Abstract
Soil, water, and air NO3− pollution is a major environmental problem worldwide. Stable isotope analysis can assess the origin of NOx because different NOx sources carry different isotope signatures. Hence, using appropriate chemical methods to determine the δ15N-NOx values in different samples is important to improve our understanding of the N-NOx pollution and take possible strategies to manage it. Two modified chemical methods, the cadmium–sodium azide method and the VCl3–sodium azide method, were used to establish a comprehensive inventory of δ15N-NOx values associated with major NOx fluxes by the conversion of NO3− into N2O. Precision and limit of detection values demonstrated the robustness of these quantitative techniques for measuring δ15N-NOx. The standard deviations of the δ15N-NO3− values were 0.35 and 0.34‰ for the cadmium–sodium azide and VCl3–sodium azide methods. The mean δ15N-NO3− values of river water, soil extracts, and summer rain were 8.9 ± 3.3, 3.5 ± 3.5, and 3.3 ± 2.1‰, respectively. The δ15N-NO3− values of low concentration samples collected from coal-fired power plants, motor vehicles, and gaseous HNO3 was 20.3 ± 4.3, 5.6 ± 2.78, and 5.7 ± 3.6‰, respectively. There was a good correlation between the δ15N-NO3− compositions of standards and samples, which demonstrates that these chemical reactions can be used successfully to assess δ15N-NO3− values in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is a key nutrient, essential to the survival of humans and all other living organisms. However, the generation of reactive N by humans has at least doubled the rate of input of reactive N to the earth system. The release of a large amount of N will overcome several thresholds set for the health of humans and the ecosystem, including those for drinking water pollution, air quality degradation, freshwater eutrophication, biodiversity loss, stratospheric ozone depletion, and climate change (Erisman et al. 2013). One of the major species of fixed N is NO3−-N, mainly from atmospheric deposition, sewage NO3 discharge, and NO3 fertilizer application (Kendall et al. 2007; Michalski et al. 2004). Therefore, understanding the origin of NO3−-N is critical to implement possible strategies for managing the N pollution problem.
Isotope analysis is a methodology used worldwide to assess the cycle of N through the environment because N-rich sources carry different stable isotope signatures (Hastings et al. 2013). For example, δ15N values of NO3− from organic fertilizers display typical values ranging from − 6 to 6‰, whereas δ15N values of atmospheric N depositions are between − 13 and 13‰. In addition, δ15N values of soil and surface groundwater have been shown to range from 0 to 8 and − 4 to 15‰, respectively (Kendall 1998; Mayer et al. 2002; Xue et al. 2009). Furthermore, δ15N values of NOx generated from natural sources are always lower than those generated by anthropogenic sources (Felix and Elliott 2014; Freyer 1978). Previous studies characterizing the δ15N composition of NOx emissions from power plants (− 9–26‰) have shown that that these are important NOx sources driving the deposition of NO3− at rural monitoring sites in the eastern USA (Elliott et al. 2007; Felix et al. 2012). Stable N isotope data of NO3− (δ15N-NO3−) can be used to trace the production and destination of NO3−-N. Thus, precise and accurate, but also inexpensive and rapid, δ15N-NO3− analyses are needed to improve the identification and assessment of NO3− in atmospheric, soil, surface water, and groundwater samples and samples from their emission sources. During the past decades, a variety of analytical methods have been developed to determine δ15N in NO3−, such as ion exchange and bacterial denitrification (Casciotti et al. 2002; Rock and Ellen 2007; Sigman et al. 2001; Xue et al. 2010). However, these methods have some associated drawbacks; e.g., Silva et al. (2000) pointed out that ion exchange or the AgNO3− method is relatively labor intensive, requires a large volume of sample (sample size of 100–200 mmol of NO3−), and is time-consuming (3–5 days for sample preparation). The bacterial denitrification method also requires a long time for bacterial growth, as long as 10–12 days from Petri dish to media bottles (Xue et al. 2009). A series of chemical methods for δ15N-NO3− analysis have been created for sea water, freshwater, and soil samples. These approaches can analyze samples with low volume, and concentrations of samples do not produce interferences at high concentrations and also have the potential to be less labor-intensive than other approaches (Braman and Hendrix 1989; Lachouani et al. 2010; Tsunogai et al. 2008).
