Abstract
Research has shown linkages between environmental exposures and population health metrics such as low birth weight and incidence of congenital anomalies. While the exact causal relationship between specific environmental teratogens and suspected corresponding congenital anomalies has largely not been established, spatial analysis of anomaly incidence can identify potential locations of increased risk. This study uses the Vital Statistics Birth Master File to map and analyze the rates of congenital anomalies of births from non-smoking mothers 15–35 years old within Los Angeles County. Hot spot analysis shows that the distribution of congenital anomalies is not randomly distributed throughout the county and identified the Antelope Valley and San Gabriel Foothills as two areas with elevated incidence rates. These results are not explained by potential confounders such as maternal age, race, smoking status, or socioeconomic status and seem to correlate well with the concentration of atmospheric ozone. This approach demonstrates the value of using spatial techniques to inform future research efforts and the need to establish and maintain a comprehensive reproductive health surveillance system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital anomalies are a heterogeneous group of birth outcomes that have a significant impact on children, families, and the public health of communities. In the USA, approximately 3% of all newborns are affected by birth defects (CDC 2008). While some birth anomalies are directly related to an identifiable genetic mutation or syndrome, data increasingly demonstrate potential environmental and epigenetic effects related to a wide range of environmental exposures. A growing number of studies have identified linkages between various congenital anomalies and environmental exposures, particularly between air pollutants and temperature in both animal and human models (Agay-Shay et al. 2013; Becerra et al. 2013; Ghosh et al. 2012; Ponce et al. 2005; Ritz et al. 2007; Ritz 2009). The relationship between the environment and reproductive health is an area of interest for scientists, public health experts, and policy makers alike. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine (ASRM) issued a Joint Committee Opinion in 2013 titled “Exposure to Toxic Environmental Agents.” The report acknowledged evidence that environmental toxins are ubiquitous, and that these toxins can negatively affect health from preconception through adult life (ACOG Committee 2013; Bergman et al. 2012).
Research on congenital anomalies and their associations with environmental exposures presents many challenges. First, congenital anomalies are a diverse set of conditions. Among the most common anomalies are cleft lip and palate, neural tube defects, chromosomal abnormalities, abdominal wall defects, hypospadias, and cardiac defects. Second, congenital anomalies are rare events and obtaining high enough statistical power to evaluate patterns for a specific condition is difficult. Thirdly, even when researchers establish an association between an environmental exposure and birth outcome, establishing causation and interaction with other confounding exposures is challenging. Furthermore, the quality of birth defect tracking data is variable, relying on medical documentation by health care professionals and their delegates. These challenges, however, are not insurmountable. Innovations in technology and spatial epidemiological research methods provide new opportunities to assess whether environmental conditions increase the rare chance that a neonate will be born with a congenital anomaly.
With over 100,000 births per year across 88 cities in both highly diverse neighborhoods and homogenous ethnic enclaves, Los Angeles County provides a unique opportunity to study complex political and economic structures. Moreover, Los Angeles County has a varied physical geography including a busy industrial port in the south, urban centers, suburban valleys with adjacent foothills, and low mountains just south of a high desert plain in the northernmost part of the county.
The topographically, racially, and economically diverse regions in the county have varying levels of air quality, water quality, and exposures to potential teratogens. Prior research in Los Angeles County has demonstrated that air quality and proximity to high-traffic areas increase the risk of prematurity and low birth weight (Wu et al. 2009) as well as childhood asthma and respiratory problems (McConnell et al. 2010; Pastor Jr. et al. 2005). However, at this time, public health resources to continue the work of understanding the effect of the environment on children are very limited. Reproductive health surveillance data are slim and not consistently maintained in Los Angeles County.
To guide further investigation into relationships between environmental exposures and congenital anomalies, this study uses the California Department of Public Health Birth Statistical Master Files, which contain data collected on each newborn at the time of newborn hospital discharge, as a means to identify spatial patterns of congenital anomalies. In the absence of a more robust reproductive health surveillance system, this database represents the single most comprehensive data available for birth outcomes in Los Angeles County. Using these data and spatial statistical methods, this study aims to identify hot spots, areas with disproportionately high concentrations of abnormal births as compared to neighboring regions. If the spatial distribution of these anomalies is significant, especially after controlling for other known risk factors such as maternal age and smoking, it will guide further environmental exposure research.
