Abstract
Heavy metal pollution has become a global concern due to accumulation in tissue and transferable effects to humans via the food chain. This study focused on monitoring the accumulation of cadmium (Cd) and lead (Pb) in surface soil and body content: bone, heart, brain, liver, lung, muscle, kidney, feathers, feces, and gizzard contents of house crow Corvus splendens in the Klang region, Malaysia. The results revealed the occurrence of Pb and Cd in all biological samples from house crows, food contents, and surface soil samples. Heart and kidney accrued high amounts of Cd, while high amounts of Pb were found to accumulate in bones and feathers. Major discrepancies were also discovered in the concentrations of metals between juvenile and adults, as well as female and male bird samples. Concentrations of Pb and Cd in house crow internal tissues correlated significantly with that of bird feathers, but none could be established with that of surface soil. In addition, a significant correlation was observed between Pb concentration in the internal tissues to that of the feces, but the same was not the case when compared with the surface soil concentration. Metal accrual in the house crows feathers and feces may be through a long-term transmission via the food chain, which are eliminated from feathers via molting. This may suggest the utility of molted breast feathers of house crow in the bio-monitoring of Cd and Pb contamination, whereas feces of house crow appear only to be suitable for the bio-monitoring of Pb contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The degradation of the natural environment and subsequent industrialization in developing countries is soaring daily, hence the need for impact assessments as animals and humans are directly and indirectly affected by this degradation. The direct effect of this degradation and subsequent industrialization is an increase in pollution by toxic heavy metals. According to Li et al. (2001) and Lee et al. (2006), the increasing heavy metals’ concentration in the environment is a serious ecological problem requiring attention. Kelly et al. (1996) noted that surface soils act as a long-term sink of heavy metals and other pollutants. The long-term exposure to heavy metals in the environment and via the food chain, resulting from destructive anthropogenic activities, poses potential threats to public health (Sheppard et al. 2009). In another view, Adams et al. (2004) opined that heavy metals are bio-accumulated in plant tissues from contaminated soil. These heavy metals accumulate in animal tissues via the food chain, and eventually, it gets bio-accumulated in the tissues of animals who are the final consumers (Bilandžić et al. 2010). Generally, heavy metals are bio-accumulated in the body organs, especially kidneys and liver (Godt et al. 2006), which could result in increased mortality rate among the animal population inhabiting contaminated environment.
Over the past decade, there has been an increased concern towards the environmental toxicity of heavy metals and their resultant effects on resident biological communities, which led to the deployment of biological based monitoring strategies, involving the use of living organisms to measure heavy metals toxicity in contaminated environments (Burger et al. 1997; Kalisinska et al. 2004). According to Erwin and Custre (2000), birds have been effectively employed globally as bio-indicators of environmental pollution since 1960s. Bird species living close to urban habitat are at higher risks of being affected by heavy metals toxicity. Therefore, they serve as potential bio-indicator organisms in eco-toxicological research. House crow (Corvus splendens) is one of the bird species frequently seen in urban areas, as it inhabits areas close to human settlements and consume human waste scattered around homes. In many countries, synanthropic organisms, such as house crow, have been widely employed as bio-indicators to assess heavy metal contamination.
In the assessment of heavy metal concentration, lead (Pb) has received more attention due to its persistent nature, being able to remain in the environment for a prolonged period even after the source of contamination has been eliminated. It is in this regard that Scheifler et al. (2006) stressed that Pb toxicity is the greatest threat faced by blackbirds. In addition, cadmium (Cd) is equally lethal to birds, such as house crows, and has been reported to act in synergy with Pb toxicity (Manjula et al. 2015). According to the findings of Manjula et al. (2015), Cd and Pb concentration in the house crows’ feathers in urban regions is higher when compared with that of the house crows’ feathers from the rural regions. Breast feathers of house crows (Janaydeh et al. 2016) and house sparrows (Baker et al. 2017) have been used for bio-monitoring of heavy metal pollution. Generally, urban areas are more imparted by anthropogenic activities and therefore have a tendency to become more polluted with heavy metals such as Cd and Pb, compared to the rural regions (Manjula et al. 2015).
Several studies have documented the heavy metals’ concentration accumulated in feathers and feces of birds (Dauwe et al. 2000, 2005; Malik and Zeb 2009; Hashmi et al. 2013). Indeed, studies have shown that the buildup of heavy metals in body tissues and organs of birds has the tendency to impede their physiological activities, thereby affecting the birds’ population in general (Gragnaniello et al. 2001; Muralidharan et al. 2004). In assessing heavy metal accumulation in birds, varying body samples, such as blood, feces, feathers, kidney, and liver, have been used. However, several reports favor the use of feathers or feces, owing to the fact it is non-destructive and non-invasive (Abdullah et al. 2015). In addition, birds excrete substantial quantity of heavy metals through feather molt, making the determination and quantification of the level of contaminants easy (Malik and Zeb 2009; Zamani-Ahmadmahmoodi et al. 2010). Several studies also demonstrated a strong correlation between heavy metal concentration in breast feathers and internal tissues in birds, which suggests that breast feathers are ideal samples for the determination of metal concentration in birds (Goede 1985; Lee et al. 1989; Burger 1993; Dmowski 1999; Ranta et al. 1978; Markowski et al. 2013; Janaydeh et al. 2016; Baker et al. 2017). Fecal samples have been successfully employed to determine the transfer of contaminants from the environment via the food chain to birds. However, the use of feathers, generally, and breast feathers particularly in determining heavy metal pollution has not been widely employed. Studies focusing on other non-invasive techniques such as the use of fecal samples in determining metal pollution are also limited (Spahn and Sherry 1999; Dauwe et al. 2004; Morrissey et al. 2005). Very few studies had focused on the correlation between the heavy metals’ concentration in food and feces (Dauwe et al. 2004; Morrissey et al. 2005), whereas studies dedicated to determining the correlation between metal concentration in feces and uptake in internal tissues are generally lacking.
