Abstract
Urbanization and anthropogenic activities create many environmental issues in urban water supply reservoirs, especially in metropolitan regions. Thus, this study was carried out aiming to evaluate the variance in the physical-chemical characteristics of bottom sediment along the Lake Guaíba, Brazil. Lake Guaíba is a freshwater lake situated in a metropolitan region in southern Brazil, being the main water supply to the region. Surface sediment was evaluated to pH, electrical conductivity, particle-size, total organic carbon and nitrogen, metals and inorganic elements (Fe, Al, Ca, Ba, Sr, Co, Tl, Zn, Cu, Cr, Ni, Pb, Cd, and Hg), and organic compounds. The sediments in the Lake Guaíba show a wide range in the physical-chemical characteristics. Metals Zn, Cu, Cr, and Ni appear in higher concentrations near to the margin of southern Porto Alegre, where there was also more clay plus silt. Sediments of Lake Guaíba have physical-chemical variability by the settle tendency and water flow from the riverine to lacustrine areas. The sediment in Lake Guaíba had a median of: Zn, 132; Cu, 78; Cr, 42; Ni, 28; Pb, 33; Cd, 0.3; and Hg, 0.07 μg/g. Bed sediments of Lake Guaíba are polluted with Zn, Cu, Cr, and Ni, major in the east margin (near to Porto Alegre). The potential toxic metals and organic compounds found in Lake Guaíba are commonly reported in urban regions around the world. Those elements and compounds derive from many anthropic activities, as industries, sewage, and vehicles. With diffuse sources in the region, the pollution control in Lake Guaíba is very complex.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The high population density in the metropolitan areas and their industrial and agricultural activities impact the local water resources that often serves as a source of water to the same population. Metals are natural elements found in the soil and mineral matrix; however, they can be accumulated in the environment through anthropic pollution. Chemicals entering the water bodies, binding suspended particles, are deposited in bottom sediments where they accumulate in concentrations many times greater than natural concentrations (Sharley et al. 2016; Zhou et al. 2017). These sediments formed by deposition of organic and inorganic particles that originate in metropolitan areas play an important role in aquatic ecosystems, affecting biogeochemical cycles, nutrients redistribution, and maintenance of environmental quality (Liu et al. 2016; Sharley et al. 2016).
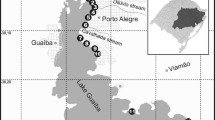
Lake Guaíba is an urban shallow open lake located in the metropolitan region of Porto Alegre; the capital of Rio Grande do Sul State (RS), southern Brazil (Fig. 1). It has served as the main water source for the capital since its foundation in the nineteenth century. Currently, the water of the Lake Guaíba has multiple uses such as water supply, wastewater dilution, recreation, fishing, and navigation. Lake Guaíba has an area (A) of 496 km2, volume (V) of 1.44 km3, mean depth (V/A) of 3 m, average discharge (Q) of 1193 m3/s, water residence time (V/Q) of 14 days, and sedimentation rate (14C and 210Pb) of 6 mm/year (Laybauer 2002). The lake is fed by the tributaries rivers “Jacuí” (almost 85% of the water), “dos Sinos,” “Caí,” and “Gravataí.” Those rivers meet at the Jacuí’s Delta, forming a transitional environment (from riverine to lacustrine), and this water flows through the lake until reaching Patos Lagoon. The lake acts as a reservoir that receives significant water and sediment load and cannot be seen merely as an extension of its branch of rivers (Laybauer 2002).
The sediment in Lake Guaíba transitional waters has physical and chemical diversity influenced by its tributaries. The objectives of this study were to characterize the physio-chemical diversity of bottom sediments in these transitional waters and to determine the influence of tributaries on sediment characteristics. Specifically, we determined the extent to which metal concentrations of bottom sediments were controlled by distance from tributaries versus sediment characteristics within the lake.
Materials and methods
Study area
Lake Guaíba is a freshwater lake situated in the metropolitan region of Porto Alegre City (state’s capital), southern Brazil (Fig. 1). It is an open shallow lake with an area (A) of 496 km2, volume of 1.44 km3, and mean depth of 3 m; the average annual discharge from the lake is 1193 m3/s, water residence time is 14 days, and the sedimentation rate is 6 mm/year (Laybauer 2002). Lake Guaíba is one of several interconnected freshwater lakes that include the Patos Lagoon (with outflow in the Atlantic Ocean), Lagoon of Casamento, and Lagoon Mirim.
The lake’s name (from the original “Guahyba”) comes from the indigenous language Tupi-Guarani (Brazilians natives) meaning “meeting of the waters,” due to the Jacuí’s Delta where are the outflow of rivers “Jacuí,” “dos Sinos,” “Caí,” and “Gravataí.” The Lake Guaíba’s drainage region covers 84,751 km2 (with 2523 km2 of specific watershed), covering 251 municipalities: 1/3 of the area, 50% of the municipality, and more than 60% of the inhabitants of the state (de Andrade et al. 2018a, b). The region has many industries and service companies (Petrochemical, Leather and Footwear, Food and Beverage, Landfills and Wastewater Treatment, Mining and Metallurgy, Pulp and Paper), creating many pollution possibilities.
Sediment sample and preparation
Surface bottom sediment (0–10 cm) composite samples (with three sub-samples) were collected between 18th and 19th of January 2017 in 27 sites of Lake Guaíba with an Ekman Bottom Grab (dredge) sampler. During those days, Guaíba had 0.51 m of water level (being 0.47 m the historical average for January) and 25.1 ± 0.1 °C of average air temperature (CEIC 2017). Sampling sections (Fig. 1) were geo-located (GPS TrackMaker®v13). Depth (Table 1) was measured with an echobathymeter (Eagle® Cuda 300).
