Abstract
In this study, the biosorption properties of Jatoba (Hymenaea courbaril) fruit shell for removal of Pb(II) and Cd(II) ions from aqueous solutions, and its potential as a low-cost biosorbent for water treatment, were investigated. The Jatoba fruit shell (JBin) was subjected to different treatments with heated water (JBH2O) and sodium hydroxide (JBNaOH) to modify its surfaces and improve its adsorption properties. The chemical modification of the surfaces of the resulting materials was confirmed by analyzing the compositions and structural features of the raw material and the chemically treated materials using SEM, FTIR, 13C NMR, and pHpzc. The ability of the biosorbents to remove the metal ions was investigated with batch adsorption procedures. The adsorption data were then examined in detail by applying adsorption models of Langmuir, Freundlich, and Dubinin-Radushkevich. The results showed that the experimental data were best described by the Langmuir model for the Pb-JBin and Cd-JBNaOH systems, the Freundlich model for the Pb-JBH2O and Pb-JBNaOH systems, and the Dubinin-Radushkevich model for Cd-JBin and Cd-JBH2O systems. The maximum adsorption capacities of JBNaOH obtained using the Langmuir model reached values of 30.27 and 48.75 mg g−1 for Cd(II) and Pb(II), respectively. The adsorption kinetic studies showed that the pseudo-second-order model was the best fitted to the experimental data, and adsorptions for Pb-JBH2O and Cd-JBH2O are controlled by intraparticle diffusion mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial development in a country almost always promotes the living standard and the economic wealth of its population. However, as it has been seen time and time again over decades, the industrial growth of a country can also lead to undesired environmental problems, especially associated with the industrial wastes generated by various industrial chemical processes. Depending on the industry type (chemical, textile, and metallurgical), industrial effluents may contain significant concentrations of heavy metals and/or organic compounds (Chakravarty et al. 2010; Palin et al. 2016). And such industrial effluents that oftentimes are discarded to the environmental without any treatment and control, as has been done in many countries, particularly pose the most significant environmental damages and health risks.
Many industrial effluents possess heavy metals that are cumulative and non-biodegradable in the environment; they can thus severely contaminate rivers and streams and harm aquatic life (Taşar et al. 2014). Among the elements, heavy metals, lead, and cadmium are considered the most toxic and to cause problems to human health even at extremely low concentrations. Notably, lead poisoning can impair the nervous system, gastrointestinal tract, kidney, and reproductive system (Palin et al. 2016) whereas cadmium poisoning can cause renal and carcinogenic disorders, pulmonary insufficiency, bone lesions, cancer, anemia, and hypertension (Jain et al. 2015).
Hence, several physicochemical methods have been in development for removal of such heavy metals from wastewaters. The most commonly considered methods include extraction, ion exchange, chemical precipitation, membrane separation, and adsorption (Witek-krowiak et al. 2011). Biosorption, which uses waste materials from renewable sources as adsorbents, has been emerging as an efficient and promising method for the removal of pollutants from wastewaters. Moreover, the method is considered as clean, efficient, and inexpensive (Mahmood-ul-Hassan et al. 2015), besides being biological and relatively more benign for environmental control (Jain et al. 2015). The effectiveness of a given biosorbent to remove pollutants may be measured by the ability of the active sites on its surfaces to bind and pull out the pollutant species from solutions (Pavan et al. 2006).
Recently, several agro-industrial residues have been evaluated as low-cost biosorbent materials for the removal of heavy metals from aqueous solutions. These materials include banana stalk, corn cob, sunflower head (Mahmood-ul-Hassan et al. 2015) peanut shell (Witek-krowiak et al. 2011), fruit wastes (Kelly-Vargas et al. 2012), rice straw and bran (Ding et al. 2012; Zafar et al. 2015), papaya seeds (Gilbert et al. 2011), and ginger residue (Kumar and Ahmand 2011) which have also been successfully employed for removal of pollutants.
With the aim of finding new renewable sources, which can be used as efficient adsorbent materials for heavy metal ions in solutions, herein we investigated Jatoba (Hymenaea courbaril) fruit shells as biosorbents. The Jatoba tree is originally found in Brazil’s Amazon and Atlantic forest regions. The tree is often planted in areas of heterogeneous reforestation and afforestation, and in places such as parks and gardens as it grows very easily. Jatoba fruit possesses indehiscent pods of hard shell, presenting dimensions of 8 to 15 cm in length, 3 to 5 cm in width, and 4 to 6 cm in thickness, with each fruit containing about two to four seeds. The fruit has a hard shell, weighing up to 66% of the fruit’s total weight. Each seed is surrounded by a yellow pulp, which when dried is edible and is often consumed with regional dishes as well as cakes, breads, and hot cereals (Silva et al. 2001). Additionally, the use of Jatoba fruit and its stem bark for treatment of gastritis, ulcers, and diarrhea and as anti-inflammatory agent has been reported in the literature (Grandi et al. 1989; Boniface et al. 2017). Thus, besides promoting an appropriate means for the disposition of Jatoba fruit wastes, the present work aims to make useful biosorbents from Jatoba fruit’s shell, studying different treatments of modification, and apply them in removal of Pb(II) and Cd(II) ions from aqueous solutions. To prepare the most effective biosorbent from Jatoba fruit’s shell, the latter was treated with basic solution and boiled water, and the adsorption property of each material was investigated. The modification techniques may reduce organic components of the raw material such as cellulose, hemicellulose, and tannins, which create a high chemical oxygen demand when used as adsorbent material, and increase surface active sites for adsorption (Pezoti et al. 2016).