Likewise, in Italy, heavy metal contamination in the environment has been detected with the use of hooded crow as a bio-indicator (Giammarino et al. 2014). Omnivorous birds such as corvids were often studied for bio-accumulation of toxic heavy metals. Horai et al. (2007) provided some notable comparative data on corvids in a research carried out in Japan, in which the concentrations of Pb and Cd metal in hepatic tissues of jungle crow were analyzed. Similarly, a polish study was conducted by Komosa et al. (2012) on heavy metal accumulations (Cd and Pb) in the liver tissues of the rook. In addition, the hooded crow was used as a bio-indicator in a study conducted by Giammarino et al. (2014) to assess the heavy metal contaminations in Italy. In Malaysia, however, only a limited information is available on heavy metal accumulation in wild birds in general and house crows in particular. It is therefore important to investigate the bio-accumulation of these metals in wild birds, particularly house crows, and especially in the highly anthropogenic impacted Malacca strait of Malaysia.
The objectives of this study are as follows:
(1) To determine the concentration of the heavy metals, Cd and Pb, in biological samples of house crow from Klang habitats to assess the level of heavy metals pollution, (2) to determine the house crow for use as suitable species for bio-monitoring programs in polluted habitats like Klang area, (3) to determine the concentration of heavy metals in house crow feces and feathers and the use of them as possible non-destructive and alternative samples to internal organs for future studies, and (4) to determine the correlation between Cd and Pb concentration in body tissues and gizzard contents or surface soil from Klang area.
Materials and methods
Geographical location
The Klang area is one of the most developing areas in the Straits of Malacca as well as in Malaysia. The current geographical location was selected based on its presence in one of the most urbanized areas with more than 16% of the country’s population (more than 4.4 million people). Klang is going through a rapid urbanization and development which made it prone to numerous anthropogenic activities resulting to the buildup of heavy metals in inhabitant regions that include both terrestrial and aquatic living organisms (Ismail et al. 1993; Zulkifli et al. 2010; Naji and Ismail 2012; Nayan and Ishak 2013; Janaydeh et al. 2016). The industrial areas of Klang account for about 70% of the anthropogenic activities, and for this, it is classified among the most developed areas of Selangor state. Huge construction works and numerous industrial activities such as power generation, smelting of iron, and chemical and plastic manufacturing are the dominant activity taking place in Klang area. The entire anthropogenic activities consequence in amassing environmental pollutant made up of heavy metals which are primarily from agricultural, transportation, and industrial activities. Naji and Ismail (2012) have ascribed the proliferation of heavy metals in the operation to corresponding activities, which they stated to be above recommended and background levels. Lately, it has been reported that the soil of Klang area is polluted with heavy metals that can posing a great risk to human life. However, children are at even greater risk compared to adult human population, among which some tolerance to the heavy metal toxicity has been observed (Yuswir et al. 2015).
Sample collection
Crow collection
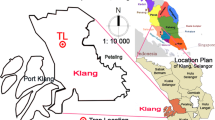
Culling method was employed, with assistance from the Klang Municipal authority’s department of public health, to collect forty-two house crows (Corvus splendens) in Klang (trap location N 03° 08′ 22.00″—E 101° 22′ 20.08″) in December 2014 (Fig. 1). Their weight was measured and classified according to their age in which 21 crows were adults and other 21 crows were juveniles, and according to gender, there were 24 male and 18 female crows. The population of the crows is being controlled and managed through culling exercise to reduce the number of bird species in the area. Cervical dislocation was used to euthanize the house crows. The method employed in sacrificing the birds was as approved by the EU Regulation 1099/2009. Samples of heart, liver, bone, brain, muscular, kidney, and lung tissues were collected (each 1–3 g approximately) and kept in clean vials in ice bath prior to being transported to the laboratory. In separate sealed plastic bags, samples of gizzard content, breast feathers, and feces were collected from all birds. Until investigation, samples were preserved under − 80 °C.
Surface soil sampling and storage
In January 2015, a total of 40 surface soil samples were collected randomly from industrial sites, residential places, agricultural areas, town area, and schools in the Klang area, Selangor (Fig. 1). The global positioning system coordinates were utilized to record all sampling locations (Table 1). Fifty grams of surface soil per sample was collected from the top 5 cm of the soil (and put into polyethylene bags) with stainless steel scoops and then sent to the laboratory. The stainless steel scoops are the preferred choice for sampling surface, as it cannot contaminate the samples with heavy metals (Mitra and Kebbekus 1997; Usepa 2011). To obtain constant dry weight, the soil samples were oven dried at 55–60 °C for 72 h, samples were then grinded with clean, dry mortar and pestle. Then, the samples were filtered through a 2-mm stainless steel sieve prior to polyethylene bag storage.
Heavy metal determination in the body contents
Until constant dry weight was achieved, the samples of lung, heart, kidney, liver, muscle, feces, brain, bone, and gizzard contents were oven dried at 50–55 °C. Meanwhile, as per Burger (1993), the house crow feathers were washed vigorously using double-distilled water swapped with acetone followed by overnight air dry. Concentrated nitric acid (HNO3 AnalaR grade, BDH 69%) was applied to digest about 0.5 g of the body tissues. Similarly, a mixture of the concentrated nitric acid and perchloric acid (AnalaR grade 60%) in a ratio 4:1 was utilized to digest about 0.5–1.0 g of homogenized feces and gizzard content samples within the range of 1 h, then at 40 °C, they were transferred into a hot-block digester and completely digested for at least 3 h at high temperature of 140 °C (Ismail 1993; Yap et al. 2003b). Double-distilled water was used to dilute the digested samples until 40 ml and filtered using Whatman filter paper no. 1. The resultant filtrate solutions were then preserved at 4 °C in clean polyethylene vials until analyzed with an air-acetylene flame atomic absorption spectrophotometer (AAS) (Perkin Elmer Model 4100). The result obtained was presented as weight per gram of dry weight (μg/g dw).