Methods
For this study, a hot spot analysis was performed on birth records from the Vital Statistics Birth Master File for Los Angeles County from 2006 to 2010. The database contains detailed demographic information related to the child, mother, and father, as well as medical data related to the birth of every newborn infant as recorded on the birth certificate. Birth outcome data are extracted from the medical record, as documented by the health care providers of both the mother and infant. UCLA IRB and Cal Protects IRB approval was obtained with a waiver for informed consent given the minimal risk nature of the study. Data from the Vital Statistics Birth Master File for this study included maternal, paternal, and child names, dates of birth, maternal home address, medical record number, and genetic and non-genetic birth anomalies, as listed in Table 1.
To control for the well-established association between advanced maternal age and chromosomal abnormalities (Hook 1981; Hook et al. 1983; Schreinemachers et al. 1982), a subgroup analysis of geocoded births from mothers aged 15–35 years was conducted. Mothers who reported smoking were also excluded since smoking is an established risk factor for birth defects (Hackshaw et al. 2011). Only those births that had a complete maternal residence address associated with the birth certificate data were evaluated. In the end, approximately 5% of births were not geocoded for this reason. This percentage was similar across the county.
Birth records were geocoded by maternal home address and aggregated to US Census Bureau public use microareas (PUMAs). PUMAs are created by state census agencies using census tracts as building blocks. Within Los Angeles County, there are 69 PUMAs with an average area of 58 mi2 and an average population of 142,299 residents according to the 2010 census. These PUMAs have 100,000–500,000 residents and provide the advantage of having relatively equal population bases.
The rate of congenital anomalies was calculated for each PUMA by comparing the number of anomalous births to total births. These rates were then analyzed using the hot spot analysis tool within ArcGIS 10.3 mapping software. The weight matrix for this analysis was defined by the polygon contiguity edges and corner rule. This parameter constrains the calculation of each polygon’s Getis-Ord Gi* statistic to only its first-order neighbors, with all the outlying polygons having no influence. An additional cluster analysis was also conducted to corroborate the hot spot analysis result. The cluster analysis tool within ArcGIS 10.3 calculates Anselin’s Local Moran’s I as a measure of spatial autocorrelation for each feature and its associated neighborhood.
Cumulative incidence rates of congenital anomalies for all major racial/ethnic groups residing both inside and outside of the hot spots, as well as odds ratios between these groups, were calculated using MedCalc statistical software. These analyses were performed to understand if ethno-cultural differences in maternal behavior or higher concentrations of populations with similar genetic backgrounds might be driving the results.
Results
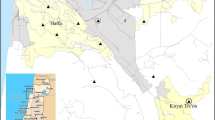
From 2006 through 2010, there were 436,218 live births of non-smoking mothers aged 15–35 years, of which 462 newborns were confirmed to have a congenital anomaly as listed on birth certificates. This number represents approximately 1.06% of all births from mothers meeting the inclusion criteria. Statistically significant hot spots were identified in the northernmost part of the county in the Antelope Valley (Fig. 1 and Table 2). Hot spots with a lower degree of confidence were present along the foothills in the more affluent areas of Pasadena, the San Gabriel Valley, and the Santa Clarita Valley. Geographically, these areas are a group of foothills and low mountains, bordered on the north by an elevated high desert plateau within the western tip of the Mojave desert. In terms of land use, this geography consists of dense suburbs in the foothills with rural and industrial land to the north. The analysis also identified statistically significant cold spots in parts of the Central and South Los Angeles regions, which are composed of densely populated working class communities alongside manufacturing and industrial centers.
The mapped results of the cluster analysis were similar to the hot spot analysis. A high-high cluster was identified in the same area as 99% confidence interval hot spots with one additional PUMA (Baldwin Park, Azusa, Duarte, and Irwindale cities) included in the cluster. A low-low cluster was identified in the same areas as the cold spots at all confidence intervals with three additional PUMAs (Huntington Park City, Florence-Graham, and Walnut Park; LA City (East Central/Silver Lake, Echo Park and Westlake); LA City (East Central/Silver Lake, Echo Park and Westlake)) included in the cluster. One high-low outlier PUMA (LA City (Southwest/Marina del Rey and Westchester) and Culver City Cities)) and one low-high outlier PUMA (Arcadia, San Gabriel, and Temple City Cities) were also identified (Appendix A).