Physicochemical analysis
Sediment samples were returned to the laboratory, dried (45 °C) and sieved (2 mm) to perform the analysis. Sieved samples were analyzed for pH in CaCl2 (ratio 1:2.5, v/v); electrical conductivity (EC; 1:5, v/v); bulk density (Ds); and particle size (pipette method). Total organic carbon (TOC) and total nitrogen (TN) were evaluated with an organic elemental analyzer (OEA; Thermo Scientific™ Flash™ 2000 NC Soil Analyzer); sulfanilamide was used as a standard to verify the process quality.
Inorganic elements (Fe, Al, Ca, Ba, Sr, Co, Tl, Zn, Cu, Cr, Ni, Pb, Cd, and Hg) were assessed via microwave-assisted acid extraction using EPA 3051A (USEPA 2007) and the quantification was performed in a inductively coupled plasma mass spectrometry (ICP-MS; PerkinElmer ELAN® 9000). The sediment samples (0.2 g) stood overnight in 5 mL of concentrated nitric acid (HNO3; Fisher® Trace Metal Grade) before the microwave extraction. Dilution (1:100) was made with ultrapure water (Barnstead®). Certified standard reference materials (NIST2711—Montana, NIST2709—San Joaquin, and EPA CRM020–50—Metals #2) were used to verify the process quality. All process was done in triplicate.
Metals were compared with the Brazilian sediment guidelines, Conama no. 454 (Brasil 2012), based from the Canadian Environmental Quality Guidelines (CEQGs)—sediment quality guidelines for the Protection of Aquatic Life (freshwater), where: the “Level 1” of Brazilian guideline is equivalent to interim sediment quality guideline (ISQG), as the threshold effect level (TEL); and the “Level 2” equivalent to probable effect level (PEL).
A geo-accumulation index (Igeo) was determined following the equation (Muller 1969): Igeo = log2 (Cn/1.5 × Bn); where Cn is the measured value of the metal in the sediment sample, Bn is the geochemical background level of element (Laybauer 2002), and 1.5 is the background matrix correction factor to lithogenic effects (Muller 1969; Chaharlang et al. 2016; Liu et al. 2016). Although the background analysis from Laybauer’s was assessed with a total extraction (HCl-HNO3-HF).
Organic compounds
The extraction of organic compounds was made by transferring 5 g of the dry sediment into 50 mL polypropylene centrifuge tubes followed by 10 mL of a mixture (1:1 v/v) of HPLC grade acetone and dichloromethane (Fisher Scientific). The tubes were placed for 30 min in ultrasonic bath, and then centrifuged for 20 min at 3500 rpm. The supernatants were transferred to glass evaporation tubes, and the residues were re-extracted by adding the solvent mixture and repeating the extraction process two more times. The collected extracts were evaporated in an evaporator workstation (TurboVap®) to near dryness at a temperature not exceeding 45 °C. The residues in the evaporation tube were dissolved by adding 0.9 mL of methanol, vortexed for 30 s and its content was transferred to 1-mL volumetric tube and brought to volume with methanol. The methanol extracts were transferred to 2-mL GC vials for analysis.
Non-target screening GC-MS analyses were performed by gas chromatography (Hewlett Packard 6890) connected to a mass selective detector (Hewlett Packard 5973). A HP-5MS capillary column (30 m × 0.25 mm × 0.50 μm) containing 5% phenyl methyl siloxane (HP 19091J-133) was programmed to start at 40 °C (2 min), followed by an increase to 280 °C (6 °C/min) holding this temperature for 5 min (total run time of 47 min; maximum temperature of 325 °C). Helium was used as the carrier gas at a flow rate of 1.0 mL/min with 7.15 psi pressure and 36 cm/s. Samples were injected in split mode (with ratio 3.679:1; split flow 3.7 mL/min; total flow 6.9 mL/min; electron energy of 70 eV.). The whole system was controlled by a ChemStation which included a version of the Wiley HP Mass Spectral Libraries containing more than 275,000 entries. The 4,4′-PCB (biphenyl, 4,4′-dichloro-) and TCDD (2,3,7,8-tetrachlorobenzo-p) follow the same methods but were evaluated as quantitative.
Statistical analysis and geoprocessing
Results were submitted to analysis of variance (ANOVA) and, when significant, means were compared by Tukey test with a 95% confidence interval (p < 0.05). All graphics and statistical analyses were developed with software TIBCO Statistica® v13.
The geoprocessing database was designed with geographic information system (GIS) software (Esri, ArcGIS® v10.4), performed using an inverse distance weighted (IDW) technique. The IDW interpolation uses a linearly weighted combination of a set of sample points. In this study, 27 neighboring sediment sample points were used for interpolation. The weight is a function of inverse distance. The spatial resolution at which the output raster was created was 30 m. A Python script was written for interpolation evaluation and systematization.
Results
The sediments in the Lake Guaíba (Fig. 1) have a wide range in the physical-chemical characteristics. Depth ranged from 1.8 m (LG02) to 5.1 m (LG21 and LG23) (Table 1); no significant correlations (r < 0.5; p > 0.05) occurred with the depth. Depth increased (Fig. 2) from the north (inflow in Jacuí’s Delta) to south (emissary to Patos Lagoon). Clay ranged from 1.9% (LG08) to 41.7% (LG21); silt ranged from 1.6% (LG08) to 77.5% (LG03); and total sand (fine, coarse, and gravel) ranged from 0.5% (LG01) to 96.5% (LG08). Clay plus silt (C + S) was high in the central and southern Guaíba, as in the “curve” (LG02 and LG03) in north area, following the natural settling of particle size (Fig. 2).