Materials and methods
Raw material
Jatoba fruits were collected from native trees of the countryside of Santa Fe City, Parana State, Brazil. First, the shells were separated from the fruits, and then washed with a copious amount of tap water, followed by a copious amount of distilled water, and dried in an oven at 100 °C for 12 h. Subsequently, the resulting material was ground using a knife mill (Marconi brand, model MA048) and submitted for granulometric separation. The resulting samples with particle diameters of 250 to 425 μm were properly stored for posterior studies.
Preparation of biosorbents
Different biosorbents were prepared from Jatoba fruit peels by washing with distilled water (JBin), boiling in distilled water (JBH2O), and boiling in 0.1 mol L−1 NaOH solution (JBNaOH). Typically, 100 g of the raw material was placed in 2 L of boiled distilled water and/or basic solution. The mixtures were magnetically stirred and separated from the solutions, after 2 h of contact time. The biosorbent obtained from the treatment under basic solution (JBNaOH) was properly rinsed with distilled water until the wash water reached to a pH value of close to 7.0. Then, all the biosorbents were dried at 105 °C for 24 h, and stored for further use.
Characterization of biosorbents
The biosorbents obtained above were characterized by pHpzc, FTIR spectroscopy, scanning electronic microscopy (SEM), and 13C nuclear magnetic resonance (NMR 13C) spectroscopy. pHpzc was determined following the methodology described by Prahas et al. (2008). pHpzc corresponds to the condition which is pHinitial = pHfinal or ∆pH = 0. FTIR spectra were obtained in the range between 4000 and 400 cm−1 (resolution of 4 cm−1 and acquisition rate of 20 scans min−1), using a Bomem MB100 spectrometer. Pellets were prepared mixing 1 mg of sample with 500 mg KBr (Merck) in an agate mortar, and then pressed at 5 t cm−2 for 5 min. The morphologies of the materials were examined from their SEM images obtained by using a Shimadzu model SS-550 Superscan instrument. The preparation of the samples for imaging was carried out by depositing gold (Au) over the samples using a Shimadzu sputter coater IC-50 ion in 3 cycles of 5 min at a current of 60 mA. Solid-state 13C NMR spectra were acquired by using cross-polarization magic angle spinning (CP/MAS) technique with a Mercury Plus 300 (Varian) NMR instrument, operating at a resonant frequency of 75.5 MHz and at 20 °C. The surface areas of materials were determined using a Quantachrome, NOVA 1200e surface area and pore size analyzer. The BET surface areas were calculated from the mathematical model proposed by Brunauer–Emmett–Teller (BET), and results were 2.07, 2.61, and 4.64 m2 g−1 for JBin, JBH2O, and JBNaOH biosorbents, respectively.
Biosorption experiments
The biosorption studies were carried out using standard solutions of metal ions, prepared from their corresponding salts: lead nitrate (Pb(NO3)2, Merck) and cadmium nitrate (Cd(NO3)2, Merck). The working solutions were prepared by diluting the standard solutions with distilled water. For each adsorption experiment, 25.0 mL of heavy metal ion solution of known concentration was placed in contact with 0.125 g of biosorbent (dosage = 5.0 g L−1) in polypropylene tubes, and subjected to agitation (at 180 rpm) at room temperature. After different contact times, aliquots of solutions were sampled and properly stored in glass recipients for further analysis. The determination of heavy metal concentrations was performed by flame atomic absorption spectrometry (FAAS).
The effect of pH on the materials’ sorption properties toward heavy metals was carried out using 500.0-mg L−1 solutions after adjusting their pH in the range of 2.0 to 6.0, with nitric acid and sodium hydroxide solutions. The mixtures, containing the biosorbent and aliquot of heavy metal solution, were agitated for 120 min at room temperature.
The biosorption kinetic studies were carried out using heavy metal ion solutions with a concentration of 500 mg L−1 at pH 5.50 and 4.00 for Pb(II) and Cd(II), respectively. The systems were kept under agitation in the interval between 5 and 360 min. The concentration of heavy metal on biosorbents at a given time, t (q t , mg g−1), was calculated from Eq. 1:
where C 0 is the initial concentration (500.0 mg L−1), C t is the heavy metal concentration in solution at any time (mg L−1), V is the solution volume (L), and W is the biosorbent mass (g).
