrations obtained in this study to those reported in different Mediterranean areas is presented in Table 3. It is clear that Cu, Ni, Pb, and Hg concentrations are quite similar to those obtained from Egyptian, Spanish, and Italian coasts. However, Cr and Mn concentrations are the highest compared to other regions. In some cases, Cd, Cu, Ni, Pb, and Zn concentrations are lower and sometimes higher. Within the Moroccan Mediterranean littoral, Ni and Cu indicate the lowest concentrations, while Cd, Pb, and Zn contents were the lowest compared to Nador and the highest with respect to Tetouan coastlines.
Assessment of sediment contamination
In the previous section, it was observed that the LGB exceeded the shale and continental crust values only for Cd and Pb, while it was lower for Cu, Cr, Ni, and Mn. Therefore, the LGB was adopted to calculate the pollution indices.
The PLI calculation results, using the nine heavy metals, are illustrated in Fig. 3 a. The obtained values are comprised between 0.12 and 1.72. In most stations, PLI values are below one. However, in the three study periods, St4 shows values greater than one during the three sampling periods similarly to St2 and St3 in the period of June 2009. According to Tomlinson et al. (1980) classification, St2, St3, and St4 are classified as polluted while other sites are significantly less polluted.
The mCd values are comprised between 0.14 and 2.95 (Fig. 3b), which indicates very low to moderate degree of contamination. In fact, majority of stations sampled during 2012 showed mCd values below 1.5, indicating nil to very low degree of contamination. During June 2009, St1, St3, and St10 show mCd values comprised between 1.5 and 2, indicating low degree of contamination. However, St2, St4, and St6, in June 2009, show mCd values between 2 and 4, suggesting the presence of moderate degree of contamination.
The contamination degrees obtained using Igeo show that most sampling sites range between uncontaminated and strongly contaminated (Fig. 4). For Cd, Cu, Ni, and Hg, Igeo is less than one in all sampling sites, which will be classified as uncontaminated to moderately contaminated. During June 2009, St3 and St4 are classified respectively as moderately to strongly contaminated and strongly contaminated in relation to Cr, while the remaining sampling sites are classified from uncontaminated to moderately contaminated. Moreover, St2 is classified as moderately contaminated in relation to Pb and Zn, whereas the remaining stations range between uncontaminated and moderately contaminated. In the case of Fe, the calculated Igeo classifies St1 and St2 respectively as moderately contaminated and moderately to strongly contaminated. However, when Igeo is calculated using Mn, St3, St4, and St6 are classified as uncontaminated to moderately contaminated, St10 is classified as moderately contaminated, while all remaining sampling stations are classified as uncontaminated.
The Fig. 5 presents the obtained EF results. With respect to Cd and Cu, all sampling sites are characterized by EF values lower than one, indicating no enrichment by these metals. Besides St3 and St4, which indicate respectively a minor enrichment over the three periods and a moderate enrichment in June 2009, most stations show no enrichment regarding Cr. All sampling sites indicate no enrichment by Ni, except St4, which shows minor enrichment in November and June 2012. Regarding Pb, St2 shows minor enrichment in June 2009 and moderate to severe enrichment in 2012; however, St3, St6, St8, and St10 show minor enrichment. On the other hand, Zn shows minor enrichment in St2, St4, St5, and St7, whereas the rest of sampling stations have no enrichment of this metal. With respect to Hg, minor enrichment is recorded in St2, St4, St6, and St9, while moderate to server enrichment is obtained at St8 during June 2009. The EF calculated using Mn indicates no enrichment to minor enrichment in all sampling sites.
Despite the PLI, mCd, Igeo and EF indices show the presence of non-significant heavy metal pollution and enrichment, the RI calculation reveals that all sampling sites are characterized by low to moderate potential ecological risk (Fig. 6). The comparison of heavy metal concentrations to ERM and ERL guidelines (Table 1) is presented in Fig. 7. This figure shows that Cd and Cu concentrations in all sampling stations are below these two guidelines, indicating that undesirable effects caused by both metals are rare. During June 2009, concentration contents of Cr in St4, Zn in St2, and Hg in St2 and St8 are above the ERM guideline. Consequently, this result suggests that undesirable effects, due to such metals, are frequent. Elsewhere, some sampling sites show a metal concentrations ranging between ERL and ERM standards; Cr in St1 and St3, Ni in St1, St2, St3, St4 and St10, Pb in St2, St3 and St6, Zn in St4, St6, and St7, and finally Hg in St3, St4, St6, and St10. This finding implies that undesirable effects caused by these heavy metals are occasional.
To summarize for all sampling periods, 20%, 50%, 30%, 30%, and 40% of sampling sites have respectively the concentrations of Cr, Ni, Pb, Zn, and Hg that fall between ERL and ERM. These outcomes indicate that undesirable effects in these sampling sites are occasional. On the other hand, 10%, 10%, and 20% of sampling stations show successively the concentrations of Cr, Zn, and Hg higher than ERM. This result suggests that undesirable effects at these sites are frequent.