Compositions of δ15N-NO3− can provide evidence of the NO3−-N sources and the mechanisms of NO3−-N transformations in terrestrial ecosystems. Although recent methods used to analyze δ15N-NO3− in surface water and soil are advanced, it is necessary to analyze δ15N-NO3− in other samples, such as δ15N-NO3− in NOx-N samples emitted from coal-fired plants and vehicles, which are absorbed by strong acid, alkali, or oxidant solutions. In addition, the chemical method is a new technique to measure δ15N-NO3−; therefore, it requires further demonstration.
Here, we present a series of chemical methods for detecting sensitive natural abundance of δ15N-NO3− in surface water, rainfall, soil, air, and coal-fired power plant and vehicular emission samples. The objectives of this study were as follows: (1) to develop improved chemical methods to determine the δ15N-NO3− composition of low-concentration samples with small volumes and complex absorbing solutions and (2) to identify the δ15N-NO3− signatures of NO3−-N at environmental concentrations.
Materials and methods
Collection of NO3 −-N in river, rain, and soil
The collection of water samples (river and rain water) were conducted at Suzhou in southeast China during summer (June–August 2014). River water samples were collected in the central stream using a polyethylene barrel at a depth of 0.5 to 1 m from the water surface. Rain water samples were collected by a sensor-controlled auto sampler (ZJC-II, Zhejiang Hengda Instrument Company, China), which collected samples during precipitation events. All samples were subsequently transferred into plastic bottles (0.2–10 L) and transported on ice to the laboratory for storage at − 5 °C prior to analysis.
Soil samples were collected from a summer rice–winter wheat rotated cropland near Suzhou after the wheat harvest in 2014, and they were extracted by 2 mol L−1 KCl solution according to a water:soil ratio of 5:1.
Collection of NOx-N in the atmospheric environment
Gaseous HNO3 and NOx emissions from coal-fired power plants and vehicles were collected in the Taihu Lake region during 2014 summer by an integrated air sampler (ZC-Q0102, Zhejiang Hengda Instrument Company, China). The sampler was operated for 24 h and gathered the ambient air by two low-volume pumps (1 L min−1). The air was passed through a filter train.
Gaseous HNO3 was absorbed by 0.5 g NaOH in 50 mL of methanol (pH > 12) following the method of Adon et al. (2010), and NOx emission from coal-fired plants was trapped by a 25-mL aliquot of absorbing solution (6 mL of H2O2 in 1 L of ~0.05 M H2SO4) following the method of Felix et al. (2012). NOx emissions from vehicles were absorbed in a tunnel (Jiuhua Tunnel, Nanjing City) by the same solution as the coal-fired power plant samples.
All the samples were stored at − 5 °C until required for further analysis. Previous studies have shown that the N isotopic fractionation resulting from the above conversions was minimal (Felix et al. 2012; Walters et al. 2015).
Analyses of NO3 −-N concentration
The water samples were filtered through glass fiber filters (Whatman, GF/F, 47 mm in diameter) and deposited in pre-cleaned polyethylene bottles. Samples collected from air and NOx emission sources were adjusted to a pH of 8.0 by adding 1 mol L−1 hydroxide solution. The NO3−-N concentrations in all of the collected samples were analyzed using a Skalar segmented flow-injection analyzer (Skalar San++ System, Breda, the Netherlands) with a relative error of ± 2% and a detection limit of 0.03 mg N L−1.
Analyses of δ15N-NO3 − in water and soil samples
Based on the methods of Stedman (1959a) and Stedman (1959b), McIlvin and Altabet (2005) developed a Cd reduction and N3− method to reduce NO3− in samples to N2O for the determination of δ15N. In brief, in weak alkaline solution, cadmium reduces NO3− to NO2−, whereas NO2− reacts with N3− to produce N2O in acidic conditions. The reaction between NO2− and N3− at a pH > 2 generates nitrous acidium ions (H2NO2+), followed by the reaction with the N3−.
The resulting N isotopic composition of the N2O product should be given by the mean of the the NO3− and N3− δ15N values since each component contributes one N atom. Hence, we obtain Eq. (1):
The expected slope of a binary plot of NO3− standard δ15N values versus the measured δ15N of the produced N2O is 0.5.
A graphical representation of the isotopic analysis method is given in Fig. 1.