Congenital anomaly incidence rates were consistently elevated for mothers of all major ethnic groups except for those classified as two or more races, or part of small ethnic populations (e.g., American Indian/Alaskan native, other, or not stated on the birth record) (Table 3). Combined, these groups represented only 3% of all births in Los Angeles County and about 4% of the births in the identified hot spots. Overall, newborns of mothers residing in hot spots were almost three times as likely to be born with a congenital anomaly when compared to newborns whose mothers resided outside of the hot spots.
To evaluate the impact of potential confounding factors, the congenital anomaly hot spot analysis was compared with the distribution of land use and median household income. No association was found between these factors and the hot spots (Appendices B and C). Additionally, to evaluate if hospital reporting was primarily responsible for elevated rates of congenital anomalies in the hot spots of the northern region of the county, we compared the rates of congenital anomalies for all mothers living within the region by their birth hospital. Results indicate that mothers who delivered at Antelope Valley Hospital (the only hospital in the northernmost region of the county) had higher rates of anomalies than those who delivered elsewhere. Mothers residing in that area who delivered elsewhere, however, still had higher rates of anomalies than the county average (Appendix D). Finally, because some of the hot spots are located on the boundary of Los Angeles County, specifically those in the northern part of the county, there is the question of possible edge effects from surrounding counties. However, the PUMAs in this area (Lancaster City, Palmdale City, and Castaic) have the first, second, and sixth highest rates of congenital anomalies in the entire county, respectively. As such, it is highly likely that these contiguous areas would remain designated as hot spots even if the surrounding counties were included in the analysis.
Discussion
The finding of hot spots in the Antelope Valley and surrounding foothills in the northernmost parts of Los Angeles County suggests that the incidence of congenital anomalies is not spatially random. Increased risk observed among all four of the largest ethnic groups residing in the hot spots also suggests that the results are associated more with the environmental conditions within the hot spots than differing racial and socioeconomic factors.
This study does not have the power to suggest potential causal relationships to specific environmental exposures. What these data do suggest, however, is that where a mother lives is associated with her risk of having a child born with a congenital anomaly. More research is needed to match specific exposures with particular anomalies.
This analysis detects clusters of congenital abnormalities in Los Angeles County corresponding to new areas of heightened environmental concern. Prior research has shown associations between environmental toxins, primarily air pollution, and poor birth outcomes such as low birth weight and preeclampsia in the Port of Los Angeles area, which is located in the southwest corner of the county (Wu et al. 2009; Moretti and Neidell 2011). Given those findings, the authors of this article suspected that the proximal area around the Port of Los Angeles would have increased levels of congenital anomalies as well. The results from this study, however, do not show a similar association. While bias and underreporting may factor in, the results from this study point to a different pattern for congenital anomalies.
An interesting finding, and plausible explanation for the distribution of anomalies, may be correlation with other air pollutants. In particular, elevated levels of ozone seem to correlate with the pattern observed. During the late spring, summer, and early fall months, elevated ozone is common in the Los Angeles area. On high ozone days, ozone is produced from industrial, commercial, residential, and transportation emission sources. These emission sources are distributed broadly over Los Angeles County, but more concentrated in the coastal and downtown areas. Because photochemical production of ozone requires several hours, and because high ozone almost always occurs on days with a persistent sea breeze (a weather pattern in Los Angeles that is associated with low mixing heights, intense sunlight, and reasonably elevated temperatures), the highest ozone levels accumulate in the inland valleys, foothills, and mountain areas downwind of the central city. Figure 2 shows the average number of days exceeding the current federal National Ambient Air Quality standard for ozone (8 h at 75 ppb) during 2005–2009 at monitors located in the study area (SCAQMD 2013). All of the hot spot areas have high ozone levels, while all but two of the cold spots and areas without significance had markedly fewer days exceeding the ozone standard. Prior studies have linked ozone and birth defects (Ritz et al. 2002), but more epidemiologic studies and research are needed in this area to further determine what factors might be driving these associations.
