Abstract
Fluoride (F) contamination in groundwater can be problematic to human health. This study evaluated the concentration of fluoride in groundwater resources of Isfahan Province, the central plateau of Iran, and its related health issues to the inhabitant populations. For this purpose, 573 drinking groundwater samples were analyzed in 2016 by using the spectrophotometric method. Non-carcinogenic health risks due to F exposure through consumption of drinking water were assessed using the US EPA method. In addition, the associated zoning maps of the obtained results were presented using geographic information system (GIS). The results indicated that F content in drinking water ranged from 0.02 to 2.8 mg/L. The F contents were less than 0.50 mg/L in 63% of the drinking groundwater samples, 0.51–1.5 mg/L in 33.15%, and higher than 1.5 mg/L in 3.85% (Iran and World Health Organization guidelines) of the drinking groundwater samples. The F levels in the west and the south groundwater resources of the study areas were lower than 0.5 mg/L, which is within the recommended values for controlling dental caries (0.50–1.0 mg/L). Therefore, these places require more attention and more research is needed to increase F intake for health benefit. The HQ index for children, teens and male and female adults had health hazards (HQ > 1) in 51, 17, 28, and 18 of samples, respectively. Groundwater resources having a risk of more than one were located in the counties of Nayin, Natanz, and Ardestan. So, in these areas, there are potential risks of dental fluorosis. The most vulnerable groups were children. The F levels must be reduced in this region to decrease endemic fluorosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride (F) is one of the essential trace nutrients. Humans are exposed to this inorganic compound through different sources such as water, soil, food, pesticide residues, pharmaceutical drugs, and toothpaste. F sources include atmospheric, precipitation, minerals, geothermal, and human sources (aluminum and coal industries, glassworks, steel mills, fertilizer use, manufacturing processes) (KheradPisheh et al. 2016; Ozsvath 2009). It enters the water mainly from natural sources (KheradPisheh et al. 2016). The concentration of F in groundwater is higher than surface waters because it is impacted by minerals and rocks such as fluorite, apatite, amphibole, and micas (Chuah et al. 2016; Irigoyen-Camacho et al. 2016; Wasana et al. 2017). Countries such as Tanzania (Bhattacharya et al. 2016), China (Gao et al. 2013), Saudi Arabia (Zabin et al. 2008), India (Raj and Shaji 2017; Shaji et al. 2007; Salve et al. 2008), Iran (KheradPisheh et al. 2016), Libya (Tejaswi et al. 2013), South Africa (Kut et al. 2016), Turkey (Oruc 2008), Pakistan (Rasool et al. 2015), and northern Thailand (Chuah et al. 2016) have reported high concentrations of F in their groundwater. Groundwater is the main source of drinking water in particular communities or villages (Huang et al. 2017; Ayoob and Gupta 2006). Drinking water is the most important source of F exposure. More ingested F in water (90%) is absorbed, particularly from the gastrointestinal tract when the F concentration increases by 1 ppm (WHO 2004).
Studies showed that F has some impacts on human health depending on its content in drinking water (Omid et al. 2017; Newton et al. 2017; Näsman et al. 2016; Li et al. 2001; Indermitte et al. 2009). F is used as a compound known for mineralization of bones and teeth. It is a halogen with a high electronegativity, which is attracted by positively charged calcium in teeth and bones (Harrison 2005). Especially in children, F is accumulated in the growing bones and teeth which helps to strengthen the tooth enamel. Also, F in adults and children acts as an antibacterial agent in the mouth, which helps in minimizing acid attack on teeth, thereby improving dental health. F exposure of 0.05–0.07 mg/kg body weight/day, in children, has been suggested as optimal for dental health benefits (Yeung 2008). When the F level in drinking water is lower than 0.5 mg/L, the risk of dental caries increases (Gao et al. 2013; Bo et al. 2003; WHO 2004; Harrison 2005; Do et al. 2017; Ozsvath 2009; Irigoyen-Camacho et al. 2016). Thus, water fluoridation is the most cost-effective method of public health that reduces the incidence of dental caries (IRIS 2016).
Dental and skeletal fluorosis are the adverse effects that occur due to long-term excessive exposure to F and its accumulation in the body (Rahmani et al. 2010; Goodarzi et al. 2016; Das and Mondal 2016). The risk exists just for children in the 8 years age range and younger when permanent teeth are developing. Concentrations between 1.5 to 4 mg/L may result in dental fluorosis (Goodarzi et al. 2017). The prevalence of dental fluorosis in 60–90% of adolescents was seen in water consumption containing 2–7 mg/L F, in Moldova and Ukraine (Fordyce et al. 2007). Ingestion of 4–10 mg/L of F via drinking water can produce skeletal deformities (Yeung 2008; Kasim and Choudhary 2017). The fluorosis by ingestion of high F containing water has been documented in various regions (Huang et al. 2017; Indermitte et al. 2009). Also, various studies, in areas where added or naturally occurring F is high, have shown that over-exposure to F in water decreased red blood cells, osteoporosis (Hillier et al. 1996), decreased thyroid function (Susheela et al. 2005), oxidative stress (Nabavi et al. 2012), nervous system impairment (Valdez-Jiménez et al. 2011), periodontal disease (Hassell and Harris 1995), renal disease (Xiong et al. 2007), decreased intelligence in children (Karinnzade et al. 2014; Trivedi et al. 2007; Das and Mondal 2016), hypertension (Sun et al. 2013), decreased fertility in animals and humans, and low birth weight infants (Aghaei et al. 2015).
