Abstract
The results of investigations about the polymerization of p-aminophenol in neutral-weak alkaline medium (pH = 7–8) in our lab showed that the produced polymer which was insoluble in water and soluble in methanol has high tendency to form selectively a blue complex with Ni(II). Investigations into the chemical structure of polymer showed that polymer has a special structure, similar to polyamine in which the aromatic rings are connected through O-bridges. Based on these data, it was decided to polymerize p-aminophenol in situ on graphene oxide (GO) and use as a new sorbent for selective separation and preconcentration of trace amount of Ni(II) from water samples. By this, the rate of sorption of Ni(II) will also be increased considerably with respect to GO alone. Resulting composite (GO-Pp-AP) was characterized by FT-IR, XRD, FE-SEM, and EDS. The obtained data confirmed the uniform growth of the polymer on the GO and the absence of granular particles. The composite shows high tendency and high rate of sorption of Ni(II) and consequently was utilized for solid phase extraction (SPE) of Ni(II) ions before its determination by flame atomic absorption (FAAS). The effects of important parameters on the recovery of Ni(II) were investigated. The presence of foreign ions has no meaningful effect on the recovery percentage of Ni(II). Under the optimum conditions, limit of detection and relative standard deviation were found to be 0.70 μg L−1 and 1.8% (for n = 6; at 20 μg L−1 of Ni(II)), respectively. Testing the standard reference material and analyzing the spiked real samples exhibit that the procedure can be successfully employed for determination of Ni(II) in natural water and wastewater samples.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The adverse effects of Ni(II) on human health have been investigated intensively (Duda-Chodak and Blaszczyk 2008; Schaumlöffel 2012). Many people, especially workers who are exposed to air-born Ni(II) are afflicted with nasal and lung cancer (Sunderman et al. 1988). For this reason, this element was classified as a human carcinogen by International Agency for Research on Cancer (IARC). To reduce the dangerous effects of Ni(II) and control the level of this element in different samples, an accurate, reliable, and sensitive method is necessary. Various techniques, such as inductively coupled plasma-optical emission spectrometry (ICP-OES) (Fabec and Ruschak 1985), inductively coupled plasma-mass spectrometry (ICP-MS) (Martín-Cameán et al. 2014), and electrochemical analysis (stripping methods) (Gonzalez et al. 2002), are available for this purpose.
Flame atomic absorption spectrometry (FAAS) is one of the most common analytical methods which has been widely employed for determination of some elements in various samples (Carter et al. 2009). But, there are two limitations of this method: One is low sensitivity and the other is matrix effect. The influence of these limitations can be decreased by preconcentration and cleaning up of the samples. Consequently, many researchers have recommended techniques for enrichment and separation of heavy metal ions before injection to FAAS (Ferreira et al. 2007).
Among various techniques, solid phase extraction (SPE) is preferred, not only due to simplicity, low cost, high efficiency in extraction, and time-saving but also because of availability of many kinds of sorbent for special purposes (Camel 2003). Therefore, a careful choice of the sorbent for SPE is crucial. Until now, many kinds of sorbent for SPE of Ni(II) have been reported. Some examples include Ambersorb-572 (Baytak and Türker 2006), gallic acid-modified silica gel 572 (Xie et al. 2008), surfactant modified sawdust (Baki and Shemirani 2013), poly(methyl methacrylate) grafted agarose (Pourmand et al. 2015), Bacillus sphaericus-loaded Chromosorb 106 (Tuzen and Soylak 2009), and Ni(II) imprinted polymers (Otero-Romaní et al. 2009).
