Abstract
Microelement (As, Cd, Cr, Cu, Ni, Pb, and Zn) concentrations were determined in the muscle, skin, gill, and liver tissues of Carassius auratus gibelio collected from subsidence pools at three different coal mines in the Huainan coalfield in China. The concentrations of elements in the water were within the allowable levels for raising fish. However, the higher levels of these metals in sediment may pose potential harm on fish. It was found that the concentrations of Cr, Ni, and Zn in all fish tissues were higher, while As, Cd, and Pb levels were relatively low. Microelement accumulation appeared to be more widespread in subsidence pools than that in natural water. Elements accumulated in fish tissues differently: the highest metal concentrations were generally found in the liver tissues of the fish analyzed, whereas the lowest were recorded in the muscles. The mean element concentrations in muscle tissue from C. auratus gibelio collected from subsidence pools (As, 0.16 mg/kg; Cd, 0.06 mg/kg; Cr, 6.21 mg/kg; Cu, 1.61 mg/kg; Ni, 3.88 mg/kg; Pb, 1.76 mg/kg; and Zn, 12.80 mg/kg dry weight) were far below the allowable limit of the hygienic standard in fish proposed by the Ministry of Health in China, suggesting that the fish were safe for human consumption. A health risk assessment also suggested there was no risk from the analyzed elements for inhabitants near the Huainan coalfield that consume fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish have been used as biomarkers for contamination in an aquatic environment at large (Alquezar et al. 2006). They usually represent the highest levels in aquatic food chains and thus may uptake and accumulate a large amount of metals (essential and non-essential) from water, food, and sediment in various organs and tissues (Canli and Atli 2003; Yılmaz et al. 2010).
A growing number of studies on the bioaccumulation of trace elements in fish have been reported over the last few decades (Chen and Chen 1999; Chi et al. 2007; Qiu et al. 2011; Abdel-Khalek 2015). It has been established that factors influencing the bioaccumulation processes in fish include metal types, fish species, and tissue types. Meanwhile, the living conditions of fish, including the water chemistry and metal levels in sediment, could affect the accumulation of metals in fish (Li et al. 2015).
Metals such as Cr, Cu, Ni, and Zn are essential for organisms because they play crucial roles in biological systems, such as enzymatic activity and other biological processes. These essential elements are beneficial for organisms within acceptable concentration, while they can be harmful if they exceed a tolerable limit. As, Cd, and Pb are non-essential metals with no known biological function and are all toxic, even on a microscale (Fernandes et al. 2008). The accumulation of metals in fish tissues depends on their physiological roles (Stanek et al. 2012). Gills and internal organs are most vulnerable to the toxic effects of contaminants. The metal concentrations in gills can reflect the metal concentration of its surrounding water, whereas the storage of metals in fish may be reflected by their bioconcentration in liver tissues (Yılmaz et al. 2007). Due to their physiological functions, including absorption, adsorption, and respiratory oxygen exchange, gill tissues can be the primary pathway for metals to enter into a fish’s body (Kargin and Erdem 1991; Klavins et al. 2009; Monikh et al. 2015). When excess or toxic elements are taken up by fish, the liver, in its role as a metabolic organ, is usually the storage sites of metals and is where they are detoxified by converting them into metallothioneins (Hogstrand and Haux 1991; Kargin and Erdem 1991; Yılmaz et al. 2007). Fish muscles are the main edible parts; however, muscles are not tissues in which trace metals are accumulated (Yilmaz 2003). However, fish muscles are the main edible parts eaten by human beings. As Kousar and Javed (2014) described, fish skin is in direct contact with metals in the surrounding water and sediment; thus, it likely adsorbs a variety of metals. In addition, people who consume fish ingest the skin and muscles together. Both the muscles and skin are important for metal accumulation and human consumption. Consistent with the previously mentioned studies (Kargin and Erdem 1991; Yilmaz 2003; Yılmaz et al. 2007; Kousar and Javed 2014), the muscles, skin, gills, and liver are selected as the tissues analyzed in this study. Trace elements accumulated by fish may be consumed by humans and eventually have an impact on human health, causing health problems such as tissue damage, cancer, and mutagenesis (Förstner and Wittmann 2012; Monikh et al. 2015). The impacts of the consumption of metals from contaminated fish flesh on human health have been reported in many studies (Suñer et al. 1999; Chan et al. 2003).
Anthropogenic contamination from mining activities resulting in high levels of trace metals in the Huainan coalfield has been reported in recent years (Sun et al. 2010a; Zhou et al. 2012; Wang et al. 2013; Fang et al. 2014). Many studies focused on the problems of trace metal contamination associated with coal mining (Tang et al. 2013; Wang et al. 2013; Lu et al. 2017). According to a prior study (Sun et al. 2010b), trace metal contamination in coal mining areas may have long-term and far-reaching environmental and health implications. Recent studies have demonstrated that metal levels in aquatic systems may be increased by mining effluent and that the consumption of polluted water originating from mining operations is an important exposure route that can result in metal accumulation in fish (Moiseenko and Kudryavtseva 2001; Zhuang et al. 2013). Metals in subsidence water may be generated by mining and agricultural activities, domestic pollution sources, and wet and dry deposition of pollutants as well as metal released into the water from sediment that originated from farmland or mines. The metal levels in fish living in these bodies of water may increase with prolonged exposure. This pattern is probably directly attributable to the discharge of contaminants from neighboring mine operations (Zhuang et al. 2013).