As a percentage, the calculated M-ERM-Q using Cd, Cu, Cr, Ni, Pb, Zn, and Hg ranges between 0.03 and 0.49 with an average value of 0.18 (Fig. 8). These values indicate that these heavy metals mixture may present between 9 and 49% of probability to be toxic. This result highlights the presence of lowest to medium-low potential of adverse effects on biota populations. In addition, an M-MRE-Q < 0.1 is recorded only in three sampling stations, which are St6, St7, and St9. Therefore, the heavy metals combination in these stations is classified as non-toxic.
Statistical analysis
The PCA was applied to normalized dataset. Initially, the Kaiser-Meyer-Olkin (KMO) and Bartlett sphericity tests were applied to all data to verify their suitability for PCA statistical analysis. In general, high KMO values, greater than 0.5 and close to 1, indicate that the data are appropriate and useful for the PCA (Jamshidi-Zanjani and Saeedi 2013; Fujita et al. 2014; Pejman et al. 2015). In addition, the level of significance of Bartlett’s sphericity tests (BST) emphasized the significant relationships between the variables. Indeed, small level of significance, less than 0.05, indicate the existence of significant relationships between variables and therefore the PCA can be useful (Kaiser and Rice 1974; Pejman et al. 2015). In this study, the KMO and BST values were equal to 0.78 and 0.01, respectively. Therefore, these values indicate that PCA would be appropriate for the purpose of dataset analysis.
Correlation analysis was conducted to explore relationships between studied heavy metals and to identify their common origins in surface sediments along the study area (Table 4). The Pearson’s matrix coefficients, calculated with 95% of confidence level, show significant and positive correlations between the studied metals: Cu and Cd (0.80), Cr and Cd (0.73), Cr and Cu (0.62), Ni and Cd (0.88), Ni and Cu (0.64), Ni and Cr (0.89), Zn and Cd (0.60), Zn and Cu (0.66), and finally between Zn and Pb (0.86). Heavy metals with significant correlations may have common sources and similar behavior during transformation or migration (Wang et al. 2012). It is clear from Table 4 that Fe has no significant correlation with other metals. This confirms also its suitability to be a reference element for EF calculation.
Otherwise, the relationship between variables was performed using cluster analysis. As shown in Fig. 9, heavy metals were grouped in three main clusters. Cluster 1 includes Cd, Cu, Hg, Pb, and Zn. Although Fe forms a single group (cluster 3), it is correlated by similarity to Mn, Cr, and Ni form cluster 2. As Fe is primarily originated from lithogenic sources, it can be assumed that heavy metals of cluster 2 are also derived from natural origin. However, the principal source of cluster 1 heavy metals is mainly anthropogenic, such as wastewater discharges, industry, agricultural activities, traffic, and transport.
In the same way, PCA was established to compare the composition of heavy metals between sampling sites and to identify responsible elements of surface sediments contamination (Fig. 10). Two factors, with eigenvalues > 1, representing a cumulative variance of 68.4% were obtained. The first factor F1, which represents 47.14% of total variance, is correlated negatively with Fe and positively with the remaining heavy metals (Fig. 10a). Since these two metal groups have unequal charge levels on F1, it can be confirmed that Fe is derived from different source, mainly lithogenic. The second factor F2, which explains 21.30% of total variance, is correlated positively with metals of anthropogenic origin (Cu, Hg, Pb, and Zn) and negatively with those of natural origin (Mn, Cr, and Ni). Regarding Cd, it is considered as typical metal associated with human activities (Zahra et al. 2014; Strbac et al. 2018). This metal shows both positive and negative charge on F1 and F2 respectively. Therefore, it can be proposed that Cd is essentially derived from both natural and anthropogenic sources.
The individual diagrams (Figs. 10 b and c) divide the sampling sites into three main groups. The group 3 sites (St3, St4, and St10) are dominated mainly by metals of natural origin, while St7 and St2 from group 2 are dominated mainly by metals of anthropogenic origin. Concerning the remaining sampling sites, heavy mental enrichment is mainly caused by the contribution of both natural and anthropogenic factors.
Discussion
This study provides an overview of heavy metals contamination in surface sediments along the Mediterranean shoreline of Morocco. The purpose was to determine heavy metal origins and to define the most vulnerable sites to this pollution. The study of heavy metal concentrations showed significant variation between the three sampling periods. This behavior could be the consequence of several chemical or environmental processes. In fact, the modification of some environmental conditions such as pH and redox potential can trigger the transfer or the mobilization of heavy metals in sediments, as well as the modification of their concentrations (Kalantzi et al. 2013; Duodu et al. 2017). In addition, it has been clearly established that organic matter and grain size are two main factors, which influence heavy metal concentrations in sediments (El Bilali et al. 2002; Kalantzi et al. 2013; Fujita et al. 2014; Li et al. 2017). Therefore, their modification induces heavy metal concentrations change. For instance, the concentrations of Cu, Zn, and Mn increase with the finest fractions while Cr decreases, whereas organic matter plays a significant role in their fixation (Chakrapani and Subramanian 1993; Rodríguez-Barroso et al. 2010). Investigating the cores executed in the lagoon of Nador, Bellucci et al. (2003) have suggested a recent decrease in metal concentrations provoked by the granulometry change.