The pH (CaCl2 1:2.5) in the surface sediments of Lake Guaíba had a median of 5.4, ranging from 4.6 (LG06) to 6.7 (LGOUT); the pH had a negative correlation with the clay plus silt (r = − 0.76). The electrical conductivity (EC) ranged from 43 μs/cm (LG08) to 410 μs/cm (LG16), with a median of 115 μs/cm (Table 2); EC was not strongly correlated with other parameters.
Carbon (TOC) and nitrogen (TN) ranged from 1.0 mg/g and 0.0 mg/g (LG08) to 55.7 mg/g and 14.7 mg/g (LG12), respectively (Table 2) and were highly correlated with one another (r = 0.99). TOC was higher near the margins of southern Porto Alegre, and also near to the cities of Guaíba and Eldorado do Sul (Fig. 2), with a tendency to increase from north to south, where the sediments contained more clay and silt.
Metal concentrations differed among the evaluated sites (Table 3). All metal concentrations in sites LG00, LG04, LG08, LG13, LG14, LG22, and LGOUT (Fig. 1) were below the average values. However, some sites had metals above the average and/or the guideline values: LG05 and LG06 (Zn, Cu, Cr, and Ni); LG09 (Fe, Ca, Ba, Sr, Co, and Zn); LG11 and LG12 (Cu, Cr, Ni, Pb, and Cd); LG15 (Ni); LG16 (Zn and Cr); LG23 (Cu); and LG24 (Pb and Hg). Although LGDS (Dilúvio Stream outflow) had most of the elements below the average, concentrations of Zn and Cd were above the average (Table 3). They also had higher values of Zn, Cu, and Cd standardized by Al (Table 4). The standardization tends to compensate for particle size differences among sites.
In many sites, metals Zn, Cu, Cr, Ni, and Pb had values above the interim sediment quality guideline (ISQG), “Level 1” in Brazilian sediment quality guidelines (Brasil 2012); however, only Ni had values above the probable effect level (PEL). Concentrations exceeding the PEL were found at sites LG06, LG10, LG11, LG12, and LG15. Comparing the median values of the sites (Table 3) with the ISQG, reference values (RV) and background values (BG) of the Lake Guaíba (Laybauer 2002): Cu and Cr were above all (ISQG, RV, and BG); Zn above the ISQG and BG, but below the RV (samples from 1998 to 1999); Ni above the ISQG and below the RV and BG; Pb above the BG and below the ISQG and RV; Hg above the RV and BG and below the ISQG; and Cd below or near all (ISQG, RV, and BG).
The geo-accumulation index (Igeo) of Zn surpasses the unpolluted values only in sites LG05, LG06, LG09, LG16, and LGDS (Fig. 3). Pb surpasses only in site LG24, and Cd only in site LGDS. Means of Cu, Cr, and Hg surpass the “unpolluted” value (zero line). Sites where none of the metals exceed the “unpolluted” include sites: LG00, LG04, LG08, LG13, LG14, LG22, and LGOUT. Most of the sites with higher Igeo were near to east margin (Porto Alegre) and those with lowest were near the west margin of the lake (Fig. 1).
Principal component analysis (PCA) indicated a relationship between metal concentrations (Al, Cr, Pb, Tl, Hg, Cu, Ni, and Cd) and clay, silt, and carbon (TOC). Distance from the first group was another relationship among the metals Fe, Co, Ba, Zn, and Sr (Fig. 4).
The 4,4′-PCB appears only in the north region of the lake (sites LG00, LG02, LG04, and LG05), with values ranging around 307 μg/kg. TCDD ranged from non-detected (ND) to 30.3 μg/kg (Table 2). TCDD present a significant (p < 0.01) correlation (r = 0.59) with TOC. A screening in the sediment of Lake Guaíba found four principal groups and more than nineteen organic compounds (Table 5), from more than 275,000 entries evaluated. Some compounds (found in most sites) were pooled: aliphatic hydrocarbons (HC), alkanes (from 10 to 44 carbons); non-aliphatic HC (mixed); cycloaliphatic and derivatives; and benzene and derivatives. Four organic compounds were found in all the sites evaluated: “14-beta-H-pregna”; “diacetone alcohol” (4-hydroxy-4-methyl-2-pentanone); “phenol, 2,4-bis TMS” [phenol, 2,4-bis(1,1-dimethylethyl)]; “nonahexacontanoic acid.”
Discussion
The bed sediments in Lake Guaíba are predominantly fine particle sizes (silt and clay). However, some sites have a high amount (> 70%) of total sand (sum of fine, coarse, and gravel). These sites were in “sandy points” (LG04 and LG08), sand deposition regions (LG09, LG13, and LG22), and areas with large water flow (LG00—Jacuí’s Delta outflow; LGDS—Dilúvio Stream outflow; LGOUT—connection with Patos Lagoon). Depth was not correlated (r < 0.5) with the particle size. Most of those sandy sites were in the west margin of the lake (on the right side of predominant water flow), the opposite of should occur in rivers (Menegat and Carraro 2009). The Lake Guaíba presents characteristics of “fluvial lakes” (Smol 2008), with the sediment deposition being dependent of the water flows and velocity.