Heavy metal determination in the surface soil
The direct aqua regia method was employed to determine the concentrations of Cd and Pb in surface soil samples. About 1 g of the sample was weighed, grinded, and then transferred into a digestion tube. A mixed solution of concentrated nitric acid (HNO3 AnalaR grade, BDH 69%) and perchloric acid (AnalaR grade 60%) in a ratio 4:1 was then employed to digest the soil samples. Samples were digested in a vertical tube placed in a hot-block digester at 40 °C for 1 h and raised up to 140 °C for at least 3 h to obtain complete digestion (Ismail 1993; Yap et al. 2003b). Subsequently, the digested samples were cooled to room temperature and diluted with 40 ml of double-distilled water. The solutions were filtered using Whatman filter paper no. 1, and the resultant filtrate solutions were then preserved at 4 °C in sterilized polyethylene vials until analyzed using an air-acetylene flame atomic absorption spectrophotometer. Result obtained were presented as weight per gram of dry weight (μg/g dw).
Quality control
To avoid contamination, 10% HCl was used to wash the glassware’s and equipment’s used for this study, followed by cleaning with deionized distilled water and air dried prior to use. Quality control calibration curves were produced to guarantee accuracy and precision of the analytical method by evaluating numerous-level calibration standards and using 1000 mg/l (BDH Spectrosol) stock solution, individual metal studied standard solutions were prepared. The analysis procedure quality is assured with certified reference material (CRM) that was earlier utilized for surface soil (International Atomic Energy Agency, Soil-5, Vienna, Austria) and for tissue (dogfish muscle; National Research Council Canada, Ottawa, ON, Canada). There were satisfactory results in metal recovery from analytical results for CRM, and the certified values showed the percentage values as 88.6% for Cd and 86.1% for Pb. Quality control samples prepared from single element standard solutions of Cd and Pb were examined in every eight samples.
Statistical analyses
All statistical analyzes were done using SPSS (version 24.0, SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk one sample test was employed to test for conformity to a normal distribution. The result revealed non-conformity to normal distribution and was then normalized through log 10 transformation. For the determination of significant levels applicable for any two variables and strength of relationships, the Pearson’s correlation coefficient was employed. In order to reveal the statistical significance between the concentrations of heavy metals in both juvenile and adult, female and male, Student’s t test was employed.
Results
The concentrations of heavy metal Cd and Pb present in 42 house crows were studied using AAS. The accruals of Cd and Pb in surface soil and house crow tissues, such as brain heart, kidney, liver, lung, muscle, bone, and breast feathers, including gizzard and feces contents, are shown in Fig. 2. The result reveals the occurrence of heavy metals in the entire biological samples of house crow as well as surface soil collected from Klang area. The amount of Pb concentrations was the highest, followed by Cd. The Cd concentrations (2.03 ± 0.30 μg/g dw; 1.78 ± 0.43 μg/g dw) were the highest in heart and kidney tissues, respectively, whereas lowest values of Cd concentration (0.66 ± 0.13 μg/g dw) were observed in muscle tissues. In addition, breast feathers, kidney, and bone recorded the highest Pb concentrations at 32.01 ± 12.16, 21.47 ± 11.48, and 23.44 ± 7.06 μg/g dw, respectively. Whereas the lowest concentration (5.06 ± 3.64 μg/g dw) was observed in muscle tissues. In surface soil samples, the mean values of Pb (56.11 ± 0.49 μg/g dw) and Cd (0.66 ± 0.23 μg/g dw) concentrations were recorded in samples collected from Kalang area (Fig. 2), while the result of heavy metals according pollution sites did not appear in this manuscript.
Figure 3 shows a comparison of the heavy metal (Cd) concentrations in the tissues of juvenile and adult and male and female house crows. In all body tissues examined, the adult house crow showed the highest values of Cd concentration while the lowest concentration of Cd was detected in the juvenile house crow. In addition, statistically significant variation were shown between Cd concentrations of the two age groups of the house crow (P < 0.05) for all body tissues except lung, muscle, and bone (P > 0.05) (Fig. 3a). The highest values of Cd concentrations were recorded in female house crows as compared to the male house crows. There was a significant difference between Cd concentration in the two sex groups of house crow (P < 0.05) for body tissues except bone (P > 0.05) (Fig. 3b). Moreover, the female house crows accumulated significantly more heavy metal (Cd) (P < 0.05) in breast feathers and gizzard than the male house crows (Fig. 3b).
Effects of heavy metal (Pb) against gender and age of house crows are shown in Fig. 4. Generally, the adult house crows were observed the highest values of Pb concentration while the lowest concentration of Pb was detected in the juvenile house crows. Statistically, Student’s t test shows significant differences between the two age groups of the Pb concentration in house crows (P < 0.05) (Fig. 4a). Moreover, the adult house crows accumulated significantly more heavy metal (Pb) (P < 0.05) in feces and gizzard content than the male house crows (Fig. 4b).
Table 2 explains the relationship between Cd concentrations in various body tissues and substances collected from the house crow that was sampled from Klang area. The amount of Cd concentrations detected in the breast feathers positively correlated with the amount of Cd detected in almost all internal tissues with the exception of bone. Concentrations of Cd in breast feathers were strongly correlated to concentrations of Cd in kidney and muscle with a positive relationship, while Cd concentrations in breast feathers reported a moderately positive correlation with Cd in brain and liver tissues. In addition, the level of Cd concentrations in gizzard content was positively correlated with concentrations of Cd in the heart, lung, liver, kidney, brain, and breast feathers tissues of the house crow (P < 0.05). Furthermore, no significant correlation was reported between Cd concentrations in the surface soil and Cd in internal organs or breast feathers of house crow from Klang area.