Temperature is a potential confounder worthy of examination. Prior studies have also linked birth defects with elevated core body temperature from fevers and external causes such as hot tubs (Moretti et al. 2005), although not with ambient temperature. Day-to-day variations in ozone concentrations in Los Angeles County are strongly correlated to daytime temperatures during the spring, summer, and fall (Lin et al. 2001). However, the ozone concentration gradients in Los Angeles County are steeper than the temperature gradients, especially in the cities on the coastal plain lying between the San Gabriel Mountains and the coast in the southern half of the county (Fig. 2). The overall ozone distribution pattern is largely controlled by the timescales of the photochemistry that forms ozone, combined with dominant wind direction, transport time, and the spatial distribution of emissions of ozone precursors. Therefore, areas farther from the foothills can be nearly as hot but have much lower ozone.
Limitations
The incidence of congenital anomalies identified in the current study is much lower than the expected 3% from the literature. In this study, overall rates were closer to 1%. This discrepancy is most likely related to reporting based only on hospital discharge data, which may not capture anomalies that were diagnosed later in infancy or childhood. Lower congenital anomaly rates in certain areas may also be associated with hospital underreporting. This study attempted to account for this limitation by analyzing data from specific hospitals used by mothers within hot spots. As there is no comprehensive birth defect registry in Los Angeles County, vital statistic records are the most practical way to identify congenital anomalies. Given this potential for underreporting, more information is needed for a complete understanding of which risks exist and which approaches work best to ameliorate them.
Although women provided their primary addresses, the duration of residence at a particular address is not ascertainable from the birth certificate data. Therefore, it cannot be determined where a woman was living at the time of conception and early pregnancy. The most likely time for potential impact of environmental exposure on congenital anomalies is in the early first trimester of pregnancy as this is the critical window for organ development. Yet, prior studies on maternal mobility in New York and Texas suggest that misclassification due to maternal mobility is low. This finding is likely because most women who move do not move great distances within a region during their pregnancy (Bell and Belanger 2012; Chen et al. 2009; Lupo et al. 2010). It is also important to mention that where a mother resides does not capture where she works and visits during her pregnancy, and subsequently all the possible exposures with which she may come into contact. However, in the absence of birth surveillance data that tracks a mother’s movement, aggregating births to large PUMAs accounts for this limitation in the data to some extent.
Another limitation in interpretation of the results of this study is the bias associated with the modifiable areal unit problem (MAUP). The MAUP refers to the bias that is introduced when point data are aggregated together and is a concern with any choice of geographic unit of analysis. While the PUMA boundaries are not randomly created, they do have desirable characteristics that mitigate some of the drawbacks and led to their selection as a unit of analysis. First, PUMAs have relatively equal population bases. Census tracts are aggregated together using a specific protocol so that the resulting area contains between 100,000 and 500,000 residents. In this way, each of the units of analysis contains roughly the same denominator to calculate birth anomaly incidence. Second, PUMAs contain a larger population than other common geographic units such as census tracts or zip codes. A larger population within each unit of analysis was preferred due to the low incident rate of congenital anomalies among neonates.
Additionally, this study limited the analyses to infants of non-smoking mothers. These smoking data rely solely on self-report and medical record and does not capture the effects of secondhand smoke or mothers who chose not to disclose their smoking behaviors.
Conclusions
From a public health and public policy perspective, several factors deserve consideration. Policy changes are needed to incorporate environmental risk in screening programs. Ongoing studies of the relationship between geography and risk of congenital abnormalities could allow public health interventions, even if the exact cause of the increased risk remains undiscovered. An example of a simple policy change is to push for insurance coverage of early prenatal screening for congenital anomalies, by ultrasound or non-invasive prenatal screening (prenatal cell-free DNA screening), in all women living in high-risk areas, regardless of age or health history.
Additionally, novel approaches must be adopted to understand the causal relationships between congenital anomalies and environmental toxins. Congenital anomalies themselves are comprised of heterogeneous groups of rare and unrelated conditions. More research is needed to classify types of anomalies and understand the potential causes of their development. The potential set of environmental toxins is very large. Potential areas for future research include determining more specific exposures of concern, routes of exposures, and populations that may be more susceptible to the effects of these exposures. With this information, researchers and epidemiologists will be better able to inform environmental, public health, and reproductive health policies.
