Abstract
The study of airborne metals in urban areas is relevant due to their toxic effects on human health and organisms. In this study, we analyzed metals including rare earth elements (REE) in particles smaller than 2.5 μm (PM2.5), collected at five sites around the Mexico City Metropolitan Area (MCMA), during three periods in 2011: April (dry-warm season, DW), August (rainy season, R), and November (dry-cold season, DC). Principal component analysis allowed identifying factors related to geogenic sources and factors related to anthropogenic sources. The recognition of the high impact of geogenic sources in PM2.5 is in agreement with the REE distribution patterns, which show similar behavior as those shown by igneous rocks, confirming the influence of the regional geogenic material. Metals associated to geogenic sources showed higher concentration (p < 0.05) at NE of the MCMA and a significant correlation with prevalent winds. Geogenic metals show similar seasonal distribution, with the highest concentration during DW (p < 0.05), suggesting a possible metal resuspension effect which affects more significantly at lower relative humidity (RH). The metals associated with anthropogenic sources are in agreement with the urban complexity of the area, showing homogenous distribution throughout MCMA (p > 0.05) and no similar seasonal pattern among them. These unexpected results exposed outstanding information regarding the identification of different geogenic sources as the main contributors of metals in the atmospheric environment in the MCMA and highlighted the importance of meteorology in the spatial and seasonal metal patterns.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last decade, atmospheric contamination in urban areas has attracted attention due to increased anthropogenic emissions. In order to evaluate the degree of air quality degradation, chemical studies on suspended particulate matter are of particular relevance because by identifying their sources, these studies can help to develop efficient strategies for air quality control. Atmospheric particulate matter (PM) is a mixture of solid particles and liquid droplets with diameter less than 100 μm. The size and composition of PM depend on the sources and formation processes (Finlayson-Pitts and Pitts 2000). This is an important issue due to the effects on human health risk, especially those particles with diameter less than 2.5 μm (PM2.5) (Dockery and Pope 1994; Pope et al. 1995; Pinkerton et al. 2000; Pope et al. 2002; Gauderman et al. 2004; Hu 2009; Feng et al. 2016; Ma et al. 2017; Zhou et al. 2017).
Many studies have focused on the determination of elemental constituents, inorganic ions, and organic components of PM2.5 (Querol et al. 2004; Moreno et al. 2006a, b; Ragosta et al. 2008; Kulshrestha et al. 2009; Ravindra et al. 2008; Zhao et al. 2009; Amador-Muñoz et al. 2011; Xu et al. 2012; Chen et al. 2014; Gao et al. 2016). Metals in PM2.5 vary over a wide range of concentrations. Furthermore, reported inhomogeneity in pollution levels in the same urban area has led to the identification of punctual sources.
Geogenic and anthropogenic emissions can contribute as sources of metals onto PM. Geogenic sources may result from volcanic activity, erosion of crustal minerals, sea salt aerosols, etc., while anthropogenic sources include vehicular traffic, fossil fuel burning, waste incineration, and industrial metallurgical processes, among others.
Although anthropogenic activities are recognized as the main contributors of PM into the urban air, metals from geogenic sources have been less highlighted. Multivariate analysis has been widely used to determine specific sources based on metal markers (Kulshrestha et al. 2009). In this context, the rare earth elements (REEs) provide relevant information. REEs have similar configuration of valence electrons and ionic ratios. As they have similar chemical and physical behavior, REE can be used as geochemical tracer to characterize igneous rocks and to determine geogenic sources. In several urban areas over the world, environmental enrichment of REE has been related to the use of products enriched with REE in different fields, including ceramics, electronics, optoelectronics, superconductors, pharmaceutical, and fertilizers. However, the genetic information of REE contained in PM2.5 can also be used to identify regional geogenic sources.