Three coal mines and a remote village used as a reference site in different parts of the Huainan coalfield were selected for this study. Each mine has the different mining age, which indicates that they may be subjected to distinct effects from the coal industry. The output of raw coal from each mine differs due to differences in production scale. A considerable number of subsidence pools are formed due to mining, and three of them were selected in each site for this study. Meanwhile, potentially toxic trace elements have migrated to the pools and affected aquatic organisms. A survey showed that the most important fish species in the subsidence pools is Carassius auratus gibelio, commonly known as “Crucian carp”, which is very important to fisheries in the region due to its size and taste. This species prefers muddy bottom conditions, is omnivorous, and feeds mainly on benthic plant matter, animals, and sediment, presenting a varied diet throughout the water column. Moreover, it is easily captured for study. Therefore, it is suitable to use this species to study the bioaccumulation of metals in the study areas. Wang et al. (2015) have reported metal accumulation in fish collected from subsidence pools in Huainan. However, only four metals (Cd, Cu, Pb, and Zn) in three different tissues were analyzed. A comparison with a reference site that had been affected a little or not at all by coal mining operations was lacking. Moreover, the effects of maturity and living habits on metal uptake were not taken into account for the tested fish. A more comprehensive and profound study is needed on the bioaccumulation or regulation of metals in fish from subsidence pools in the Huainan coalfield.
Carp are an important component of human diet in this region; therefore, the aim of this study is to determine the concentrations and variations of trace metals (As, Cd, Cr, Cu, Ni, Pb, and Zn) in different tissues of the crucian carp collected from subsidence pools located in three sites in the Huainan coalfield. Meanwhile, the relationships between metal levels in fish and in their surrounding ecosystem (water or sediment) are analyzed. Furthermore, the health risks from the consumption of fish muscle by different exposure groups are estimated.
Materials and methods
Study sites
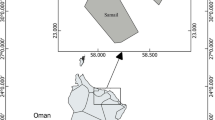
The Huainan coalfield is situated in the Anhui Province at the southeast corner of the North China Plateau and covers a total area of 3200 km2 (Fig. 1). It is an elongated field with a mean length of 180 km (W–E) and a mean width of 15–25 km (N–S). The area has a typical seasonal temperate semi-humid monsoon climate with a mean annual temperature of 15.1 °C and an average annual rainfall of 920 mm. The primary wind direction is from south to north.
The expansion of the coal mining subsidence zone is increasing with the development of the coal industry (Yao and Gui 2007). The coal mining subsidence zone has increased from 6709 to 11,800 hm2 from 1992 to 2005 (Yao and Gui 2008). Generally, the depth of the mine subsidence district is approximately 3–10 m. As a result, a considerable amount of subsidence pools and lakes have formed. Many subsidence pools are used by fishermen to raise fish (C. auratus gibelio, mainly) for economic benefits. Nevertheless, the pools are surrounded by several smelting plants and large thermal power plants that may be sources of heavy metal pollution (Yao and Gui 2007).
Three coal mines were selected in the study. Coal mine XXZ is located at latitude 32° 35′ 41″ N and longitude 116° 49′ 38″ E. Mining operations there have continued for more than 50 years. Coal mine PY is located in the northern part of the city of Huainan. It was constructed in 1973, and exploration began in 1983. Coal mine GQ, latitude 32° 50′ 11″ N and longitude 116° 32′ 43″ E, commenced mining in 2008. Their scale of production is in the order of GQ > PY > XZZ. There are several subsidence pools with different deposition times located in these sampling sites. Three typical pools of each mine were selected for sampling. The reference site is a remote village far away from any mines that is exposed to less effluent from the mining industry than the other three sampling sites.
Sample collection and analysis
Field sampling
Crucian carp, surface water, and sediment samples were collected in 2014 from subsidence pools at the three coal mine sites and a natural body of water at the reference site in the Huainan coalfield: XZZ, PY, and GQ coal mines and RS (Fig. 1). Three typical subsidence pools were chosen near each coal mine. At each selected subsidence pool, three water samples were collected and every water sample consisting of three subsamples obtained from different depths was collected and homogenized. Nine water samples were collected from different locations in a river at the reference site. The collected 36 water samples were transferred into labeled polyethylene bottles and adjust pH to less than 2 with HNO3. Meanwhile, 36 surface sediment samples (the upper 0–5 cm) were collected from the same points corresponding to their water stations using grab-type sampling instruments. The sediment samples were placed into polyethylene bags. Both the water and sediment samples were brought to a laboratory and stored under appropriate conditions for analysis. Fish samples were collected randomly from the subsidence pools with the help of local fishermen based on their availability at the sites. Thirty-six fish samples (3 fish from each pool and 9 fish samples for each mine) whose weights were all greater than 350 g were selected to ensure that they were all mature and met the size required for commercialization.
To avoid contamination, all sample collection devices and storage containers were soaked overnight in 10% nitric acid (AnalaR) and rinsed with deionized water (Milli-Q; 18 MΩ cm). Conductivity, pH, and temperature were measured in situ at each site (1 m below surface) to confirm that the physicochemical properties were similar among each site. Total N and total P in the water samples were determined using the standard method.
Sample treatment
The water samples were filtered through 0.45-μm-pore-size cellulose acetate filters prior to analysis. All sediment samples were air-dried, ground up, and sieved to < 63 μm. Next, 0.1 g of each sediment sample was placed in a Teflon vessel and digested with aqua regia on a hot plate until the solutions were evaporated near dryness. The abovementioned procedures were repeated until the solution was almost clear. Finally, the digested material was dissolved in 2% (v/v) HNO3 to give a final volume of 25 mL.