Finally, it is important for public health activists and elected legislators to work together and commit to the maintenance of a comprehensive reproductive health surveillance system. Data infrastructure is essential for future research regarding environmental effects on reproductive health outcomes. One of the greatest challenges of this study was finding the best way to identify both exposures as well as anomalies. The California Birth Defects Monitoring Program, which is no longer available in Los Angeles County, serves as a substantive example of an important data set vulnerable to shifts in political priorities. Without such data, researchers are required to expend much more energy and resources to capture limited and incomplete information (Croen et al. 1991; Ritz et al. 2007). This study aims to reveal whether the use of spatial methods limited to existing health records might be a viable substitute to a more robust birth outcome surveillance system; however, a more robust surveillance system should be a public health priority. This study also highlights that regional environmental conditions, and not merely local situations, can impact reproductive health. By highlighting these regional environmental threats, scientists, public health experts, and policy makers can collaborate to mitigate hidden hazards to the health of the community at large.
References
ACOG (American College of Obstetricians and Gynecologists) Committee. (2013). Exposure to toxic environmental agents. Fertility and Sterility, 100(4), 931–934. https://doi.org/10.1016/j.fertnstert.2013.08.043.
Agay-Shay, K., Friger, M., Linn, S., Peled, A., Amital, Y., & Peretz, C. (2013). Ambient temperature and congenital heart defects. Human Reproduction, 28(8), 2289–2297. https://doi.org/10.1093/humrep/det244.
Becerra, T. A., Wilhelm, M., Olsen, J., Cockburn, M., & Ritz, B. (2013). Ambient air pollution and autism in Los Angeles County, California. Environmental Health Perspectives, 121(3), 380–386. https://doi.org/10.1289/ehp.1205827.
Bell, M., & Belanger, K. (2012). Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environment exposures. Journal of Exposure Science & Environment Epidemiology, 22(5), 429–438. https://doi.org/10.1038/jes.2012.42.
Bergman, A., Heindel, J.J., Jobling, S., Kidd, K.A., Zoeller, R.T., & Jobling, S.K. (2012). State of the science of endocrine disrupting chemicals—2012. United Nations Environment Programme and World Health Organization. http://www.who.int/ceh/publications/endocrine/en/. Accessed 30 January 2017.
CDC (Centers for Disease Control and Prevention). (2008). Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978–2005. Morbidity and Mortality Weekly Report, 57(01), 1–5 https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5701a2.htm. Accessed 6 March 2017.
Chen, L., Bell, E. M., Caton, A. R., Druschel, C. M., & Lin, S. (2009). Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environmental Research, 110(2), 162–168. https://doi.org/10.1016/j.envres.2009.11.001.
Croen, L. A., Shaw, G. M., Jensvold, N. G., & Harris, J. A. (1991). Birth defects monitoring in California: a resource for epidemiological research. Paediatric and Perinatal Epidemiology, 5(4), 423–427. https://doi.org/10.1111/j.1365-3016.1991.tb00728.x.
Ghosh, J. K. C., Wilhelm, M., Su, J., Goldberg, D., Cockburn, M., Jerrett, M., & Ritz, B. (2012). Assessing the influence of traffic related air pollution on risk of term low birth weight on the basis of land-use-based regression models and measures of air toxics. American Journal of Epidemiology, 175(12), 1262–1274. https://doi.org/10.1093/aje/kwr469.
Hackshaw, A., Rodeck, C., & Boniface, S. (2011). Maternal smoking in pregnancy and birth defects: a systematic review based on 173,687 malformed cases and 11.7 million controls. Human Reproductive Update, 17(5), 589–604. https://doi.org/10.1093/humupd/dmr022.
Hook, E. B. (1981). Rates of chromosome abnormalities at different maternal ages. Obstetrics & Gynecology, 58(3), 282–285.
Hook, E. B., Cross, P. K., & Schreinemachers, D. M. (1983). Chromosomal abnormality rates at amniocentesis and in live-born infants. Journal of the American Medical Association, 249(15), 2034–2038. https://doi.org/10.1001/jama.1983.03330390038028.
Lin, C. Y. C., Jacob, D. J., & Fiore, A. M. (2001). Trends in exceedances of the ozone air quality standard in the continental United States, 1980–1998. Atmospheric Environment, 35(19), 3217–3228. https://doi.org/10.1016/S1352-2310(01)00152-2.