The Mexico City Metropolitan Area (MCMA) has experienced a substantial uncontrolled development in the suburban part of the city over the past 30 years. The impact of the urbanization and industrialization growth in the urban area of the MCMA has increased the metal content in the environment, as in the case for soils (Morton-Bermea et al. 2009a, b; Rodríguez-Salazar et al. 2011), biological material (Guzmán-Morales et al. 2011), and air (Rosas et al. 1995; Miranda et al. 2000; Chow et al. 2002; Querol et al. 2008). Regarding atmospheric contamination in MCMA, Rosas et al. (1995) reported lead on particles smaller than 10 μm (PM10) during 1990–1991, originated by the use of leaded gasoline (used in Mexico up to 1989). Miranda et al. (2000) reported 15 elements in particles < 15 μm (coarse fraction) and PM2.5 (fine fraction) collected during summer of 1995 in the south of Mexico City. Chow et al. (2002) described 36 metals, ions, and carbon collected in PM2.5 and PM10 around Mexico City during winter 1992. Miranda et al. (2000; 2005) and Chow et al. (2002) associated the metals in the air with the emission from natural (suspended dust) and anthropogenic sources (vehicular traffic and industrial sources). Moreover, Querol et al. (2008) published chemical speciation of PM10, PM2.5, and PM1, collected during March 2006 at different sites in the MCMA, as part of the MILAGRO (Megacity Initiative: Local and Global Research Observations) campaign. They associated the high metal levels with resuspension of mineral dust, as well as with industrial emissions, local traffic, and oil combustion.
Since 1986, the local government authorities of Mexico City, through the Dirección de Monitoreo Atmosférico (SEDEMA), has established a network of air quality monitoring stations, including PM2.5 among other parameters. Nowadays, there are more than 13 stations collecting TSP (total suspended particles), PM10, and PM2.5 around MCMA. Despite the big effort done by the air quality monitoring program, no extensive data on spatial and temporal variations of PM2.5 elemental composition have been published. The objective of this study was to investigate the chemical components (Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Rb, Sr, Cd, Sb, Cs, Th, Pb, Tl, La, Ce, Sm, and Eu) of PM2.5 to evaluate, in terms of meteorological parameters, their temporal and spatial variation. The assessment of this data is a key point to identify source apportionment and their contribution.
Experimental method
PM2.5 sampling
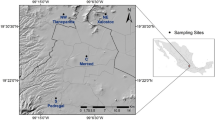
A PM2.5 sampling campaign was conducted in the MCMA in 2011. Twenty-four-hour PM2.5 samples were collected simultaneously every sixth day, at five representative sites with different urban conditions in the MCMA (Table 1 and Fig. 1): northwest (NW, Tlalnepantla), northeast (NE, San Agustin), center (C Merced), southwest (SW, Coyoacán), and southeast (SE, Universidad Autónoma Metropolitana, Iztapalapa).
Sampling was performed during three seasons in 2011: April (dry-warm season, DW), August (rainy season, R), and November (dry-cold season, DC). Thereby, 73 PM2.5 samples were collected.
The airborne particles were accumulated with a high volume sampler (Tisch and Andersen General Metal Works) with a flow rate of 1.13 m3 min−1 ± 10% on Teflon-coated glass fiber filters (20 cm × 25 cm) pre-baked to 180 °C for at least 24 h. Blank test background contamination was monitored by using operational blanks (unexposed filter), which were processed in parallel with field samples.
Analytical treatment
All samples and blank filters were kept in a desiccator for 48 h. A fraction (about 1/20) was exactly weighted and digested in Teflon vessels where 8 mL aqua regia and 2 mL HF were added. The mixture was left to stand overnight. Thereafter, the mixture was subjected to microwave-assisted digestion procedure using an ETHOS ONE (Millipore) microwave oven equipped with a rotor system for 10 Teflon vessels (PRO-24). The digestion temperature ranged from room temperature to 190 °C in 25 min and maintained for 15 min. After cooling, the solution was transferred to a Teflon vessel and evaporated to dryness. To ensure full removal of HF, 3 mL HCl was added to the digestate and evaporated twice. The final digested samples were taken to a volume of 50 mL with 2% HNO3 (v/v).
Instrumental analysis
Metal concentration measurements (Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Rb, Sr, Cd, Sb, Cs, Th, Pb, Tl, La, Ce, Sm, and Eu) were carried out using an ICP-MS (iCAP Q Thermo) at the Instituto de Geofísica, Universidad Nacional Autónoma de México. Prior to ICP-MS analysis, a calibration curve was performed using a five-point curve (0, 0.01, 0.1, 1, and 5 ng mL−1) with standard solutions that were prepared by diluting a multi-element standard solution (High Purity Standard) with 2% HNO3 (v/v). Instrumental drift was corrected using 115In as internal standard, prepared from a certified stock solution of 1000 mg L−1 (Merck). Urban dust reference material 1649a (National Institute for Standard and Technology) was used to evaluate the method’s efficiency.