The fish were euthanized and frozen (− 20 °C) prior to being thawed and dissected into various tissues (muscle, skin, gill, and liver tissues). All fish tissues were ovened at 80 °C. The muscle tissues were weighed before and after the oven process. The average dry weight to wet weight ratio was calculated for the fish muscle in order to convert the two types of values and compared them to published results. The conversion value was approximately 5.00 (ww/dw). Digestion was performed on a hot plate using a 1:1 mixture of concentrated nitric acid and hydrogen peroxide (v/v) until a clear yellow and nearly dry solution was obtained.
Sample analysis
The concentrations of As, Cd, Cr, Cu, Ni, Pb, and Zn in the water, sediment, and fish samples were determined using inductively coupled plasma mass spectrometry (ICP-MS, Thermo Fisher Scientific X Series 2) in a certified laboratory, the Hefei National Laboratory for Physical Sciences at the Microscale. Standard curves were produced from certified aqueous standards of multiple elements, including the elements analyzed in this study. One of the standard solutions with a known concentration was detected 10 times, then the detection limits of the metals were calculated by multiplying the standard deviation of the results by 3. The detection limits of the seven metals are listed in Table 1.
Quality assurance
Analytical grade reagents and deionized water were used throughout the analysis. All plastic- and glassware used were immersed in 10% nitric acid overnight and rinsed with deionized water before use. For quality control, reagent blanks and duplicate samples were included. To verify the accuracy and precision of the digestion procedure and subsequent analyses, certified references of sediment (GBW07309, GSD-9) and animal samples (GBW10050, GSB-28), purchased from the National Research Center for Standards in China, were included in each analytical run of the sediment and fish samples, respectively. The bias of the chemical analysis was within 10%. Satisfied recoveries of CRM were obtained and shown in Table 1. Statistical analysis of the data was carried out with the SPSS statistical package. One-way ANOVA was used to compare data among sites or tissues.
Determination of target hazard quotient
The target hazard quotient method, which could provide quantitative estimation for the risk level associated with pollutant exposure, has been used recently by many researchers (Chien et al. 2002; Wang et al. 2005; Yi et al. 2011).
The target hazard quotient (THQ), which is the ratio between an exposure and a reference dose (RfDo), is used to express the risk of non-carcinogenic effects. The method for the determination of THQ is provided in the US EPA Region III risk-based concentration table (USEPA 2009). To assess the potential health risks posed to local people by consuming C. auratus, THQs (USEPA 2000) were calculated by
where THQ is the target hazard quotient; EFr is the exposure frequency (365 days/year); EDtot is the exposure duration (70 years), which is equivalent to the average human lifetime (USEPA 1991); FIR is the food ingestion rate (g/person/day); C is the heavy metal concentration in fish (mg/kg, dry weight; the average concentration of each metal in fish flesh was used in this study); RfDo is the oral reference dose (mg/kg/day, the values of RfDo for the analyzed trace elements are as follows: As = 3 × 10−4, Cd = 1 × 10−3, Cr = 1.5, Cu = 4 × 10−2, Ni = 2 × 10−2, Pb = 4 × 10−3, and Zn = 3 × 10−1); BWa is the average adult body weight; and ATn is the average exposure time to non-carcinogens (365 days/year/number of exposure years, assuming a lifespan of 70 years). It is assumed that cooking has no effect on the toxicity of heavy metals in aquatic products (Chien et al. 2002). If the THQ is less than 1, there is no measurable risk. If the THQ value is greater than 1, there is a concern for potential non-carcinogenic exposure.
Hallenbeck (1993) reported that exposure to two or more pollutants may result in additive and/or interactive effects. In this study, the total THQ is calculated from the arithmetic sum of the individual metal THQ values (Chien et al. 2002):
Results and discussion
Metal concentrations in the medium
Differences in water physicochemistry (temperature, pH, conductivity, etc.) may influence the uptake and bioavailability of metals in fish (Playle 1998; Jezierska and Witeska 2006). Therefore, sites with similar physicochemical parameters were selected to minimize variability. The physicochemical parameters of the surface water in each subsidence pool are listed in Table 1. The results indicated that there were no large variation on temperature and pH values, ranging from 13.96 to 15.02 °C and 7.44 to 7.5, respectively. Moreover, total nitrogen (TN) and total phosphorus (TP) of the water from the XZZ and GQ coal mines were similar, with both being in the range of 1.11 ~ 1.36 mg/L and 0.60 ~ 0.67 mg/L, respectively, whereas those of the water from the PY mine were different (TN = 0.69 mg/L, TP = 1.18 mg/L).
The details of the metal concentrations in subsidence waters from the different sites are summarized in Table 2. The environmental quality standard for surface water in China (GB3838-2002) is used to classify the quality of surface water, divided into five levels. Level refers to natural water that can be used for National Natural Reserve, while level II denotes clean water that can be used for drinking and domestic purposes. The water quality standard for fisheries (GB11607-89) is also shown in Table 2. It refers to the safe element levels in water used to raise fish. Meanwhile, the global average background concentrations of elements in bodies of water are listed in Table 2 as well.
According to these criteria, the levels of Zn, Pb, and Cd in this study belong in level I, while Cu and Cr concentrations were within level II. However, As levels in the subsidence pools were all in level III or beyond. In spite of the levels being higher than the global average background concentrations, all of the trace element concentrations in the sampling sites were within the permissible limits of water quality standard for fisheries (GB11607-89) except Cu concentration, which is slightly higher. Therefore, the pools are suitable for raising fish.