Aluminum (Al) had the highest values in the sites LG01, LG06, LG07, LG11, LG12, LG16, and LG24 (Table 3) All these sites had high values of clay plus silt (> 85%). The Al (most abundant metal of lithosphere) has a high correlation with clay (r = 0.88; R2 = 0.78) (Fig. 5a) and silt (r = 0.76). Aluminum (Al) follows the same tendency of clay plus silt (C + S), with higher values in the central and southern zones of Lake Guaíba, as in the “curve” in north area (Fig. 2), due to the high correlation. These sites also show high values of total organic carbon (TOC) that has a strong correlation with the Al (r = 0.93). All sites with more than 25% of clay had also high values (> 25 mg/g) of TOC (Tables 1 and 2). However, despite the expected relationship between particle size and carbon (Liu et al. 2016), the linear correlation of clay and TOC was reduced due to one site (LG12) that was well off the trend line (Fig. 5b).
The electrical conductivity (EC) had higher values in the margin of Porto Alegre, major in the sites LGDS, LG05, LG06, LG11, LG12, and LG16 (Table 2), creating a plume to the south Guaíba (Fig. 1). High values appear also in the site LG07 (216 μs/cm), near to Guaíba City.
Site LG12 had the highest carbon (TOC) and nitrogen (TN) concentrations, and the lowest relation C:N (3.8), in an average of 10 (Table 2). The C:N ratio is an indicator about the organic matter sources and fate and reflect changes in the nutrient status (Lucas et al. 2015). Site LG12 was near of Belém Novo neighborhood, with large presence of residences near the lakeshore and a wastewater treatment plant (WWTP). This site had also higher values of Zn, Cu, Cr, Ni, Pb, and Cd (Table 3).
Metals Zn, Cu, Cr, and Ni appear in higher concentrations near to the margin of southern Porto Alegre (Fig. 7), where was more clay plus silt (Fig. 2). It is not possible to say if those metals came directly from Porto Alegre or if those metals just accumulate in these areas due to the clay and silt fraction. Metals in bed sediments of Lake Guaíba have a strong relationship most with clay fractions, but direct or indirect also with the silt fractions and TOC (Fig. 4). Metals and organic matters can enter on Lake Guaíba in many ways (Fig. 6).
Due the strong relation between clay and aluminum, the standardization (subdivision) of metals by Al can compensate the variability of particle sizes. Despite the high values of some standardized metals (Table 4) in sites LG08, LG09, and LG13, these values are overestimated due the low clay plus silt (< 10%) or due to the high clay/silt (Table 1). Site LG17 show high standardized values to some metals (Cu, Cr, Ni, and Pb), although the low bulk average concentrations (Table 3).
Site LGDS (Dilúvio Stream outflow) had very high standardized values to metals Zn, Cu, and Cd (Table 4), confirming the high bulk concentrations of the same metals (Table 3). The LGDS had the highest concentration of Cd (0.51 μg/g) and the second highest of Zn (231 μg/g). Metals Zn and Cd have visible concentrations near to the outflow of the stream (Fig. 7). Dilúvio Stream have a watershed of 83.7 km2, with a high population density (6400 hab/km2), and a length of 17.6 km (Menegat et al. 2006). The stream is a well-known polluted water body that flows over Porto Alegre with outflow in the Lake Guaíba, polluting the sediments in the margins with TOC, TN, Zn, Cu, Pb, Cr, and Ni (de Andrade et al. 2018a, b). Due to the outflow, the local accumulate more fine and coarse sand and gravel (Table 1), carrying out the silt and clay (and, consequently, the pollution).
Site LG06 (with high values of Zn, Cu, Cr, and Ni) is near to the outflow of Salso Stream. Similar to Dilúvio Stream (LGDS), the Salso suffers the effects in a region with high population density (626 hab/km2) and unlawful discharges of raw sewage. Salso Stream has a length of 16.7 km and a drainage basin of 93 km2 (Menegat et al. 2006). Despite the coverage of treatment systems in the region, many households do not make proper connection to the system. Salso Stream have high average amounts of clay plus silt (75%) and organic matter (8%) near to the outflow, as metals Zn (134), Cu (35), Cr (33), Pb (32), and Ni (11 μg/g). Previous studies reported indicatives of contamination with Zn, Cu, and Ni in the Salso Stream (Soares et al. 2004). Site LG11 is near to the outflow of Guabiroba Stream (with a watershed of 10 km2 and popular density of 426 hab/km2), but this local may accumulate the water flows from LG06.
The sediment particle size is inversely proportional to their sorption capacity of metals and compounds; in the meantime, smaller particles can be resuspended in and moving with the water column (Sangster et al. 2015; Tansel and Rafiuddin 2016; Zhou et al. 2017). Thus, polluted sediments may act as a potential release source of those elements and compounds (Zhou et al. 2017).
The geo-accumulation index (Igeo) indicates an empirical relationship of metal enrichment degree, comparing to the natural (background) concentrations (Muller 1969; Chaharlang et al. 2016; Liu et al. 2016). However, Igeo (Fig. 2) do not compensate the variability of particle size (Table 1) or TOC (Table 2). Comparing those values, all sites with positive Igeo had high values of clay plus silt and TOC, unless LGDS (Zn = 0.49; Cu = 0.88; and Cd = 0.18) and LG09 (Zn = 0.66; Cu = 0.91; and Hg = 0.46). Sites LG00, LG04, and LGOUT (other sites with minor clay plus silt and TOC) did not show any positive Igeo.
Major part of locations with lowest (negative) Igeo was near the west margin of the lake, and most locations with higher Igeo were near the east margin (close to Porto Alegre City). Some of the sites with high metals concentrations (Table 3) were in the central area of the lake and east margin (near to Porto Alegre City): LG05 and LG06 (Zn, Cu, Cr, and Ni); LG11 and LG12 (Cu, Cr, Ni, Pb, and Cd); LG16 (Zn and Cr). Sites LG05, LG06, LG11, and LG12 had the highest Igeo of Cu (> 1.5) and Cr (> 0.85). Metals Cu, Cr, and Hg surpass the “unpolluted” value in the average (Fig. 3).