Statistical analysis
Statistica Software version 10.0 (Statsoft, Dell Inc.) was used to calculate all statistical metrics. Median comparison was made by Mann–Whitney U test. Correlation analysis was carried out by Spearman R. Factor analysis with PCA as an extraction method and varimax rotation was used to identify the sources. Results from PCA were compared using either the Spearman or Pearson correlation matrix showing coherent results.
Meteorological data
The weather in MCMA can be divided in three climatic periods per year: warm-dry season (DW) from April to June, rainy season (R) from July to October, and cold-dry season (DC) from November to February (Jáuregui 2000). Data of relative humidity (RH, %), temperature (T, °C), wind speed (m s−1), wind direction (grades), and PM2.5 mass concentration for the corresponding sampling days were downloaded from the Automatic Monitoring Network website (http://www.aire.cdmx.gob.mx/default.php?opc='aKBhnmI='&opcion=Zw==).
Results and discussion
Quality control
The analytical procedure of simultaneous analysis of metals contained in particulate matter was evaluated. Detection limits were calculated as three times the standard deviation of 10 replicates of the blank filter. Blank test background contamination was monitored using operational blanks (unexposed filter papers), which were processed with field samples. All analyzed metals are above the detection limits. Accuracy was evaluated by means of recovery rates of 22 aliquots of SRM NIST 1649a, loaded in Teflon-coated glass fiber filters (the same filter employed to collect PM2.5). Good agreement is observed between the data and reference concentration for all metals. The average recoveries ranged between 72 and 124%. Results obtained from ICP-MS metal analysis and recovery percentages as well as detection limits are reported in Table 2.
Good recoveries are due to combined effect of an optimum digestion procedure and the optimal ICP-MS instrumental conditions that lead to obtaining low detection limits and low production of mass interferences. It must be mentioned that Ti, Sr, and Tl are not included in as certified elements in NIST 1649a; the analytical method showed good reproducibility, with a relative standard deviation (RSD = 0.42–1.7%, n = 22). Therefore, they were analyzed routinely in PM2.5 samples. Recoveries in Table 2 are in the same range than those reported elsewhere by other groups using different digestion parameters (concentration and volume of acids and oxidizing agents), as well as microwave oven settings (Karanasiou et al. 2005; Karthikeyan et al. 2006).
PM2.5 mass concentration and meteorological conditions
Seasonal PM2.5 concentrations and meteorological parameters are summarized in Table 3. During DW season, the PM2.5 median was the highest (31 μg m−3, p < 0.05), when RH was the lowest (45.8%, p < 0.05). The associations between concentration and chemical composition of PM2.5 with meteorological conditions are well known. For instance, Kulshrestha et al. (2009) in a study of both urban and rural environment of Agra, India, found that PM10 and PM2.5 concentrations were determined by temperature, relative humidity, and wind speed; this explains the high concentration found during the winter months. Zhao et al. (2009) point out that seasonal variations of temperature and relative humidity can be responsible for changes in concentrations of PM2.5 collected in Beijing. Nonetheless, they highlight the importance of wind patterns in those changes. Moreover, for Fuzhou, China, Xu et al. (2012) report that higher PM2.5 concentrations are associated with low temperatures, high relative humidity, and low wind speed prevailing during winter. However, the effect of cold season in the increase of PM2.5 mass concentration was not observed in our study. The increase of PM2.5 concentration during DW seems to be influenced by low RH, with no spatial variation of observed in the MCMA (p > 0.05). The prevailing wind direction in the sampling area during 2011 was from north to south as shown in Fig. 2. Wind speed median values ranged from 1.7 to 2.4 m s−1, with maximum speeds around 6 m s−1. PM2.5 was positively correlated with T (R = 0.31) and inversely with RH (R = − 0.24) and Wsp (− 0.57) (p < 0.05) (Table 8).
Metal concentration in PM2.5
Table 4 shows the metal concentration in PM2.5 as well as annual median concentration of PM2.5 in the MCMA during 2011. High variability was observed among metals, rising up to four orders of magnitude. The most abundant metals were Fe (67.7%), Ti (10.1%), and Sr (8.4%), while the least abundant were Th (0.01%) and Tl (0.01%). This suggests geogenic emission as an important source to the PM2.5 of MCMA.
