Abstract
We investigated the temporal and among-site differentiation of soil functionality properties in fields under different grazing intensities (heavy and light) and compared them to those found in their adjacent hedgerows, consisting either of wooden shrubs (Rubus canescens) or of high trees (Populus sp.), during the cold and humid seasons of the year. We hypothesized that greater intensity of grazing would result in higher degradation of the soil system. The grazing factor had a significant effect on soil organic C and N, microbial biomass C, microbial biomass N, microbial activity, and β-glucosidase, while acid phosphatase and urease activity were not found to differ significantly among the management systems. The intensity of grazing affected mostly the chemical properties of soil (organic C and N) and altered significantly the composition of the soil microbial community, as lower C:N ratio of the microbial biomass indicates the dominance of bacteria over fungi in the heavily grazed fields. All estimated biological variables presented higher values in the humid period, although the pattern of differentiation was similar at both sampling times, revealing that site-specific variations were more pronounced than the time-specific ones. Our results indicate that not all C, N, and P dynamics were equally affected by grazing. Management plans applied to pastures, in order to improve soil quality properties and accelerate passive reforestation, should aim at the improvement of soil parameters related primarily to C and secondly to N cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grazing of pasture and range lands by livestock constitutes a common practice of land management that can alter the spatial heterogeneity of vegetation, influencing ecosystem processes and biodiversity (Adler et al. 2001). Livestock grazing is often regarded as one of the principal causes of vegetation and soil degradation (Wu et al. 2009; Deng et al. 2014). Grazing has been found to affect the physical, chemical, and biological properties of soil (Altesor et al. 2006; Stephens et al. 2008; Zhu et al. 2016). The effects of grazing pressure on soil quality variables are consequences from several activities of domestic herbivores, e.g., defoliation, excreta deposition, and trampling (Cingolani et al. 2003; Köhler et al. 2005; Wang et al. 2011). Soil biological properties, such as the size and the activity of the soil microbial biomass (Liu et al. 2015), as well as the activities of soil enzymes that are fundamental to the cycling of C, N, and P (Su et al. 2004), have been found to be affected by grazing. Additionally, changes on litter accumulation and decomposition have a strong effect on the amount and composition of soil organic matter (Nautiyal et al. 2010).
The intensity of grazing is a significant factor affecting soil properties and functions; light grazing, compared with grazing exclusion, is generally believed to be beneficial, enhancing water retention capacity and soil fertility (Su et al. 2005; Peco et al. 2006, 2012), as it affects the spatial distribution of nutrients in soil by altering the composition of the natural vegetation as well as by contributing to the redistribution of nutrients through feces from sheltered areas under shrubs to exposed locations between shrubs (Papatheodorou 2008). On the other hand, heavy grazing can cause severe loss of soil fertility and productivity, as a consequence of reduction in vegetation cover and may finally lead to soil degradation (Thornes 2007; Deng et al. 2014; Zhu et al. 2016). However, it is not yet clear whether different levels of grazing intensity affect similarly soil microbiological properties that determine soil processes. Different studies that have focused on the effect of grazing intensity on soil characteristics have shown contradictory results (Peco et al. 2006; Deng et al. 2014; Zhu et al. 2016). Therefore, further research should be conducted to study the influence of grazing intensity on soil ecosystem processes.
Since 1988, in the European Union, a common practice for enlarging the areas of natural vegetation is the set-aside of agricultural land (Matthews 2013). This is considered to be one of the most widely applied measures to counteract the rapid loss of species diversity and conserve the remaining diversity, especially within the zones of protected areas (Wade 1997). Previous studies have shown that plant species succession towards naturalized grasslands strongly correlates with the soil ecosystem and nutrient availability (Berg and Hemerik 2004; Wan et al. 2010). Long-term grazing management practices are known to affect negatively many soil biochemical characteristics related to soil functionality and nutrient cycles (Prieto et al. 2011). Consequently, it is important to know the level of soil degradation, in terms of functionality, in order to suggest the proper management plans that could accelerate the restoration of these systems in terms of soil quality.