The World Health Organization (WHO) within the international program on chemical safety classifies F as one of the ten chemicals of major public health concern (Antonijevic et al. 2016). The maximum contaminant level (MCL) of 4 mg/L for F in drinking water and 2 mg/L as the secondary maximum contaminant level (SMCL) has been set by the US Environmental Protection Agency (EPA 2011). WHO and national standard of Iran recommend 0.5–1.5 mg/L of F in drinking water (WHO 2004). When water is boiled or frozen, its F content is not released. Removal of F from water is a difficult water treatment process that mostly includes activated carbon filtration, reverse osmosis, and ion exchange or distillation (KheradPisheh et al. 2016; IRIS 2016).
It is essential to estimate F concentration in drinking water sources and assesses its potential health risks (Ozsvath 2009). Several research studies have performed the risk assessment for F exposure mostly from drinking water (Augustsson and Berger 2014; Fordyce et al. 2007; Gao et al. 2013; Guissouma et al. 2017; Huang et al. 2017; Shyam and Kalwania 2012). Many regions of Iran, such as Borazjan, Hormozgan, Khurram Abad, Lar (southern Iran), Maku, and Kuhbanan (center of Iran), are at risk of fluorosis (Keshavarz et al. 2015; Dindarloo et al. 2016; Goodarzi et al. 2016).
The Human Health Risk Assessment (HHRA) model is used to assess the adverse health effects on humans, now or in the future, through exposures to chemicals and environmental agents (Patterson et al. 2002). Risk assessment is a four-step process which involves hazard identification, dose-response, exposure assessment, and risk characterization. Non-carcinogenic risk assessments are typically based on the use of the hazard quotient (HQ), a ratio of the estimated dose of a contaminant to the reference dose (EPA 2011). Reference dose (RfD) is estimated from the daily exposure level without significant risk of harmful effects during the lifetime. When the exposure level exceeds the reference dose, the probability of adverse effects in human population increases (EPA 2004). According to the Integrated Risk Information System (IRIS) database of the US EPA, the oral RfD of F for drinking water consumption is 0.06 mg/kg/day, which comprises both the daily intake from ingestion of F through drinking water (50 μg/kg/day) and dietary intake of F (10 μg/kg/day) (EPA 2011).
Isfahan Province, central plateau of Iran, is located in a semi-arid and dry desert climate, and its water consumption is relatively high. In this province, groundwater resources serve as the main source of drinking water source in the rural regions. There is no basic information on the safety of rural drinking water in Isfahan. The importance of groundwater requires research into groundwater contamination and its associated health risks in the less-populated and small rural area. This study aims to investigate the distributions of F level in drinking groundwater resources and its effect on human health in Isfahan Province by using GIS for the identification of risk areas. The results of this study would be useful for all stakeholders, national and regional decision-making organizations in safe drinking water supply for both excessive and insufficient F content and in Isfahan to prevent long-term potential health risks. Also, this information may also be useful for future water resource planning for this area.
Materials and methods
Study area
Isfahan Province with an area of 107,027 km2 is located in the central plateau of Iran around the Zayandehroud river, which flows from the west to southeast in central Iran. It has 23 counties and 33 aquifers. The area extends from east of 49° 38′ to 55° 32′ longitude and north of 30° 42′ to 34° 27′ latitude. This area includes agricultural, industrial, urban, and uncultivated lands. Isfahan Province is located in a semi-arid and dry desert climate. The height of the province’s various regions varies from less than 500 m in the east to more than 4000 m in the west. Most precipitations occur in the western regions. The average annual rainfall and temperature of the region are 140 mm and 14.5 °C, respectively. In Isfahan Province, there are extended sequences of sedimentary deposits, metamorphic rocks, and igneous sediments of different ages. The geology of Isfahan is dominated by primary granites, which is locally rich, especially in alkali-rich granites, red-colored conglomerate without fossil, red or grained sandstone and gray schist, gray-limy grayish or yellow marl, lime, and the stones of calcareous dolomite. Water is supplied for urban, agricultural, industrial use from surface water and underground water. In the rural areas, drinking water source is provided from the groundwater, whereas the main water source of the urban areas is surface water, Zayandehroud river, which is supplied from the Babashekhali water treatment plant (Chavoshi et al. 2011).