Table 5 shows a comparison of metal concentration in our study versus other urban areas. Most of the metals in our study were in the concentration range reported in other parts of the world (Moreno et al. 2006a, b; Cheng et al. 2010; Hieu and Lee 2010; Xu et al. 2012). However, Ti and Sr were the higher in our study, suggesting that geogenic emissions in the MCMA are more important sources than in the other cities.
Spatial and seasonal variation
Spatial, seasonal, and annual distribution patterns of metals are depicted in box plots (Fig. 3). Spatial and seasonal comparisons are described in Tables 6 and 7, respectively. Two metal groups with similar behavior among them were observed: Ti, Mn, Fe, Co, and Cu; and Rb, Sr, Cs, Th, La, Ce and Sm. The highest concentrations for both groups were observed at NE (p < 0.05) (Table 6). This two elements groups are related to geogenic sources. The recognition of geogenic sources as contributors in PM has been reported for other urban areas worldwide (Moreno et al. 2006a, b; Zhao et al. 2009; Hieu and Lee 2010; Xu et al. 2012). In particular, for the MCMA, Miranda et al. (2000, 2005) had also identified soil-derived dusts as the main contributor for metallic components in PM2.5, regardless of the site where sampling was carried out. Most of the detected elements show a similar seasonal distribution (Fig. 3, Table 7), with the highest concentration during DW (p < 0.05). This pattern suggests a possible resuspension of metals by the wind from the top soil, stimulated by dry conditions where the lowest RH and the highest T values (p < 0.05) were observed (Table 3). This behavior is opposite to what has been observed during cool season in other parts of the world (Kulshrestha et al. 2009; Zhao et al. 2009; Xu et al. 2012).
Europium presented a different pattern as compared to the other metals, with the highest concentration observed at south of MCMA and during rainy season. V, Cr, Ni, Pb, Cd, and Tl are a group of metals showing also a different pattern, with a homogenous distribution throughout the studied area (p > 0.05) (Fig. 3, Table 6), suggesting similar sources around the MCMA or a regular transport from the emitting sources. Sb was also homogeneously distributed, except at NW, where the lowest concentration was found. Eu, V, Cr, Ni, Pb, Cd, Tl, and Sb did not show the same seasonal pattern than the other metals, neither among them.
Source recognition
The analysis of the information in Table 8, which shows the Spearman correlation coefficients between the concentration of the metals and the PM2.5, allows us to recognize at least two groups of elements with different origin.
Principal component analysis (PCA) was used to provide information for the identification of major metal sources. PCA results are consistent regardless of the nature of the database (raw or normalized). The number of factors retained was selected based on the scree test, which yielded six meaningful factors (Costello & Osborne 2005). Factor loadings had eigenvalues higher than 0.8. This number of factors provided the best solution, with eigenvalues above 1 for factors 1 to 5 and 0.87 for factor 6.
A total explained variance of 84.14% was observed. The main three factors explained 64.91% of the total variance, while the rest only explained 19.24%. Chromium, Cs, La, Ce, Sm, and Th were related with factor 1 (36.36%); Ti, Fe, Mn, Co, and Cu with factor 2 (17.44%); and Rb, Sr, and Eu with factor 3 (11.11%). Factors 4 to 6 clustered Ni, V, Cd, Pb, and Sb (Table 9).
Understanding results from similar works (Amato et al. 2009; Chen et al. 2014; Moreno-Rodríguez et al. 2015), along with the clear evidence gained from previous works related to the identification of the main sources of metals in MCMA (Morton-Bermea et al. 2009a, b; Guzmán-Morales et al. 2011), allows us to attribute the metals contained in factors 1 to 3 to geogenic sources.
The metals identified in factor 4 (Ni and V) are typically attributed to sources associated with fossil fuel combustion (Yuan et al. 2006; Dongarrà et al. 2010). On the other hand, previous works reported evidence associating these metals with geogenic sources of the studied area (Morton-Bermea et al. 2009a, b; Guzmán-Morales et al. 2011). Based on these two issues, we classify elements grouped in factor 4 as product of mixed sources.
The metals contained in factors 5 and 6 are interpreted as resulting from anthropogenic emissions, possibly related to vehicular traffic and/or industrial activities (Zhai et al. 2014; Zhang et al. 2013).