In our study, we assessed how different levels of grazing affect soil variables related to soil quality compared to those found in their adjacent hedgerows, within a protected area. Fields under heavy and light grazing management, located within the protected zone around Pamvotis Lake, were selected. Hedgerows, on the border line of these fields, remnants of pre-existing natural vegetation, consisted either of wooden shrubs (Rubus canescens) or of high trees (Populus sp.) were chosen to represent two types of natural ecosystems. We studied soil characteristics related to soil functionality (nutrient concentrations, the activities of urease, alkaline phosphatase, and dehydrogenase, which are involved in N, P, and C transformations in soil) and microbial structure (microbial biomass C and N) and activity. Our aims were to examine (i) the temporal and among-management system variability of soil enzyme activities, microbial biomass and activity, and available nutrients, (ii) the magnitude of soil degradation based on the level of grazing, and (iii) which of the biological C, N, and P cycles is most influenced by grazing. We hypothesized that greater intensity of grazing would result in higher degradation of the soil system, modifying accordingly the soil biochemistry of these fields. We further hypothesized that grazing would have an equal negative effect on soil C, N, and P dynamics.
Materials and methods
Study site
The study area is located in the surrounding land region of Lake Pamvotis, situated at 470 m a.s.l. in northwestern Greece (39° 40′ N, 20° 51′ E). The lake occupies an area of 22.8 km2 and is located in the vicinity of the city of Ioannina. The soil of the surrounding land areas is alluvial and originates from the surrounding hills where the parental rock is mainly limestone (Tzialla et al. 2006). Ioannina area belongs to the humid Mediterranean bioclimatic belt. The mean annual precipitation is about 1100 mm, falling mostly during winter and spring, and the mean monthly temperature is 14.3 °C, while the mean January–June temperature difference is 20.1 °C (Hellenic National Meteorological Service 2011). For the past 50 years, a large part of the area around the lake (495 ha) has been developed in a mosaic of small grazed fields (0.7–3.6 ha), interspersed with remnants of natural vegetation. The latter, although covering areas almost equal to those of the grazed fields, they serve as hedgerows, separating pasture lands. The vegetation of these hedgerows is similar and consists either of Populus sp. or of R. canescens. The vegetation of the grazed fields is dominated by herbs like Mentha aquatica, Geranium columbinum, Galium aparyne, Epilobium sp., Rapistrum sp., Senecio sp., Capsella bursapastoris, and Urtica dioica (Buka 2012).
Lake Pamvotis and its surrounding region have been recognized as an internationally important conservation site and are listed in the Special Conservation Areas (SCAs) under the European Community Council Directive on the conservation of natural habitats and of wild fauna and flora (Habitats Directive, EC, 92/43). A wetland reserve program is designed for the protection and restoration of the area, which includes the retiring of any future use of agricultural land.
Experimental design and sampling
Soil samples were collected from lightly grazed fields (five sheep per day per hectare), heavily grazed fields (18 sheep per day per hectare), and the adjacent hedgerows, consisted either of Populus sp. or of R. canescens. For each management system (heavily grazed, lightly grazed, R. canescens hedgerows, Populus sp. hedgerows), four replicate fields were randomly selected. On each sampling occasion, five soil samples were taken randomly from each replicate field (grazed or hedgerow) with a cylindrical auger (12-cm depth and 7.5-cm diameter). In all cases, litter layer was removed before sampling. Immediately after collection, soil samples were intermingled so that a single composite sample was formed for each replicate field. Samples were stored in plastic bags and transported to the laboratory. They were sieved through a 2-mm mesh for the removement of stones, roots, and organic debris, stored at a constant temperature of 4 °C, and analyzed within the same week. Samples were taken in January (winter sampling—trees without foliage) and May (spring sampling—after foliation of the trees) 2013. The overall full factorial experimental design consisted of four replicate fields × four management regimes × two sampling occasions.