In site LG00 (Lake Guaíba water inflow, from Jacuí’s Delta) there was no any bulk concentrations of metal above the average (Table 3), and the local show low values of clay, silt, and carbon (Tables 1 and 2); however, the site had positive values of Zn/Al and Cd/Al (Table 4). Major part of the pollution of the Lake Guaíba came from the polluted water bodies that flow to the lake (de Andrade et al. 2018a, b), as the rivers Sinos, Caí, and Gravataí (in Jacuí’s Delta) and the urban streams. In order to solve these environmental liabilities, public actions should not focus only on Guaíba, but also in those water bodies (de Andrade et al. 2018a, b).
The sediment in site LG09 had the highest concentrations of Fe (191 mg/g), Ba (1.45 mg/g), Sr (143 μg/g), Co (98 μg/g), and Zn (261 μg/g), and a high Ca (6.1 mg/g). Calcium (Ca), barium (Ba), and strontium (Sr) are alkaline earth metals. LG09 and LGDS (Dilúvio Stream outflow) were the only places with low clay plus silt and TOC, but high Igeo (Zn, 0.66; Cu, 0.91; and Hg, 0.46). LG09 was near to the outflow of Petim Stream, close to Guaíba City (in the west margin). In the drainage basin of the stream, there are many land uses: natural areas; production of eucalyptus, rice, and other grains; and historic coal mining areas.
Site LG24 had the highest concentration of Hg (0.10 μg/g; Igeo = 1.19) and Pb (53 μg/g; Igeo = 0.39). This high contamination should come from the deposition along the time, the same in site LG15 (Ni) and LG23 (Cu). Differently, the high value of Ca in LGOUT occurred due to the large presence of shells of native bivalves in the place. Pb had higher concentrations in the south part of Lake Guaíba and where was more clay plus silt (Fig. 7). Lead (Pb) is predominantly a legacy contaminant, with significantly reducing across the world over time (Sharley et al. 2016); sources of Pb include coal combustion, leaded gasoline, ore smelting, and resuspension of soil, sediment, and road dust (Wan et al. 2016). The phasing out of Pb pollution was correlated with the abolition of leaded fuels in many countries during the 1970s (Sharley et al. 2016).
Generally, there is a risk of pollution by Zn, Cu, Cr, and Ni in the sediments of Lake Guaíba. Metals Zn and Ni reduced the concentrations along the time, but it is still high comparing to ISQG; Cu and Cr increased the concentrations. Metals Pb, Cd, and Hg are more controlled, below the guideline; however, Pb shows values above the BG and ISQG, and Hg above BG and RV, in many sites (Table 3). Even that Zinc (Zn) and copper (Cu) can be naturally found in high concentrations in some soils in the Lake Guaíba drainage region (FEPAM 2014), they can pose risks to organisms in the freshwater ecosystems (Fu et al. 2016). Cooper (Cu) had century-old use in the southern Brazil as Cu-based pesticides in vineyards (Patinha et al. 2018).
According to Laybauer (2002), the balance of metals in the Lake Guaíba had a constant trend between the beginning of the twentieth century and the 1960s, with a strong increase (2–3 × in the contents of Cr, Ni, and Zn) between the 1980s and 1990s due to the increase in industrial activities in the region (like the Leather and Footwear industry). After this time period, concentrations were reduced and were probably linked to improvements in environmental processes and public oversight. Considering the short-term average sedimentation rate of sediments in Lake Guaíba (6 mm/year) and the collected layer (0–10 cm), the samples could represent a history of about 15 years.
The industrial revolution generated increases in global pollution, although late development countries show this same pattern with a certain delay. China had a second high pollution trend from the 1980s and stabilizing in the 2000s, caused by the political reform in the country (Wan et al. 2016; Bing et al. 2016). Toxic metal pollution is usually related to industrial development and its subsequent reduction is generally related to prevention laws and consequent investment in emission control (Wan et al. 2016).
Sediment material is divided into two broad categories based on its sources: “allochthonous,” from outside; and “autochthonous,” from inside (Smol 2008). The sediments (and consequently the pollution) that get in the Lake Guaíba came from the water bodies that flow to the lake, from the urban dust, from the runoff, and other sources (Fig. 6). Lake sediments accumulated significant fractions of organic matter (increasing oxygen demand and nutrient regeneration), that came from external inputs (from the catchment), internal biota (phytoplankton, macrophytes, bacteria), and from pollution sources (Zhang et al. 2017).
In Porto Alegre, the concentrations of Zn, Cr, Cu, Ni, and Pb in urban dust were high in comparison with background values for southern Brazil; the means (± standard deviation) values were Zn, 256 ± 128; Cr, 157 ± 53; Cu, 114 ± 46; Ni, 62 ± 24; and Pb, 52 ± 31 μg/g (Poleto et al. 2009). In this same region, higher metal concentrations (Zn, Pb, Ni, and Cd) were found (in order) in areas with predominant commercial, residential, and industrial activities; this occurs due to the higher vehicular traffic in the commercial and residential areas (Martínez and Poleto 2014). Atmospheric deposition reflects the regional anthropogenic metal emissions, although xenobiotics can be found even in remote alpine lakes, as in the Tibetan Plateau (Bing et al. 2016). Chromium (Cr) pollution is most often associated with industrial activity and processes such as metal finishing or electroplating and is not considered a diffuse pollutant (Sharley et al. 2016).