It is evident that metals associated with geogenic sources explained the maximum variance and allowed the differentiation of several geogenic sources as responsible for the enrichment of a large group of metals present in PM2.5. Such enrichment may be interpreted as a consequence of regional transport of geogenic material, influenced by the direction of the prevalent winds coming from the north (Fig. 2), as well as to the contribution of local geogenic material and/or resuspension of soil dust/geogenic material. The high concentration of geogenic metals at NE (Fig. 2, Table 6) confirms this argument.
The recognition of the high impact of geogenic sources in PM2.5 collected in MCMA is in agreement with the information obtained by means of REE assessment. Figure 4 shows the REE distribution patterns considering all REEs (normalized to chondrite, Evensen et al. 1978), in spite that only La, Ce, Sm, and Eu were included in the validation of the analytical procedure applied in this study. The low concentration of REE limited the assessment of REE pattern behavior in eight samples; all of them were collected during DW, when PM2.5 is higher and RH lower. The REE patterns in PM2.5 collected in MCMA clearly show a similar behavior as those shown by igneous rocks, confirming in this case, the influence of the regional geogenic material.
Distribution patterns of REEs normalized to chondrite (Evensen et al. 1978) in PM2.5. Patterns were calculated for samples collected during DW
The metals in factors 4–6 were associated with different anthropogenic sources, not necessarily common among them. Anthropogenic sources can be associated to industrial activities and traffic emissions, which show up the complexity of the urban air in the MCMA.
Conclusion
We showed the importance of local geogenic material and/or resuspension of soil dust/geogenic material as the main source of metals associated to the PM2.5 in the MCMA. Anthropogenic metal sources are minor sources to the PM2.5 of MCMA. Geogenic metal concentrations identified in the factors 1, 2, and 3 were found at NE of the MCMA which is in agreement with the dominant wind direction from north to south during 2011. The contribution of geogenic materials transported by prevalent winds represents a significant influence on the air quality in the MCMA. Although previous studies in the MCMA had already signaled the importance of geogenic sources in PM2.5, the use of ICP-MS might allow identifying the emitting sources in a more definite way, due to the detection of REE. Dry-warm season was the period with the highest geogenic metal concentrations. No significant differences in the spatial distribution of metals from anthropogenic sources were observed, suggesting common sources around MCMA.
Because the MCMA is a complex area with more than 20 million inhabitants exposed to this environment, the information obtained from this study, related to the impact of geogenic sources, the spatial and temporal behavior of metals in PM2.5, and the influence of meteorological parameters must be confirmed with further studies, in order to establish public policies aimed to improve the air quality of the area.
References
Amador-Muñoz, O., Villalobos-Pietrini, R., Miranda, J., & Vera-Avila, L. E. (2011). Organic compounds of PM2.5 in Mexico Valley: spatial and temporal patterns, behavior and sources. Science of the Total Environment, 409(8), 1453–1465. https://doi.org/10.1016/j.scitotenv.2010.11.026.
Amato, F., Pandolfi, M., Viana, M., Querol, X., Alastuey, A., & Moreno, T. (2009). Spatial and chemical patterns of PM 10 in road dust deposited in urban environment. Atmospheric Environment, 43(9), 1650–1659. https://doi.org/10.1016/j.atmosenv.2008.12.009.
Chen, Y., Schleicher, N., Chen, Y., Chai, F., & Norra, S. (2014). The influence of governmental mitigation measures on contamination characteristics of PM2.5 in Beijing. Science of the Total Environment, 490, 647–658. https://doi.org/10.1016/j.scitotenv.2014.05.049.
Cheng, Y., Lee, S. C., Ho, K. F., Chow, J. C., Watson, J. G., Louie, P. K. K., Cao, J. J., & Hai, X. (2010). Chemically-speciated on-road PM2.5 motor vehicle emission factors in Hong Kong. Science of the Total Environment, 408(7), 1621–1627. https://doi.org/10.1016/j.scitotenv.2009.11.061.
Chow, J. C., Watson, J. G., Edgerton, S. A., & Vega, E. (2002). Chemical composition of PM2.5 and PM10 in Mexico City during winter 1997. Science of the Total Environment, 287(3), 177–201. https://doi.org/10.1016/S0048-9697(01)00982-2.
Costello, A. B., & Osborne, J. W. (2005). Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Practical Assessment, Research & Evaluation, 10(7), 1–9.