Recently, numerous nano-materials, especially carbon-based, have been developed (Latorre et al. 2012), among which graphene oxide (GO) is one of the most important due to the huge surface, hydrophilic properties, and presence of functional groups on its structure (Sitko et al. 2013). Some research papers show that GO is an efficient sorbent for extraction of Cu(II), Co(II), Zn(II), Ni(II), Pb(II), Hg(II), and Cd(II) from aqueous samples (Yang et al. 2010; Zhao et al. 2011; Shirkhanloo et al. 2015; Henriques et al. 2016; Ren et al. 2016), but it is not a selective and competitive sorbent, particularly when the rate of sorption of some ions on it is not suitable (Shirkhanloo et al. 2015). Consequently for selective separation, GO must be modified through synthesis of GO-based composites with special chelating polymers; accordingly, the sorbent capacity and selectivity toward the analyte will be improved. Some examples of these hybrid materials which have been used are as follows: aminosilanized GO (Sitko et al. 2014), mercapto-GO (Sitko et al. 2015), ethylenediamine-GO (Zawisza et al. 2016), poly(amidoamine)-GO (Yuan et al. 2013), polypyrrole-reduced GO (Chandra and Kim 2011), and GO-poly(2,6-diaminopyridine) (Kojidi and Aliakbar 2017).
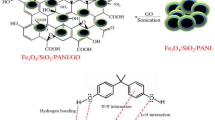
In this work, p-aminophenol (p-AP) was selected as a suitable monomer for in situ oxidative polymerization on GO surfaces, considering the selectivity and high tendency of poly(p-aminophenol) (Pp-AP) for chelating with Ni(II) ions. Resulting GO-based-poly(p-aminophenol) composite (GO-Pp-AP) was characterized with Fourier transform infrared (FT-IR), X-ray diffraction (XRD), field emission electron microscopy (FE-SEM), and energy-dispersive X-ray spectroscopy (EDS) techniques; then it was packed in a glass tube and utilized for quick SPE of Ni(II) in aquatic samples.
Experimental
Chemicals and materials
All chemicals were of analytical grade and used without further purification. Various kinds of salts and acids such as ammonium persulfate (APS), sodium acetate, sodium hydroxide, phosphoric acid, hydrochloric acid, nitric acid, and sulfuric acid were purchased from Merck (Darmstadt, Germany). The standard solution of Ni(II) and other used cations were prepared by dilution of 1000 mg L−1 atomic absorption standards (Bern Kraft, Duisburg, Germany). Fine powder of graphite (~ 50 μm ≥ 99%) and p-aminophenol (≥ 98%) was available from Sigma-Aldrich (Saint Louis, USA). To confirm the efficiency of the method, a standard reference material of natural water (SRM 1640a, CNi(II) = 25.32 ± 0.14 μg L−1) was used and supplied by NIST (Gaithersburg, USA). The buffers were prepared using phosphoric acid (0.3 mol L−1) and sodium hydroxide.
Instrumentation
FT-IR spectra were recorded by a Bruker ALPHA spectrometer (Rosenheim, Germany) using pressed KBr pellets. All UV-Vis spectra were prepared by using spectrophotometer model CARY 100 Conc (Varian, Australia). An Equinox XRD 3000 diffractometer (INEL, France Company) with a rotating anode and a cupper Kα source (λ = 0.15406 nm) was used for recording the XRD patterns. SEM images were taken by AIS-2100 (Seron, South Korea) scanning electron microscope. The surface of morphology of the synthesized composite was investigated using FE-SEM (MIRA3TESCAN-XMU, Czech Republic). EDS analysis was taken by SAMX (Levens, France). A flame atomic absorption spectrometer (Varian, Spectra AA 220, Australia) was used for determination of Ni(II) and other used cations, and the parameters of hollow cathode lamp, slit, and condition of air-acetylene burner were according to the recommendation of the supplier of the instrument.
Synthesis of GO
Modified Hummers method (Marcano et al. 2010) was used for oxidation of graphite powder for synthesis of GO. In brief, after dispersion of graphite (5 g) in a mixture of concentrated H2SO4/H3PO4 (9:1 v/v, 400 mL), it was stirred for 1 h in an ice bath. Then, 15 g of potassium permanganate was added slowly, while the temperature was maintained at < 5 °C. Afterward, the ice bath was removed and the mixture was stirred overnight at room temperature. The resulting brownish paste was slowly added with 350 mL of water with vigorous agitation. After cooling the mixture to room temperature, it was treated with 10 mL H2O2. Subsequently, the color of the solution turned from dark brown to yellow. The mixture was washed and raised with 100 mL of HCl 3 mol L−1. This was repeated several times with double distilled water until the pH of the supernatant was nearly neutral. After filtration and drying under vacuum, the GO was used for further reaction.