Analyses of soil chemical and biochemical variables
Soil pH was determined using an electrode pH meter in a 1:2 soil/water suspension (McLean 1982). Soil organic C was determined by a wet oxidation titration procedure using an acid dichromate system (Allen 1974). Soil organic N was measured by the Kjeldahl method. Soil microbial biomass C (MBC) was measured by the fumigation incubation method of Jenkinson (1976), as modified by Ross (1990) in samples adjusted to 60% of their water holding capacity. The Kc factor was 0.45 (Jenkinson 1976). Soil microbial biomass N (MBN) was determined by the fumigation–extraction method using a factor of 0.54 to convert N-flush to MBN (Brookes et al. 1985).
Acid phosphatase and β-glucosidase activities were determined according to the procedures of Allison and Jastrow (2006), modified for 96-well microplates. Approximately 1–2-g fresh soil (0.5-g dry weight equivalent) were added in 60 ml of 50-mM sodium acetate buffer, pH 5, and homogenized in a blender for 1 min. Then 50 μL of homogenized soil slurry was combined with 150-μL substrate solution and incubated for 2 h (β-glucosidase) or 1 h (acid phosphatase) at 21 °C under constant shaking. Substrate solutions were 5-mM p-nitrophenyl-β-glucopyranoside for β-glucosidase and 5-mM p-nitrophenyl-phosphate for acid phosphatase, all in acetate buffer. After incubation, 100 mL of the slurry-substrate supernatant (without soil particles) was carefully transferred to another microplate for colorimetric determination of product concentrations. The p-nitrophenol (pNP) reaction product from the acid phosphatase and β-glucosidase assays was measured at 405 nm after addition of sodium hydroxide. Eight replicates were run per sample; in each case, we included appropriate controls to estimate the background absorbance of the substrate and homogenate. The activity of the two enzymes is presented in units of μmol pNP g−1 dry soil h−1.
For the estimation of urease activity, the method of Sinsabaugh et al. (2000) was used. The microplate configuration was similar to that described for the NAG assay. The concentration of urea in the assay wells was 20 mM. The plates were incubated at 20 °C for approximately 18 h. Ammonium released by the reaction was quantified using colorimetric salicylate and cyanurate reagent packets from Hach. Urease activity was measured spectrophotometrically at 610 nm. Activity is expressed as micromole of ammonium released per hour per gram soil (μmol NH4 + g−1 h−1).
Statistical analyses
One-way ANOVA was used to identify differences in soil texture (percentage contribution of sand, clay, and silt) among the study management systems. Additionally, to explore temporal and among-system variations of soil biochemical variables, a two-way ANOVA was used to determine the effect of sampling time, management system, and their interaction. In the case of significant effects, a least significant difference test was performed. When the assumptions of the ANOVA (normal distribution, independence between means, and variance) were not met, data were transformed properly.
A classification tree was applied to our dataset in order to address the role of grazing intensity. More specifically, we (a) examined whether soil samples from the grazed fields were clearly separated from those from the hedgerows, in terms of soil biological and physicochemical variables, (b) evaluated whether the management system effect masked the temporal variability on soil biochemical and microbial variables or vice versa, and (c) assessed the importance of soil variables in this separation. Decision tree modeling is a form of classification that involves the use of an algorithm to obtain repeated partitions of the dataset into increasingly homogeneous subsets with respect to the predictor variables (Breiman et al. 1984); in this case, they were the soil variables. The process is recursive, growing from the root node (the complete dataset) to the terminal nodes (Friedl and Brodley 1997). To group the samples, a variable combining the sampling date and site was used, while the soil biochemical variables were used as continuous predictors.
During the classification tree analysis, decision tree algorithms go through a process of “pruning” the tree, where the model is refined in order to result in the simplest possible model given the constraints. Variables that have a minimal predictive effect are pruned away and the remaining variables are able to be ranked according to their importance. In a bar graph, the importance of ranking on a 0–100 scale for each predictor variable considered in the analysis can easily be pictorially shown. This plot can be used for visual inspection of the relative importance of the predictor variables used in the analysis and, thus, helps in concluding as to which predictor variable is the most important predictor (Breiman et al. 1984).