According to the results of one-way ANOVA for the levels of elements in water from four sampling sites, all element levels showed significant differences among the sites, except Cr level. In general, element levels in the water from subsidence pools were higher than those in the water from the reference site, likely indicating the effects of mining operations on water quality. Nevertheless, no pattern was found for the element distribution among the subsidence pools at the selected three coal mines. Moreover, the mean levels of heavy metals in the subsidence waters from different sampling sites tested were ranked as Cr > Cu > Zn > As > Ni > Pb > Cd. According to Yao and Gui (2007, 2008), metals from coal can migrate to the water through several means, such as the processes of coal transportation, cleaning, storage, and combustion. Moreover, there are many factors that can affect metal concentrations in subsidence water, such as the activation, migration, and redistribution of heavy metals in water, sediment, and organisms; agricultural pollution; the chemical properties of metals; and groundwater conditions (Abernathy et al. 1984; Borg 1987; Yao and Gui 2008). Thus, the factors that can influence metal transportation should all be considered in the Huainan coalfield.
The concentrations of elements in sediment samples are indicated in Table 3. There were significant differences between the As, Cd, Cr, and Pb levels in the sediment samples from the natural body of water and subsidence pools, i.e., those in the former were lower than those in the latter. This indicated that sediment in the subsidence pools suffered more pollution than that in the river. Moreover, a majority of the element levels (As, Cd, Cr, and Zn) were highest in sediment samples from the XZZ coal mine. Compared to toxicological reference values (threshold effect level, TEL; probable effect level, PEL) and background values in Anhui and China, the contents of As, Cr, and Ni in sediment from all sites slightly exceeded the threshold effect levels, which is defined as the concentration of contaminants that have a relatively low effect on biological communities (Shakeri, Shakeri et al. 2016). Only Cr levels in sediment from XZZ and GQ were higher than the probable effect levels, above which adverse effects are likely to occur (Long and MacDonald 1998). The levels of trace elements (except Ni and Pb) in sediment from the Huainan coalfield were above the background values in the Anhui province, especially Cd, which was approximately two times higher.
Trace element distribution in fish tissues
Trace element concentrations in fish muscle
To explore the metal levels in fish flesh from subsidence pools in the Huainan coalfield, metal concentrations in muscles of the same fish species from the published data in other studies on waters from the same and different areas were collected to be compared to our results. These data are listed in Table 4.
Compared to the results from the same sampling sites conducted by Wang et al. (2015), the metal levels in fish muscle in this study were apparently higher. This may be explained by fluctuations in the living conditions of the fish and the individual sampling season of our study. The growth rate of fish would be higher in summer, resulting in higher trace element accumulation (Zubcov et al. 2012). The comparative concentrations of these elements in fish muscle from the subsidence pools in Huainan were almost all the highest, with the exception of Cu, Pb, and Zn. The Pb level in the muscle tissue from fish in the Dniester River was much higher than that from the fish in this study. Meanwhile, Cu and Zn levels in fish flesh from Lake Taihu, which suffers from algal blooms, were elevated in comparison to those in this study (Chi et al. 2007; Zeng et al. 2012). Cyanobacterial cells are ingested by fish in the lake. Moreover, the intracellular metals in cyanobacterial cells especially the comparatively higher essential metals Cu and Zn enter into the fish and are accumulated in the fish muscle. The deficiency of As levels in this fish species in the literature exactly reflects the importance of this type of study.
As demonstrated by Tao et al. (2012), the metal levels in fish muscle from developed industrial areas and mining areas are usually higher than those from other areas. Our results are higher than those reported by Has-Schön et al. (2008) (from the “Hutovo Blato” Nature Park), as well as those reported by Sapozhnikova et al. (2005) (the Dniester River is surrounded by developed industrial and agricultural areas). As it is seen in Table 5, the average levels of metal in fish flesh from subsidence pools in the Huainan coalfield were mostly at high levels compared to those from other bodies of water.
The different element concentrations in the tissues of fish from the four sites are presented in Fig. 2. All elements in muscle tissue collected from subsidence pools were slightly higher than those from the reference site, except Cd. However, there was no significant difference among the sites. In contrast, the Cd level in muscle from the GQ mine was significant lower than that from the others. Generally, the accumulation of trace elements in muscle is a comparatively steady process. Therefore, the ranges in the levels of the analyzed elements in fish muscle from the three mines are narrow, and their concentrations were comparative low. The different capacities of fish muscles to absorb trace elements are due to the various roles of these elements in fish metabolism, resulting in the considerable differences in their concentration levels. The average concentrations in muscle were ranked as Zn > Cr > Ni > Pb > Cu > As > Cd (Table 6). Generally, the levels of elements essential for vital activities were higher than those of non-essential elements.
Metal concentrations in fish skin and gills
The skin covers the fish body and is in direct contact with the water and sediment. Moreover, it is often consumed together with fish muscle by human beings. As shown in Fig. 2 and Table 4, the element levels in fish skin were in the following descending order: Zn > Cr > Ni > Pb > Cu > As > Cd, which is in accordance with the element contents of the muscles. According to the results of ANOVA, only Cd, Cu, and Pb showed statistically significant differences among the sites, with the lowest values in fish skin from the natural body of water. There was no pattern for which element had the highest content in the skin among the sites. This indicates that living conditions have a significant impact on Cd, Cu, and Pb accumulation in fish skin.
The gills are also important tissues that are in direct contact with the conditions in which the fish live. The surface of the gills may absorb metals from the surroundings. Statistical differences were observed for Cd, Cr, and Ni levels in gills from the four sites, with the lowest levels found in gills from the reference site. The other element contents in gills showed no significant differences among the sites. Meanwhile, element levels (with the exception of Cr and Ni) in fish gills from the XZZ coal mine were the highest, in accordance with this mine having the longest operation time. It is assumed that element accumulation in fish tissues may be affected by the history of mining operations. The order of element concentrations was slightly inconsistent with those found in the muscle and skin, following the order of Zn > Cr > Cu > Ni > Pb > As > Cd. The concentrations of all metals in the gills in this study were significantly higher than those in muscle, which agrees with the findings of many other researchers (Has-Schön et al. 2008; Yılmaz and Doğan 2008; Rajkowska and Protasowicki 2013).