We used a cadmium column to connect to a peristaltic pump, and the flow was set at 5 mL/min to perform the NO3− to NO2− reduction step. Furthermore, unlike the study of McIlvin and Altabet (2005), a pH of 8.0 was demonstrated to be optimal for the NO3− to NO2− conversion in our study (Fig. 2). Several drops of 0.5 M HCl were then added to decrease the pH to 2–3. Blanks require one drop of 0.5 M HCl to attain a pH of 3. The pH of blanks and samples were subsequently increased to 8.0 using a 1 M imidazole (C3H4N2) solution. In cases in which too much imidazole was added, we added some drops of HCl to augment the pH again.
To convert NO2− to N2O, we improved the methods developed by McIlvin and Altabet (2005) and Schilman and Teplyakov (2007). In brief, we poured 16 mL of sample remaining from the above procedure into a 50-mL headspace vial tightly capped with a Teflon-lined septum. The vial was put under vacuum and purged with He to eliminate the negative pressure effect. Then, 0.8 mL of N3− and acetic acid (CH3COOH) buffer (15 mL of 2 mol L−1 N3− and 15 mL of 20% acetic acid) was added to vials via a syringe, and the vials were shaken vigorously for 1 min. According to McIlvin and Altabet (2005), the reaction must be kept at a pH between 4 and 5. All vials were put in a 30 °C water bath for 30 min. After 30-min quiescence, 0.5 mL of 6 M NaOH solution was injected into the vial with a syringe to produce a basic solution and stop the reaction. Afterwards, the pH of the reaction should be > 10. Three blanks were included in each sample batch to test the seals of the vial and the value of the reagent blank.
The vial’s N2O concentration was analyzed by an automated PT-IRMS which included a continuous flow IRMS (IsoPrime100, IsoPrime Limited, UK) with a cryo-focusing unit (Trace Gas Preconcentrator, IsoPrime Limited, UK). The sample entered the IRMS via a capillary from an open split directly to the ion source. Each sample was accompanied by a direct inlet injection of pure N2O. Data were collected throughout the run for masses 44, 45, and 46. The area under each peak was calculated for each mass by Ionvantage software.
For the calculation of δ values, the δ notation is specified for a particular element X, where the superscript H gives the heavy isotope mass of that element,
where Rsample is the ratio of the heavy isotope to the light isotope of the sample and Rstandard is the isotope ratio of the international standard (0.0036765). In this study, R was calculated by
where area45 is the peak area of the m/z 45 of N2O that contains a 15N atom, while the area44 is the peak area of the m/z 44 of N2O that does not contain a 15N atom.
Analyses of δ15N-NO3 − in air samples
We can use the cadmium–sodium azide method to analyze the N natural isotopic abundance of freshwater and soil extracts, but this method is not suitable for samples absorbed with strong acid, alkali, or oxidant solutions because of the difficulty to adjust the pH in the cadmium–sodium azide process.
Therefore, we tried to use the chemical method defined by Lachouani et al. (2010) to determine the δ15N-NOx values for coal-fired power plant, motor vehicles, and gaseous HNO3 samples those absorbed by strong acid, alkali, or oxidant solutions in this study.
The chemical reactions involved in this method are as follows:
The measured N2O consists of two N atoms, one originating from the sample and the other from the sodium azide.
Lachouani et al. (2010) determined that the influence of blank δ15N on the sample δ15N composition should be corrected by the following mass balance equation:
where δ15Nblank corr is the δ15N value of the samples after correction and δ15Nsample and δ15Nblank are the δ15N values of samples and blanks directly measured by PT-IRMS. The areasample and areablank were also directly measured by PT-IRMS.
Samples collected from air and NOx emission samples were evaporated to dryness in a water bath at 80 °C to remove the influence of H2O2 and filled with 5 mL deionized water before performing the 15N-NO3− isotopic analysis.
Results and discussion
Precision of δ15N-NO3 − analyses by chemical methods
The international δ15N-NO3− reference standards USGS34 and USGS32 were used to define the analytical precision. Standards for concentration analyses were produced in duplicate with 100 μmol L−1 solutions mixed together according to different volume ratios to generate distinct δ15N-NO3− compositions (Table 1). The mixed standard solutions and sample solutions were prepared from the same matrix to provide a comparable precision in the δ15N-NO3− results. There are good linear correlations between δ15N-NO3− values of standard solutions and measured δ15N-N2O values of samples using the two methods (Fig. 3). The linear regression slopes for the cadmium–sodium azide and VCl3–sodium azide methods were 0.45 and 0.44, respectively.