A correlation between Pb concentrations in different body tissues and contents of house crow collected from Klang area is illustrated in Table 3. The level of Pb concentrations in breast feathers showed a positive correlation with concentrations in all internal organs except bone tissues. Concentrations of Pb in breast feathers were strongly correlated to concentrations of Pb in lung and muscle with a positive relationship, while Pb concentrations in breast feathers reported as positive correlation with Pb in the heart and kidney. There is a positive correlation between Pb concentrations in feces and Pb concentrations in heart, lung, kidney, muscle, breast feathers, and gizzard contents. In addition, the Pb concentrations in gizzard content show a positive correlation with concentrations of Pb in heart and breast feathers of house crow. Also, Pb in house crow internal tissues was correlated significantly with a breast feathers, feces, and gizzard contents but did not show any correlation with the surface soil.
Discussion
Heavy metal concentrations in Malaysia especially Klang area at the west shoreline of Peninsular Malaysia were previously reported in the literature in sediments and bio-monitor organisms (Ismail et al. 1993; Ismail and Ramli 1997; Yap et al. 2002, 2003a, 2005). However, there is very few available reported data on heavy metal pollution in house crow at Peninsular Malaysia, especially in the Klang area at the Straits of Malacca. Results from the current study are important in providing information on heavy metal accumulation in house crow in general and in food and feces contents of house crow, specifically in Klang habitat at the Straits of Malacca, Malaysia. These results describe the relationship between heavy metal concentrations in breast feathers and internal tissues or between metal concentrations in food, surface soil, and feces in wild birds, suggesting the importance of the use of breast feathers or feces as a substitute sample to internal tissue organs, in analyzing heavy metals buildup in house crow. Furthermore, the outcomes of this study will help in assessing the degree of vulnerability of house crow to the level of ecological contamination and potential health risk of heavy metals in the habitat, which is vital to the environmental risk assessment on human toxicity effect.
In this study, high concentrations of Cd and Pb metals were detected in all body content samples from studied house crows sampled from Klang area at the Straits of Malacca and differs according to body tissue of origin (Fig. 2). Kidney and liver are the major internal organs for heavy metal detoxification in house crow, while the molting breast feathers serve as external tissues for storage and elimination of heavy metals. Furthermore, the highest concentrations of Pb were reported in bone tissues, which confirmed that the bone tissues are a place of final lead deposition (Scheuhammer 1987). The concentrations of Cd and Pb in feces of house crow from Klang area, Malaysia, are higher than those present in house crow, common myna, and ring dove from Punjab, India (Kler et al. 2014) and also higher than those reported in great tits from Portugal (Costa et al. 2013). Detectable concentrations of Cd and Pb in feces of the chicks of great tit have been previously reported by Nyholm (1995), great blue heron (Fitzner et al. 1995) and little blue heron (Spahn and Sherry 1999). Lead and cadmium levels of 1.59 and 0.11 ppm were reported by Spahn and Sherry (1999). Meanwhile, 6 and 0.5 ppm, respectively, were detected in feces and feathers by Fitzner et al. (1995). Pb and Cd levels in our study were markedly higher. In addition, the levels of heavy metals in gizzard contents of house crows are higher than those recorded in gizzard contents of spruce grouse from North Central Ontario, Canada (Bendell-Young and Bendell 1999). The amount of heavy metals in house crow is dependent on food chains and pollutants from the environment, due to the opportunist and scavenging nature of house crow it feeds mainly on refuse around human habitats. In addition, the Klang habitat has huge anthropogenic activities (Yap et al. 2003a; Ismail and Naji 2011; Yuswir et al. 2015; Janaydeh et al. 2016), this suggest that the highest Cd and Pb concentrations in the body contents of house crow are caused by ingestion of contaminated food by surface soil, which is the major origin of heavy metal contamination (Conner et al. 1994). The categorization of Pb concentrations in Galliformes liver organ by Franson (1996) into three groups; 2–6 μg/g is a subclinical exposure, 3–6 μg/g to be toxic, and > 5 to > 20 as fatal. The lethal dose (LD50) for Pb in laboratory studied rats was shown to be 70 μg/g of the body weight (Kotsonis and Klaassen 1977). In this study, 7.14% of the house crows demonstrated liver Pb concentrations more than the three category reported by Franson (1996) (> 5 to > 20, fatal). High concentrations of Cd were found in the kidney and liver tissues (1.78 and 1.38 μg/g dw respectively). Eisler (1985) concluded in his study that Cd concentrations > 10 μg/g ww (approximately 30–40 μg/g dw) are poisonous in the kidney and liver organs of wild birds. Thus, the Cd concentration for liver and kidney organs in this present study is not beyond the poisonous point.
The significant observation of this research is the correlation between metals (Cd and Pb) level and bird age factor in diverse body contents of house crow. The concentration of the heavy metals appears to be age dependent, as a higher metal concentration is detected in adult organs compared to juvenile organs (Fig. 3). The findings of this study support the report of Swaileh and Sansur (2006). In addition, the accumulation of Cd and Pb metals in molted breast feathers and gizzard contents from the body of house crow is age dependent. The high energy requirement of the adult house crow which is as a result of energy required for mating, egg laying, and other physiological activities, leading to the ingestion of more contaminated food resulting to increase in heavy metals in the adults compare to the juveniles. Certain discoveries have revealed major difference in the concentrations of Cd and Pb metals among different sex groups (Burger 1993; Naccari et al. 2009; Kekkonen et al., 2012), which is comparable to the major difference in the amount of Cd and Pb metals detected in female and male (P < 0.05) in this study. The present study revealed the comparison of the males’ body contents, the females’ stored higher Cd, and Pb metals. Heavy metals were also stored in the feces, molted breast feathers, and gizzard contents from female house crows body, subsequently haboring high amount of the heavy metals (Cd and Pb) in comparison with the molted feathers, gizzard, and feces from the body content of male house crow.