Dockery, D. W., & Pope, C. A. (1994). Acute respiratory effects of particulate air pollution. Annual Review of Public Health, 15, 107–132.
Dongarrà, G., Manno, E., Varrica, D., Lombardo, M., & Vultaggio, M. (2010). Study on ambient concentrations of PM 10, PM 10–2.5, PM 2.5 and gaseous pollutants. Trace elements and chemical speciation of atmospheric particulates. Atmospheric Environment, 44(39), 5244–5257.
Evensen, N. M., Hamilton, P. J., & O'nions, R. K. (1978). Rare-earth abundances in chondritic meteorites. Geochimica et Cosmochimica Acta, 42(8), 1199–1212. https://doi.org/10.1016/0016-7037(78)90114-X.
Feng, S., Gao, D., Liao, F., Zhou, F., & Wang, X. (2016). The health effects of ambient PM 2.5 and potential mechanisms. Ecotoxicology and Environmental Safety, 128, 67–74. https://doi.org/10.1016/j.ecoenv.2016.01.030.
Finlayson-Pitts, B.J., Pitts, Jr. J.N. (2000). Chemistry of the upper and lower atmosphere-. Theory, experiments, and applications. Academic Press. USA. ISBN 0-12-257060-x
Gao, Y., Guo, X., Ji, H., Li, C., Ding, H., Briki, M., Tang, L., & Zhang, Y. (2016). Potential threat of heavy metals and PAHs in PM2. 5 in different urban functional areas of Beijing. Atmospheric Research, 178, 6–16.
Gauderman, W. J., Avol, E., Gilliland, G. F., Vora, H., Duncan Thomas, M. S., Berhane, K., McConnel, R., Kuenzli, M., Lurmann, F., Rappaport, E., Margolis, H., Bates, D., & Peters, J. (2004). The effect of air pollution on lung development from 10 to 18 years of age. New England Journal of Medicine, 351, 1057–1067.
Guzmán-Morales, J., Morton-Bermea, O., Hernández-Álvarez, E., Rodríguez-Salazar, M. T., García-Arreola, M. E., & Tapia-Cruz, V. (2011). Assessment of atmospheric metal pollution in the urban area of Mexico City, using Ficus benjamina as biomonitor. Bulletin of Environmental Contamination and Toxicology, 86(5), 495–500. https://doi.org/10.1007/s00128-011-0252-9.
Hieu, N. T., & Lee, B. K. (2010). Characteristics of particulate matter and metals in the ambient air from a residential area in the largest industrial city in Korea. Atmospheric Research, 98(2), 526–537. https://doi.org/10.1016/j.atmosres.2010.08.019.
Hu, Z. (2009). Spatial analysis of MODIS aerosol optical depth, PM2.5 and chronic coronary heart disease. International Journal of Health Geographics, 8(1), 27. https://doi.org/10.1186/1476-072X-8-27.
Jáuregui, O. E. (2000). El clima de la Ciudad de México (p. 2000). UNAM, México: Plaza y Valdés.
Karanasiou, A. A., Thomaidis, N. S., Eleftheriadis, K., & Siskos, P. A. (2005). Comparative study of pretreatment methods for the determination of metals in atmospheric aerosol by electrothermal atomic absorption spectrometry. Talanta, 65(5), 1196–1202. https://doi.org/10.1016/j.talanta.2004.08.044.
Karthikeyan, S., Joshi, U. M., & Balasubramanian, R. (2006). Microwave assisted sample preparation for determining water-soluble fraction of trace elements in urban airborne particulate matter: evaluation of bioavailability. Analytica Chimica Acta, 576(1), 23–30. https://doi.org/10.1016/j.aca.2006.05.051.
Kulshrestha, A., Satsangi, P. G., Masih, J., & Taneja, A. (2009). Metal concentration of PM2.5 and PM10 particles and seasonal variations in urban and rural environment of Agra, India. The Science of the Total Environment, 407(24), 6196–6204. https://doi.org/10.1016/j.scitotenv.2009.08.050.
Ma, Q. Y., Huang, D. Y., Zhang, H. J., Wang, S., & Chen, X. F. (2017). Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. International Immunopharmacology, 50, 139–145. https://doi.org/10.1016/j.intimp.2017.06.019.