Sample collection and analysis
The 573 samples were collected from all drinking groundwater resources, including 473 tube wells, 62 springs, and 38 qanats in the region, during March 2016 to February 2017. Water samples were analyzed at 40, 40, 5.6, and 13.4% in spring, summer, fall, and winter, respectively. The samples belonged to the rural areas and only 33 samples were from groundwater resource located in urban area. Temperature and geographical positions during the sampling were recorded by using GPS. The samples were taken under standard conditions and transferred to the laboratory in 1-l polyethylene bottles. 4The concentration of F was measured by the SPADNS method using a DR-5000 Hach-Lange spectrophotometer, USA. Electrical conductivity (EC) and pH were measured by EC meter (wtw), and chloride ion was measured by following the standard method (APHA 2005).
Statistical analysis
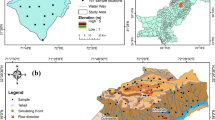
The SPSS 20 software was used for data analysis. Correlation calculations and basic statistics were carried out. Study area map with sampling location points, fluoride distribution, and other parameters was created by using ArcGIS 10.2. The inverse distance weighting (IDW) method was chosen for interpolation and the best prediction approach for concentration zoning map of fluoride in the study area. For the other maps, the classification by size and color symbol was used. The sampling locations are shown in Fig. 1.
Exposure and health risk assessment
Human health risk assessment is a methodology used to evaluate the potentially harmful effects on human health upon exposure to certain chemical agents for a certain period (Means 1989). This approach has been used and confirmed in several cases of health risk assessment in different environments (Huang et al. 2017). There are various methods to categorize carcinogenic and non-carcinogenic effects and to estimate potential health hazards of pollutants. Non-carcinogenic risk assessments are usually based on the use of HQ, which is defined as the ratio of the estimated dose of a contaminant to the reference dose. This method was established by US EPA in 1988 (Barnes et al. 1988).
In the study area, calculations for non-carcinogenic health risks associated with the consumption of groundwater containing F by the local residents were made using the standard assumptions used in US EPA risk analysis. The population was divided into the following four age groups because of physiological and behavioral differences: children (0–10 years old), teens (11–20 years old), and male and female adults (21–72 years old). The exposure parameters and their probability distributions for the four age groups are presented in Table 1.
Human exposure to F can be achieved in oral intake by drinking water and diet, dermal absorption, and inhalation. Considering that drinking water is the main source of human exposure to F, it was studied based on water consumption in the region. The estimated daily intake (EDI) was calculated for various populations that received F from drinking water and body weight using Eq. (1) (EPA 1989):
where EDI is estimated as daily intake of F through ingestion (mg/kg/day), IR is the drinking water ingestion rate (L/day), C is the F concentration in drinking water (mg/L), and BW is body weight (kg).
An estimation of non-carcinogenic risks from F exposure through different exposure routes was calculated by dividing the EDI by the safe dose (RfD). Then, HQ that represents the fluorosis risk from drinking water was calculated using Eq. (2):
where HQ is considered to be the hazard quotient, of which a value lower than 1 implies a negligible risk of non-carcinogenic effects, whereas HQ greater than 1 indicates potential non-cancer causing health effects.
Results and discussion
The F concentration was monitored in 573 water samples taken from all rural drinking groundwater sources in Isfahan Province during March 2016 to February 2017.
Figure 2 clearly shows the results of the F concentrations in the groundwater sources of the study area. The sources with a concentration of less than 0.5 mg/L are represented with yellow and 0.5–1.5 mg/L with green, and sources with a concentration more than 1.5 mg/L are represented with red. F concentration was not related to the type of sources like wells, springs, and qanats (p = 0.802). One-sample T test showed that there was a significant difference between the F concentration in the samples with respect to the WHO (2004) guidelines and also the Iranian national standard (0.5–1.5 mg/L) (p < 0.001), and 22 samples had concentrations above 1.5 mg/L. High F concentrations were located in Nayin (11), Natanz (1), and Ardestan (10) counties. This may be due to the geological structure because of gypsum and salt tissue present in their soils, and the correlation between F and calcium, sodium, sulfate ions, or depth of the well can be the reason for increasing F in groundwater in these regions (Chuah et al. 2016; KheradPisheh et al. 2016). Nouri et al. (2006) reported that the F content of deep aquifers was lower than that of shallow ones. Their results also showed that the clay content of the soil can lead to the replacement of F− ions by OH− ions and cause F to dissolve, thereby increasing its concentration. Gypsum and salt contents of soil in Zarrin Dasht County of Fars Province caused high F in groundwater (KheradPisheh et al. 2016). In the present study also, 63% (361 samples) of the samples had F concentrations of less than 0.5 mg/L, which their distributions were more in the whole province, especially in the west and south. In these areas, probably the risk of dental caries is high. Studies showed that when F level in drinking water is lower than 0.5 mg/L, the risk of dental caries increases (Gao et al. 2013; WHO 2004). Also KheradPisheh et al. (2016) found F concentration in Isfahan Province to be lower than 1.5 mg/L (0.02–1.5 mg/L, mean = 0.29 mg/L). In a study conducted by Chavoshi et al. (2011), the groundwater F concentration of Isfahan was in spring (0.09–0.4 mg/L) and in summer (0.05–0.1 mg/L). The concentration of F in drinking water can vary according to socioeconomic and weather conditions, health habits, and dietary populations in countries. The optimum F concentration in water (D as mg/L) can be calculated by [Eq. (3)]:
where Tm is the maximum average temperature (in °F).