Equilibrium studies of the biosorption processes were performed using heavy metal ion solutions with concentrations ranging from 50.0 to 800.0 mg L−1 at pH 5.5 and 4.0 for Pb(II) and Cd(II), respectively, and an agitation time of 180 min. The adsorption capacity of biosorbents to retain heavy metal ions (q e , mg g−1) was then calculated by using Eq. 2:
where C 0 and C e correspond to initial and final equilibrium concentrations, respectively, V is the solution volume (L), and W is the biosorbent mass (g).
To gain insight into the interaction between adsorbates and biosorbents, and to obtain the parameters that can provide information about adsorption mechanisms, the models of Langmuir (1916; Pezoti et al. 2016), Freundlich (Freundlich 1906; Pezoti et al. 2014), and Dubinin–Radushkevich (1960; Gautam et al. 2014) were applied. In addition, for evaluation of the adsorption dynamic in relation to time, kinetic models of pseudo-first order (Lagergren 1898; Salem and Awwad 2014) and pseudo-second order (Pezoti et al. 2014) and intraparticle diffusion (Ofomaja 2010) were employed. Equations of models are shown in Table 1.
The kinetic and isotherm models were fitted to the experimental data, using Origin 6.1® software. The selections of mathematic models, which explain the adsorption processes, were performed by analysis of determination coefficients (R 2) and variation from the normal standard deviation (Δ qe ). The Δ qe values were calculated applying Eq. 3:
where N is the data number and q e , exp and q e,cal (mg g−1) are values of experimental and calculated adsorption capacities, respectively.
Desorption procedure
Desorption experiments were carried out after biosorption assays. Typically, the biosorbents were loaded with the heavy metal (Pb(II) and Cd(II) ions using the same condition for the equilibrium studies (biosorbent dosage of 5.0 g L−1 and Pb(II) and Cd(II) solutions with concentrations of 100.0 and 200.0 mg L−1, respectively). The materials obtained after filtration were then washed with distilled water to remove any non-adsorbed species and dried at 40 °C for 24 h. Then, 0.125 g of biosorbents was placed in contact with 25.0 mL of HCl 0.1 mol L−1 and/or NaOH 0.1 mol L−1 and stirred for 2 h. The concentrations of the heavy metal ions in the liquid phase were determined, and percentages of the metal ions removed were then calculated.
Results and discussion
Characterization of biosorbents
The pHpzc values of sorbent materials can provide important information about the possible biosorption mechanisms occurring on the material. Figure 1 shows plots constructed from the pHfinal values and ∆pH vs. pHinitial values for biosorbents JBin, JBH2O, and JBNaOH. The results indicate that the pHpzc values of JBin, JBH2O, and JBNaOH were found to be 3.4, 3.0, and 6.0, respectively. It is known that biosorption processes of heavy metals are favored in pH values higher than those of pHpzc, where the surface of the biosorbent material is negatively charged. This negative surface of the material enables metal ions to anchor on it, favoring the biosorption processes. On the other hand, at pH values lower than those of pHpzc, the functional groups present on the biosorbent surfaces are protonated and can generate a positive liquid charge, which can repel metal ions and create an unfavorable biosorption process (Salem and Awwad 2014).
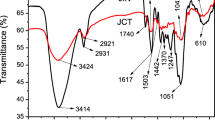
The biosorbents are constituted mainly of macromolecules of cellulose surrounded by hemicelluloses and lignin molecules. The main functional groups that may exist on the biosorbent surfaces are hydroxyl, carbonyl, carboxylic, and phenolic. Figure 2 shows FTIR spectra of biosorbents obtained. As can be seen, the spectra have a strong band at 3400 cm−1, which can be attributed to stretching of O–H and N–H bonds characteristic of phenolic compounds, carboxylic acids, and alcohol, amide, and amine groups (Chakravarty et al. 2010; Salem and Awwad 2014). The band at 2930 cm−1 can be assigned to C–H stretching methyl groups. The peaks at 1730 and 1620 cm−1 can be assigned to C–O stretching vibration of ester and carboxyl groups (Nagy et al. 2014; Rangabhashiyam and Selvaraju 2015). Additionally, bands between 1651 and 1421 cm−1 can be attributed to the vibrational stretching of the C–O bond of amide and carboxylic groups, whereas the peak at 1250 cm−1 may be assigned to C–O stretching of phenols and carboxylic acids (Nagy et al. 2014). The band at 1058 cm−1 has been attributed to C–O stretching, suggesting the presence of lignin (Palin et al. 2016). According to the results, it can be seen that the JBNaOH spectrum showed changes when compared to other materials. The peaks at 1730, 1455, 1400, and 1250 cm−1 decreased significantly, which suggests that the performed treatment could have removed amide and carboxylic compounds, as well as phenolic compounds.