Synthesis of GO-Pp-AP
GO-Pp-AP was prepared similar to in situ polymerization of other kinds of monomers on GO (Chandra and Kim 2011; Kojidi and Aliakbar 2017; Xu et al. 2010; Zhang et al. 2010). In a typical procedure, 800 mg of GO was fully dispersed in 100 mL of water with the aid of an ultrasonic bath. Two hundred milligrams of monomer was added and stirred vigorously for at least 30 min to form a uniform mixture. Afterward, the pH of the solution was raised to about 8.0 using 100 mg of sodium acetate. The polymerization was performed in the conventional method (Liu et al. 2013) by dropwise addition of the oxidant (APS solution, 20 mL of 0.1 mol L−1) into the mixture over for 4 h, and the reaction was continued with constant stirring at room temperature for 12 h. At the end, the black precipitate was separated by filtration and washed with 50 mL of HCl 1 mol L−1 (to remove soluble oligomers and other materials), and then with a large amount of double distilled water. The yield of the polymerization and the polymer content in the composite were approximately 78.5% and 16.4%, respectively.
Column preparation and SPE procedure
A glass tube (20 × 1.0 cm, i.d.) equipped with a porous glass at the end was used as an analytical column. Then, 150 mg prepared sorbent powder of (GO-Pp-AP) was slurred and transferred into column. After swelling the sorbent, the bed height was about 1 cm. The column was preconditioned by phosphate buffer (pH = 7). It has to be mentioned that choosing 150 mg of the sorbent was after some experiments with different amounts of composite (50–200 mg).
In the SPE procedure, 200 mL of water samples was adjusted to pH = 7 by adding the phosphate buffer and then passed through the column using a controllable vacuum pump. The flow rate of the samples was 10 mL min−1. After loading, the column was washed with 20 mL of double distilled water (clean up). Three milliliters of HCl (2 mol L−1, 1 mL min−1) was used as eluent, and after elution of Ni(II), it was injected to a FAAS. However, in some more experiments, the column method was compared with batch method (dispersive method). It was found that the column method was easier and quicker.
Sample preparation
The water samplings were carried out three times with the interval of 10 days during the May of 2017. The samples were transferred to the lab, and after filtration, they were conditioned and kept in acid-cleaned polyethylene bottles. Tap water was collected from a city pipeline system of Rasht (North of Iran). River sample, lagoon water, and wastewater were sampled from Sefidrud River (Rudbar, North of Iran), Anzali Lagoon (at South of Caspian Sea), and zeolite production unit in Behdash Chemical Co. (Qazvin industrial city), respectively.
Results and discussion
Characterization of Pp-AP
In our previous work (Dizavandi et al. 2017), Pp-AP was prepared at pH = 8 electrochemically. Then, it was characterized by cyclic voltammetry (CV), FT-IR, hydrogen-nuclear magnetic resonance (H-NMR), and a suitable mechanism for electrode reaction was proposed. The obtained results showed that a simple repetitive structure of the trimmer could be as Fig. 1 (Dizavandi et al. 2017). As it can be seen, the aromatic rings are connected together through O-bridges. The achieved results of some other experiments showed that the structure of the prepared polymer by chemical polymerization is the same as electrochemical polymerization production. The results of some experiments showed that the prepared polymer is able to form a blue complex with Ni(II) selectively. The tendency toward the nickel ion was investigated by recording the UV-Vis spectra of a solution (25 mL) containing 1 mL of methanolic solution of polymer (1.2 mg mL−1) and Ni(II) ion (0.5 mg L−1) in the pH range of 4–10. It was found that there is a λmax at about 620 nm (at pH 6–8). The same experiments were carried out with Co(II), Cu(II), Hg(II), Zn(II), Pb(II), Cd(II), and Fe(II). No difference was found for spectra of these cations with comparison by the spectra of Pp-AP alone (related data not shown). Therefore, it was decided to use this polymer and GO for preparation of a new composite for extraction of Ni(II) by SPE. In previously published paper, the p-nitrophenol was polymerized by electro reduction of NO2 group as a chelate for Ni(II). The produced polymer was also a kind of polyaminophenol in which the aromatic rings are connected together through N-bridges (Jalali and Aliakbar 2013).