Variations in the urban dust between the cities reflect differences in the land uses, such as the degree of industrialization and patterns of traffic movements (Poleto et al. 2009). Urban and motorway road dust are highly contaminated with potential toxic metals (as Zn, Cu, Cr, Ni, and Pb) than non-urban regions; high vehicular traffic has been reported as a major source of metal diffuse pollution around the world (Zhang et al. 2016; Adamiec et al. 2016; Sharley et al. 2016). The wear and tear of vehicles pieces (emission, brake pads, tires, oil, grease, rust), as well the pavement paint and degradation, release dust and debris that are carried by stormwater runoff to roadside soils and waterbodies, accumulating in the sediments (Zhang et al. 2016; Sharley et al. 2016). Braking system and tires are exposed to frictions, emitting particles. Brake pads and linings present several metals in addiction to Iron (Zn, Cu, Cd, Fe, Ni, and Pb); tires present large amounts of Zn, due to ZnO and ZnS added to activate vulcanization in the tire tread (Zhang et al. 2016; Adamiec et al. 2016; Sharley et al. 2016).
Persistent organic pollutants (POPs) is a generic name to a group of organic chemicals with characteristics of long half-life times, present in the environment even without current use and remaining very long in sediment (Sakan et al. 2017). Examples of POPs are polychlorinated biphenyls (PCBs), polyaromatic hydrocarbons (PAHs), and organochlorine pesticides (OCPs). Generally, POPs are hydrophobic, binding the particle fraction in the water bodies and depositing in the bed. Those organic micropollutants have multiple sources, as industry, agriculture, traffic, domestic sewage, industrial wastewater, and runoff from nonpoint sources (Sakan et al. 2017).
Aliphatic hydrocarbons (alkanes) were found in higher values in sites LG02, LG06, LG10, LG11, LG15, and LG18. Site LG11 had the highest amount of aliphatic hydrocarbons (31%). Benzene and derivatives were found in higher values in sites LG02, LG03, LG16, LG17, and LG18 (Table 5). Site LG00 (Dilúvio Stream outflow) had higher values of organic compounds: cycloaliphatic and derivatives; phenol, 2,4-bis (TMS); phthalic acid; and fluoranthene. Fluoranthene is a PAH found in many combustion products, being a marker of pyrogenic sources (Sakan et al. 2017). Hydrocarbons (HC) and derivatives (aliphatic, benzene, PAH) are commonly found in petroleum products (as vehicular fuels); those entering in the aquatic ecosystems by surface runoff, urban sewage, industrial discharges, and dry or wet air deposition, accumulating in the sediments due to the hydrophobicity (Nascimento et al. 2017).
The phenol, 2,4-bis(1,1-dimethylethyl) is an alkyl phenol formed in the degradation of pesticides and agricultural products (being commonly found in the sediment and water), presenting characteristics of POPs (as high toxicity, persistence, and bioaccumulation) and acting as endocrine disruptor (Kee et al. 2015). Endocrine disrupting chemicals (EDCs) are exogenous agents that can alter endocrine system functions, being found in personal care products, fuels, pharmaceuticals, and other chemicals (Scognamiglio et al. 2016; Wirbisky et al. 2016). Phenol, 2,4-bis (TMS) was found in all the evaluated sites (Table 5). Previous studies reported the presence of phenol in toxicity analysis in Lake Guaíba watershed (Gonçalves et al. 2012; Terra and Gonçalves 2013).
Esters of phthalic acid, also known as phthalates, are commonly used as additives or plasticizers in a wide range of industrial applications (Scognamiglio et al. 2016). The phthalic acid esters (PAEs) have been widely used in common products as plasticizers (as in PVC), being easily released and accumulated in the environment (due to their hydrophobicity) and transferred to organisms (Tan et al. 2017). PAEs are also considered to be EDCs (Scognamiglio et al. 2016; Tan et al. 2017).
Site LG07 (near to a historical cellulose factory, in Guaíba City) had higher amounts of the follows organic compounds: 14-beta-H-pregna, carbamic acid-, and atrazine-desisopropyl (Table 5). The “14-beta-H-pregna” is naturally found in plants (Koldaş et al. 2015); TOC had a high correlation (r = 0.86) with the phytochemical, and a linear correlation (R2 = 0.71) when excluded the site LG12 (highest TOC). Carbamic acids are sub-products of many compounds, as pesticides or polymers (Wang et al. 2003).
The atrazine-desisopropyl (1,3,5-triazine-2,4-diamine, 6-chloro-N-ethyl-) is a degradation product of the herbicide atrazine (a pre-emergent herbicide to control broadleaf and grassy weeds), and also a EDC (Wirbisky et al. 2016). Atrazine runs off with soil in rainfall or irrigation water to aquatic ecosystems and their degradation products can cause ecological damage (Qu et al. 2017). In a survey about the presence of emerging contaminants in Brazilian’s drinking water sources, atrazine was detected in the Lake Guaíba in concentration of 3 ng/L (Machado et al. 2016).
Conclusions
Sediments of Lake Guaíba have physical-chemical variability by the settle tendency and water flow from the riverine to lacustrine areas. The particle sizes have a high correlation with the metal concentrations. Bed sediments of Lake Guaíba are polluted with Zn, Cu, Cr, and Ni, major in the east margin (near to Porto Alegre). The potential toxic metals and organic compounds found in Lake Guaíba are commonly reported in urban regions around the world. Those elements and compounds derive from many anthropic activities, as industries, sewage, and vehicles. With diffuse sources in the region, the pollution control in Lake Guaíba is very complex.
References
Adamiec, E., Jarosz-Krzemińska, E., & Wieszała, R. (2016). Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environmental Monitoring and Assessment, 188, 369. https://doi.org/10.1007/s10661-016-5377-1.