In fact, the theory of reaction mechanisms reveals that the slope of the linear relation between the true δ15N of a NO3− standard (or sample) and the measured δ15N of the produced N2O should to be 0.5, since one N atom in the N2O product originates from NO3− and the other one from N3−. The intercept reflects the combination of the initial δ15N of N3− values with the isotopic fractionation associated with the reactions from NO3− to N2O.
In our study, the slopes of the regression lines were close to the 0.5 theoretical value predicted from the 1:1 mixture of NO2−-N and N3−-N. The correlation between concentration and signal intensity for NO3− reveals 3.8 nmol NO3− in the 5 mL blank samples (0.76 μmol L−1), which was mainly derived from sodium azide in the VCl3–sodium azide solution. Furthermore, the correlation of the δ15N-NO3− values in standard solutions with the measured δ15N-N2O values of samples also revealed the role of blank samples. Figure 4a shows an augmentation in the linear regression slope when the sample concentrations increased, whereas when the blank correction was applied through the mass balance equation, the slopes of the linear regression approached 0.5 (Fig. 4b).
Except for blanks, other effects such as the IRMS precision could be responsible for the results of measured δ15N-NO3− values (Lachouani et al. 2010). However, the excellent correlation of standard and measured δ15N demonstrated the methods to be robust and good quantitative techniques for measurement of δ15N-NO3− in the environmental samples.
δ15N-NO3 − precision and accuracy
To evaluate the accuracy and precision of both methods of δ15N isotopic analyses, we analyzed laboratory and international δ15N-NO3− standards with each batch of submitted samples. Results showed that both methods presented accurate δ15N-NO3− values defined by the international and in-lab standard numbers (Table 2). The δ15N-NO3− standard deviations for the cadmium–sodium azide and the VCl3–sodium azide methods were 0.34 and 0.33‰, respectively, which is excellent. Standards are important not only to assess the precision and accuracy of δ15N-NO3− compositions and subsequent concentrations but also to determine the sample concentration limit of detection (LOD).
Throughout the course of this study, the blank NO3− concentration was 0.76 μmol L−1, obtained by the VCl3–sodium azide method. The LOD was determined by taking 10 times the NO3− concentrations of the blank (e.g., 7.6 μmol L−1) in a 5 mL solution. The result produced an adequate accuracy and precision in the determination of the natural δ15N-NO3−N from NOx emissions from coal-fired power plants and motor vehicles, and low concentrations of gaseous NO2 and HNO3 (Table 3). The LOD value was much lower to that given by Lachouani et al. (2010).
The LOD associated with the cadmium–sodium azide method generated a PT-IRMS baseline signal intensity of m/z 44 corresponding to a concentration of 0.82 μmol L−1. The accuracy and precision are acceptable when measuring the natural δ15N-NO3− in surface water and soil extract samples and testing a LOD of 8.2 μmol L−1 through standards (Table 3).
Characterizations of δ15N-NO3 − values in different samples
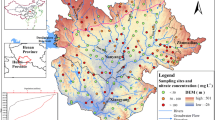
Analytical results showed that the δ15N-NO3− values of river water samples ranged from − 1.4 to 14.4‰, with a mean of 8.9 ± 3.3‰ (Fig. 5). This is comparable to the range of water δ15N-NO3− values previously published in this region (Chen et al. 2012). However, our values were much higher relative to typical δ15N-NO3− values taken at watershed outlets in predominantly forested land (Mayer et al. 2002). The variability in the δ15N values of riverine NO3− could be sufficiently explained by mixing of NO3− from various sources. For example, river water originating from manure and sewage is much more enriched in 15N relative to other N sources.
Soil extracts present an NO3− isotopic composition varying from − 4.3 to 8.6‰ (mean of 3.5 ± 3.5‰), which overlaps the range of typical soil δ15N values provided by Xue et al. (2009). The rate of mineralization and nitrification, soil depth, vegetation, climate, and site history may also affect the δ15N composition in soil (Kendall 1998; Mayer et al. 2002).