The average annual temperature in Isfahan Province is 14.5 °C (58.2 °F). So, the optimum F content in drinking water of Isfahan should be 1 mg/L (KheradPisheh et al. 2016).
The minimum and the maximum F concentrations in the groundwater resource of each county are shown in Fig. 3. The results show that F concentrations varied from 0.02 to 2.8 mg/L, with a mean of 0.52 ± 0.46 mg/L, while the Nayin (0.04–2.8 mg/L), Ardestan (0.05–1.92 mg/L), and Natanz (0.16–1.72 mg/L), respectively, had the highest concentrations and Falavarjan (0.21–0.33 mg/L) and Barkhar (0.02–0.36 mg/L) had the lowest concentrations.
One-way analysis of variance (ANOVA) showed that there was seasonal significant difference in groundwater F concentrations (p < 0.001). The mean concentrations of F in samples in spring (0.63 mg/L) and winter (0.55 mg/L) were higher than those in summer (0.42 mg/L) and fall (0.27 mg/L). These high concentrations might be due to precipitation in spring and winter which dissolve the F contents of soils in groundwater resources.
Chavoshi et al. (2011) found that due to more precipitation in spring, the F content of water was higher than that in summer. However, Keshavarz et al. (2015) in a study implemented in Bushehr Province, south of Iran, found that there was no seasonal significant difference in groundwater F concentrations in spring and summer.
Figure 4 shows the F concentration in aquifers in the study area. As can be seen, Dahag sorkh (4804) and Ardestan (4801) aquifers, with high F concentration, are also located in Ardestan County.
The water quality parameters (electrical conductivity, temperature, pH, and chloride ions) are shown in Fig. 5. The data in resource is classified by size symbol. The temperature which was between 0 and 34 °C is shown in Fig. 5a. The pH was in the range of 6 to 9 and is also shown in Fig. 5b. Figure 5c shows that the EC varies between 0 and 5770 (μmhos/cm). The concentration of chloride ions in the samples was found to be in the range of 4–1225 mg/L, shown in Fig. 5d. Statistical analysis demonstrated that there was a low significant correlation between groundwater F content and EC (p < 0.001 and r = 0.418), Cl (p < 0.001 and r = 0.361), pH (p = 0.006 and r = 0.077), and temperature (p < 0.001 and r = 0.246). This result contradicts the findings of Nouri et al. (2006) who found a significant negative correlation between F content and pH of groundwater in Shush, south-west of Iran. It is obvious that high pH values promote the dissolution of CaF2 in groundwater and could increase F content of the groundwater (Chen et al. 2016). Tiwari et al. (2017) also found a positive correlation between groundwater F− content by pH (p = 0.36).
In this study, hazard quotients (HQs) by F concentrations in drinking groundwater were calculated for residents of Isfahan Province. Reference dose of 0.06 mg/kg/day by the IRIS database of the US EPA was used. Figure 6 shows the spatial variation of health risk that resulted from the groundwater F concentrations for four age groups: children, teens, and adults (males, females). The HQs show two classes to be lower than one and more than in the resources. Based on the obtained results, the HQ values of F were reported in children (0.01–2.033), teens (0.06–1.36), adult males (0.08–1.626), and adult females (0.06–1.4). One-way ANOVA also showed that there was a significant difference in HQs between group ages (p < 0.001(. The HQ index for children, teens and male and female adults had health hazard (HQ > 1) in 51, 17, 28, and 18 of samples, respectively. Groundwater resources having a risk more than one were located in the counties of Nayin, Natanz, and Ardestan. So, in these areas, there are potential risks of dental fluorosis.
Table 2 also compares the HQ values of studied groups with respect to the seasonal variations. As can be seen here, the highest HQs for non-carcinogenic risk are related to children group in the spring with the lowest body weight, as well as years of growth. However, in the rest of the season, the highest HQs were related to this age group.
In the study of Chen et al. (2016) infants and children, the most vulnerable groups were exposed through the oral route. The risk index for infants and children had health hazard in the majority of samples (72 and 60%) than that for males and females (28 and 22%). Erdal and Buchanan (2005) examined F absorption through fluoridated drinking water, cow’s milk, beverage, food, and F supplementation and found the F risk for children and other groups to be safe. Battaleb-Looie et al. (2013) found that F intakes (from dates and drinking water) for children and adults were 3.4 and 1.6 times higher than safe dose, respectively.