Bing, H., Wu, Y., Zhou, J., et al. (2016). Historical trends of anthropogenic metals in Eastern Tibetan Plateau as reconstructed from alpine lake sediments over the last century. Chemosphere, 148, 211–219. https://doi.org/10.1016/j.chemosphere.2016.01.042.
Brasil (2012) Resolução CONAMA n° 454, de 01 de novembro de 2012. Diário Oficial [da] República Federativa do Brasil, Brasil.
CEIC (2017) Centro Integrado de Comando da Cidade de Porto Alegre: Dados Históricos das Estações Meteorológicas. http://www2.portoalegre.rs.gov.br/ceic/default.php?p_secao=52. Accessed 26 Jan 2017.
Chaharlang, B. H., Bakhtiari, A. R., Mohammadi, J., & Farshchi, P. (2016). Geochemical partitioning and pollution assessment of Ni and V as indicator of oil pollution in surface sediments from Shadegan wildlife refuge, Iran. Marine Pollution Bulletin, 111, 247–259. https://doi.org/10.1016/j.marpolbul.2016.06.109.
de Andrade, L. C., Andrade, R. D. R., & Camargo FA de, O. (2018a). The historical influence of tributaries on the water and sediment of Jacuí’s Delta, Southern Brazil. Ambiente & Água, 13, 12. https://doi.org/10.4136/ambi-agua.2150.
de Andrade, L. C., Tiecher, T., de Oliveira, J. S., et al. (2018b). Sediment pollution in margins of the Lake Guaíba, Southern Brazil. Environmental Monitoring and Assessment, 190, 3. https://doi.org/10.1007/s10661-017-6365-9.
FEPAM - Fundação Estadual de Proteção Ambiental Henrique Luiz Roessler (2014) Portaria FEPAM No 85. Dispõe sobre o estabelecimento de Valores de Referência de Qualidade (VRQ) dos solos para 09 (nove) elementos químicos naturalmente presentes nas diferentes províncias geomorfológicas/geológicas do Estado do Rio Grande do Sul. FEPAM, Porto Alegre, RS.
Fu, Z., Wu, F., Chen, L., et al. (2016). Copper and zinc, but not other priority toxic metals, pose risks to native aquatic species in a large urban lake in Eastern China. Environmental Pollution, 219, 1069–1076. https://doi.org/10.1016/J.ENVPOL.2016.09.007.
Gonçalves, S. P., Lucheta, F., de, S. V. K., & Terra, N. R. (2012). The influence of xenobiotics in river sediment on the reproduction and survival of Daphnia magna, 1820, Straus. Acta Limnologica Brasiliensia, 24, 220–234. https://doi.org/10.1590/S2179-975X2012005000040.
Kee, Y. L., Mukherjee, S., & Pariatamby, A. (2015). Effective remediation of phenol,2,4-bis(1,1-dimethylethyl) and bis(2-ethylhexyl) phthalate in farm effluent using guar gum – a plant based biopolymer. Chemosphere, 136, 111–117. https://doi.org/10.1016/J.CHEMOSPHERE.2015.04.074.
Koldaş, S., Demirtas, I., Ozen, T., et al. (2015). Phytochemical screening, anticancer and antioxidant activities of Origanum vulgare L. ssp. viride (Boiss.) Hayek, a plant of traditional usage. Journal of the Science of Food and Agriculture, 95, 786–798. https://doi.org/10.1002/jsfa.6903.
Laybauer, L. (2002). Estudo do Risco ambiental e da dinâmica sedimentológica e geoquímica da contaminação por metais pesados nos sedimentos do Lago Guaíba, Rio Grande do Sul, Brasil. Universidade Federal do Rio Grande do Sul.
Liu, J., Yin, P., Chen, B., et al. (2016). Distribution and contamination assessment of heavy metals in surface sediments of the Luanhe River Estuary, northwest of the Bohai Sea. Marine Pollution Bulletin, 109, 633–639. https://doi.org/10.1016/j.marpolbul.2016.05.020.
Lucas, B. T., Liber, K., & Doig, L. E. (2015). Spatial and temporal trends in reservoir physicochemistry and phosphorus speciation within Lake Diefenbaker, a Great Plains reservoir, as inferred from depositional sediments. Journal of Great Lakes Research, 41, 67–80. https://doi.org/10.1016/j.jglr.2015.07.009.
Machado, K. C., Grassi, M. T., Vidal, C., et al. (2016). A preliminary nationwide survey of the presence of emerging contaminants in drinking and source waters in Brazil. Science of the Total Environment, 572, 138–146. https://doi.org/10.1016/J.SCITOTENV.2016.07.210.
Martínez, L. L. G., & Poleto, C. (2014). Assessment of diffuse pollution associated with metals in urban sediments using the geoaccumulation index (Igeo). Journal of Soils and Sediments, 14, 1251–1257. https://doi.org/10.1007/s11368-014-0871-y.
Menegat R, Carraro CC (2009) Manual para saber por que o Guaíba é um lago: Análise integrada de geologia, geomorfologia, hidrografia, estratigrafia e história da ciência, 1st edn. Armazém Digital, Porto Alegre.
Menegat, R., Porto, M. L., Carraro, C. C., & Fernandes, L. A. D. (2006). Atlas Ambiental de Porto Alegre (3rd ed.). Porto Alegre: UFRGS.
Muller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. Geological, 2, 108–118.
Nascimento, R. A., de Almeida, M., Escobar, N. C. F., et al. (2017). Sources and distribution of polycyclic aromatic hydrocarbons (PAHs) and organic matter in surface sediments of an estuary under petroleum activity influence, Todos os Santos Bay, Brazil. Marine Pollution Bulletin, 119, 223–230. https://doi.org/10.1016/J.MARPOLBUL.2017.03.069.