Likewise, meteorological fluctuations influence significantly heavy metal concentrations in surface sediments. Indeed, dilution due to unpolluted rainfall during wet periods reduces heavy metal concentrations, while they increase during dry periods (Collvin 1985; Gupta et al. 2009). Therefore, the higher concentrations of heavy metals observed in the study area sediments could be the consequence of drought periods. On the other hand, these important concentrations may be induced by industrial development recorded during the beginning of the century in Morocco, while decreasing phases can be the consequence of environmental strategies imposed by the Moroccan government.
The study of heavy metal concentrations in sediment, compared to different geochemical background values, is not sufficient to assess the pollution status (Zhu et al. 2012; El Zrelli et al. 2015; Pejman et al. 2015; Strbac et al. 2018). For this reason, quantitative methods were used to assess the pollution level and the possible origin of heavy metals.
The calculation of mCd index demonstrated that heavy metal contamination ranges from very low to moderate levels. According to this index, St2, St4, and St6 showed the presence of moderate contamination by heavy metals. Alternatively, using the Igeo index, heavy metal contamination ranges from uncontaminated to strongly contaminated. Otherwise, the FE calculation indicated that most heavy metals showed no enrichment to minor enrichment except for some ones, which indicated severe enrichment. It has been reported that EF values ranging between 0.5 and 1.5 indicate natural origin of heavy metal, while values above 1.5 suggest anthropogenic sources (Birth 2003; Wang et al. 2016; Malvandi 2017). In this study, Cr in St3 and St4, Pb in St2, Zn in St2, Hg in St8, and Mn in St6 and St10 showed EF values > 1.5. This result indicates that human activities are involved in the enrichment process. However, the remaining sampling sites imply the dominance of geological character in the enrichment process. Overall, it can be deduced that the obtained information about pollution status depends mainly on the geochemical background value and on the chosen contamination index. Indeed, Strbac et al. (2018) have confirmed the influence of geochemical background values in the establishment of sediment quality criteria.
The statistical analysis conducted in this study supports the hypothesis that Cd, Cu, Hg, Pb, and Zn are derived mainly from anthropogenic origin, while Fe, Mn, Cr, and Ni are derived from natural sources. As a result, the contamination of surface sediments along the Mediterranean coasts of Morocco is particularly due to the combination between anthropogenic and natural factors. Indeed, Bloundi et al. (2009) and Ben Omar et al. (2015) have endorsed this result on the northern Moroccan coast. Thus, the study area can be considered as not significantly enriched by human activities.
In the study area, the probable anthropogenic sources of surface sediment contamination by heavy metals can be grouped into different types. Leachate released from landfills and agriculture activities can contribute in part to sediments enrichment with heavy metals such as Cr, Mn, Ni, Cu, Zn, Cd, and Pb (Zhang and Shan 2008; Fujita et al. 2014). Road traffic can contribute to significant enrichment by Cd, Pb, Zn, and Hg (Duodu et al. 2017) as well as Ni that can be derived from trace elements contained in gasoline (Wang et al. 2016). Otherwise, Cd and Hg can also be associated to suspended particles within vehicle emissions and lubricating oils (Duodu et al. 2017).
Furthermore, the geological character of the study area influences significantly metal composition of coastal surface sediments. The geological formations are formed mainly by peridotite, kinzigite, and granulite rich in certain metals such as Cr, Fe, Mn, and Ni (Gonzalez et al. 2007; Ben Omar et al. 2015; Hajjar et al. 2017). Silicate minerals such as orthopyroxene and muscovite as well as oxides like spinel, widely present along the study area’s watershed, are well known to contain these metals (Hajjar et al. 2017). Similarly, amphibolites, which are composed of amphibole, feldspar, plagioclase, and pyroxene, also known for their Cr and Ni content (Hajjar et al. 2017; Ben Omar et al. 2015). Black shales and sea sprays are the most important natural sources of Cd. In addition, crustal materials weathering releases this metal to soils and aquatic systems (Hutton 1983). These processes play an important role in Cd enrichment; however, they rarely produce high concentrations in the environment (Holmes 1975; Hutton 1983). Consequently, the very low concentrations obtained in this study (lower than DL) support the natural origin of Cd metal.