All statistical analyses were conducted using Statistica 7 for Windows (StatSoft, Tulsa, USA).
Results
In all sampling systems, the soil texture was similar, as no statistically significant difference was revealed by ANOVA among the percentages of sand, clay, and silt of the soil samples. Soils could be characterized as loamy soils (Table 1).
The effects of sampling time, the management system, and their interactions on all soil variables are shown in Table 2 and Fig. 1. The interactive effect of the two independent variables did not affect any of the estimated soil variables. Six out of ten estimated soil variables were significantly affected by time. The values of MBC, MBN, MBC/MBN, microbial activity, as well as the activity of β-glucosidase and urease increased significantly from February to June, while the concentrations of organic C, organic N, the activity of acid phosphatase, and pH values did not differ. Regarding the effect of management system, the activity of urease and pH were the only soil variables that did not present any significant difference. MBC, microbial activity, organic C, and organic N presented a similar pattern as the highest values were recorded in the hedgerow soil samples and the lowest in the grazed fields, especially in the heavily grazed fields. MBC differed slightly from this pattern since the heavily grazed fields presented higher values than the lightly grazed ones. MBN and MBC/MBN ratio followed the opposite pattern as the highest values were recorded in the heavily grazed fields and differed significantly compared to the other three systems, either hedgerows or grazed.
Mean values (±SE) of soil biochemical variables recorded in January and May at the four management systems. “Time” and “system,” on the top of the graphs, indicate a significant effect of sampling time and management system, respectively as revealed by two-way ANOVA (* P < 0.05, ** P < 0.01, *** P < 0.001, for all cases n = 4)
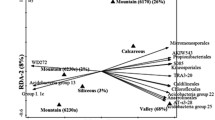
The results of the classification tree are presented in Fig. 2. The tree consisted of eight terminal nodes. All management system × time combinations of samples constituted very cohesive groups (75–100% right classification). Three or four out of the four samples from each system were classified in the same terminal node. Populus sp. soil samples were characterized by high acid phosphatase activity values and were clearly separated from the other samples, where acid phosphatase activity values were lower. MBC/MBN ratio further discriminated R. canescens samples from the grazed field samples. The grazed samples branch was further dichotomized due to organic C concentrations, as the heavily grazed field samples exhibited lower values of organic C and were classified at the left, while the lightly grazed samples with higher values of organic C formed the right branch. In all cases, the branches from all four management systems were further dichotomized based on the sampling date. The temporal discrimination of the Populus sp. and R. canescens samples was based on the values of urease activity, while the grazed field samples from the two samplings were discriminated on the basis of MBN concentrations. Predictor importance analysis showed that among all soil variables, those with the greatest classification power were MBC/MBN ratio, MBN, organic C, acid phosphatase, and urease (Fig. 3).
Classification tree of soil samples collected from different management systems (P Populus sp., R R. canescens, LG light grazing, HG heavy grazing) on two sampling occasions (1 January, 2 May). The variables used for the classification of the soil samples were the soil biochemical parameters. The number of samples in each terminal node is indicated. Bold letters indicate the fraction of samples that were classified correctly (for all cases n = 4)
Discussion
The effect of grazing on soil pH has been extensively discussed in the literature. Shand et al. (2002) have indicated that the addition of urine in grazed soils results in elevated pH levels, especially in heavily grazed fields, while others have found no significant difference across sampling fields, no matter the intensity level of grazing (Somda et al. 1997; Liu et al. 2005). In the present study, the variability of soil pH (6.89–7.65) across management systems and sampling times was not statistically significant, indicating that alkalinity is not an important issue that could affect soil fertility of the grazed fields.