The analyzed δ15N-NO3− composition of summer rain water varied from − 4.6 to 9.5‰, with a mean of 3.3 ± 2.1‰ (Fig. 5). These values fall well within the range of previously reported values for atmospheric NO3− (Hastings et al. 2013; Elliott et al. 2007; Tobari et al. 2010; Russell et al. 1998), and are comparable to the results reported in southern China and in urban and suburban sites in the North China Plain (Fang et al. 2011). However, our results are higher than those for NO3− precipitates from two rural sites in the Hebei Province of northern China, and Japan, where most values of δ15N-NO3− in rain water were negative(Fukuzaki and Hayasaka 2009). The higher values could result from higher contributions of NO3−-N derived from vehicle exhausts and coal-fired boilers. For example, 33 sites across the mid-western and northeastern USA yielded δ15N composition of wet NO3− depositions that ranged from − 8.1 to 3.2‰ with a mean value of − 1.5‰, and the highest δ15N values in NO3− close to major stationary emission sources (Elliott et al. 2007).
The δ15N-HNO3 values of representative emission sources are summarized in Fig. 5. Samples collected from coal-fired power plants yielded a mean δ15N-NO3− value of 20.3 ± 4.3‰ with concentrations of HNO3 ranging from 60 to 80 mg m−3, close to the mean value of coal fire reported by Felix et al. (2012). The δ15N-NO3− values of vehicular emissions collected in this study ranged from 0.4 to 9.4‰, with a mean of 5.7 ± 3.6‰. The range and standard deviation of δ15N-NO3− of the vehicle emissions in this study are lower than those published by Felix and Elliott (2014). The values are also higher than the values obtained from 26 gasoline and diesel-powered vehicles controlled by the NO2/NOx molar ratios (Walters et al. 2015)
Gaseous HNO3 measurements produced concentrations with a range of 1.71–16.07 μg N m−3 during the summer. The δ15N-HNO3 values varied from 2.2 to 10.8‰, with a mean value of 5.9 ± 2.4‰, higher than that of other studies. For example, in Ohio, New York, and Pennsylvania, 96 measurements of gaseous δ15N-HNO3 ranged from − 4.9 to 10.8‰ with a mean of 3.2‰ (Elliott et al. 2009). In addition, a study on variation of gaseous δ15N-NO3− values at Akita in Japan was even lower than 0‰ (Kawashima 2014). Concentrations of HNO3 are also correlated with stationary source emissions, suggesting that the spatial distribution of stationary sources is a primary control on HNO3 formation and associated δ15N-HNO3 values (Elliott et al. 2009).
Isotopes as tracers of anthropogenic NO3 −-N sources and impacts
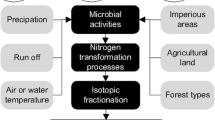
Excess N causes environmental pollution and threatens agricultural productivity, food security, ecosystem and human health, and economic prosperity (Zhang et al. 2015). The isotopic composition of NO3− is not only a powerful tool to determine its origin but can also provide clues about the N transformation processes such as NH3 volatilization and denitrification (Mayer et al. 2002). Furthermore, stable isotopes contained in reactive N compounds are providing new insights into the environmental N cascade, since N sources present different stable isotope signatures that can be used to assess the N flow (Hastings et al. 2013).
Isotopic studies have helped determine the source, origin, and cycle of N. The δ15N-NO3− composition confirmed that the source of NO3− deposited across the northeastern USA came principally from power plant NOx emissions (Elliott et al. 2007; Felix et al. 2012). The isotopic composition of NO3−, combined with Δ17NO3 and δ18NO3 values, established the main terrestrial sources of NO3− to be the sewage and manure effluents in the upstream areas of the Yellow River in China (Liu et al. 2013).
The sources of N carry distinct stable isotope signatures. The δ15N-NO3− analysis of water and air samples in this study showed that the measured δ15N-NO3− values of coal-fired power plants were much higher than those other power plants (Fig. 5). The δ15N-NO3− data demonstrated that NOx emitted from motor vehicles and soil extracts has relatively low δ15N values. Although various processes such as fractionation and seasonality of oxidation pathways can influence the isotopic values of N compounds, the range of δ15N-NO3− values coming from motor vehicle samples was close to that of gaseous HNO3, and thus, motor vehicle emissions can be considered the major source of gaseous HNO3.