Characterization of GO and GO-Pp-AP
The FT-IR spectra of graphite, GO, p-AP, and GO-Pp-AP are shown in Fig. 2a–d. As it can be seen in Fig. 2b, the bands at 1726 cm−1, 1411 cm−1, 1225 cm−1, and 1056 cm−1 can be assigned to C=O (carbonyl/carboxyl), carboxyl C–O, epoxy C–O, and stretching vibration of alkoxy C–O, respectively. The band of aromatic C=C is observed at 1624 cm−1. All these bands confirmed that the oxidation of graphite was carried out successfully. The band at 3418 cm−1 is attributed to the O–H stretching vibration of adsorbed water molecules and hydroxyl groups of GO (Marcano et al. 2010). Figure 2c, d shows the FT-IR spectra of p-AP and GO-Pp-AP. The comparison of these spectra shows some differences, and the spectrum of GO-Pp-AP is less crowded. The weak bands at 3381 and 3264 cm−1 (Fig. 2d) are due to the NH2 asymmetric and symmetric vibrations, while the band at 1613 cm−1 (Fig. 2c) which is attributed to NH2 scissor mode is weakened sharply. The bands at 1557 and 1502 cm−1 can be assigned to aromatic C=C of GO-Pp-AP. The observed band at 1224 cm−1 is due to absorption of aryl = C–O– (etheric band) (Dizavandi et al. 2017; Taj et al. 1992). By considering the low percentage of polymer in composite (16.4%) (see “Synthesis of GO-Pp-AP” section), it can be seen that some bands of GO are weakened which means the suitable covering of GO by in situ polymerization process.
The XRD patterns of graphite, GO, and GO-Pp-AP are shown in Fig. 3a–c. The peak at 2θ = 26.36° (Fig. 3a) is donated to the normal graphite spacing (002) (Dimiev and Tour 2014). This peak is disappeared in the pattern of GO because of oxidation. But, a new diffraction peak at 2θ = 11.46° (Fig. 3b) is appeared which means an increasing interlayer distance to 0.77 nm which can be due to the production of oxygen-containing functional groups on the surfaces of GO sheets (Dimiev and Tour 2014). In the XRD pattern of GO-Pp-AP (Fig. 3c), because of the low polymer content, the peak intensity at 11.46° is decreased and also a small shift to a lower degree (2θ = 11.19°) can be observed.
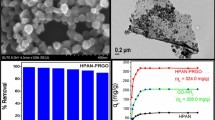
Figure 4a, b shows the SEM images of GO. This figure exhibits a typically curved and layer-like structure. The FE-SEM images of GO-Pp-AP composite (Fig. 4c, d) show porous morphology, and there are no granular particles of polymer which indicates the growth of polymer along GO sheets. Also, the related elemental mapping images (Fig. 4e, h) exhibit homogeneous distribution of carbon, oxygen and nitrogen in the entire range. EDS analysis of composite (Fig. 4i) shows the presence of about 61% C, 22% O, and 7% N. All of these data confirm that the GO sheets are finely coated by polymer through in situ polymerization process, and thus it is believed that the adsorption probability of p-AP monomer on the whole surface of GO is equipotent, resulting in the formation of a uniform polymer coating on the surface of GO sheets (Xu et al. 2010; Chiou et al. 2007; Kumar et al. 2012).
Optimization of SPE
Some parameters affecting the efficiency of the SPE were studied and optimized. The pH of the samples, sample flow rate, sample volume, and the elution conditions such as the kind and concentrate of acid and time of elution were assumed to be the most effective parameters. Respective data and figures are given in the ESM (Electronic supplementary material). And, also the parameters and values tested during optimization of SPE and the selected value are shown in Table 1. The optimal values of these parameters are as follows: The pH of the sample was 7, sample flow rate through column was 10 mL min−1, sample volume was 200 mL, and the best eluent was HCl with concentration of 2 mol L−1 (1 mL min−1). At these steps, the recovery percentages were calculated by measuring the nickel ion concentration in eluate and comparing it with the initial concentration in standard solution considering the preconcentration factor.