In the study of Chavoshi et al. (2011) in the center of Iran, the total HQ for both population groups (children and adults) via consumption of cereals, water, soil, and vegetables was less than 1.0, resembling no obvious risk. For 68% of the total population of the western plains area in Jilin Province, China, the HQ through drinking water was also above 1 (Bo et al. 2003).
Conclusion
This study was carried out in the central plateau of Iran, Isfahan Province, to estimate the non-carcinogenic risk posed by F ingestion via drinking water from groundwater resources. Results showed that the calculated mean non-carcinogenic risk was lower than 1. However, in some of the areas, the F concentrations in groundwater were higher than those in the WHO or Iranian guidelines. These findings indicated the absence of potential health effects at the province level. However, F may pose possible health risks to local residents, because the HQ of F was > 1 in some counties, including Nayin, Natanz, and Ardestan, where the highest levels of F contamination were found.
Moreover, children are a vulnerable population in most of the studied areas, and they are at a high risk of non-carcinogenic hazards from exposure to drinking water with high F levels, which should be addressed. Also, in cities in the west and south of the province, low F and high dental caries are high.
Uncertainty during the risk assessment includes the variables used in calculations, such as the difference in body weight of individuals. In addition, weather conditions can greatly affect drinking water consumption. The consumption rate of drinking water in a hot climate is higher than that in a mild climate. As a result, the F intake can be different.
Although F in drinking water usually takes into account the most effective daily intake, other F sources, including beverages (such as tea) or foods and F supplements, can also significantly contribute to a daily intake of F. In this study, the non-carcinogenic risk for Isfahan residents’ exposure to F could be underestimated because only the drinking water exposure pathway was considered. Therefore, an accurate estimate of other sources of exposure to additional F should be considered.
References
Aghaei, M., Derakhshani, R., Raoof, M., Dehghani, M., & Mahvi, A. H. (2015). Effect of fluoride in drinking water on birth, height and weight: an ecological study in Kerman Province, Zarand County, Iran. Fluoride, 48, 160–168.
Antonijevic, E., Mandinic, Z., Curcic, M., Djukic-Cosic, D., Milicevic, N., Ivanovic, M., Carevic, M. & Antonijevic, B. 2016. “Borderline” fluorotic region in Serbia: correlations among fluoride in drinking water, biomarkers of exposure and dental fluorosis in schoolchildren. Environmental geochemistry and health, 38, 885-896.
APHA. (2005). Standard Methods for the Examination of Water and Wastewater, 21st ed (pp. 1220). Washington DC: American Public Health Association.
Augustsson, A., & Berger, T. (2014). Assessing the risk of an excess fluoride intake among Swedish children in households with private wells—expanding static single-source methods to a probabilistic multi-exposure-pathway approach. Environment International, 68, 192–199. https://doi.org/10.1016/j.envint.2014.03.014.
Ayoob, S., & Gupta, A. K. (2006). Fluoride in drinking water: a review on the status and stress effects. Critical Reviews in Environmental Science and Technology, 3 6(6), 433–487. https://doi.org/10.1080/10643380600678112.
Barnes, D. G., Dourson, M., Preuss, P., Bellin, J., Derosa, C., Engler, R., Erdreich, L., Farber, T., Fenner-Crisp, P., & Francis, E. (1988). Reference dose (RfD): description and use in health risk assessments. Regulatory Toxicology and Pharmacology, 8(4), 471–486. https://doi.org/10.1016/0273-2300(88)90047-5.
Bhattacharya, P., Lesafi, F., Filemon, R., Ligate, F., Ijumulana, J. & Mtalo, F. (2016). Geogenic fluoride and arsenic contamination in the groundwater environments in Tanzania. EGU General Assembly Conference Abstracts, 18, 16677.
Battaleb-Looie, S., Moore, F., Malde, M., & Jacks, G. (2013). Fluoride in groundwater, dates and wheat: estimated exposure dose in the population of Bushehr, Iran. Journal of Food Composition and Analysis, 29(2), 94–99. https://doi.org/10.1016/j.jfca.2012.08.001.
Bo, Z., Mei, H., Yongsheng, Z., Xueyu, L., Xuelin, Z., & Jun, D. (2003). Distribution and risk assessment of fluoride in drinking water in the west plain region of Jilin province, China. Environmental Geochemistry and Health, 25(4), 421–431. https://doi.org/10.1023/B:EGAH.0000004560.47697.91.
Chavoshi, E., Afyuni, M., Hajabbasi, M., Khoshgoftarmanesh, A., Abbaspour, K., Shariatmadari, H., & Mirghafari, N. (2011). Health risk assessment of fluoride exposure in the soil, plants, and water at Isfahan, Iran. Human and Ecological Risk Assessment, 17(2), 414–430. https://doi.org/10.1080/10807039.2011.552397.