The 13C NMR analyses have been used for identification of functional groups present in hemicellulose, lignin, and cellulose structures. Figure 3 shows 13C NMR spectra for biosorbents herein obtained. According to the results, it is possible to identify characteristic peaks of the functional groups present in the biosorbent structure, including the peak at 21 ppm that can be attributed to acetyl and methyl groups of hemicelluloses, and the peak at 56 ppm that can be assigned to methoxy groups of lignin (Fu et al. 2015). The peaks at 65 and 73 ppm were attributed to aliphatic carbon 6 (C6) of crystalline cellulose, and to carbons 2 (C2), 3 (C3), and 5 (C5) of the cellulose, respectively, while the peak at 105 ppm was assigned to carbon 1 (C1) of the anomeric cellulose. The peaks in the range of 82 to 89 ppm have been assigned to C4 of the amorphous and crystalline cellulose, respectively (Cao et al. 2016). At lower intensity, the peaks are observed from 135 ppm which indicates the presence of aromatic lignin (Fu et al. 2015). The spectra of JBin and JBH2O showed peaks at 38 ppm corresponding to amide and carboxylate groups, which is not observed in the case of JBNaOH, indicating that the chemical modification carried out with NaOH alters the surface composition of material. This result is consistent with those obtained from the FTIR spectra.
Figure 4 shows the SEM images of JBin (Fig. 4a), JBH2O (Fig. 4b), and JBNaOH (Fig. 4c). As can be observed from Fig. 4, the biosorbents show a heterogeneous structure with cavities and cracks on its surfaces, which are distributed from unorganized forms (De Souza et al. 2012; Peláez-Cid et al. 2013). The SEM image of JBNaOH (Fig. 4c) shows more cracks and pores in comparison with the images of JBin and JBH2O (Fig. 4a, b), indicating that the chemical modification promotes changes on surface features of the material, possibly due to removal of phenolic compounds present in the precursor, as can be seen in FTIR spectra, and increasing the values of the BET surface area. This alteration in the surface characteristic of the JBNaOH is in accordance with results of 13C NMR and FTIR analyses.
Adsorption studies
Effect of pH
Figure 5 shows the effect of pH on the ability of the biosorbent to remove Pb(II) and Cd(II) ions from aqueous solutions. According to the results, the pH effect on biosorption is more evidenced for JBNaOH, which shows the highest q e values. These highest values of heavy metal removal for JBNaOH prove the influence of chemical treatment performed. The q e of JBNaOH increases with pH until values of about 5.5, which was 5.6 and 2.1 times higher than those found at pH 2.0, for biosorption of Pb(II) and Cd(II), respectively. It was observed that at high pH values, solutions show lowest hydrogen ion concentrations, consequently producing a smaller competition between hydrogen ions and metal ions by the adsorption sites on the biosorbents, leading to improvement in biosorption capacities. Additionally, the pH values that lead to greater removal of heavy metal ions by JBNaOH were found to be lower than those of pHpzc, under conditions in which the biosorbent surface is protonated. This suggests that the modification from chemical treatment improved the material’s porosity, and consequently its biosorption capacity. The BET surface area value of JBNaOH is 2.24 times higher than that of JBin and 1.77 higher than that of JBH2O. The results obtained herein agree with those in other studies involving biosorption of metal ions. Palin et al. (2016) studied the biosorption of Pb(II) by quebracho tannin resin, and they observed that the best removal occurs at pH 5.0. Using rice straw as biosorbent, Ding et al. (2012) obtained the best adsorption of ion Cd(II) in pH about 5.0.
Adsorption kinetic
With the aim of understanding the adsorption dynamic of heavy metal ions on the materials, pseudo-first- and pseudo-second-order models and intraparticle diffusion model were fitted to the experimental data, and the results are shown in Fig. 6. It can be observed from the figures that the equilibriums were established in about 75 min for all biosorbents. The kinetic parameters obtained from the fits of models to the experimental data for adsorption of Pb(II) and Cd(II) ions on different biosorbents are listed in Table 2. According to results for both heavy metals, the pseudo-second-order model presented highest values of R 2 and lowest values of ∆ qe , when compared with the values observed for the pseudo-first-order model. The pseudo-second-order model may be considered a special kind of Langmuir kinetic, which assumes that the concentration of adsorbate is constant in relation to time and the total number of available binding sites depends on the adsorbed amount at equilibrium (Gupta and Bhattacharyya 2011). As shown in Table 2, R 2 and ∆ qe values ranged from 0.9612 to 0.9906 and 2.94 to 6.47, respectively, for the adsorption of Pb(II) on different JB biosorbents. For adsorption of Cd(II), the values of parameters R 2 and ∆ qe ranged from 0.9728 to 0.9844 and 3.72 to 4.04, respectively. Additionally, it is possible to observe that the values of q e,exp and q e,cal were close to each other, confirming the best fit of the pseudo-second-order model to experimental data. These results are in agreement with works reported in the literature on the adsorption of Pb(II) and Cd(II) on other biosorbents (Huang and Liu 2013; Palin et al. 2016).
The intraparticle diffusion model has been applied to investigate the occurrence of diffusion mechanism in the biosorption system. The model is represented by linear fits of a graphic of q t vs. t 0.5. Figure 7 shows the intraparticle diffusion model for biosorption kinetics of Pb(II) and Cd(II) for different biosorbents, respectively, and Table 3 presents the calculated parameters. The parameter K id is obtained from a straight slope, and the intercept corresponds to the value of C i .