Conclusions
The cadmium–sodium azide and the VCl3–sodium azide chemical methods were successfully used to determine the δ15N-NO3− composition of samples with low N concentration, small N volume, and complex absorbing solutions based on the conversion of NO3− into N2O, analyzed by PT-IRMS. The good linear correlation of measured δ15N values between standard and sample demonstrated the soundness of both chemical methods and of the quantitative measurement technique for δ15N-NO3−. The low standard deviations provided by the δ15N-NO3− values indicated excellent precision. The sample concentration LOD obtained by the VCl3–sodium azide and cadmium–sodium azide methods were 7.6 and 8.2 μmol L−1, respectively.
Results indicated that river water samples collected in this study showed a δ15N-NO3− value from − 1.4 to 14.4‰ with a mean of 8.9 ± 3.3‰. The δ15N-NO3 of soil extracts varied from − 4.3 to 8.6‰, with a mean of 3.5 ± 3.5‰. The analyzed δ15N-NO3− values in summer rain water samples varied from − 4.6 to 9.5‰. The δ15N values of HNO3 sampled from coal-fired power plants display a mean value of 20.3 ± 4.3‰, and δ15N-NO3− values of motor vehicle emissions collected in a tunnel ranged from 0.4 to 9.4‰ with a mean of 5.7 ± 3.6‰. Measurements of gaseous HNO3 yielded δ15N-NO3− values ranging from 2.2 to 10.8‰, with a mean of 5.9 ± 2.4‰.
In summary, the NOx emitted from motor vehicles and soil extracts exhibited relatively low δ15N values, whereas the vehicle values were close to those of gaseous HNO3. The isotopic signatures presented in this study can be used to identify and trace the transport of N.
References
Adon, M., Galy-Lacaux, C., Yoboue, V., Delon, C., Lacaux, J. P., Castera, P., et al. (2010). Long term measurements of sulfur dioxide, nitrogen dioxide, ammonia, nitric acid and ozone in Africa using passive samplers. Atmospheric Chemistry and Physics, 10(15), 7467–7487. https://doi.org/10.5194/acp-10-7467-2010.
Braman, R. S., & Hendrix, S. A. (1989). Nanogram nitrite and nitrate determination in environmental and biological-materials by vanadium(Iii) reduction with chemi-luminescence detection. Analytical Chemistry, 61(24), 2715–2718. https://doi.org/10.1021/ac00199a007.
Casciotti, K. L., Sigman, D. M., Hastings, M. G., Bohlke, J. K., & Hilkert, A. (2002). Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Analytical Chemistry, 74(19), 4905–4912. https://doi.org/10.1021/ac020113w.
Chen, Z. X., Liu, G., Liu, W. G., Lam, M. H. W., Liu, G. J., & Yin, X. B. (2012). Identification of nitrate sources in Taihu Lake and its major inflow rivers in China, using δ15N-NO3 − and δ18O-NO3 − values. Water Science and Technology, 66(3), 536–542. https://doi.org/10.2166/wst.2012.193.
Elliott, E. M., Kendall, C., Boyer, E. W., Burns, D. A., Lear, G. G., Golden, H. E., et al. (2009). Dual nitrate isotopes in dry deposition: Utility for partitioning NOx source contributions to landscape nitrogen deposition. Journal of Geophysical Research-Biogeosciences, 114, doi:Artn G04020https://doi.org/10.1029/2008jg000889.
Elliott, E. M., Kendall, C., Wankel, S. D., Burns, D. A., Boyer, E. W., Harlin, K., Bain, D. J., & Butler, T. J. (2007). Nitrogen isotopes as indicators of NOx source contributions to atmospheric nitrate deposition across the Midwestern and northeastern United States. Environmental Science & Technology, 41(22), 7661–7667. https://doi.org/10.1021/es070898t.
Erisman, J. W., Galloway, J. N., Seitzinger, S., Bleeker, A., Dise, N. B., Petrescu, A. M. R., Leach, A. M., & de Vries, W. (2013). Consequences of human modification of the global nitrogen cycle. Philosophical Transactions of the Royal Society B-Biological Sciences, 368(1621), 20130116. https://doi.org/10.1098/rstb.2013.0116.