Chen, J., Wu, H., Qian, H., & Gao, Y. (2017). Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of northwest China. Exposure and Health, 9(3): 183-95. https://doi.org/10.1007/s12403-016-0231-9.
Chuah, C. J., Lye, H. R., Ziegler, A. D., Wood, S. H., Kongpun, C., & Rajchagool, S. (2016). Fluoride: a naturally-occurring health hazard in drinking-water resources of Northern Thailand. The Science of the Total Environment, 545, 266–279.
Das, K., & Mondal, N. K. (2016). Dental fluorosis and urinary fluoride concentration as a reflection of fluoride exposure and its impact on IQ level and BMI of children of Laxmisagar, Simlapal Block of Bankura District, W.B., India. Environmental Monitoring and Assessment, 188(4), 218. https://doi.org/10.1007/s10661-016-5219-1.
Dindarloo, K., Jamali, H. A., Lakbala, P., Valizade, H., Azad, M., & Mahmodi, H. (2016). Determination of fluoride concentration in drinking water and its relation with DMFT: a case study in Hormozgan, Iran. Journal of Basic Research in Medical Sciences, 3, 28–36.
Do, L., Ha, D., Peres, M. A., Skinner, J., Byun, R., & Spencer, A. J. (2017). Effectiveness of water fluoridation in the prevention of dental caries across adult age groups. Community Dentistry and Oral Epidemiology, 45(3):225–232.
EPA (1989). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part A) (pp. 20450). Washington, DC: Office of Emergency and Remedial Response U.S. Environmental Protection Agency. http://www.epa.gov/swerrims/riskassessment/risk_superfund.html. Accessed 31 August 2017.
EPA (2004). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) Final. Washington, DC: Office of Superfund Remediation and Technology Innovation U.S. Environmental Protection Agency. https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part-e. Accessed 31 August 2017.
EPA (2011). The United States Environmental Protection Agency. EPA 635/R-03/007. http://www.epa.gov/iris. In support of summary information on the Integrated Risk Information System (IRIS). Exposure factors handbook 2011 edition (Final). Washington, DC: Chapter 8―Body Weight Studies.
Erdal, S., & Buchanan, S. N. (2005). A quantitative look at fluorosis, fluoride exposure, and intake in children using a health risk-assessment approach. Environmental Health Perspectives, 113(1), 111–117.
Fordyce, F., Vrana, K., Zhovinsky, E., Povoroznuk, V., Toth, G., Hope, B., Iljinsky, U., & Baker, J. (2007). A health risk assessment for fluoride in Central Europe. Environmental Geochemistry and Health, 29(2), 83–102. https://doi.org/10.1007/s10653-006-9076-7.
Gao, H. J., Jin, Y. Q., & Wei, J. L. (2013). Health risk assessment of fluoride in drinking water from Anhui Province in China. Environmental Monitoring and Assessment, 185(5), 3687–3695. https://doi.org/10.1007/s10661-012-2820-9.
Goodarzi, F., Mahvi, A. H., Hosseini, M., Nodehi, R. N., Kharazifard, M. J., & Parvizishad, M. (2016). Fluoride concentration of drinking water and dental fluorosis: a systematic review and meta-analysis in Iran. Dental Hypotheses, 7, 81.
Goodarzi, F., Mahvi, A. H., Hosseini, M., Nodehi, R. N., Kharazifard, M. J., & Parvizishad, M. (2017). Prevalence of dental caries and fluoride concentration of drinking water: a systematic review. Dental Research Journal, 14, 163.
Guissouma, W., Hakami, O., Al-Rajab, A. J., & Tarhouni, J. (2017). Risk assessment of fluoride exposure in drinking water of Tunisia. Chemosphere, 177, 102–108. https://doi.org/10.1016/j.chemosphere.2017.03.011.
Harrison, P. T. (2005). Fluoride in water: a UK perspective. Journal of Fluorine Chemistry, 126(11-12), 1448–1456. https://doi.org/10.1016/j.jfluchem.2005.09.009.
Hassell, T. M., & Harris, E. L. (1995). Genetic influences in caries and periodontal diseases. Critical Reviews in Oral Biology & Medicine, 6(4), 319–342. https://doi.org/10.1177/10454411950060040401.
Hillier, S., Inskip, H., Coggon, D., & Cooper, C. (1996). Water fluoridation and osteoporotic fracture. Community Dental Health, 13, 63–68.
Huang, D., Yang, J., Wei, X., Qin, J., Ou, S., Zhang, Z. & Zou, Y. (2017). Probabilistic risk assessment of Chinese residents' exposure to fluoride in improved drinking water in endemic fluorosis areas. Environmental Pollution, 222, 118–125. https://doi.org/10.1016/j.envpol.2016.12.074.
Indermitte, E., Savva, A., & Karro, E. (2009). Exposure to high fluoride drinking water and risk of dental fluorosis in Estonia. International Journal of Environmental Research and Public Health, 6(2), 710–721. https://doi.org/10.3390/ijerph6020710.