Figure 7 shows different fits, indicating that performed treatments promoted significant changes on the surface of biosorbents, which influenced directly on the biosorption mechanisms of Pb(II) and Cd(II) ions. The intraparticle diffusion is considered an important step of the biosorption process, when the straight line of fit passes through the origin (Palin et al. 2016). As can be seen from the figures, Pb-JBin, Cd-JBin, and Cd-JBNaOH systems showed graphics with two steps, which are related to processes of gradual biosorption and final equilibrium, respectively. On the other hand, Pb-JBH2O and Cd-JBH2O systems showed three steps, where the first step could be attributed to the diffusion of heavy metal from the solution to the external surface of the biosorbent, the second step to intraparticle diffusion, and the third step to the equilibrium (Gupta and Bhattacharyya 2011). These profiles indicate that intraparticle diffusion is not the rate-limiting step in the biosorption of Pb(II) and Cd(II) on JBH2O.
The Pb-JBNaOH system exhibited a different fit profile of all other materials. The system presented only one step, which can be attributed to the equilibrium state. In this state, a decrease in the diffusion rate of the adsorbate occurs, due to its low concentration in solution (Gao et al. 2013). The Pb-JBNaOH system presented a low value of K id constant (low slope), added to a high value of C i . Additionally, biosorption kinetic (Fig. 7) showed that the system reached equilibrium at a time of about 25 min, indicating a high initial rate of adsorbate transference from the solution to the biosorbent surface. These characteristics demonstrate that the chemical modification of JB shells with NaOH improved the adsorption capacity of the biosorbent, mainly for the Pb(II) ions.
Adsorption isotherms
Figure 8 shows experimental data and the non-linear fitting of Langmuir, Freundlich, and Dubinin–Radushkevich models for biosorption of Pb(II) and Cd(II), respectively. The Langmuir model assumes that the adsorption process occurs in monolayer on a surface that exhibits a finite number of sites, which are equally available for interaction with the adsorbate molecule. The empirical model of Freundlich considers that the adsorption process occurs in multilayer on a heterogeneous surface, with non-uniform distribution of energetic sites that present different heats of adsorption. The Dubinin–Radushkevich equation is an empirical model used to distinguish the physical and chemical adsorption of metal ions from the analysis of free energy per molecule of adsorbate, which can be calculated by the use of the B DR constant. The adsorption process occurs by chemisorption when 8 < E < 16 kJ mol−1 and by physisorption when E < 8 kJ mol−1 (Pezoti et al. 2016).
In Table 4, the biosorption isotherm parameters calculated from non-linear fits are presented. As can be seen, the Langmuir model gives the best fit for the experimental data of the system Pb-JBin, with the highest value of R 2 (0.9313) and lowest value of ∆ qe (14.08). On the other hand, the Freundlich model described the systems Pb-JBH2O and Pb-JBNaOH better, giving R 2 and ∆ qe values of 0.9733 and 8.81 for JBH2O and 0.9190 and 14.37 for JBNaOH, respectively. The separation factor (R L ) calculated from the Langmuir model allows to estimate if the adsorption is favorable or unfavorable. For values 0 < R L < 1, the process is favorable; for R L > 1, the adsorption is unfavorable; for R L = 1, the process is linear; and for R L = 0, the adsorption occurs in an irreversible way (Pillai et al. 2013; Pezoti et al. 2016). The initial concentrations of Pb(II) in this study were from 50.0 to 800.0 mg L−1 for all adsorbents used, and values of R L ranged from 0.5 to 0.06 for JBin, 0.77 to 0.17 for JBH2O, and 0.13 to 8.85 × 10−3 for JBNaOH, indicating that Pb(II) biosorption on different JB biosorbents is favorable. The same behaviors were reported by Yuvaraja et al. (2014) who investigated the biosorption of Pb(II) on Solanum melongena. Additionally, the parameters n F and 1/n F of the Freundlich model provides information related to the intensity and spontaneity of the biosorption process, respectively (Mahapatra et al. 2012; Sivasankar et al. 2012). As shown in Table 4, values of n F ranged from 2.57 to 4.99 for Pb(II) biosorption on the different biosorbents, indicating that the process is favorable, which is in concordance with that observed from parameter R L of Langmuir. The parameter 1/n F , which allows estimation of the surface heterogeneity of material, gives values ranging from 0 to 1. This means that the surface becomes more heterogeneous when its value gets closer to zero. 1/n F values ranged from 0.39 to 0.20, indicating high degree of heterogeneity of the JB biosorbents. The Pb-JBin and Pb-JBNaOH systems exhibit the highest values of free energy (E) of 9.13 and 25.00 kJ mol−1, respectively, indicating that the biosorption of Pb(II) on JBin and JBNaOH occurs by a chemisorption process. On the other hand, for Pb-JBH2O, the E value was 5.00 kJ mol−1, indicating that the biosorption may occur by physisorption. Anayurt et al. (2009) investigated the adsorption of Pb(II) on Lactarius scrobiculatus biomass, obtaining a value of E = 10.3 kJ mol−1.