Miranda, J., Crespo, I., & Morales, M. A. (2000). Absolute principal component analysis of atmospheric aerosols in Mexico City. Environemental Science and Pollution Research, 7(1), 14–18. https://doi.org/10.1065/espr199910.006.
Miranda, J., Barrera, V. A., Espinosa, A. A., Galindo, O. S., & Meinguer, J. (2005). PIXE analysis of atmospheric aerosols in Mexico City. X-Ray Spectrometry, 34(3), 315–319. https://doi.org/10.1002/xrs.823.
Moreno, T., Querol, X., Alastuey, A., Viana, M., Salvador, P., De la Campa, A., Artiñano, B., de la Rosa, J., & Gibbons, W. (2006a). Variations in atmospheric PM trace metal content in Spanish towns: illustrating the chemical complexity of the inorganic urban aerosol cocktail. Atmospheric Environment, 40(35), 6791–6803. https://doi.org/10.1016/j.atmosenv.2006.05.074.
Moreno, T., Querol, X., Castillo, S., Alastuey, A., Cuevas, E., Herrmann, L., Mounkaila, M., Josef, E., & Gibbons, W. (2006b). Geochemical variations in aeolian mineral particles from the Sahara–Sahel Dust Corridor. Chemosphere, 65(2), 261–270. https://doi.org/10.1016/j.chemosphere.2006.02.052.
Moreno-Rodríguez, V., Del Rio-Salas, R., Adams, D. K., Ochoa-Landin, L., Zepeda, J., Gómez-Alvarez, A., Palafox-Reyes, J., & Meza-Figueroa, D. (2015). Historical trends and sources of TSP in a Sonoran desert city: Can the North America Monsoon enhance dust emissions? Atmospheric Environment, 110, 111–121. https://doi.org/10.1016/j.atmosenv.2015.03.049.
Morton-Bermea, O., Hernández-Álvarez, E., González-Hernández, G., Romero, F., Lozano, R., & Beramendi-Orosco, L. E. (2009a). Assessment of heavy metal pollution in urban topsoils from the metropolitan area of Mexico City. Journal of Geochemical Exploration, 101(3), 218–224. https://doi.org/10.1016/j.gexplo.2008.07.002.
Morton-Bermea, O., Hernandez, E., Martinez-Pichardo, E., Soler-Arechalde, A. M., Santa-Cruz, R. L., Gonzalez-Hernandez, G., Beramendi-Orosco, L., & Urrutia-Fucugauchi, J. (2009b). Mexico City topsoils: heavy metals vs. magnetic susceptibility. Geoderma, 151(3), 121–125. https://doi.org/10.1016/j.geoderma.2009.03.019.
Pinkerton, K. E., Green, F. H. Y., Saiki, C., Vallyathan, V., Plopper, C. G., Gopal, V., Hung, D., Bahne, E. B., Lin, S., Ménache, M. G., & Schenker, M. B. (2000). Distribution of particulate matter and tissue remodeling in the human lung. Environmental Health Perspectives, 108(11), 1063–1069. https://doi.org/10.1289/ehp.001081063.
Pope, C. A., Dockery, D., & Schwartz, J. (1995). Review of epidemiological evidence of health effects of particulate air pollution. Inhalation Toxicology, 7, 1–18.
Pope, C. A., Burnett, R. T., Thun, M. J., Calle, E. E., Krewski, D., Ito, K., & Thurston, G. D. (2002). Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA, 287(9), 1132–1141. https://doi.org/10.1001/jama.287.9.1132.
Querol, X., Alastuey, A., Viana, M. M., Rodriguez, S., Artiñano, B., Salvador, P., Garcia Dos Santos, S., Fernandez-Patier, R., Ruiz, C. R., de la Rosa, J., Sánchez de la Campa, A., Menendez, M., & Gil, J. I. (2004). Speciation and origin of PM10 and PM2.5 in Spain. Journal of Aerosol Science, 35(9), 1151–1172. https://doi.org/10.1016/j.jaerosci.2004.04.002.
Querol, X., Pey, J., Minguillón, M. C., Pérez, N., Alastuey, A., Viana, M., Moreno, T., Bernabé, R. M., Blanco, S., Cárdenas, B., Vega, E., Sosa, G., Escalona, S., Ruiz, H., & Artiñano, B. (2008). PM speciation and sources in Mexico during the MILAGRO-2006 campaign. Atmospheric Chemistry and Physics, 8(1), 111–128.