Irigoyen-Camacho, M., Pérez, A. G., González, A. M., & Alvarez, R. H. (2016). Nutritional status and dental fluorosis among schoolchildren in communities with different drinking water fluoride concentrations in a central region in Mexico. The Science of the Total Environment, 541, 512–519. https://doi.org/10.1016/j.scitotenv.2015.09.085.
IRIS (2016). Integrated Risk Information System, Fluorine (Soluble Fluoride). https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr¼53. Accessed 16 July 2016.
Karinnzade, S., Aghaei, M., & Mahvi, A. H. (2014). IQ of 9-12-year-old chldren in high-and low-drinking water fluoride areas in west azerbaijan province, Iran: further information on the two villages in the study and the confounding factors considered. Fluoride, 47, 266–271.
Kasim, M., & Choudhary, A. (2017). Long-term effects due to high fluoride concentration in drinking water and socio-economic status of the rural population in district pale, rajasthan: a case study. Journal of Global Biosciences, 6, 4689–4694.
Keshavarz, S., Ebrahimi, A., & Nikaeen, M. (2015). Fluoride exposure and its health risk assessment in drinking water and staple food in the population of Dayyer, Iran, in 2013. Journal of Education and Health Promotion, 4, 72.
KheradPisheh, Z., Ehrampoush, M., Montazeri, A., Mirzaei, M., Mokhtari, M., & Mahvi, A. (2016). Fluoride in drinking water in 31 provinces of Iran. Exposure and Health, 8(4), 465–474. https://doi.org/10.1007/s12403-016-0204-z.
Kut, K. M. K., Sarswat, A., Srivastava, A., Pittman, C. U., & Mohan, D. (2016). A review of fluoride in African groundwater and local remediation methods. Groundwater for Sustainable Development, 2, 190–212.
Li, Y., Liang, C., Slemenda, C. W., Ji, R., Sun, S., Cao, J., Emsley, C. L., Ma, F., Wu, Y., & Ying, P. (2001). Effect of long-term exposure to fluoride in drinking water on risks of bone fractures. Journal of Bone and Mineral Research, 16(5), 932–939. https://doi.org/10.1359/jbmr.2001.16.5.932.
Means, B. 1989. Risk-assessment guidance for superfund. Volume 1. Human health evaluation manual. Part A. Interim report (Final). Environmental Protection Agency, Office of Solid Waste and Emergency Response, Washington, DC.
Nabavi, S. M., Nabavi, S. F., Eslami, S., & Moghaddam, A. H. (2012). In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chemistry, 132(2), 931–935. https://doi.org/10.1016/j.foodchem.2011.11.070.
Näsman, P., Granath, F., Ekstrand, J., Ekbom, A., Sandborgh-Englund, G., & Ford, C. M. (2016). Natural fluoride in drinking water and myocardial infarction: a cohort study in Sweden. The Science of the Total Environment, 562, 305–311. https://doi.org/10.1016/j.scitotenv.2016.03.161.
Newton, J. N., Verne, J., Dancox, M., & Young, N. (2017). ‘Are fluoride levels in drinking water associated with hypothyroidism prevalence in England? A large observational study of GP practice data and fluoride levels in drinking water’: comments on the authors’ response to earlier criticism. Journal of Epidemiology and Community Health, 71(4), 315–316. https://doi.org/10.1136/jech-2016-208649.
Nouri, J., Mahvi, A. H., Babaei, A., & Ahmadpour, E. (2006). Regional pattern distribution of groundwater fluoride in the Shush aquifer of Khuzestan County, Iran. Fluoride, 39, 321.
Omid, N., Maguire, A., O'Hare, W. T., & Zohoori, F. V. (2017). Total daily fluoride intake and fractional urinary fluoride excretion in 4-to 6-year-old children living in a fluoridated area: weekly variation? Community Dentistry and Oral Epidemiology, 45(1), 12–19. https://doi.org/10.1111/cdoe.12254.
Oruc, N. (2008). Occurrence and problems of high fluoride waters in Turkey: an overview. Environmental Geochemistry and Health, 30(4), 315–323. https://doi.org/10.1007/s10653-008-9160-2.
Ozsvath, D. L. (2009). Fluoride and environmental health: a review. Reviews in Environmental Science and Bio/Technology, 8(1), 59–79. https://doi.org/10.1007/s11157-008-9136-9.
Patterson, J., Hakkinen, P. B., & Wullenweber, A. E. (2002). Human health risk assessment: selected Internet and World Wide Web resources. Toxicology, 173(1–2), 123–143. https://doi.org/10.1016/S0300-483X(02)00027-6.
Rahmani, A., Rahmani, K., Dobaradaran, S., Mahvi, A. H., Mohamadjani, R., & Rahmani, H. (2010). Child dental caries in relation to fluoride and some inorganic constituents in drinking water in Arsanjan, Iran. Fluoride, 43, 179–186.