The effect of co-exiting ions
Table S2 (ESM) shows the effect of potentially interfering ions. To study the applicability of this method to real samples such as lagoon and river, the concentrations of Na+, K+, Mg2+, and Ca2+ were selected at high values. The obtained results confirm the selectivity of prepared composite toward Ni(II), and the presence of some heavy metal ions has no meaningful effect on the SPE process. According to the obtained results by using the synthesized composite in this paper (GO-Pp-AP) and SPE procedure under optimum condition for Ni(II), the recovery percentage for the mentioned ions with concentration of 20 μg L−1 (with presence of Ni(II)) were in the range of 42–66%. The decrease of recovery percentage for these metal ions could be due to covering of GO surfaces by polymer, and on the other hand, the Pp-AP is selective for Ni(II).
Comparison between GO and GO-Pp-AP in SPE procedure of Ni(II)
As it was mentioned in introduction, GO is behaved as an efficient sorbent for some heavy metal ions samples (Yang et al. 2010; Zhao et al. 2011; Shirkhanloo et al. 2015; Henriques et al. 2016; Ren et al. 2016). Referring to these published papers and showing the advantage of the prepared composite, the rate of sorption of Ni(II) (20 μg L−1) was compared between these two sorbents using column method. The recovery percentage of Ni(II) for two sorbents was measured vs the rate of sample flow rate through the column. Figure 5 shows the obtained results. As it can be seen, due to the high tendency of Pp-AP toward the Ni(II), the recovery percentage changed slightly at higher flow rate of sample. This decreases the time of analysis with respect to using the GO. Although at low flow rate, the recovery percentage is high for both sorbents, the time of analysis will be about 2–3 h (for 200 mL of sample). On the other hand, at higher flow rate (10 mL min−1), time of preconcentration of ion decreases to 20 min with high recovery percentage for GO-Pp-AP. It has to be mentioned that at high flow rate, the GO will be packed strongly and it was useless for repetitive of SPE. This case was not happened to GO-Pp-AP composite.
Figures of merit
Some solutions with different concentration of Ni(II) in the range of 3–70 μg L−1 (C0) were prepared, and under optimum conditions, the SPE processes were carried out (see “Column preparation and SPE procedure” section). After elution, the amounts of Ni(II) were measured by FAAS. The standard plot was established according to the concentration of Ni(II) in initial solutions (C0) and the obtained absorbance of Ni(II) in eluate (Abs). The related equation with correlation coefficient (r2) are as follows:
The limit of detection (LOD) was measured according to the procedure recommended in some text books (Harris 2010; Skoog et al. 1998). In brief, the standard deviation of blank solution was calculated by seven replicate measurements. Three times of blank standard deviation was chosen as detection limit of signal. Using the slope of calibration plot, the LOD concentration was determined and it was found to be 0.70 μg L−1. The RSD (relative standard deviation) for six replicate determinations of 20 μg L−1 of Ni(II) was found to be 1.8%. Table 2 shows some figures of merit of this method and previously reported paper with SPE/FAAS techniques for determination of Ni(II) in water samples (Baytak and Türker 2006; Xie et al. 2008; Baki and Shemirani 2013; Ngeontae et al. 2007; Yilmaz and Soylak 2014).
Reusability of column
To evaluate the reusability and stability of the GO-Pp-AP packed column, several loadings with the sample solutions (Vsample = 200 mL, CNi(II) = 20 μg L−1) and subsequent elutions were carried out under optimum conditions. The column was used repeatedly after washing with 5 mL HCl (2 mol L−1), 20 mL double distilled water, and precondition using phosphate buffer (pH = 7). It was found that the average recovery percentage was 97.0 ± 2.3% after 7 cycles of sorption and desorption.
Validation of method
To investigate the accuracy and validation of the proposed method, a standard solution (SRM 1640a, CNi(II) = 25.32 ± 0.14 μg L−1) was analyzed. The obtained result (24.86 ± 0.42 μg L−1) in Table 3 shows that there is no significant difference between the certified and the achieved result.