In the study area, rivers traverse geological formations, agricultural lands, urban centers, industrial zones as well as contaminated lands. Consequently, these principal watercourses will transport heavy metals from watershed to the shoreline. Overall, the calculated values of contamination indices, PLI and mCd, indicate that metal pollution was higher in St2, St3, St4, and St6, which correspond successively to Oued Souani, Oued Mghogha, Oued Martil, and Oued Cabaillo. Previous studies have shown that Oued Martil receives significant amount of urban and industrial effluents enriched by heavy metals (Lahbabi and Anouar 2005; Ben Omar et al. 2015). Additionally, Bloundi et al. (2009) have found high concentrations of Zn, Pb, Cu, Ni, and Cr near the estuary of Cabaillo and Selouane rivers. These latter drain the water flowing through an industrial park and urban centers. Therefore, these results confirm the involvement of principal rivers in heavy metal mobilization from watershed to the shoreline. However, the obtained conclusions about pollution status may depend mainly on the used geochemical background values and the selected contamination index. Eventually, in order to estimate the contribution rate of anthropogenic or natural factors to heavy metals enrichment, additional detailed studies are required. This perspective requires further investigations including contamination change along main rivers from upstream to downstream.
Conclusion
This article discusses heavy metal contamination in surface sediments along the Mediterranean coast of Morocco. To investigate the degree and possible sources of such contamination, the concentrations of nine heavy metals (Cd, Cu, Cr, Ni, Pb, Zn, Hg, Fe, and Mn) were measured at ten sampling stations. The order of average contents of these metals, without taking into account sampling periods, was as follows Fe > Mn > Zn > Cr > Pb > Ni > Cu > Hg > Cd. The obtained concentrations showed significant variabilities, with respect to sampling periods, indicating the governance of several chemical or environmental processes in this coastal area.
Environmental and ecological contamination indices, calculated using heavy metal concentrations, suggested that pollution was higher at sampling sites corresponding to Oued Souani, Oued Mghogha, Oued Martil, and Oued Cabaillo. These rivers drain heavy metals from urban and industrial effluent, and agricultural lands as well as through geological formations, which are enriched by these elements.
The RI index showed that majority of sampling sites present a low ecological risk except Oued Souani, which showed a moderate potential ecological risk. With respect to ERL and ERM guidelines, it is found that 20%, 50%, 30%, 30%, and 40% of sampling sites indicate that undesirable effects, associated to Cr, Ni, Pb, Zn, and Hg respectively, are occasional. While, 10%, 10%, and 20% of sampling sites showed that undesirable effects, associated successively to Cr, Zn, and Hg, are frequent. The calculated M-ERM-Q for all sampling sites indicated that heavy metals combination may have between 9 and 49% of probability to be toxic. This finding suggest lowest to medium-low potential of adverse effects to biota populations.
Statistical analysis showed that Cd, Cu, Hg, Pb, and Zn are primarily produced from anthropogenic sources, while Fe, Mn, Cr, and Ni are mostly derived from natural source. Consequently, it can be concluded that anthropogenic and natural factors contribute mutually to heavy metal enrichment in surface sediments along the Mediterranean coast of Morocco. Despite these results, no intense heavy metal pollution was detected in the surface sediments of the study area. However, non-controlled pollution and contamination of surface sediments by heavy metals can have adverse effects on the biota, and subsequently a direct or indirect impact on human health. As a result, particular attention should be given to this contamination, particularly in urban areas, heavily populated, and also in those with minor or no pollution.
This study contributes to an effective environmental monitoring along the Mediterranean coast of Morocco and supports the establishment of public policies to ensure the protection of coastal environments. The obtained findings will be used as a database for further detailed researches. With a view to this research, the investigation of heavy metal evolution along main rivers, from upstream to downstream, would be crucial. Such further investigation will enable differentiation between contribution rate of anthropogenic and natural factors in surface sediments contamination along this coastal area.
References
Abrahim, G. M. S., & Parker, R. J. (2008). Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environmental Monitoring and Assessment, 136(1–3), 227–238.
Accornero, A., Gnerre, R., & Manfra, L. (2008). Sediment concentrations of trace metals in the Berre lagoon (France): an assessment of contamination. Archives of Environmental Contamination and Toxicology, 54(3), 372–385.
Alexander, C. R., Smith, R. G., Calder, F. D., Schropp, S. J., & Windom, H. L. (1993). The historical record of metal enrichment in two Florida estuaries. Estuaries, 16, 627–637.
Alomary, A. A., & Belhadj, S. (2007). Determination of heavy metals (Cd, Cr, Cu, Fe, Ni, Pb, Zn) by ICP-OES and their speciation in Algerian Mediterranean Sea sediments after a five-stage sequential extraction procedure. Environmental Monitoring and Assessment, 135(1–3), 265–280.
Alves, S. I. R., Sampaio, F. C., Nadal, M., Schuhmacher, M., Domingo, L. J., & Segura-Muñoz, I. (2014). Metal concentrations in surface water and sediments from Pardo River, Brazil: Human health risks. Environmental Research, 133, 149–155.
Andrieux, J., Fontboté, J. M., & Mattauer, M. (1971). Sur un modèle explicatif de l’arc de Gibraltar. Earth and Planetary Science Letters, 12, 191–198.