Few studies assessed the correlation between the heavy metals of molting breast feathers and the internal organs in wild birds from the Klang region (Janaydeh et al. 2016). Therefore, the breast feathers in house crows are employed as a good indicator of heavy metal contamination, suggesting the relevance of estimating quantitatively the levels of heavy metals in molting breast feathers against to the body internal organs like the kidney and liver tissues (Baker et al. 2017; Janaydeh et al. 2016). In this study, Pearson’s correlation test showed numerous significant positive relationships between the levels of heavy metals (Cd and Pb) in the house crows’ breast feather and internal tissues. The metal concentration in breast feathers of the house crows is reflected by the history of internal exposure and accumulation. The correlation of metals in internal tissues and breast feathers in wild birds had been predicted in few findings (e.g., Goede 1985; Lee et al. 1989; Burger 1993; Dmowski 1999; Ranta et al. 1978; Markowski et al. 2013). Also, Lee et al. (1989) revealed major positive correlations in heavy metal concentration in internal organs and its level in breast feathers, this is in accordance with the findings of this research.
Bird feces can provide valuable information on the concentration of heavy metals in the environment and the corresponding concentration available in the bird’s food (Spahn and Sherry 1999; Dauwe et al. 2004; Morrissey et al. 2005). Employing feces in metal bio-monitoring has tremendous advantages. Apart from the fact that it is a non-destructive and non-invasive approach, feces usually contain higher amount of the heavy metals compared to the amount in the food consumed, which simplifies quantification (Morrissey et al. 2005). In addition, Pb in house crow tissues correlated significantly with its levels in feces and gizzard contents but showed no correlation with surface soil samples from Klang area. Cd concentration in feces significantly correlated with its level surface soil only. Therefore, it is safe to suggest that feces are suitable as an indicator of Pb metal accumulation in house crows, to estimate quantitatively the concentrations of Pb metals in feces related to the body organs. The heavy metal concentration in feces of house crows reflects adequately internal tissue levels or food exposure levels. Moreover, Berglund et al. (2011) reported a few significant correlations between metal concentration in feces and its level liver tissues, which is comparable with our results. In general, this study shows that the feces can be used to estimate internal levels of Pb metal in internal organs of house crow.
Food is the major route of contamination in house crows. Therefore, the high concentration of heavy metals in the food of house crow suggests higher concentration of heavy metal pollution in Klang habitat (surface soil). Analyzing the concentration of heavy metals in the body organs of house crows in this study allowed us to assess the environmental pollution of metals in bird and their immediate habitat (surface soil). The chronic effect of exposure of internal body organs to heavy metal is reflected in their accumulations. This is dependent on the level of contaminants in consumed food, for instance, that the brain and muscle are the accumulation sites, while kidney and liver are the detoxification sites. These later organs perform a vital role in the conversion of food and xenobiotic bio-transformation. Likewise, molted breast feathers and lung tissues are the locations for excretion and exposure. The buildup of heavy metals in feces and breast feathers shows the occurrence of pollutants in the environmental habitats. A very important bio-indicator for heavy metal pollution in birds are the birds’ breast feathers (Veerle et al. 2004; Naccari et al. 2009; Janaydeh et al. 2016), this is true owing to the fact that heavy metals (Cd and Pb) are accumulated and excreted in molted breast feather tissues. Similarly, it is dependent on age due to contact with environmental habitat contamination. Our results recommend the house crows as a perfect model species in ecological research which is cost-effective and suitable bio-monitor for environmental pollution.
Conclusion
In Klang area, (Cd) and (Pb) were reported as highly accumulated heavy metals in house crows at significant levels and adequate for analysis and identification. In addition, sex and age are relevant factors that can account for variation in the pattern of bio-accumulation in wild populations with recent exposure to high concentrations of these metals. Furthermore, this research recommends the use of feces and breast feathers of house crow as a substitute sample to internal organs in the study of metals buildup in house crow. Through food contamination, heavy metals in the environment are conveyed to individuals and chronic exposure can cause severe health problems to house crows. Therefore, heavy metal accumulation in generally can pose a health risk at both the individual and the population level, particularly if exposure is prolonged. However, increased heavy metals in feces and breast feathers may indicate an active removal of excess heavy metals from the body and therefore stresses the importance of feces and breast feathers as potential suitable non-destructive and non-invasive sample for bio-monitoring. The high trophic position of crows as well as their abundance make them a suitable species for bio-monitoring programs especially in areas with high anthropogenic activities such as Klang area.
References
Abdullah, M., Fasola, M., Muhammad, A., Malik, S. A., Bostan, N., Bokhari, H., Kamran, M. A., Shafqat, M. N., Alamdar, A., Khan, M., & Ali, N. (2015). Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: a case study from severely contaminated areas. Chemosphere, 119, 553–561. https://doi.org/10.1016/j.chemosphere.2014.06.068.
Adams, M. L., Zhao, F. J., McGrath, S. P., Nicholson, F. A., & Chambers, B. J. (2004). Predicting cadmium concentrations in wheat and barley grain using soil properties. Journal of Environmental Quality, 33(2), 532–541. https://doi.org/10.2134/jeq2004.5320.
Baker, N. J., Dahms, S., Gerber, R., Maina, J., & Greenfield, R. (2017). Metal accumulation in house sparrow (Passer domesticus) from Thohoyandou, Limpopo province, South Africa. African Zoology, 52(1), 43–53. https://doi.org/10.1080/15627020.2017.1293491.