Analytical application
To study the applicability of the offered method, some real samples were analyzed. Percentages of recoveries were determined by standard addition (20 μg L−1). All the analytical data can be seen in Table 4.
Conclusion
In the present work, GO was synthesized and then covered by poly(p-aminophenol) through in situ oxidative polymerization. Resulting composite (GO-Pp-AP), after characterization by FT-IR, XRD, FE-SEM, and EDS, was utilized as an efficient and selective sorbent for SPE of Ni(II) in water samples prior to its determination by FAAS. The major advantages of this method are high recovery percentage, low consumption of sorbent, low volume of eluent, high preconcentration factor, applicability in salty media, acceptable LOD, good RSD, high accuracy, and reusability. By GO as a solid support, the stability of the composite for analytical aims will be increased considerably, especially when the eluent is acidic solution. All these points demonstrate clearly the potential of the offered method for monitoring the trace nickel concentration in aquatic samples.
References
Baki, M. H., & Shemirani, F. (2013). Surfactant modified walnut sawdust as an alternative green support for efficient preconcentration of nickel ions from different real samples. Analytical Methods, 5(13), 3255–3263.
Baytak, S., & Türker, A. R. (2006). Determination of lead and nickel in environmental samples by flame atomic absorption spectrometry after column solid-phase extraction on Ambersorb-572 with EDTA. Journal of Hazardous Materials, 129(1–3), 130–136.
Camel, V. (2003). Solid phase extraction of trace elements. Spectrochimica Acta Part B: Atomic Spectroscopy, 58(7), 1177–1233.
Carter, S., Fisher, A. S., Goodall, P. S., Hinds, M. W., Lancaster, S., & Shore, S. (2009). Atomic spectrometry update. Industrial analysis: metals, chemicals and advanced materials. Journal of Analytical Atomic Spectrometry, 24(12), 1599–1656.
Chandra, V., & Kim, K. S. (2011). Highly selective adsorption of Hg 2+ by a polypyrrole–reduced graphene oxide composite. Chemical Communications, 47(13), 3942–3944.
Chiou, N.-R., Lu, C., Guan, J., Lee, L. J., & Epstein, A. J. (2007). Growth and alignment of polyaniline nanofibres with superhydrophobic, superhydrophilic and other properties. Nature Nanotechnology, 2(6), 354–357.
Dimiev, A. M., & Tour, J. M. (2014). Mechanism of graphene oxide formation. ACS Nano, 8(3), 3060–3068.
Dizavandi, Z. R., Aliakbar, A., & Sheykhan, M. (2017). Electrocatalytic determination of clopidogrel using Bi2O3-Pp-AP/GCE by differential pulse voltammetry in pharmaceutical productions. Journal of Electroanalytical Chemistry, 805, 24–31.
Duda-Chodak, A., & Blaszczyk, U. (2008). The impact of nickel on human health. Journal of Elementology, 13(4), 685–693.
Fabec, J. L., & Ruschak, M. L. (1985). Determination of nickel, vanadium, and sulfur in crudes and heavy crude fractions by inductively coupled argon plasma atomic emission spectrometry and flame atomic absorption spectrometry. Analytical Chemistry, 57(9), 1853–1863.
Ferreira, S. L., de Andrade, J. B., Maria das Graças, A. K., Pereira, M. d. G., Lemos, V. A., dos Santos, W. N., et al. (2007). Review of procedures involving separation and preconcentration for the determination of cadmium using spectrometric techniques. Journal of Hazardous Materials, 145(3), 358–367.
Gonzalez, P., Cortınez, V., & Fontan, C. (2002). Determination of nickel by anodic adsorptive stripping voltammetry with a cation exchanger-modified carbon paste electrode. Talanta, 58(4), 679–690.
Harris, D. C. (2010). Quantitative chemical analysis. New York: W. H. Freeman and Company.
Henriques, B., Gonçalves, G., Emami, N., Pereira, E., Vila, M., & Marques, P. A. (2016). Optimized graphene oxide foam with enhanced performance and high selectivity for mercury removal from water. Journal of Hazardous Materials, 301, 453–461.