Ayadi, N., Aloulou, F., & Bouzid, J. (2015). Assessment of contaminated sediment by phosphate fertilizer industrial waste using pollution indices and statistical techniques in the Gulf of Gabes (Tunisia). Arabian Journal of Geosciences, 8(3), 1755–1767.
Bastami, K. D., Bagheri, H., Haghparast, S., Soltani, F., Hamzehpoor, A., & Bastami, M. D. (2012). Geochemical and geo-statistical assessment of selected heavy metals in the surface sediments of the Gorgan Bay, Iran. Marine Pollution Bulletin, 64(12), 2877–2884.
Bastami, K. D., Neyestani, M. R., Shemirani, F., Soltani, F., Haghparast, S., & Akbari, A. (2015). Heavy metal pollution assessment in relation to sediment properties in the coastal sediments of the southern Caspian Sea. Marine Pollution Bulletin, 92(1–2), 237–243.
Bellucci, L. G., El Moumni, B., Collavini, F., Frignani, M., & Albertazzi, S. (2003). Heavy metals in Morocco Lagoon and river sediments. Journal de Physique IV (Proceedings), 107, 139–142 EDP sciences.
Ben Omar, M. B., Mendiguchía, C., Er-Raioui, H., Marhraoui, M., Lafraoui, G., Oulad-Abdellah, M. K., Oulad-Abdellah, M. G.-V., & Moreno, C. (2015). Distribution of heavy metals in marine sediments of Tetouan coast (North of Morocco): natural and anthropogenic sources. Environmental Earth Sciences, 74(5), 4171–4185.
Birth, G. (2003). A scheme for assessing human impacts on coastal aquatic environments using sediments. In C. D. Woodcoffe & R. A. Furness (Eds.), Coastal GIS (p. 14). Australia: Wollongong University Papers in Center for Maritime Policy.
Bloundi, M. K., Duplay, J., & Quaranta, G. (2009). Heavy metal contamination of coastal lagoon sediments by anthropogenic activities: the case of Nador (East Morocco). Environmental Geology, 56(5), 833–843.
Buatmenard, P., & Chesselet, R. (1979). Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth and Planetary Science Letters, 42, 399–411.
Buccolieri, A., Buccolieri, G., Cardellicchio, N., Dell'Atti, A., Di Leo, A., & Maci, A. (2006). Heavy metals in marine sediments of Taranto Gulf (Ionian Sea, southern Italy). Marine Chemistry, 99(1–4), 227–235.
Carman, C. M., Xiang-Dong, L., Gan, Z., Onyx, W. H., & Wai, Y. L. (2007). Trace metal distribution in sediments of the Pearl River Estuary and the surrounding coastal area, South China. Environmental Pollution, 147, 311–323.
Castillo, M. A., Trujillo, I. S., Alonso, E. V., de Torres, A. G., & Pavón, J. C. (2013). Bioavailability of heavy metals in water and sediments from a typical Mediterranean Bay (Málaga Bay, Region of Andalucía, Southern Spain). Marine Pollution Bulletin, 76(1–2), 427–434.
Chakrapani, G. L., & Subramanian, V. (1993). Heavy metals distribution and fractionation in sediments of the Mahanadi River basin, India. Environmental Geology, 22, 80–87.
Chapman, P. M., Wang, F., Janssen, C., Persoone, G., & Allen, H. E. (1998). Ecotoxicology of metals in aquatic sediments: binding and release, bioavailability, risk assessment, and remediation. Canadian Journal of Fisheries and Aquatic Sciences, 55(10), 2221–2243.
Cheggour, M., Chafik, A., Fisher, N. S., & Benbrahim, S. (2005). Metal concentrations in sediments and clams in four Moroccan estuaries. Marine Environmental Research, 59(2), 119–137.
Chen, J. S., Wang, F. Y., Li, X. D., & Song, J. J. (2000). Geographical variations of trace elements in sediments of the major rivers in eastern China. Environmental Geology, 39(12), 1334–1340.
Christophoridis, C., Dedepsidis, D., & Fytianos, K. (2009). Occurrence and distribution of selected heavy metals in the surface sediments of Thermaikos Gulf, N. Greece. Assessment using pollution indicators. Journal of Hazardous Materials, 168, 1082–1091.
Collvin, L. (1985). The effect of copper on growth, food consumption and food conversion of perch Perca fluviatilis L. offered maximal food rations. Aquatic Toxicology, 6(2), 105–113.
de Paula Filho, F. J., Marins, R. V., de Lacerda, L. D., Aguiar, J. E., & Peres, T. F. (2015). Background values for evaluation of heavy metal contamination in sediments in the Parnaíba River Delta estuary, NE/Brazil. Marine Pollution Bulletin, 91(2), 424–428.
Díaz-de Alba, M., Galindo-Riano, M. D., Casanueva-Marenco, M. J., García-Vargas, M., & Kosore, C. M. (2011). Assessment of the metal pollution, potential toxicity and speciation of sediment from Algeciras Bay (South of Spain) using chemometric tools. Journal of Hazardous Materials, 190(1–3), 177–187.