Bendell-Young, L. I., & Bendell, J. F. (1999). Grit ingestion as a source of metal exposure in the spruce grouse, Dendragapus canadensis. Environmental Pollution, 106(3), 405–412. https://doi.org/10.1016/S0269-7491(99)00101-3.
Berglund, Å. M., Koivula, M. J., & Eeva, T. (2011). Species-and age-related variation in metal exposure and accumulation of two passerine bird species. Environmental Pollution, 159(10), 2368–2374. https://doi.org/10.1016/j.envpol.2011.07.001.
Bilandžić, N., Sedak, M., Đokić, M., & Šimić, B. (2010). Wild boar tissue levels of cadmium, lead and mercury in seven regions of continental Croatia. Bulletin of Environmental Contamination and Toxicology, 84(6), 738–743. https://doi.org/10.1007/s00128-010-9999-7.
Burger, J. (1993). Metals in avian feathers: bioindicators of environmental pollution. Reviews in environmental toxicology, 5, 203–311.
Burger, J., Kennamer, R. A., Brisbin, I. L., & Gochfeld, M. (1997). Metal levels in mourning doves from South Carolina: potential hazards to doves and hunters. Environmental Research, 75(2), 173–186. https://doi.org/10.1006/enrs.1997.3789.
Connor, E. E., Scanlon, P. F., & Kirkpatrick, R. L. (1994). Bioavailability of lead from contaminated sediment in northern bobwhites, Colinus virginianus. Archives of Environmental Contamination and Toxicology, 27(1), 60–63.
Costa, R. A., Eeva, T., Eira, C., Vaqueiro, J., & Vingada, J. V. (2013). Assessing heavy metal pollution using Great Tits (Parus major): feathers and excrements from nestlings and adults. Environmental Monitoring and Assessment, 185(6), 5339–5344. https://doi.org/10.1007/s10661-012-2949-6.
Dauwe, T., Bervoets, L., Blust, R., Pinxten, R., & Eens, M. (2000). Can excrement and feathers of nestling songbirds be used as biomonitors for heavy metal pollution? Archives of Environmental Contamination and Toxicology, 39(4), 541–546. https://doi.org/10.1007/s002440010138.
Dauwe, T., Janssens, E., Bervoets, L., Blust, R., & Eens, M. (2004). Relationships between metal concentrations in great tit nestlings and their environment and food. Environmental Pollution, 131(3), 373–380. https://doi.org/10.1016/j.envpol.2004.03.009.
Dauwe, T., Janssens, E., Pinxten, R., & Eens, M. (2005). The reproductive success and quality of blue tits (Parus caeruleus) in a heavy metal pollution gradient. Environmental Pollution, 136(2), 243–251. https://doi.org/10.1016/j.envpol.2005.01.009.
Dmowski, K. (1999). Birds as bioindicators of heavy metal pollution: review and examples concerning European species. Acta Ornithologica-Polska Akademia, 34, 1–26.
Eisler, R. (1985). Cadmium hazards to fish, wildlife, and invertebrates: a synoptic review Contaminant Hazards Reviews Report, vol. 85 (1.2), Washington, DC.
Erwin, M., & Custer, T. W. (2000). Herons as indicators. In J. A. Kushlan & H. Hanfer (Eds.), Heron conservation. (pp. 310–330). San Diego: Academic Press.
Fitzner, R. E., Gray, R. H., & Hinds, W. T. (1995). Heavy metal concentrations in great blue heron fecal castings in Washington State: a technique for monitoring regional and global trends in environmental contaminants. Bulletin of Environmental Contamination and Toxicology, 55(3), 398–403.
Franson, J. C (1996) Interpretation of tissue lead residues in birds other than waterfowl. In Environmental contaminants in wildlife: interpreting tissue concentrations, W. N. Beyer, G. H. Heinz, and A. W. Redmon-Norwood (eds.). Society of Environmental Toxicology and Chemistry Special Publication, CRC Press, Inc., Boca Raton, Florida, 265–279 pp.
Giammarino, M., Quatto, P., Squadrone, S., & Abete, M. C. (2014). The hooded crow (Corvus cornix) as an environmental bioindicator species of heavy metal contamination. Bulletin of Environmental Contamination and Toxicology, 93(4), 410–416. https://doi.org/10.1007/s00128-014-1362-y.
Godt, J., Scheidig, F., Grosse-Siestrup, C., Esche, V., Brandenburg, P., Reich, A., & Groneberg, D. A. (2006). The toxicity of cadmium and resulting hazards for human health. Journal of Occupational Medicine and Toxicology, 1(1), 1–6.
Goede, A. A. (1985). Mercury, selenium, arsenic and zinc in waders from the Dutch Wadden Sea. Environmental Pollution Series A, Ecological and Biological, 37(4), 287–309. https://doi.org/10.1016/0143-1471(85)90119-9.
Gragnaniello, S., Fulgione, D., Milone, M., Soppelsa, O., Cacace, P., & Ferrara, L. (2001). Sparrows as possible heavy-metal biomonitors of polluted environments. Bulletin of Environmental Contamination and Toxicology, 66(6), 719–726. https://doi.org/10.1007/s001280068.
Hashmi, M. Z., Malik, R. N., & Shahbaz, M. (2013). Heavy metals in eggshells of cattle egret (Bubulcus ibis) and little egret (Egretta garzetta) from the Punjab province, Pakistan. Ecotoxicology and Environmental Safety, 89, 158–165. https://doi.org/10.1016/j.ecoenv.2012.11.029.
Horai, S., Watanabe, I., Takada, H., Iwamizu, Y., Hayashi, T., Tanabe, S., & Kuno, K. (2007). Trace element accumulations in 13 avian species collected from the Kanto area, Japan. Science of the Total Environment, 373(2), 512–525. https://doi.org/10.1016/j.scitotenv.2006.10.010.