Jalali, M., & Aliakbar, A. (2013). Electrochemical synthesis and characterization of a new selective chelating agent for Ni (II) and its environmental analytical application. Analytical Methods, 5(22), 6352–6359.
Kojidi, M. H., & Aliakbar, A. (2017). A graphene oxide based poly (2, 6-diaminopyridine) composite for solid-phase extraction of Cd (II) prior to its determination by FAAS. Microchimica Acta, 184(8), 2855–2860.
Kumar, N. A., Choi, H.-J., Shin, Y. R., Chang, D. W., Dai, L., & Baek, J.-B. (2012). Polyaniline-grafted reduced graphene oxide for efficient electrochemical supercapacitors. ACS Nano, 6(2), 1715–1723.
Latorre, C. H., Méndez, J. Á., García, J. B., Martín, S. G., & Crecente, R. P. (2012). Carbon nanotubes as solid-phase extraction sorbents prior to atomic spectrometric determination of metal species: A review. Analytica Chimica Acta, 749, 16–35.
Liu, Z., Liu, Q., Dai, X., Shen-Tu, C., Yao, C., & Kong, Y. (2013). Synthesis of poly (2, 6-diaminopyridine) using Interface polymerization and the electrochemical properties of poly (2, 6-diaminopyridine). ECS Electrochemistry Letters, 2(7), G1–G4.
Marcano, D. C., Kosynkin, D. V., Berlin, J. M., Sinitskii, A., Sun, Z., Slesarev, A., Alemany, L. B., Lu, W., & Tour, J. M. (2010). Improved synthesis of graphene oxide. ACS Nano, 4(8), 4806–4814.
Martín-Cameán, A., Jos, A., Calleja, A., Gil, F., Iglesias-Linares, A., Solano, E., & Cameán, A. M. (2014). Development and validation of an inductively coupled plasma mass spectrometry (ICP-MS) method for the determination of cobalt, chromium, copper and nickel in oral mucosa cells. Microchemical Journal, 114, 73–79.
Ngeontae, W., Aeungmaitrepirom, W., & Tuntulani, T. (2007). Chemically modified silica gel with aminothioamidoanthraquinone for solid phase extraction and preconcentration of Pb (II), Cu (II), Ni (II), Co (II) and Cd (II). Talanta, 71(3), 1075–1082.
Otero-Romaní, J., Moreda-Piñeiro, A., Bermejo-Barrera, P., & Martin-Esteban, A. (2009). Ionic imprinted polymer for nickel recognition by using the bi-functionalized 5-vinyl-8-hydroxyquinoline as a monomer: Application as a new solid phase extraction support. Microchemical Journal, 93(2), 225–231.
Pourmand, N., Sanagi, M. M., Naim, A. A., Ibrahim, W. A. W., & Baig, U. (2015). Dispersive micro-solid phase extraction method using newly prepared poly (methyl methacrylate) grafted agarose combined with ICP-MS for the simultaneous determination of Cd, Ni, Cu and Zn in vegetable and natural water samples. Analytical Methods, 7(7), 3215–3223.
Ren, X., Wu, Q., Xu, H., Shao, D., Tan, X., Shi, W., Chen, C., Li, J., Chai, Z., Hayat, T., & Wang, X. (2016). New insight into GO, cadmium (II), phosphate interaction and its role in GO colloidal behavior. Environmental Science & Technology, 50(17), 9361–9369.
Schaumlöffel, D. (2012). Nickel species: Analysis and toxic effects. Journal of Trace Elements in Medicine and Biology, 26(1), 1–6.
Shirkhanloo, H., Khaligh, A., Mousavi, H. Z., & Rashidi, A. (2015). Graphene oxide-packed micro-column solid-phase extraction combined with flame atomic absorption spectrometry for determination of lead (II) and nickel (II) in water samples. International Journal of Environmental Analytical Chemistry, 95(1), 16–32.
Sitko, R., Zawisza, B., & Malicka, E. (2013). Graphene as a new sorbent in analytical chemistry. TrAC Trends in Analytical Chemistry, 51, 33–43.