Raj, D., & Shaji, E. (2017). Fluoride contamination in groundwater resources of Alleppey, southern India. Geoscience Frontiers, 8(1), 117–124. https://doi.org/10.1016/j.gsf.2016.01.002.
Rasool, A., Xiao, T., Baig, Z. T., Masood, S., Mostafa, K. M., & Iqbal, M. (2015). Co-occurrence of arsenic and fluoride in the groundwater of Punjab, Pakistan: source discrimination and health risk assessment. Environmental Science and Pollution Research, 22(24), 19729–19746. https://doi.org/10.1007/s11356-015-5159-2.
Salve, P., Maurya, A., Kumbhare, P., Ramteke, D., & Wate, S. (2008). Assessment of groundwater quality with respect to fluoride. Bulletin of Environmental Contamination and Toxicology, 81(3), 289–293. https://doi.org/10.1007/s00128-008-9466-x.
Shaji, E., Viju, J., & Thambi, D. (2007). High fluoride in groundwater of Palghat District, Kerala. Current Science, 92, 240–245.
Shyam, R., & Kalwania, G. (2012). Health risk assessment of fluoride with other parameters in ground water of Sikar city (India). Environmental Earth Sciences, 65(4), 1275–1282. https://doi.org/10.1007/s12665-011-1375-3.
Sun, L., Gao, Y., Liu, H., Zhang, W., Ding, Y., Li, B., Li, M., & Sun, D. (2013). An assessment of the relationship between excess fluoride intake from drinking water and essential hypertension in adults residing in fluoride endemic areas. Science of the Total Environment, 443, 864–869. https://doi.org/10.1016/j.scitotenv.2012.11.021.
Susheela, A., Bhatnagar, M., Vig, K., & Mondal, N. (2005). Excess fluoride ingestion and thyroid hormone derangements in children living in Delhi, India. Fluoride, 38, 98–108.
Tejaswi, S. K., Shetty, S., Annapoorna, B., Pujari, S. C., Reddy, S. & Nandlal, B. 2013. A pioneering study of dental fluorosis in the libyan population. Journal of international oral health: JIOH, 5, 67.
Tiwari, A. K., Singh, A. K., & Mahato, M. K. (2017). GIS-based evaluation of fluoride contamination and assessment of fluoride exposure dose in groundwater of a district in Uttar Pradesh, India. Human and Ecological Risk Assessment: An International Journal, 23(1), 56–66. https://doi.org/10.1080/10807039.2016.1220824.
Trivedi, M., Verma, R., Chinoy, N., Patel, R., & Sathawara, N. (2007). Effect of high fluoride water on intelligence of school children in India. Fluoride, 40, 178–183.
US EPA. (2000). Risk-based concentration table. Washington, DC: Office of Health and Environmental Assessment.
Valdez-Jiménez, L., Fregozo, C. S., Beltrán, M. M., Coronado, O. G., & Vega, M. P. (2011). Effects of the fluoride on the central nervous system. Neurología (English Edition), 26(5), 297–300. https://doi.org/10.1016/S2173-5808(11)70062-1.
Wasana, H. M., Perera, G. D., Gunawardena, P. D. S., Fernando, P. S., & Bandara, J. (2017). WHO water quality standards vs synergic effect (s) of fluoride, heavy metals and hardness in drinking water on kidney tissues. Scientific Reports, 7, 42516. https://doi.org/10.1038/srep42516.
WHO (2004). World Health Organization Guidelines for drinking-water quality, World Health Organization. Third Edition, Vol 1. Recommendations, Geneva.
Xiong, X., Liu, J., He, W., Xia, T., He, P., Chen, X., Yang, K. & Wang, A. (2007). Dose-effect relationship between drinking water fluoride levels and damage to liver and kidney function s in children. Environmental Research, 103(1), 112-116. https://doi.org/10.1016/j.envres.2006.05.008.
Yeung, C. A. (2008). A systematic review of the efficacy and safety of fluoridation. Evidence-Based Dentistry, 9(2), 39–43. https://doi.org/10.1038/sj.ebd.6400578.
Zabin, S. A., Foaad, M., & Al-Ghamdi, A. Y. (2008). Non-carcinogenic risk assessment of heavy metals and fluoride in some water wells in the Al-Baha Region, Saudi Arabia. Human and Ecological Risk Assessment, 14(6), 1306–1317. https://doi.org/10.1080/10807030802494667.
Acknowledgements
This paper is a part of an approved MSc Thesis (#395894). The authors acknowledge funding provided by the Isfahan University of Medical Science, School of Health. The help and cooperation of rural Isfahan Water & Wastewater Company are also appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghapour, S., Bina, B., Tarrahi, M.J. et al. Distribution and health risk assessment of natural fluoride of drinking groundwater resources of Isfahan, Iran, using GIS. Environ Monit Assess 190, 137 (2018). https://doi.org/10.1007/s10661-018-6467-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6467-z