Fang, Y. T., Koba, K., Wang, X. M., Wen, D. Z., Li, J., Takebayashi, Y., Liu, X. Y., & Yoh, M. (2011). Anthropogenic imprints on nitrogen and oxygen isotopic composition of precipitation nitrate in a nitrogen-polluted city in southern China. Atmospheric Chemistry and Physics, 11(3), 1313–1325. https://doi.org/10.5194/acp-11-1313-2011.
Felix, J. D., & Elliott, E. M. (2014). Isotopic composition of passively collected nitrogen dioxide emissions: Vehicle, soil and livestock source signatures. Atmospheric Environment, 92, 359–366. https://doi.org/10.1016/j.atmosenv.2014.04.005.
Felix, J. D., Elliott, E. M., & Shaw, S. L. (2012). Nitrogen isotopic composition of coal-fired power plant NOx: Influence of emission controls and implications for global emission inventories. Environmental Science & Technology, 46(6), 3528–3535. https://doi.org/10.1021/es203355v.
Freyer, H. D. (1978). Seasonal trends of NH4 + and NO3- nitrogen isotope composition in rain collected at Julich, Germany. Tellus, 30(1), 83–92. https://doi.org/10.1111/j.2153-3490.1978.tb00820.x.
Fukuzaki, N., & Hayasaka, H. (2009). Seasonal variations of nitrogen isotopic ratios of ammonium and nitrate in precipitations collected in the Yahiko-Kakuda mountains area in Niigata prefecture, Japan. Water Air and Soil Pollution, 203(1–4), 391–397. https://doi.org/10.1007/s11270-009-0026-8.
Hastings, M. G., Casciotti, K. L., & Elliott, E. M. (2013). Stable isotopes as tracers of anthropogenic nitrogen sources, deposition, and impacts. Elements, 9(5), 339–344. https://doi.org/10.2113/gselements.9.5.339.
Kawashima, H. (2014). Formation mechanism and source apportionment of ammonium and nitrate ions in aerosol using nitrogen isotopes. Earozoru Kenkyu, 29(s1), 110–116.
Kendall, C. (1998). Tracing sources and cycling of nitrate in catchments. In C. Kendall & J. J. McDonnell (Eds.), Isotope tracers in catchment hydrology (pp. 519–576). Amsterdam: Elsevier.
Kendall, C., Elliott, E. M., & Wankel, S. D. (2007). Tracing anthropogenic inputs of nitrogen to ecosystems. Stable Isotopes in Ecology and Environmental Science, 2nd Edition, 375–449, doi:DOI https://doi.org/10.1002/9780470691854.ch12.
Lachouani, P., Frank, A. H., & Wanek, W. (2010). A suite of sensitive chemical methods to determine the δ15N of ammonium, nitrate and total dissolved N in soil extracts. Rapid Communications in Mass Spectrometry, 24(24), 3615–3623. https://doi.org/10.1002/rcm.4798.
Liu, T., Wang, F., Michalski, G., Xia, X. H., & Liu, S. D. (2013). Using 15N, 17O, and 18O to determine nitrate sources in the Yellow River, China. Environmental Science & Technology, 47(23), 13412–13421. https://doi.org/10.1021/es403357m.
Mayer, B., Boyer, E. W., Goodale, C., Jaworski, N. A., Van Breemen, N., Howarth, R. W., et al. (2002). Sources of nitrate in rivers draining sixteen watersheds in the northeastern US: Isotopic constraints. Biogeochemistry, 57(1), 171–197. https://doi.org/10.1023/A:1015744002496.
McIlvin, M. R., & Altabet, M. A. (2005). Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Analytical Chemistry, 77(17), 5589–5595. https://doi.org/10.1021/ac050528s.
Michalski, G., Meixner, T., Fenn, M., Hernandez, L., Sirulnik, A., Allen, E., & Thiemens, M. (2004). Tracing atmospheric nitrate deposition in a complex semiarid ecosystem using Δ17O. Environmental Science & Technology, 38(7), 2175–2181. https://doi.org/10.1021/es034980+.
Rock, L., & Ellen, B. H. (2007). Nitrogen-15 and oxygen-18 natural abundance of potassium chloride extractable soil nitrate using the denitrifier method. Soil Science Society of America Journal, 71(2), 355–361. https://doi.org/10.2136/sssaj2006.0266.