Sitko, R., Janik, P., Feist, B., Talik, E., & Gagor, A. (2014). Suspended aminosilanized graphene oxide nanosheets for selective preconcentration of lead ions and ultrasensitive determination by electrothermal atomic absorption spectrometry. ACS Applied Materials & Interfaces, 6(22), 20144–20153.
Sitko, R., Janik, P., Zawisza, B., Talik, E., Margui, E., & Queralt, I. (2015). Green approach for ultratrace determination of divalent metal ions and arsenic species using total-reflection X-ray fluorescence spectrometry and mercapto-modified graphene oxide nanosheets as a novel adsorbent. Analytical Chemistry, 87(6), 3535–3542.
Skoog, D. A., Holler, F. J., & Nieman, T. A. (1998). Principles of instrumental analysis. Saunders College Publishing.
Sunderman, F. W., Jr., Dingle, B., Hopfer, S. M., & Swift, T. (1988). Acute nickel toxicity in electroplating workers who accidently ingested a solution of nickel sulfate and nickel chloride. American Journal of Industrial Medicine, 14(3), 257–266.
Taj, S., Ahmed, M., & Sankarapapavinasam, S. (1992). Poly (para-aminophenol): a new soluble, electroactive conducting polymer. Journal of Electroanalytical Chemistry, 338(1–2), 347–352.
Tuzen, M., & Soylak, M. (2009). Column solid-phase extraction of nickel and silver in environmental samples prior to their flame atomic absorption spectrometric determinations. Journal of Hazardous Materials, 164(2–3), 1428–1432.
Xie, F., Lin, X., Wu, X., & Xie, Z. (2008). Solid phase extraction of lead (II), copper (II), cadmium (II) and nickel (II) using gallic acid-modified silica gel prior to determination by flame atomic absorption spectrometry. Talanta, 74(4), 836–843.
Xu, J., Wang, K., Zu, S.-Z., Han, B.-H., & Wei, Z. (2010). Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano, 4(9), 5019–5026.
Yang, S.-T., Chang, Y., Wang, H., Liu, G., Chen, S., Wang, Y., Liu, Y., & Cao, A. (2010). Folding/aggregation of graphene oxide and its application in Cu2+ removal. Journal of Colloid and Interface Science, 351(1), 122–127.
Yilmaz, E., & Soylak, M. (2014). Solid phase extraction of Cd, Pb, Ni, Cu, and Zn in environmental samples on multiwalled carbon nanotubes. Environmental Monitoring and Assessment, 186(9), 5461–5468.
Yuan, Y., Zhang, G., Li, Y., Zhang, G., Zhang, F., & Fan, X. (2013). Poly (amidoamine) modified graphene oxide as an efficient adsorbent for heavy metal ions. Polymer Chemistry, 4(6), 2164–2167.
Zawisza, B., Baranik, A., Malicka, E., Talik, E., & Sitko, R. (2016). Preconcentration of Fe (III), Co (II), Ni (II), Cu (II), Zn (II) and Pb (II) with ethylenediamine-modified graphene oxide. Microchimica Acta, 183(1), 231–240.
Zhang, K., Zhang, L. L., Zhao, X., & Wu, J. (2010). Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chemistry of Materials, 22(4), 1392–1401.
Zhao, G., Li, J., Ren, X., Chen, C., & Wang, X. (2011). Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environmental Science & Technology, 45(24), 10454–10462.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• GO-Pp-AP composite was prepared by in situ polymerization of p-aminophenol on graphene oxide as a solid support
• FE-SEM and nitrogen mapping analysis confirmed that the GO sheets are finely coated by poly(p-aminophenol)
• It was utilized as a sorbent for SPE of Ni(II) before FAAS determination
• It shows selectivity for Ni(II), rapid mass transfer, high recovery, and reusability
Electronic supplementary material
ESM 1
(DOCX 123 kb)
Rights and permissions
About this article
Cite this article
Kojidi, M.H., Aliakbar, A. Synthesis of graphene oxide-based poly(p-aminophenol) composite and its application in solid phase extraction of trace amount of Ni(II) from aquatic samples. Environ Monit Assess 191, 145 (2019). https://doi.org/10.1007/s10661-019-7282-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7282-x