Didon, J., Durand-Delga, M., & Kornprobst, J. (1973). Homologies géologiques entre les deux rives du détroit de Gibraltar. Bulletin de la Societe Geologique de France, 15, 79–105.
Duodu, G. O., Goonetilleke, A., & Ayoko, G. A. (2017). Potential bioavailability assessment, source apportionment and ecological risk of heavy metals in the sediment of Brisbane River estuary, Australia. Marine Pollution Bulletin, 117(1–2), 523–531.
Durand Delga, M., & Fontbote, J. M. 1980. Le cadre structurale de la Méditerranée occidentale: In: Géologie des chaînes alpines issues de la Téthys. 26ème Congr. Géol. Fr. Mém. B.R.G.M. 15, 67–85.
El Bilali, L., Rasmussen, P. E., Hall, G. E. M., & Fortin, D. (2002). Role of sediment composition in trace metal distribution in lake sediments. Applied Geochemistry, 17(9), 1171–1181.
El Nemr, A. M., El Sikaily, A., & Khaled, A. (2007). Total and leachable heavy metals in muddy and sandy sediments of Egyptian coast along Mediterranean Sea. Environmental Monitoring and Assessment, 129(1–3), 151–168.
El Zrelli, R., Courjault-Radé, P., Rabaoui, L., Castet, S., Michel, S., & Bejaoui, N. (2015). Heavy metal contamination and ecological risk assessment in the surface sediments of the coastal area surrounding the industrial complex of Gabes city, Gulf of Gabes, SE Tunisia. Marine Pollution Bulletin, 101(2), 922–929.
Ergin, M., Saydam, C., Baştürk, Ö., Erdem, E., & Yörük, R. (1991). Heavy metal concentrations in surface sediments from the two coastal inlets (Golden Horn Estuary and Izmit Bay) of the northeastern Sea of Marmara. Chemical Geology, 91(3), 269–285.
Er-Raioui, H., Bouzid, S., Marhraoui, M., & Saliot, A. (2009). Hydrocarbon pollution of the Mediterranean coastline of Morocco. Ocean & Coastal Management, 52(2), 124–129.
Feng, H., Jiang, H., Gao, W., Weinstein, M. P., Zhang, Q., Zhang, W., Yu, L., Yuan, D., & Tao, J. (2011). Metal contamination in sediments of the western Bohai Bay and adjacent estuaries, China. Journal of Environmental Management, 92, 1185–1197.
Frémion, F., Bordas, F., Mourier, B., Lenain, J. F., Kestens, T., & Courtin-Nomade, A. (2016). Influence of dams on sediment continuity: a study case of a natural metallic contamination. Science of the Total Environment, 547, 282–294.
Fujita, M., Ide, Y., Sato, D., Kench, P. S., Kuwahara, Y., Yokoki, H., & Kayanne, H. (2014). Heavy metal contamination of coastal lagoon sediments: Fongafale Islet, Funafuti Atoll, Tuvalu. Chemosphere, 95, 628–634.
Gao, X., & Chen, C. T. A. (2012). Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Research, 46(6), 1901–1911.
Gaur, V. K., Gupta, S. K., Pandey, S. D., Gopal, K., & Misra, V. (2005). Distribution of heavy metals in sediment and water of River Gomti. Environmental Monitoring and Assessment, 102, 419–433.
Gonzalez, I., Águila, E., & Galán, E. (2007). Partitioning, bioavailability and origin of heavy metals from the Nador Lagoon sediments (Morocco) as a basis for their management. Environmental Geology, 52(8), 1581–1593.
Gueddari, K., Piboule, M., & Amossé, J. (1996). Differentiation of platinum-group elements (PGE) and of gold during partial melting of peridotites in the lherzolitic massifs of the Betico-Rifean range (Ronda and Beni Bousera). Chemical Geology, 134(1–3), 181–197.
Gupta, A., Rai, D. K., Pandey, R. S., & Sharma, B. (2009). Analysis of some heavy metals in the riverine water, sediments and fish from river Ganges at Allahabad. Environmental Monitoring and Assessment, 157(1–4), 449.
Hajjar, Z., Gervilla, F., Essaifi, A., & Wafik, A. (2017). Mineralogical and geochemical features of the alteration processes of magmatic ores in the Beni Bousera ultramafic massif (north Morocco). Journal of African Earth Sciences, 132, 47–63.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control, a sedimentological approach. Water Research, 14, 975–1001.
Holmes, R. (1975). The regional distribution of cadmium in England and Wales. Ph.D. thesis, University of London.
Hortellani, M. A., Sarkis, J. E., Abessa, D., & Sousa, E. C. (2008). Assessment of metallic element contamination in sediments from the Santos-São Vicente estuarine system. Quimica Nova, 31(1), 10–19.
Hussain, R., Khattak, S. A., Shah, T. M., & Ali, L. (2015). Multistatistical approaches for environmental geochemical assessment of pollutants in soils of Gadoon Amazai industrial Estate, Pakistan. Journal of Soils and Sediments, 15, 1119–1129.