Metal concentrations in fish liver
Figure 2 shows the element levels in liver tissues from the four sampling sites. Although all elements in the liver were observed to be the lowest from the reference site, only Cd and Ni presented significant differences between the mine sites and reference site. Consistent with the gills, liver samples from the XZZ mine displayed the highest contents for a majority of the elements. However, the elements that were exceptions were Cu and Pb, which was not in agreement with the trends observed in the gills, indicating that different accumulation processes of elements exist in different tissues.
Comparison of metal levels in different tissues
The mean concentrations of As, Cd, Cr, Cu, Ni, Pb, and Zn in fish tissues from subsidence pools, irrespective of the coal mine sampled, are listed in Table 4. According to the results of ANOVA, the element concentrations in muscle were significantly lower than those in liver tissue. In contrast, As, Cd, and Cu levels showed no significant difference between the muscle and skin. There was also no significant difference in Ni and Zn levels between the skin and gills. However, significant differences were obtained among each type of tissue for Cr and Pb contents. The metal concentrations in different tissues in this study differed in the general order of liver > gill > skin > muscle. The results show that lower concentrations of elements were always observed in the muscle when compared to those of the skin and gills, while the liver presented comparatively higher values. The lowest levels being in fish muscle reflected its rather lower affinity to trace elements in the surrounding environment. A modest element accumulation in the skin and gills of fish was established in the literature. Chen and Chen (1999) stated that element concentrations in fish gills could reflect the availability of waterborne elements to fish because of the direct exposure of gills to water and sediment. The concentrations of metals in the liver were the highest among all the tissues tested. Its higher metal-accumulating abilities make the liver the most important target and storage tissue in fish. Meanwhile, the liver is considered as a good indicator of water pollution by metals (Jezierska and Witeska 2006). The metallothioneins and proteins in the liver can bind heavy metals and reduce their toxicity, resulting in high accumulation of metal pollutants from the surroundings (Usero et al. 2004).
Correlation between trace elements in fish and media
The element contents in water columns could directly affect metal bioaccumulation in fish (Wang et al. 2015). Meanwhile, food sources play an important role in element accumulation in fish. C. auratus gibelio is omnivorous, and it prefers muddy bottom conditions. Sediment associated with trace elements might be one of the food sources for this species. Therefore, Pearson’s correlation analysis was performed to obtain the relationship between the element levels in fish tissues and those in their living medium, both the water and sediment. Fish and water are always in motion in the pools, making it inappropriate to analyze the correlation of element levels in fish and the medium at one sampling point. In this study, each of the subsidence pools was considered as a closed integral object; thus, average element levels in fish or medium samples from each pool (n = 9) were used in the calculation.
The coefficients are listed in Table 5. Only significant correlation coefficients (p < 0.05 or p < 0.01) are identified in the table. The results indicated that significant positive relationships were found between Ni contents in water and gills and the similar results also happened between Zn concentrations in the water and liver. However, there were no obvious relationships between the water and tissues for the other elements. The results of this study are inconsistent with the previous study of Wang et al. (2015), which can likely be attributed to variations in living conditions and seasonal changes. In addition, a considerable number of significant positive relationships were observed between element levels in sediment and tissues. Only concentrations in the skin and sediments showed no relationship for all elements. These results confirm that this fish species has a high affinity for sediment. The trace elements in sediment affected element accumulation in fish more severely. Therefore, in this study, Ni in the gills and Pb in the liver may originate from both the water and sediment. As, Cd, and Cu in the muscles were mainly from sediment; the same is true for Cd, Cr, Pb, and Zn levels in liver tissue. The elements in tissues that have no relationship with the media may have multiple origins, such as benthic plant matter, animals, water, and sediment.
Health risk assessment
The Chinese national food safety standards (GB2762-2012) for fish are also listed in Table 6. They give the hygienic standard for the allowable limit of the four non-essential elements in fish. When converted into the same units, the mean As, Cd, Cr, and Pb contents recorded in the muscle tissues of fish were lower than these standards. Overall, the levels of these four elements were within the safe ranges. However, the maximum Pb level of muscle samples from subsidence pools slightly exceeded the safety limit, suggesting that individual fish may be not safe for human consumption. In addition, the excess levels of Pb in the skin demonstrated that residents may suffer health risks associated with Pb when consuming fish skin. The unsafe element levels in the gills and liver confirmed their inedible nature. Although the trace element levels in the edible parts of fish were not high, it is necessary to consider that some people consume larger quantities of fish than others. It is especially important to take into account the comparatively high concentration of Pb compared to the standards and the toxicity of some of the analyzed elements. Continuous monitoring should be performed to ensure the safe concentrations of trace elements in the edible parts of fish, especially the muscles.
Many people raise fish in subsidence pools or catch fish from such waters. The studied species, C. auratus gibelio, is one of the dominant species in the study area. This species enters the market and is consumed by members of the general population, fisherman and children. As a favorite daily food for local people, it is necessary to assess the potential health risks caused by the consumption of the edible parts of fish from subsidence pools, including muscle and skin tissues. However, the ingestion rate of fish skin only is much lower than that of fish muscle and has not been estimated in this study or in the literature. Therefore, only the risks associated with consuming muscle tissues were calculated in this study. It is necessary to analyze homogeneous muscle and skin samples to achieve a more accurate risk assessment for the consumption fish in future studies.