Investigation of the adsorption of Cd(II) ions indicated that the Dubinin–Radushkevich model showed the best fit for systems Cd-JBin and Cd-JBH2O, with the highest R 2 value (0.9436 and 0.9369, respectively) and lowest ∆q e value (13.93 and 15.07, respectively). For system Cd-JBNaOH, the Langmuir model presented the best fit to experimental data, with R 2 of 0.8980 and ∆q e of 15.21. The separation factor (R L ) ranged between 0.83 and 0.24 for JBin; 0.80 and 0.20 for JBH2O, and 0.53 and 0.072 for JBNaOH, indicating that the biosorption of Cd(II) on different materials is favorable. The R L values were also evaluated by Pillai et al. (2013) and Awwad and Salem (2014), which showed the biosorption of Cd(II) on loquat (Eriobotrya japonica) leaves and xanthated nano banana cellulose, respectively. As shown in Table 4, for systems Cd-JBin, Cd-JBH2O, and Cd-JBNaOH, the values of n F ranged between 1.96 and 3.72, characteristic of a favorable process, such as that observed from R L values. Additionally, the values of 1/n F range between 0.51 and 0.27, indicating that the modification of the JB biosorbents increases the degree of heterogeneity of the surface of the materials. The values of biosorption free energy (E) (Table 4) for Cd(II) ranged from 2.50 to 5.00 kJ mol−1, demonstrating that the physical interaction between biosorbent and adsorbate is favorable. Chakravarty et al. (2010) studied the biosorption of Cd(II) on heartwood powder of Areca catechu biosorbent and verified from the Dubinin–Radushkevich model that the adsorption process followed a physisorption mechanism.
The adsorption capacity (Q m ) obtained for the materials based on the Langmuir model (Table 4) demonstrated that there was an increase in the biosorption capacity of Pb(II) after the treatment with heat water (JBH2O) and NaOH (JBNaOH), reaching values of 19.60 and 48.75 mg g−1, respectively. The results of Cd(II) biosorption showed that Q m values for JBin and JBH2O were similar, and JBNaOH showed the highest value corresponding to 30.27 mg g−1.
In Table 5, the values of Q m reported in literature for different biosorbents toward adsorption of Pb(II) and Cd(II) are compiled. As can be seen, the values of Q m obtained in the present study are in agreement with the values reported in the literature, indicating that the Jatoba fruit shell can result in a good biosorbent.
Desorption
Desorption studies were carried out to verify the regeneration of biosorbents. Table 6 shows desorption results of Pb(II) and Cd(II) from the JBin, JBH2O, and JBNaOH biosorbents. According to results, the use of HCl 0.1 mol L−1 as eluent removed more than 92% of heavy metal ions uptaken by the biosorbents studied, indicating the efficiency of this procedure. On the other hand, NaOH (0.1 mol L−1) solution showed low removal of heavy metal ions with values less than 35%. Therefore, under acid conditions, hydrogen ions substitute the heavy metal ions uptaken through the ion exchange mechanism (Gautam et al. 2014). The greater efficient of HCl solution when comprising NaOH solution as eluent of heavy metal uptake on biosorbents was also observed in other studies reported in literature (Pillai et al. 2013; Gautam et al. 2014).
Conclusion
We have demonstrated the use of Jatoba fruit’s shell as an effective biosorbent for removal of heavy metal ions from aqueous solutions. By comparing various materials, including those chemically treated, it was found that the NaOH-treated Jatoba fruit’s shell (JBNaOH) not only showed different surface characteristics compared to the original material, according to analyses of SEM, FTIR, 13C-NMR, and pHpzc, but also improved adsorption properties toward heavy metal ions compared with the latter. The biosorption equilibrium studies of Pb(II) and Cd(II) showed that the Langmuir model had the best fit for the Pb-JBin and Cd-JBH2O systems, the Freundlich model for Pb-JBH2O and Pb-JBNaOH, and the Dubinin–Radushkevich model for Cd-JBin and Cd-JBH2O, indicating the heterogeneity on the surface of biosorbents. The biosorption maximum capacity values of Langmuir ranged from 19.60 to 48.75 mg g−1 for Pb-JB systems and from 25.68 to 30.27 mg g−1 for Cd-JB systems. The pseudo-second-order kinetic model presented the best fit for all systems studied. Additionally, kinetic studies showed that the intraparticle diffusion is not rate-limiting for the biosorption of heavy metal ions and that the process involves different mechanisms. These results indicate that the JB shell may be applied as biosorbent for removal of heavy metals, such as Cd(II) and Pb(II) from aqueous solutions.
References
Anayurt, R. A., Sari, A., & Tuzen, M. (2009). Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chemical Engineering Journal, 151, 255–261.
Awwad, A. M., & Salem, N. M. (2014). Kinetics and thermodynamics of Cd(II) biosorption onto loquat (Eriobotrya japonica) leaves. Journal of Saudi Chemical Society, 18, 486–493.