Hutton, M. (1983). Sources of cadmium in the environment. Ecotoxicology and Environmental Safety, 7(1), 9–24.
Jamshidi-Zanjani, A., & Saeedi, M. (2013). Metal pollution assessment and multivariate analysis in sediment of Anzali international wetland. Environmental Earth Sciences, 70(4), 1791–1808.
Kaiser, H. F., & Rice, J. (1974). Little jiffy, mark IV. Educational and Psychological Measurement, 34(1), 111–117.
Kalantzi, I., Shimmield, T. M., Pergantis, S. A., Papageorgiou, N., Black, K. D., & Karakassis, I. (2013). Heavy metals, trace elements and sediment geochemistry at four Mediterranean fish farms. Science of the Total Environment, 444, 128–137.
Lahbabi, A., & Anouar, K. (2005). Mandat de l’expert national chargé d’élaborer le plan d’action national dans le cadre du PAS. Ministère de l’aménagement du territoire, de l’eau et de l’environnement. Royaume du Maroc.
Laissaoui, A., Mas, J. L., Hurtado, S., Ziad, N., Villa, M., & Benmansour, M. (2013). Radionuclide activities and metal concentrations in sediments of the Sebou Estuary, NW Morocco, following a flooding event. Environmental Monitoring and Assessment, 185(6), 5019–5029.
Li, H., Kang, X., Li, X., Li, Q., Song, J., Jiao, N., & Zhang, Y. (2017). Heavy metals in surface sediments along the Weihai coast, China: distribution, sources and contamination assessment. Marine Pollution Bulletin, 115(1–2), 551–558.
Long, E. R., & MacDonald, D. D. (1998). Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems. Human and Ecological Risk Assessment, 4, 1019–1039.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
Long, E. R., MacDonald, D. D., Severn, C. G., & Hong, C. B. (2000). Classifying the probabilities of acute toxicity in marine sediments with empirically-derived sediment quality guidelines. Environmental Toxicology and Chemistry, 19, 2598–2601.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31.
Malvandi, H. (2017). Preliminary evaluation of heavy metal contamination in the Zarrin-Gol River sediments, Iran. Marine Pollution Bulletin, 117(1–2), 547–553.
Marrugo-Negrete, J., Pinedo-Hernández, J., & Díez, S. (2017). Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environmental Research, 154, 380–388.
McCready, S., Birch, G. F., & Long, E. R. (2006). Metallic and organic contaminants in sediments of Sydney Harbour, Australia and vicinity—a chemical dataset for evaluating sediment quality guidelines. Environment International, 32, 455–465.
Michard, A., & Chalouan, A. (1978). Présence de l’Orogene varisque dans le socle interne rifo-kabyle (microplaque d’Alboran). Comptes Rendus. Académie des Sciences, 287, 903–906.
Miller, B. S., Pirie, D. J., & Redshaw, C. J. (2000). An assessment of the contamination and toxicity of marine sediments in the Holy Loch, Scotland. Marine Pollution Bulletin, 40(1), 22–35.
Muller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. Geojournal, 2, 108–118.
Neşer, G., Kontas, A., Ünsalan, D., Uluturhan, E., Altay, O., Darılmaz, E., Küçüksezgin, F., Tekoğul, N., & Yercan, F. (2012). Heavy metals contamination levels at the Coast of Aliağa (Turkey) ship recycling zone. Marine Pollution Bulletin, 64(4), 882–887.
Niencheski, L. F., Windom, H. L., & Smith, R. (1994). Distribution of particulate trace metal in Patos Lagoon Estuary (Brazil). Marine Pollution Bulletin, 28(2), 96–102.
Olivier, P. (1984). Evolution de la limite entre zones internes et zones externes dans l'arc de Gibraltar (Maroc-Espagne) (doctoral dissertation).
Pejman, A., Bidhendi, G. N., Ardestani, M., Saeedi, M., & Baghvand, A. (2015). A new index for assessing heavy metals contamination in sediments: a case study. Ecological Indicators, 58, 365–373.
Quevauviller, P., Lavigne, R., & Cortez, L. (1989). Impact of industrial and mine drainage wastes on the heavy-metal distribution in the drainage-basin estuary of the Sado River (Portugal). Environmental Pollution, 59, 267–286.
Rabaoui, L., El Zrelli, R., Mansour, M. B., Balti, R., Mansour, L., Tlig-Zouari, S., & Guerfel, M. (2015). On the relationship between the diversity and structure of benthic macroinvertebrate communities and sediment enrichment with heavy metals in Gabes Gulf, Tunisia. Journal of the Marine Biological Association of the United Kingdom, 95(2), 233–245.
Reimann, C., & Caritat, P. (2005). Distinguishing between natural and anthropogenic sources for elements in the environment: regional geochemical surveys versus enrichment factors. Science of the Total Environment, 337, 91–107.