Lupo, P. J., Symanski, E., Chan, W., Mitchell, L. E., Waller, D. K., Canfield, M. A., & Langlois, P. H. (2010). Differences in exposure assignment between conception and delivery: the impact of maternal mobility. Paediatric and Perinatal Epidemiology, 24(2), 200–208. https://doi.org/10.1111/j.1365-3016.2010.01096.x.
McConnell, R., Islam, T., Shankardass, K., Jerrett, M., Lurmann, F., Gilliland, F., Gauderman, J., Avol, E., Künzli, N., Yao, L., Peters, J., & Berhane, K. (2010). Childhood incident asthma and traffic-related air pollution at home and school. Environmental Health Perspectives, 118(7), 1021–1026. https://doi.org/10.1289/ehp.0901232.
Moretti, M. E., Bar-Oz, B., Fried, S., & Koren, G. (2005). Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology, 16(2), 216–219. https://doi.org/10.1097/01.ede.0000152903.55579.15.
Moretti, E., & Neidell, M. (2011). Pollution, health, and avoidance behavior: evidence from the ports of Los Angeles. Journal of Human Resources, 46, 176–202. https://doi.org/10.3368/jhr.46.1.154.
Pastor Jr., M., Morello-Frosch, R., & Sadd, J. L. (2005). The air is always cleaner on the other side: race, space, and air toxics exposures in California. Journal of Urban Affairs, 27(2), 127–148. https://doi.org/10.1111/j.0735-2166.2005.00228.x.
Ponce, N. A., Hoggatt, K. J., Wilhelm, M., & Ritz, B. (2005). Preterm birth: the interaction of traffic related air pollution with economic hardship in Los Angeles neighborhoods. American Journal of Epidemiology, 162(2), 140–148. https://doi.org/10.1093/aje/kwi173.
Ritz, B. (2009). Air pollution and congenital anomalies. Occupational Environmental Medicine, 67(4), 221–222. https://doi.org/10.1136/oem.2009.051201.
Ritz, B., Wilhelm, M., Hoggatt, K. J., & Ghosh, J. K. C. (2007). Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. American Journal of Epidemiology, 166(9), 1045–1052. https://doi.org/10.1093/aje/kwm181.
Ritz, B., Yu, F., Fruin, S., Chapa, G., Shaw, G. M., & Harris, J. A. (2002). Ambient air pollution and risk of birth defects in Southern California. American Journal of Epidemiology, 155(1), 17–25. https://doi.org/10.1093/aje/155.1.17.
SCAQMD (South Coast Air Quality Management District). (2013). AQMP Appendix II: current air quality. http://www.aqmd.gov/docs/default-source/clean-air-plans/air-quality-management-plans/2012-air-quality-management-plan/final-2012-aqmp-(february-2013)/appendix-ii-final-2012.pdf. Accessed 30 January 2017.
Schreinemachers, D. M., Cross, P. K., & Hook, E. B. (1982). Rates of trisomies 21, 18, 13 and other chromosome abnormalities in about 20 000 prenatal studies compared with estimated rates in live births. Human Genetics, 61(4), 318–324. https://doi.org/10.1007/BF00276595.
Wu, J., Ren, C., Delfino, R. J., Chung, J., Wilhelm, M., & Ritz, B. (2009). Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environmental Health Perspectives, 117(11), 1773–1779. https://doi.org/10.1289/ehp.0800334.
Acknowledgements
This study was funded by the David and Lucile Packard Foundation and the Executive Advisory Board of the Iris Cantor-UCLA Women’s Health Center.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Appendix A
Cluster Analysis: Births with Congenital Anomalies, 2006–2010 (Non-Smoking Mothers Ages 15 through 34 Years Old) (PDF 1055 kb)
Appendix B
Suspected Toxic Parcels and EPA Superfund Sites (PDF 1116 kb)
Appendix C
Median Household Income, American Community Survey, 2006–2010 (PDF 1201 kb)
Appendix D
Rates of Congenital Anomalies for Mothers Residing in Hot Spot PUMAs (99% Confidence) by Birth Hospital (DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Rible, R., Aguilar, E., Chen, A. et al. Exploration of spatial patterns of congenital anomalies in Los Angeles County using the vital statistics birth master file. Environ Monit Assess 190, 184 (2018). https://doi.org/10.1007/s10661-018-6539-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6539-0