The average ingestion (consumption) rate of freshwater fish for the general population is 43.95 g/d (FAO 2013). Fishermen are assumed to consume 2.4 times more fish than the general population (Yi et al. 2011), i.e., 105.48 g/d. According to Shao et al. (2011), the average consumption rate of fish for children is assumed to be 54% of adult consumption. Nationwide surveys on physiques in 2014 showed that the average body weights of adults and children were 64.06 and 32.49 kg, respectively. Table 7 lists the estimated target hazard quotients (THQ) for individual metals and the total THQ for the consumption of fish muscle from all the subsidence pools, irrespective of sampling sites, by different exposure groups in the Huainan coalfield area.
The THQ of each metal due to fish consumption by all exposure groups was generally less than 1, suggesting that the inhabitants would not experience significant health risks from the intake of individual metals through fish consumption (Table 7). For different exposure groups, different potential risks are exhibited by the THQ values. The general population and children experience almost equal potential risk, which are always no more than 1. These results imply that fishermen (including their families) experience a risk two more times greater than that of the other two groups due to their eating habits. These results are similar to those obtained by Tao et al. (2012). The estimated THQs for individual metals decreased in the following sequence: As > Pb > Ni > Cd > Zn > C > Cr. The highest THQ values were observed for As and Pb, which are considered to be non-essential and toxic elements. The potential health risks of Cu, Ni, and Zn were low, which may be ascribed to their higher oral reference doses and moderate concentrations. However, a low THQ value was found in Cd, which is considered more toxic. This may be explained by the fact that Cd concentrations were much lower than those of the other investigated metals.
The relative contributions of the analyzed elements to the total metal THQ for fish consumption are shown in Fig. 3. As and Pb, two major risk contributors for all exposure groups in the Huainan coalfield area, account for 41.11 and 33.02% of the total THQ, respectively. Ni is a moderate risk contributor, representing approximately 14.58% of the total THQ. The risk contributions of Cd, Cu, and Zn were relatively low at approximately 4.74, 3.21, and 3.02%, respectively. The lowest risk contributor is Cr, which is only 0.31% of the total THQ. These data demonstrate the dominant risk contribution from As and Pb compared to the relatively minor risk from Cd, Cu, Ni, and Zn for the inhabitants of the study areas. It should be noted that only one kind of fish and one kind of edible tissue was considered in this assessment and that the consumption of fish skin with higher element levels as well the consumption of water, vegetables, and rice was not calculated. Therefore, the actual health risk for local people through dietary intake could be higher (Tao et al. 2012).
Conclusion
This study was conducted to provide useful information about trace element levels in water, sediment, and C. auratus gibelio from subsidence pools in the Huainan coalfield.
In the Huainan coalfield, the physicochemical parameters of all subsidence pool water investigated were similar. In general, the trace element concentrations in the water and sediment samples from the subsidence pools at three sites were higher than those in the reference natural water. Compared to the required standards, the majority of the analyzed elements in subsidence water were within allowable levels. Thus, these subsidence pools are suitable for raising fish. In addition, the element levels in sediment were apparently higher than those in water. Some elements even exceeded the threshold effect levels, which could result to a certain extent in harmful effects on aquatic organisms.
The analyzed trace elements accumulated differently in the same tissues, with the highest levels being shown by Zn and the lowest ones by As and Cd. The levels of elements in different tissues in this study differed, following the general sequence: liver > gills > skin > muscles. The higher microelement contents in the liver were attributed to its important role in storage and metabolism in fish, while the toxicity of the elements in muscle could be reduced by other metabolic organs. The lower levels of elements in fish tissues collected from the reference site compared to those in fish tissues collected from the subsidence pools were statistically significant, while no obvious trend in the bioaccumulation of metals in fish tissues among the mines studied was observed. The comparatively comprehensive variation in element content in tissues among the sites may reflect varying temporal exposures and intake patterns for the elements investigated. The latter represents the combined effects of mining operations, metal properties, and fish metabolism.
The investigation of the correlations between element concentrations in fish tissues and in the environmental medium suggests that sediment had a more significant effect on microelement accumulation in fish. The trace elements in fish may originate from multiple sources.
Analysis of the health risks from consuming fish suggests that residents of this area do not face significant potential health risks from the intake of any of the microelements in fish. However, fishermen might be subject to greater potential health risks due to their eating habits.
Overall, microelement accumulation in C. auratus gibelio was more intensive in subsidence pools than in a natural body of water. Microelement pollution derived from mining operations may become a severe problem. Fish are a valuable and nutritious food for humankind and should be incorporated into a balanced diet. The human health risks associated with fish consumption are noteworthy. The potential health risks of microelement pollution in fish from subsidence pools should be taken into account in the Huainan mining area.
References
Abdel-Khalek, A. A. (2015). Risk assessment, bioaccumulation of metals and histopathological alterations in nile ilapia (Oreochromis niloticus) facing degraded aquatic conditions. Bulletin of Environmental Contamination and Toxicology, 94(1), 77–83.
Abernathy, A., Larson, G., & Mathews Jr., R. (1984). Heavy metals in the surficial sediments of Fontana Lake, North Carolina. Water Research, 18(3), 351–354.
Alquezar, R., Markich, S. J., & Booth, D. J. (2006). Metal accumulation in the smooth toadfish, Tetractenos glaber, in estuaries around Sydney, Australia. Environmental Pollution, 142(1), 123–131.
Borg, H. (1987). Trace metals and water chemistry of forest lakes in northern Sweden. Water Research, 21(1), 65–72.
Canli, M., & Atli, G. (2003). The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environmental Pollution, 121(1), 129–136.