Ismail, A. (1993). Heavy metal concentrations in sediments off Bintulu, Malaysia. Marine Pollution Bulletin, 26(12), 706–707. https://doi.org/10.1016/0025-326X(93)90556-Y.
Ismail, A., & Naji, A. (2011). Assessment of metals contamination in Klang River surface sediments by using different indexes. Environment Asia, 4(1), 30–38.
Ismail, A., & Ramli, R. (1997). Trace metals in sediments and molluscs from an estuary receiving pig farms effluent. Environmental Technology, 18(5), 509–515. https://doi.org/10.1080/09593331808616566.
Ismail, A., Badri, M. A., & Ramlan, M. N. (1993). The background levels of heavy metal concentration in sediments of the west coast of Peninsular Malaysia. Science of the Total Environment, 134, 315–323. https://doi.org/10.1016/S0048-9697(05)80032-4.
Janaydeh, M., Ismail, A., Zulkifli, S. Z., Bejo, M. H., Aziz, N. A. A., & Taneenah, A. (2016). The use of feather as an indicator for heavy metal contamination in house crow (Corvus splendens) in the Klang area, Selangor, Malaysia. Environmental Science and Pollution Research, 23(21), 22059–22071. https://doi.org/10.1007/s11356-016-7223-y.
Kalisińska, E., Salicki, W., Mysłek, P., Kavetska, K. M., & Jackowski, A. (2004). Using the mallard to biomonitor heavy metal contamination of wetlands in North-Western Poland. Science of the Total Environment, 320(2), 145–161. https://doi.org/10.1016/j.scitotenv.2003.08.014.
Kekkonen, J., Hanski, I. K., Väisänen, R. A., & Brommer, J. E. (2012). Levels of heavy metals in House Sparrows (Passer domesticus) from urban and rural habitats of southern Finland. Ornis Fennica, 89(2), 91–98.
Kelly, J., Thornton, I., & Simpson, P. R. (1996). Urban geochemistry: a study of the influence of anthropogenic activity on the heavy metal content of soils in traditionally industrial and non-industrial areas of Britain. Applied Geochemistry, 11(1), 363–370. https://doi.org/10.1016/0883-2927(95)00084-4.
Kler, T. K., Vashishat, N., & Kumar, M. (2014). Heavy metal contamination in excreta of avian species from Ludhiana district of Punjab. International Journal, 2(7), 873–879.
Komosa, A., Kitowski, I., & Komosa, Z. (2012). Essential trace (Zn, Cu, Mn) and toxic (Cd, Pb, Cr) elements in the liver of birds from Eastern Poland. Acta Veterinaria (Beograd), 62(5–6), 579–589. https://doi.org/10.2298/AVB1206579K.
Kotsonis, F. N., & Klaassen, C. D. (1977). Toxicity and distribution of cadmium administered to rats at sublethal doses. Toxicology and Applied Pharmacology, 41(3), 667–680. https://doi.org/10.1016/S0041-008X(77)80020-3.
Lee, D. P., Honda, K., Tatsukawa, R., & Won, P. O. (1989). Distribution and residue level of mercury, cadmium and lead in Korean birds. Bulletin of Environmental Contamination and Toxicology, 43(4), 550–555. https://doi.org/10.1007/BF01701934.
Lee, S. W., Lee, B. T., Kim, J. Y., Kim, K. W., & Lee, J. S. (2006). Human risk assessment for heavy metals and as contamination in the abandoned metal mine areas, Korea. Environmental Monitoring and Assessment, 119(1–3), 233–244. https://doi.org/10.1007/s10661-005-9024-5.
Li, X., Poon, C. S., & Liu, P. S. (2001). Heavy metal contamination of urban soils and street dusts in Hong Kong. Applied Geochemistry, 16(11), 1361–1368. https://doi.org/10.1016/S0883-2927(01)00045-2.
Malik, R. N., & Zeb, N. (2009). Assessment of environmental contamination using feathers of Bubulcus ibis L., as a biomonitor of heavy metal pollution, Pakistan. Ecotoxicology, 18(5), 522–536. https://doi.org/10.1007/s10646-009-0310-9.
Manjula, M., Mohanraj, R., & Devi, M. P. (2015). Biomonitoring of heavy metals in feathers of eleven common bird species in urban and rural environments of Tiruchirappalli, India. Environmental Monitoring and Assessment, 187(5), 1–10.
Markowski, M., Kaliński, A., Skwarska, J., Wawrzyniak, J., Bańbura, M., Markowski, J., & Bańbura, J. (2013). Avian feathers as bioindicators of the exposure to heavy metal contamination of food. Bulletin of Environmental Contamination and Toxicology, 91(3), 302–305. https://doi.org/10.1007/s00128-013-1065-9.
Mitra, S., &Kebbekus, B. B. (1997). Environmental chemical analysis. CRC -Press.
Morrissey, C. A., Bscendell-Young, L. I., & Elliott, J. E. (2005). Assessing trace-metal exposure to American dippers in mountain streams of southwestern British Columbia, Canada. Environmental Toxicology and Chemistry, 24(4), 836–845. https://doi.org/10.1897/04-110R.1.
Muralidharan, S., Jayakumar, R., & Vishnu, G. (2004). Heavy metals in feathers of six species of birds in the District Nilgiris, India. Bulletin of Environmental Contamination and Toxicology, 73(2), 285–291. https://doi.org/10.1007/s00128-004-0425-x.
Naccari, C., Cristani, M., Cimino, F., Arcoraci, T., & Trombetta, D. (2009). Common buzzards (Buteo buteo) bio-indicators of heavy metals pollution in Sicily (Italy). Environment International, 35(3), 594–598. https://doi.org/10.1016/j.envint.2008.11.002.
Naji, A., & Ismail, A. (2012). Metals fractionation and evaluation of their risk connected with urban and industrial influx in the Klang River surface sediments, Malaysia. Environment Asia, 5, 17–25.