Russell, K. M., Galloway, J. N., Macko, S. A., Moody, J. L., & Scudlark, J. R. (1998). Sources of nitrogen in wet deposition to the Chesapeake Bay region. Atmospheric Environmental, 32(14–15), 2453–2465. https://doi.org/10.1016/S1352-2310(98)00044-2.
Schilman, B., & Teplyakov, N. (2007). Detailed protocol for nitrate chemical reduction to nitrous oxide for δ15N and δ18O analysis of nitrate in fresh and marine waters. Annual Report Submitted to the Earth Science Research Administration, Ministry of National Infrastructures Jerusalem, December 2007.
Sigman, D. M., Casciotti, K. L., Andreani, M., Barford, C., Galanter, M., & Bohlke, J. K. (2001). A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Analytical Chemistry, 73(17), 4145–4153. https://doi.org/10.1021/ac010088e.
Silva, S. R., Kendall, C., Wilkison, D. H., Ziegler, A. C., Chang, C. C. Y., & Avanzino, R. J. (2000). A new method for collection of nitrate from fresh water and the analysis of nitrogen and oxygen isotope ratios. J Hydrology, 228(1–2), 22–36. https://doi.org/10.1016/S0022-1694(99)00205-X.
Stedman, G. (1959a). Mechanism of the Azide-Nitrite Reaction .1. Journal of the Chemical Society(Sep-O), 2943–2949, doi:DOI https://doi.org/10.1039/jr9590002943.
Stedman, G. (1959b). Mechanism of the Azide-Nitrite Reaction .2. Journal of the Chemical Society(Sep-O), 2949–2954, doi:DOI https://doi.org/10.1039/jr9590002949.
Tobari, Y., Koba, K., Fukushima, K., Tokuchi, N., Ohte, N., Tateno, R., Toyoda, S., Yoshioka, T., & Yoshida, N. (2010). Contribution of atmospheric nitrate to stream-water nitrate in Japanese coniferous forests revealed by the oxygen isotope ratio of nitrate. Rapid Communications in Mass Spectrometry, 24(9), 1281–1286. https://doi.org/10.1002/rcm.4498.
Tsunogai, U., Kido, T., Hirota, A., Ohkubo, S. B., Komatsu, D. D., & Nakagawa, F. (2008). Sensitive determinations of stable nitrogen isotopic composition of organic nitrogen through chemical conversion into N2O. Rapid Communications in Mass Spectrometry, 22(3), 345–354. https://doi.org/10.1002/rcm.3368.
Walters, W. W., Goodwin, S. R., & Michalski, G. (2015). Nitrogen stable isotope composition (δ15N) of vehicle-emitted NOx. Environmental Science & Technology, 49(4), 2278–2285. https://doi.org/10.1021/es505580v.
Xue, D. M., Botte, J., De Baets, B., Accoe, F., Nestler, A., Taylor, P., et al. (2009). Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater. Water Research, 43(5), 1159–1170. https://doi.org/10.1016/j.watres.2008.12.048.
Xue, D. M., De Baets, B., Botte, J., Vermeulen, J., Van Cleemput, O., & Boeckx, P. (2010). Comparison of the silver nitrate and bacterial denitrification methods for the determination of nitrogen and oxygen isotope ratios of nitrate in surface water. Rapid Communications in Mass Spectrometry, 24(6), 833–840. https://doi.org/10.1002/rcm.4445.
Zhang, X., Davidson, E. A., Mauzerall, D. L., Searchinger, T. D., Dumas, P., & Shen, Y. (2015). Managing nitrogen for sustainable development. Nature, 528(7580), 51–59. https://doi.org/10.1038/nature15743.
Acknowledgements
This study was supported by the Key Research Program of the Chinese Academy of Sciences (Grant No. ZDRW-ZS-2016-5-1), the Natural Science Foundation of Jiangsu Province (Grant No. BK20131047), and the National Natural Science Foundation of China (Grant No. 41301578).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ti, C., Wang, X. & Yan, X. Determining δ15N-NO3− values in soil, water, and air samples by chemical methods. Environ Monit Assess 190, 341 (2018). https://doi.org/10.1007/s10661-018-6712-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6712-5