Chan, H., Scheuhammer, A., Ferran, A., Loupelle, C., Holloway, J., & Weech, S. (2003). Impacts of mercury on freshwater fish-eating wildlife and humans. Human and Ecological Risk Assessment, 9(4), 867–883.
Chen, M.-H., & Chen, C.-Y. (1999). Bioaccumulation of sediment-bound heavy metals in grey mullet, Liza macrolepis. Marine Pollution Bulletin, 39(1), 239–244.
Chi, Q.-q., Zhu, G.-w., & Langdon, A. (2007). Bioaccumulation of heavy metals in fishes from Taihu Lake, China. Journal of Environmental Sciences, 19(12), 1500–1504.
Chien, L.-C., Hung, T.-C., Choang, K.-Y., Yeh, C.-Y., Meng, P.-J., Shieh, M.-J., & Han, B.-C. (2002). Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Science of the Total Environment, 285(1), 177–185.
FAO (2013). http://www.fao.org/faostat/en/#data/CL. (accessed on 22 August, 2015).
Fang, T., Liu, G., Zhou, C., Yuan, Z., & Lam, P. K. S. (2014). Distribution and assessment of Pb in the supergene environment of the Huainan coal mining area, Anhui, China. Environmental Monitoring and Assessment, 186(8), 4753–4765.
Fernandes, C., Fontainhas-Fernandes, A., Cabral, D., & Salgado, M. A. (2008). Heavy metals in water, sediment and tissues of Liza saliens from Esmoriz-Paramos lagoon, Portugal. Environmental Monitoring and Assessment, 136(1–3), 267–275.
Förstner, U., & Wittmann, G. T. (2012). Metal pollution in the aquatic environment, Springer Science & Business Media.
Hallenbeck, W. H. (1993). Quantitative risk assessment for environmental and occupational health, CRC Press.
Has-Schön, E., Bogut, I., Rajković, V., Bogut, S., Čačić, M., & Horvatić, J. (2008). Heavy metal distribution in tissues of six fish species included in human diet, inhabiting freshwaters of the Nature Park “Hutovo Blato” (Bosnia and Herzegovina). Archives of Environmental Contamination and Toxicology, 54(1), 75–83.
Hogstrand, C., & Haux, C. (1991). Binding and detoxification of heavy metals in lower vertebrates with reference to metallothionein. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 100(1), 137–141.
Jezierska, B., & Witeska, M. (2006). The metal uptake and accumulation in fish living in polluted waters. Soil and water pollution monitoring, protection and remediation, Springer, pp 107–114.
Kargin, F., & Erdem, C. (1991). Accumulation of copper in liver, spleen, stomach, intestine, gill and muscle of Cyprinus carpio. Doga Turkish Journal of Zoology, 15, 306–314.
Klavins, M., Potapovics, O., & Rodinov, V. (2009). Heavy metals in fish from lakes in Latvia: concentrations and trends of changes. Bulletin of Environmental Contamination and Toxicology, 82(1), 96–100.
Kousar, S., & Javed, M. (2014). Heavy metals toxicity and bioaccumulation patterns in the body organs of four fresh water fish species. Pakistan Veterinary Journal, 34(2), 161–164.
Li, P. F., Zhang, J., Xie, H. J., Liu, C., Liang, S., Ren, Y. G., & Wang, W. X. (2015). Heavy metal bioaccumulation and health hazard assessment for three fish species from Nansi Lake, China. Bulletin of Environmental Contamination and Toxicology, 94(4), 431–436.
Long, E., & MacDonald, D. (1998). Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems. Human and Ecological Risk Assessment, 4(5), 1019–1039.
Lu, L., Liu, G., Wang, J., Wu, Y. (2017). Bioavailability and mobility of heavy metals in soil in vicinity of a coal mine from Huaibei, China. Human and Ecological Risk Assessment: An International Journal 23(5), 1164–1177
Moiseenko, T., & Kudryavtseva, L. (2001). Trace metal accumulation and fish pathologies in areas affected by mining and metallurgical enterprises in the Kola Region, Russia. Environmental Pollution, 114(2), 285–297.
Monikh, F. A., Maryamabadi, A., Savari, A., & Ghanemi, K. (2015). Heavy metals’ concentration in sediment, shrimp and two fish species from the northwest Persian gulf. Toxicology and Industrial Health, 31(6), 554–565.
Playle, R. (1998). Modelling metal interactions at fish gills. Science of the Total Environment, 219(2), 147–163.
Qiu, Y. W., Lin, D., Liu, J. Q., & Zeng, E. Y. (2011). Bioaccumulation of trace metals in farmed fish from South China and potential risk assessment. Ecotoxicology and Environmental Safety, 74(3), 284–293.
Rajkowska, M., & Protasowicki, M. (2013). Distribution of metals (Fe, Mn, Zn, Cu) in fish tissues in two lakes of different trophy in northwestern Poland. Environmental Monitoring and Assessment, 185(4), 3493–3502.
Sapozhnikova, Y., Zubcov, N., Hungerford, S., Roy, L. A., Boicenco, N., Zubcov, E., & Schlenk, D. (2005). Evaluation of pesticides and metals in fish of the Dniester River, Moldova. Chemosphere, 60(2), 196–205.
Shakeri, A., Shakeri, R., & Mehrabi, B. (2016). Contamination, toxicity and risk assessment of heavy metals and metalloids in sediments of Shahid Rajaie Dam, Sefidrood and Shirinrood rivers, Iran. Environmental Earth Sciences, 75(8), 679–691.