Patinha, C., Durães, N., Dias, A. C., et al. (2018). Long-term application of the organic and inorganic pesticides in vineyards: environmental record of past use. Applied Geochemistry, 88, 226–238. https://doi.org/10.1016/J.APGEOCHEM.2017.05.014.
Poleto, C., Bortoluzzi, E. C., Charlesworth, S. M., & Merten, G. H. (2009). Urban sediment particle size and pollutants in Southern Brazil. Journal of Soils and Sediments, 9, 317–327. https://doi.org/10.1007/s11368-009-0102-0.
Qu, M., Li, H., Li, N., et al. (2017). Distribution of atrazine and its phytoremediation by submerged macrophytes in lake sediments. Chemosphere, 168, 1515–1522. https://doi.org/10.1016/J.CHEMOSPHERE.2016.11.164.
Sakan, S., Ostojić, B., & Đorđević, D. (2017). Persistent organic pollutants (POPs) in sediments from river and artificial lakes in Serbia. Journal of Geochemical Exploration, 180, 91–100. https://doi.org/10.1016/J.GEXPLO.2017.06.008.
Sangster, J. L., Oke, H., Zhang, Y., & Bartelt-Hunt, S. L. (2015). The effect of particle size on sorption of estrogens, androgens and progestagens in aquatic sediment. Journal of Hazardous Materials, 299, 112–121. https://doi.org/10.1016/j.jhazmat.2015.05.046.
Scognamiglio, V., Antonacci, A., Patrolecco, L., et al. (2016). Analytical tools monitoring endocrine disrupting chemicals. TrAC Trends in Analytical Chemistry, 80, 555–567. https://doi.org/10.1016/J.TRAC.2016.04.014.
Sharley, D. J., Sharp, S. M., Bourgues, S., & Pettigrove, V. J. (2016). Detecting long-term temporal trends in sediment-bound trace metals from urbanised catchments. Environmental Pollution, 219, 705–713. https://doi.org/10.1016/j.envpol.2016.06.072.
Smol, J. P. (2008). Pollution of lakes and rivers: a paleoenvironmental perspective (2nd ed.). Malden: Blackwell Publishing.
Soares, M. C. C., Mizusaki, A. M. P., Guerra, T., & Vignol, M. L. M. (2004). Análise geoquímica dos sedimentos de fundo do Arroio do Salso, Porto Alegre-RS-Brasil. Pesqui em Geociências, 31, 39–50.
Tan, S., Wang, D., Chi, Z., et al. (2017). Study on the interaction between typical phthalic acid esters (PAEs) and human haemoglobin (hHb) by molecular docking. Environmental Toxicology and Pharmacology, 53, 206–211. https://doi.org/10.1016/J.ETAP.2017.06.008.
Tansel, B., & Rafiuddin, S. (2016). Heavy metal content in relation to particle size and organic content of surficial sediments in Miami River and transport potential. International Journal of Sediment Research, 31, 324–329. https://doi.org/10.1016/j.ijsrc.2016.05.004.
Terra, N. R., & Gonçalves, S. P. (2013). Daphnia magna Straus, 1820 response to sediment samples from a contaminated river ( Rio Grande do Sul, Brazil). Acta Limnologica Brasiliensia, 25, 19–33. https://doi.org/10.1590/S2179-975X2013000100004.
USEPA - United States Environmental Protection Agency (2007) Method 3051A: microwave assisted acid digestion of sediments, sludges, soils, and oils. 30.
Wan, D., Song, L., Yang, J., et al. (2016). Increasing heavy metals in the background atmosphere of central North China since the 1980s: evidence from a 200-year lake sediment record. Atmospheric Environment, 138, 183–190. https://doi.org/10.1016/j.atmosenv.2016.05.015.
Wang, H., Wang, C., Wu, W., et al. (2003). Persistent organic pollutants in water and surface sediments of Taihu Lake, China and risk assessment. Chemosphere, 50, 557–562. https://doi.org/10.1016/S0045-6535(02)00484-8.
Wirbisky, S. E., Weber, G. J., Sepúlveda, M. S., et al. (2016). An embryonic atrazine exposure results in reproductive dysfunction in adult zebrafish and morphological alterations in their offspring. Scientific Reports, 6, 21337. https://doi.org/10.1038/srep21337.
Zhang, Z., Wang, J. J., Ali, A., & DeLaune, R. D. (2016). Heavy metals and metalloid contamination in Louisiana Lake Pontchartrain Estuary along I-10 bridge. Transportation Research Part D: Transport and Environment, 44, 66–77. https://doi.org/10.1016/j.trd.2016.02.014.
Zhang, Y., Su, Y., Liu, Z., et al. (2017). Lipid biomarker evidence for determining the origin and distribution of organic matter in surface sediments of Lake Taihu, Eastern China. Ecological Indicators, 77, 397–408. https://doi.org/10.1016/J.ECOLIND.2017.02.031.
Zhou, Z., Huang, T., Li, Y., et al. (2017). Sediment pollution characteristics and in situ control in a deep drinking water reservoir. Journal of Environmental Sciences, 52, 223–231. https://doi.org/10.1016/j.jes.2016.05.006.
Funding
This work had financial support from the Brazilian National Counsel of Technological and Scientific Development (CNPq), as scholarship (doctoral) for the first author; and from the Coordination for the Improvement of Higher Education Personnel (CAPES), as scholarship (PDSE) for the first author at University of Georgia (UGA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Andrade, L.C., Coelho, F.F., Hassan, S.M. et al. Sediment pollution in an urban water supply lake in southern Brazil. Environ Monit Assess 191, 12 (2019). https://doi.org/10.1007/s10661-018-7132-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-7132-2