Ragosta, M., Caggiano, R., Macchiato, M., Sabia, S., & Trippetta, S. (2008). Trace elements in daily collected aerosol: level characterization and source identification in a four-year study. Atmospheric Research, 89(1), 206–217. https://doi.org/10.1016/j.atmosres.2008.01.009.
Ravindra, K., Stranger, M., & Van Grieken, R. (2008). Chemical characterization and multivariate analysis of atmospheric PM2.5 particles. Journal of Atmospheric Chemistry, 59(3), 199–218. https://doi.org/10.1007/s10874-008-9102-5.
Rodríguez-Salazar, M. T., Morton-Bermea, O., Hernández-Álvarez, E., Lozano, R., & Tapia-Cruz, V. (2011). The study of metal contamination in urban topsoils of Mexico City using GIS. Environment and Earth Science, 62(5), 899–905. https://doi.org/10.1007/s12665-010-0584-5.
Rolph, G., Stein, A., & Stunder, B. (2017). Real-time Environmental Applications and Display system: READY. Environmental Modelling and Software, 95, 210–228. https://doi.org/10.1016/j.envsoft.2017.06.025.
Rosas, I., Belmont, R., & Jauregui, E. (1995). Seasonal variation of atmospheric lead levels in three sites in Mexico City. Atmosfera, 8(4).
Stein, A. F., Draxler, R. R., Rolph, G. D., Stunder, B. J. B., Cohen, M. D., & Ngan, F. (2015). NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bulletin of the American Meteorological Society, 96(12), 2059–2077. https://doi.org/10.1175/BAMS-D-14-00110.1.
Xu, L., Chen, X., Chen, J., Zhang, F., He, C., Zhao, J., & Yin, L. (2012). Seasonal variations and chemical compositions of PM2.5 aerosol in the urban area of Fuzhou, China. Atmospheric Research, 104, 264–272.
Yuan, Z., Lau, A. K. H., Zhang, H., Yu, J. Z., Louie, P. K., & Fung, J. C. (2006). Identification and spatiotemporal variations of dominant PM 10 sources over Hong Kong. Atmospheric Environment, 40(10), 1803–1815. https://doi.org/10.1016/j.atmosenv.2005.11.030.
Zhai, Y., Liu, X., Chen, H., Xu, B., Zhu, L., Li, C., & Zeng, G. (2014). Source identification and potential ecological risk assessment of heavy metals in PM 2.5 from Changsha. Sci. The Science of the Total Environment, 493, 109–115. https://doi.org/10.1016/j.scitotenv.2014.05.106.
Zhang, R., Jing, J., Tao, J., Hsu, S. C., Wang, G., Cao, J., Lee, C. S. L., Zhu, L., Chen, Z., Zhao, Y., & Shen, Z. (2013). Chemical characterization and source apportionment of PM2.5 in Beijing: seasonal perspective. Atmospheric. Chemical Physics, 13(14), 7053–7074.
Zhao, X., Zhang, X., Xu, X., Xu, J., Meng, W., & Pu, W. (2009). Seasonal and diurnal variations of ambient PM2.5 concentration in urban and rural environments in Beijing. Atmospheric Environment, 43(18), 2893–2900. https://doi.org/10.1016/j.atmosenv.2009.03.009.
Zhou, Z., Shao, T., Qin, M., Miao, X., Chang, Y., Sheng, W., Wu, F., & Yu, Y. (2017). The effects of autophagy on vascular endothelial cells induced by airborne PM2.5. Journal of Environmental Sciences. https://doi.org/10.1016/j.jes.2017.05.019.
Acknowledgements
The authors gratefully acknowledge the NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model and/or READY website (http://www.ready.noaa.gov) used in this publication.
Funding
This study was performed with financial support of Project 166295 from CONACyT (Consejo Nacional de Ciencia y Tecnología) and Project CPSG/087A/2014 FA from the SECyTI (Secretaría de Ciencia y Tecnología e Inovacción) as well as from Project IN116614 from DGAPA (Dirección General de Personal Académico, UNAM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morton-Bermea, O., Garza-Galindo, R., Hernández-Álvarez, E. et al. Recognition of the importance of geogenic sources in the content of metals in PM2.5 collected in the Mexico City Metropolitan Area. Environ Monit Assess 190, 83 (2018). https://doi.org/10.1007/s10661-017-6443-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6443-z