Shao, D., Liang, P., Kang, Y., Wang, H., Cheng, Z., Wu, S., Shi, J., Lo, S. C. L., Wang, W., & Wong, M. H. (2011). Mercury species of sediment and fish in freshwater fish ponds around the Pearl River Delta, PR China: human health risk assessment. Chemosphere, 83(4), 443–448.
Stanek, M., Stasiak, K., Janicki, B., & Bernacka, H. (2012). Content of selected elements in the muscle tissue and gills of perch (Percafluviatilis L.) and water from a Polish lake. Polish Journal of Environmental Studies, 21(4), 1033–1038.
Sun, R., Liu, G., Zheng, L., & Chou, C.-L. (2010a). Geochemistry of trace elements in coals from the Zhuji mine, Huainan coalfield, Anhui, China. International Journal of Coal Geology, 81(2), 81–96.
Sun, Y., Zhou, Q., Xie, X., & Liu, R. (2010b). Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang, China. Journal of Hazardous Materials, 174(1–3), 455–462.
Suñer, M., Devesa, V., Munoz, O., López, F., Montoro, R., Arias, A., & Blasco, J. (1999). Total and inorganic arsenic in the fauna of the Guadalquivir estuary: environmental and human health implications. Science of the Total Environment, 242(1), 261–270.
Tang, Q., Liu, G., Zhou, C., Zhang, H., & Sun, R. (2013). Distribution of environmentally sensitive elements in residential soils near a coal-fired power plant: potential risks to ecology and children’s health. Chemosphere, 93(10), 2473–2479.
Tao, Y., Yuan, Z., Xiaona, H., & Wei, M. (2012). Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotoxicology and Environmental Safety, 81, 55–64.
USEPA. (1991). Technical support document for water quality-based toxics control (EPA/505/2-90-001). Washington, DC: United States Environmental Protection Agency.
USEPA. (2000). Risk-based concentration table. Philadelphia: United States Environmental Protection Agency.
USEPA. (2009). Risk-based concentration table. Philadelphia: United States Environmental Protection Agency.
Usero, J., Izquierdo, C., Morillo, J., & Gracia, I. (2004). Heavy metals in fish (Solea vulgaris, Anguilla anguilla and Liza aurata) from salt marshes on the southern Atlantic coast of Spain. Environment International, 29(7), 949–956.
Wang, X., Sato, T., Xing, B., & Tao, S. (2005). Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Science of the Total Environment, 350(1–3), 28–37.
Wang, X., Zhou, C., Liu, G., & Dong, Z. (2013). Transfer of metals from soil to crops in an area near a coal gangue pile in the Guqiao coal mine, China. Analytical Letters, 46(12), 1962–1977.
Wang, X., Chu, Z., Zha, F., Liu, S., Liu, G., & Dong, Z. (2015). Determination of heavy metals in water and tissues of Crucian carp (Carassius auratus Gibelio) collected from subsidence pools in Huainan coal fields (China). Analytical Letters, 48(5), 861–877.
Yao, E., & Gui, H. (2007). Composition and distribution of main pollution trace element in water environment of mining subsidence. Journal of Coal Science and Engineering (China), 14(1), 97–102.
Yao, E.-q., & Gui, H.-r. (2008). Four trace elements contents of water environment of mining subsidence in the Huainan diggings, China. Environmental Monitoring and Assessment, 146(1–3), 203–210.
Yi, Y., Yang, Z., & Zhang, S. (2011). Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environmental Pollution, 159(10), 2575–2585.
Yilmaz, A. B. (2003). Levels of heavy metals (Fe, Cu, Ni, Cr, Pb, and Zn) in tissue of Mugil cephalus and Trachurus mediterraneus from Iskenderun Bay, Turkey. Environmental Research, 92(3), 277–281.
Yılmaz, A. B., & Doğan, M. (2008). Heavy metals in water and in tissues of himri (Carasobarbus luteus) from Orontes (Asi) River, Turkey. Environmental Monitoring and Assessment, 144(1–3), 437–444.
Yılmaz, F., Özdemir, N., Demirak, A., & Tuna, A. L. (2007). Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chemistry, 100(2), 830–835.
Yılmaz, A. B., Sangün, M. K., Yağlıoğlu, D., & Turan, C. (2010). Metals (major, essential to non-essential) composition of the different tissues of three demersal fish species from İskenderun Bay, Turkey. Food Chemistry, 123(2), 410–415.
Zeng, J., Yang, L. Y., Wang, X., Wang, W. X., & Wu, Q. L. L. (2012). Metal accumulation in fish from different zones of a large, shallow freshwater lake. Ecotoxicology and Environmental Safety, 86, 116–124.
Zhou, C., Liu, G., Yan, Z., Fang, T., & Wang, R. (2012). Transformation behavior of mineral composition and trace elements during coal gangue combustion. Fuel, 97, 644–650.
Zhuang, P., Li, Z. A., McBride, M. B., Zou, B., & Wang, G. (2013). Health risk assessment for consumption of fish originating from ponds near Dabaoshan mine, South China. Environmental Science and Pollution Research International, 20(8), 5844–5854.
Zubcov, E., Zubcov, N., Ene, A., & Biletchi, L. (2012). Assessment of copper and zinc levels in fish from freshwater ecosystems of Moldova. Environmental Science and Pollution Research, 19(6), 2238–2247.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, 2014CB238900), the National Natural Science Foundation of China (No. 41672144). We acknowledge editors and reviewers for polishing the language of the paper and for in-depth discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, L., Liu, G., Wang, J. et al. Accumulation and health risk assessment of trace elements in Carassius auratus gibelio from subsidence pools in the Huainan coalfield in China. Environ Monit Assess 189, 479 (2017). https://doi.org/10.1007/s10661-017-6178-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6178-x