Boniface, P. K., Ferreira, S. B., & Kaiser, C. R. (2017). Current state of knowledge on the traditional uses, phytochemistry, and pharmacology of the genus Hymenaea. Journal of Ethnopharmacology, 206, 193–223.
Cao, W., Wang, Z., Zeng, Q., & Shen, C. (2016). 13C NMR and XPS characterization of anion adsorbent with quaternary ammonium groups prepared from rice straw, corn stalk and sugarcane bagasse. Applied Surface Science, 389(2016), 404–410.
Chakravarty, P., Sen Sarma, N., & Sarma, H. P. (2010). Biosorption of cadmium(II) from aqueous solution using heartwood powder of Areca catechu. Chemical Engineering Journal, 162, 949–955.
De Souza, J. V. T., Diniz, K. M., Massocato, C. L., Tarley, C. R. T., Caetano, J., & Dragunski, D. C. (2012). Removal of Pb(II) from aqueous solution with orange sub-products chemically modified as biosorbent. BioResources, 7(2), 2300–2318.
Ding, Y., Jing, D., Gong, H., Zhou, L., & Yang, X. (2012). Biosorption of aquatic cadmium(II) by unmodified rice straw. Bioresource Technology, 114, 20–25.
Dubinin, M.M. (1960). The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chemical Reviews, 60: 235–266.
Freundlich, H. M. F. (1906). Uber die adsorption in losungen. The Journal of Physical Chemistry, 57(A), 385–470.
Fu, L., McCallum, S. A., Miao, J., Hart, C., Tudryn, G. J., Zhang, F., & Linhardt, R. J. (2015). Rapid and accurate determination of the lignin content of lignocellulosic biomass by solid-state NMR. Fuel, 141, 39–45.
Gao, Y., Yue, O., Gao, B., Sun, Y., Wang, W., Li, Q., & Wang, Y. (2013). Preparation of high surface area-activated carbon from lignin of papermaking black liquor by KOH activation for Ni(II) adsorption. Chemical Engineering Journal, 217, 345–353.
Gautam, R. K., Mudhoo, A., Lofrano, G., & Chattopadhyaya, M. C. (2014). Biomass-derived biosorbents for metal ions sequestration: adsorbent modification and activation methods and adsorbent regeneration. Journal of Environmental Chemical Engineering, 2, 239–259.
Gilbert, U. A., Emmanuel, I. U., Adebanjo, A. A., & Olelare, G. A. (2011). Biosorptive removal of Pb2+ and Cd2+ onto novel biosorbent: defatted Carica papaya seeds. Biomass & Bioenergy, 35, 2517–2525.
Grandi, J. M., Trindade, J. A., Pinto, M. J. F., Ferreira, L. L., & Catella, A. C. (1989). Plantas medicinais de Minas Gerais. Acta Botânica Brasileira, 3, 185–224.
Gupta, S. S., & Bhattacharyya, K. G. (2011). Kinetics of adsorption of metal ions on inorganic materials: a review. Advances in Colloid and Interface Science, 162, 39–58.
Huang, W., & Liu, Z. (2013). Biosorption of Cd(II)/Pb(II) from aqueous solution by biosurfactant-producing bacteria: Isotherm kinetic characteristic and mechanism studies. Colloids and Surfaces B: Biointerfaces, 105, 113–119.
Jain, J., Johnson, T. A., Kumar, A., Mishra, S. V., & Gupta, N. (2015). Biosorption of Cd(II) on jatropha fruit coat and seed coat. Environmental Monitoring and Assessment, 187, 411.
Kelly-Vargas, K., Cerro-Lopes, M., Reyna-Tellez, S., Randall, E. T., & Sanchez-Salas, J. L. (2012). Biosorption of heavy metals in polluted water, using different waste fruit cortex. Physics and Chemistry of the Earth, 37–39, 26–29.
Kumar, R., & Ahmand, R. (2011). Biosorption of hazardous crystal violet dye from aqueous solution onto treated ginger waste (TGW). Desalination, 265, 112–118.
Lagergren, S. (1898). Zur theorie der sogenannten adsorption gelöster stoffe. K. Sven. Vetenskapsakad. The Hand, 24(4), 1–39.
Langmuir, I. (1916). The constitution and fundamental properties of solids and liquids. Part I: solids. Journal of the American Chemical Society, 38, 2221–2295.
Mahapatra, K., Ramteke, D. S., & Paliwal, L. J. (2012). Production of activated carbon from sludge of food processing industry under controlled pyrolysis and its application for methylene blue removal. Journal of Analytical and Applied Pyrolysis, 95, 79–86.
Mahmood-ul-Hassan, M., Suthor, V., Rafique, E., & Yasin, M. (2015). Removal of Cd, Cr, and Pb from aqueous solution by unmodified and modified agricultural wastes. Environmental Monitoring and Assessment, 187, 19.
Nagy, B., Maicaneanu, A., Indolean, C., Mânzatu, C., Silaghi-Dumitrescu, L., & Majdik, C. (2014). Comparative study of Cd(II) biosorption on cultivated Agaricus bisporus and wild Lactarius piperatus baqsed biodecomposition. Linear and nonlinear equilibrium modeling and kinetics. Journal of the Taiwan Institute of Chemical Engineers, 45, 921–929.