Nayan, D. S., & Ishak, C. F. (2013). Soil factors influencing heavy metal concentrations in medicinal plants. Pertanika Journal of Tropical Agricultural Science, 36(2), 161–178.
Nyholm, N. E. I., Sawicka-Kapusta, K., Swiergosz, R., & Laczewska, B. (1995). Effects of environmental pollution on breeding populations of birds in southern Poland. Water, Air, and Soil Pollution, 85(2), 829–834. https://doi.org/10.1007/BF00476932.
Ranta, W. B., Tomassini, F. D., & Nieboer, E. (1978). Elevation of copper and nickel levels in primaries from black and mallard ducks collected in the Sudbury district, Ontario. Canadian Journal of Zoology, 56(4), 581–586. https://doi.org/10.1139/z78-083.
Scheifler, R., Coeurdassier, M., Morilhat, C., Bernard, N., Faivre, B., Flicoteaux, P., Giraudoux, P., Noël, M., Piotte, P., Rieffel, D., de Vaufleury, A., & Badot, P. M. (2006). Lead concentrations in feathers and blood of common blackbirds (Turdus merula) and in earthworms inhabiting unpolluted and moderately polluted urban areas. Science of the Total Environment, 371(1), 197–205. https://doi.org/10.1016/j.scitotenv.2006.09.011.
Scheuhammer, A. M. (1987). The chronic toxicity of aluminium, cadmium, mercury, and lead in birds: a review. Environmental Pollution, 46(4), 263–295. https://doi.org/10.1016/0269-7491(87)90173-4.
Sheppard, S. C., Grant, C. A., Sheppard, M. I., De Jong, R., & Long, J. (2009). Risk indicator for agricultural inputs of trace elements to Canadian soils. Journal of Environmental Quality, 38(3), 919–932. https://doi.org/10.2134/jeq2008.0195.
Spahn, S. A., & Sherry, T. W. (1999). Cadmium and lead exposure associated with reduced growth rates, poorer fledging success of little blue heron chicks (Egretta caerulea) in south Louisiana wetlands. Archives of Environmental Contamination and Toxicology, 37(3), 377–384.
Swaileh, K. M., & Sansur, R. (2006). Monitoring urban heavy metal pollution using the House Sparrow (Passer domesticus). Journal of Environmental Monitoring, 8(1), 209–213. https://doi.org/10.1039/B510635D.
US Environmental Protection Agency (USEPA). (2011). Exposure factors handbook. Washington, DC: National Center for Environmental Assessment (EPA/600/R-09/052F).
Veerle, J., Tom, D., Rianne, P., Lieven, B., Ronny, B., & Marcel, E. (2004). The importance of exogenous contamination on heavy metal levels in bird feathers. A field experiment with free-living great tits, Parus major. Journal of Environmental Monitoring, 6(4), 356–360. https://doi.org/10.1039/b314919f.
Yap, C. K., Ismail, A., & Tan, S. G. (2003a). Cd and Zn concentrations in the straits of Malacca and intertidal sediments of the west coast of Peninsular Malaysia. Marine Pollution Bulletin, 46(10), 1349–1353. https://doi.org/10.1016/S0025-326X(03)00193-0.
Yap, C. K., Ismail, A., & Tan, S. G. (2003b). Background concentrations of Cd, Cu, Pb and Zn in the green-lipped mussel Perna viridis (Linnaeus) from Peninsular Malaysia. Marine Pollution Bulletin, 46(8), 1044–1048. https://doi.org/10.1016/S0025-326X(03)00163-2.
Yap, C. K., Ismail, A., Tan, S. G., & Omar, H. (2002). Concentrations of Cu and Pb in the offshore and intertidal sediments of the west coast of Peninsular Malaysia. Environment International, 28(6), 467–479. https://doi.org/10.1016/S0160-4120(02)00073-9.
Yap, C. K., Rahim-Ismail, A., Ismail, A., & Tan, S. G. (2005). Analysis of heavy metal concentration data (Cd, Cu, Pb and Zn) in different geochemical fractions of the surface sediments in the Straits of Malacca by the use of correlation and multiple linear stepwise regression analyses. Malaysian Applied Biology, 34(2), 51.
Yuswir, N. S., Praveena, S. M., Aris, A. Z., Ismail, S. N. S., & Hashim, Z. (2015). Health risk assessment of heavy metal in urban surface soil (Klang District, Malaysia). Bulletin of Environmental Contamination and Toxicology, 95(1), 80–89. https://doi.org/10.1007/s00128-015-1544-2.
Zamani-Ahmadmahmoodi, R., Esmaili-Sari, A., Savabieasfahani, M., Ghasempouri, S. M., & Bahramifar, N. (2010). Mercury pollution in three species of waders from Shadegan wetlands at the head of the Persian Gulf. Bulletin of Environmental Contamination and Toxicology, 84(3), 326–330. https://doi.org/10.1007/s00128-010-9933-z.
Zulkifli, S. Z., Mohamat-Yusuff, F., Arai, T., Ismail, A., & Miyazaki, N. (2010). An assessment of selected trace elements in intertidal surface sediments collected from the Peninsular Malaysia. Environmental Monitoring and Assessment, 169(1–4), 457–472. https://doi.org/10.1007/s10661-009-1189-x.
Acknowledgments
Special thanks to the Department of Public Health at Majlis Perbandaran Klang city for the invaluable support in collection of crows and samples. In addition, we wish to express sincere gratitude to the Universiti Putra Malaysia for providing sampling and infrastructural support.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Janaydeh, M., Ismail, A., Omar, H. et al. Relationship between Pb and Cd accumulations in house crow, their habitat, and food content from Klang area, Peninsular Malaysia. Environ Monit Assess 190, 47 (2018). https://doi.org/10.1007/s10661-017-6416-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6416-2