Rodríguez-Barroso, M. R., García-Morales, J. L., Oviedo, M. C., & Alonso, J. Q. (2010). An assessment of heavy metal contamination in surface sediment using statistical analysis. Environmental Monitoring and Assessment, 163(1–4), 489–501.
Royaume du Maroc. (2014). Haut-commissariat au plan, les résultats de la population l’égale issue du recensement général de la population et de l’habitat (RGPH). Avalable in internet http://www.hcp.ma
Ruiz, F., Abad, M., Olías, M., Galán, E., Gonzalez, I., Aguilá, E., et al. (2006). The present environmental scenario of the Nador lagoon (Morocco). Environmental Research, 102(2), 215–229.
Saddiqui, O. (1988). Tectonique de la remontée du manteau: les peridotites des Beni Bousera et leur enveloppe métamorphique, Rif interne, Maroc (Doctoral dissertation, Université Louis Pasteur (Strasbourg)).
Sakan, S. M., Đorđević, D. S., Manojlović, D. D., & Predrag, P. S. (2009). Assessment of heavy metal pollutants accumulation in the Tisza river sediments. Journal of Environmental Management, 90(11), 3382–3390.
Sakan, S., Dević, G., Relić, D., Anđelković, I., Sakan, N., & Đorđević, D. (2015). Evaluation of sediment contamination with heavy metals: the importance of determining appropriate background content and suitable element for normalization. Environmental Geochemistry and Health, 37(1), 97–113.
Schiff, K. C., & Weisberg, S. B. (1999). Iron as a reference element for determining trace metal enrichment in Southern California coastal shelf sediments. Marine Environmental Research, 48(2), 161–176.
Soliman, N. F., Nasr, S. M., & Okbah, M. A. (2015). Potential ecological risk of heavy metals in sediments from the Mediterranean coast, Egypt. Journal of Environmental Health Science and Engineering, 13(1), 70.
Strbac, S., Grubin, M. K., & Vasić, N. (2018). Importance of background values in assessing the impact of heavy metals in river ecosystems: case study of Tisza River, Serbia. Environmental Geochemistry and Health, 40(4), 1247–1263.
Suresh, G., Sutharsan, P., Ramasamy, V., & Venkatachalapathy, R. (2012). Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicology and Environmental Safety, 84, 117–124.
Taylor, S. R. (1964). Abundance of chemical elements in the continental crust: a new table. Geochimica et Cosmochimica Acta, 28(8), 1273–1285.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer meeresuntersuchungen, 33(1), 566–575.
Turekian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the earth’s crust. Geological Society of America Bulletin, 72(2), 175–192.
Violintzis, C., Arditsoglou, A., & Voutsa, D. (2009). Elemental composition of suspended particulate matter and sediments in the coastal environment of Thermaikos Bay, Greece: delineating the impact of inland waters and wastewaters. Journal of Hazardous Materials, 166, 1250–1260.
Wang, C., Liu, S., Zhao, Q., Deng, L., & Dong, S. (2012). Spatial variation and contamination assessment of heavy metals in sediments in the Manwan Reservoir, Lancang River. Ecotoxicology and Environmental Safety, 82, 32–39.
Wang, Y., Wei, Y., Guo, P., Pan, J., Wu, Q., & Liu, N. (2016). Distribution variation of heavy metals in maricultural sediments and their enrichment, ecological risk and possible source—a case study from Zhelin bay in Southern China. Marine Pollution Bulletin, 113(1–2), 240–246.
Wang, N., Wang, A., Kong, L., & He, M. (2018). Calculation and application of Sb toxicity coefficient for potential ecological risk assessment. Science of the Total Environment, 610, 167–174.
Zahra, A., Hashmi, M. Z., Malik, R. N., & Ahmed, Z. (2014). Enrichment and geo-accumulation of trace metals and risk assessment of sediments of the Kurang Nallah-feeding tributary of the Rawal Lake Reservoir, Pakistan. Science of the Total Environment, 470–471, 925–933.
Zhang, H., & Shan, B. (2008). Historical records of heavy metal accumulation in sediments and the relationship with agricultural intensification in the Yangtze–Huaihe region, China. Science of the Total Environment, 399(1–3), 113–120.
Zhu, H. N., Yuan, X. Z., Zeng, G. M., Jiang, M., Liang, J., Zhang, c., ... & Jiang, H. W. (2012). Ecological risk assessment of heavy metals in sediments of Xiawan Port based on modified potential ecological risk index. Transactions of Nonferrous Metals Society of China, 22(6), 1470–1477.
Zhu, X., Ji, H., Chen, Y., Qiao, M., & Tang, L. (2013). Assessment and sources of heavy metals in surface sediments of Miyun Reservoir, Beijing. Environmental Monitoring and Assessment, 185(7), 6049–6062.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saddik, M., Fadili, A. & Makan, A. Assessment of heavy metal contamination in surface sediments along the Mediterranean coast of Morocco. Environ Monit Assess 191, 197 (2019). https://doi.org/10.1007/s10661-019-7332-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7332-4