Ofomaja, A. E. (2010). Intraparticle diffusion process for lead(II) biosorption onto mansonia wood sawdust. Bio/Technology, 101, 5868–5876.
Palin Jr., D., Rufato, K. B., Linde, G. A., Colauto, N. B., Caetano, J., Alberton, O., Jesus, D. A., & Dragunski, D. C. (2016). Evaluation of Pb (II) biosorption utilizing sugarcane bagasse colonized by Basidiomycetes. Environmental Monitoring and Assessment, 188, 279.
Pavan, F. A., Lima, I. S., Lima, E. C., Airold, C., & Gushikemb, Y. (2006). Use of Ponkan mandarin peels as biosorbent for toxic metals uptake from aqueous. Journal of Hazardous Materials B, 137, 527–533.
Peláez-Cid, A. A., Velázquez-Ugalde, I., Herrera-González, A. M., & García-Serrano, J. (2013). Textile dyes removal from aqueous solution using Opuntia ficus-indica fruit waste as adsorbent and its characterization. Journal of Environmental Management, 130, 90–97.
Pezoti, O., Cazetta, A. L., Gomes, R. C., Barizão, E. O., Souza, I. P. A. F., Martins, A. C., Asefa, T., & Almeida, V. C. (2014). Synthesis of ZnCl2-activated carbon from macadamia nut endocarp (Macadamia integrifolia) by microwave-assisted pyrolysis: optimization using RSM and methylene blue adsorption. Journal of Analytical and Applied Pyrolysis, 105, 166–176.
Pezoti, O., Cazetta, A. L., Bedin, K. C., Souza, L. S., Souza, R. P., Melo, S. R., & Almeida, V. C. (2016). Percolation as new method of preparation of modified biosorbents for pollutants removal. Chemical Engineering Journal, 283, 1305–1314.
Pillai, S. S., Deepa, B., Abraham, E., Girija, N., Geetha, P., Jacob, L., & Koshy, M. (2013). Biosorption of Cd(II) from aqueous solution using xanthated nano banana cellulose: equilibrium and kinetic studies. Ecotoxicology and Environmental Safety, 98, 352–360.
Prahas, D., Kartika, Y., Iindraswati, N., & Ismadji, S. (2008). Activated carbon from jackfruit peel waste by H3PO4 chemical activation: pore structure and surface chemistry characterization. Chemical Engineering Journal, 140, 32–42.
Rangabhashiyam, S., & Selvaraju, N. (2015). Evaluation of the biosorption potential of a novel Caryota urens inflorescence waste biomass for the removal of hexavalent chromium from aqueous solutions. Journal of the Taiwan Institute of Chemical Engineers, 47, 59–70.
Salem, N. M., & Awwad, A. M. (2014). Biosorption of Ni(II) from electroplating wastewater by modified (Eriobotrya japonica) loquat bark. Journal of Saudi Chemical Society, 18, 379–386.
Silva, M. R., Silva, M. S., Martins, K. A., & Borges, S. (2001). Utilização tecnológica dos frutos de jatobá-do-cerrado e de jatobá-da-mata na elaboração de biscoitos fontes de fibra alimentar e isentos de açúcares. Ciência e Tecnologia de Alimentos, 21, 176–182.
Sivasankar, V., Rajkumar, S., Murugesh, S., & Darchen, A. (2012). Tamarind (Tamarindus Indica) fruit shell carbon: a calcium-rich promising adsorbent for fluoride removal from groundwater. Journal of Hazardous Materials, 225–226, 164–172.
Taşar, S., Kaya, F., & Ozer, A. (2014). Biosorption of lead(II) ions from aqueous solution by peanut shells: equilibrium, thermodynamic and kinetic studies. Journal of Environmental Chemical Engineering, 2, 1018–1026.
Witek-krowiak, A., Szafran, R. G., & Modelski, S. (2011). Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination, 265, 126–134.
Yuvaraja, G., Krishnaiah, N., Subbaiah, M. V., & Krishnaiah, A. (2014). Biosorption of Pb(II) from aqueous solution by Solanum melongena leaf powder as a low-cost biosorbent prepared from agricultural waste. Colloids and Surfaces B: Biointerfaces, 114, 75–81.
Zafar, M. N., Aslam, I., Nadeem, R., Munir, S., Ali Rana, U., & Ud-Din Khan, S. (2015). Characterization of chemically modified biosorbents from rice bran for biosorption of Ni(II). Journal of the Taiwan Institute of Chemical Engineers, 46, 82–88.
Acknowledgments
The authors acknowledge the financial support provided by CAPES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souza, I.P.A.F., Cazetta, A.L., Pezoti, O. et al. Preparation of biosorbents from the Jatoba (Hymenaea courbaril) fruit shell for removal of Pb(II) and Cd(II) from aqueous solution. Environ Monit Assess 189, 632 (2017). https://doi.org/10.1007/s10661-017-6330-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6330-7