It is generally well-documented that grazing reduces C and N stocks of grassland ecosystems (Pineiro et al. 2009; Wang et al. 2014). Similar results were recorded in our study as the soil organic C and N content of the hedgerows was significantly higher than the grazed fields. Higher organic C and organic N values in Populus sp. and R. canescens soil samples were probably due to the constant addition of organic matter inputs from plant residues and root exudates to the soil (Rutigliano et al. 2004; Singh et al. 2004). On the other hand, the reduction in vegetation by herbivores (Gao et al. 2008) and the lack of a widely developed root system (Fterich et al. 2012) in the grazed fields might result to reduced plant inputs and lower soil organic C and N concentrations. Microbial activity is also affected by soil organic matter quality (Liu et al. 2000). The constantly higher amounts of easily degradable organic inputs, in the form of animal feces, in the heavily grazed fields resulted in greater soil emissions of CO2. The decomposition of organic matter was faster than the accumulation rate of carbon in these fields which caused a greater net loss of organic matter (Morgan et al. 2004)
MBC concentration presented the same pattern with organic C, higher values in the hedgerows, and lower in the grazed fields. This was partly expected based on the high correlation between these two soil variables (Papatheodorou et al. 2013). On the contrary, heavy grazing proved to enhance soil MBC compared to light grazing. This finding could be explained in correspondence with the high MBN values found in the heavily grazed soil samples, indicating mainly an increase in bacterial biomass, as bacteria accumulate in their biomass greater amounts of nitrogen than fungi (Alexander 1977). MBC/MBN ratio has been extensively used as an indicator of changes in soil microbial community composition. The grazed fields presented significantly lower values of MBC/MBN ratio compared to the hedgerow sites. Plant residues, like dead leaves and roots, which are incorporated in the hedgerow soil systems consist of more complex C compounds that favor fungi growth, while in the grazed fields, the addition of more labile substrates (animal excreta) favors bacteria. Additionally, it has been reported that trampling can change microbial community structure by destroying fungi micelles (Hiltbrunner et al. 2012). Our results are in agreement with those of Bardgett et al. (2001), who found that, along a sequence of samples under different levels of grazing, proportion of fungi relative to bacteria was at its lowest in treatments that were most intensively grazed.
Soil enzyme activities are directly connected with the functionality of the soil systems. β-Glucosidase participates in the decomposition of cellulose and other carbohydrate polymers that derive from plant litter (Prieto et al. 2011) and thus reflects the state of soil organic matter in the ecosystem. Populus sp. and R. canescens hedgerow samples exhibited higher β-glucosidase activity compared to the grazed fields and this could be attributed to the high amounts of organic C concentrations and soil microbial biomass (Chodak and Niklińska 2010; Mastrogianni et al. 2014). Woody shrubs and high trees produce recalcitrant litter for decomposers enhancing the activity of β-glucosidase, while the perennial grasses, along with animal excreta, supply a more labile C-rich substrate of cellulolytic nature that results in the decline in the activity of hydrolytic enzymes such as β-glucosidase (Aerts and Chapin 2000; Carrera et al. 2005).
Urease is responsible for the decomposition of urea to CO2 and ammonia and is a very important enzyme in the N cycle since the availability of ammonia to plants is a definitive parameter for proper plant growth (Craine et al. 2009). Generally, urease activity is strongly correlated with organic matter content and higher urease activity is expected in rich soils (Monokrousos et al. 2014). Ηowever, Katsalirou et al. (2010) reported that soils subjected to different levels of grazing had higher values of urease activity compared to ungrazed soils, while Trasar-Cepeda et al. (2008) found that the nitrogen cycle enzymes show higher activities in pastures than in oak soils. Our results showed that urease activity presented similar values in all management systems, despite the low soil organic matter content of the grazed sites. The incorporation of animal urine in these systems, throughout the year, rich in readily decomposable nitrogen (Diaz-Fierros et al. 1988), probably resulted in high urease activity. The presence of animals in the grazing fields preserved properties related to the functionality of N cycle at a high level. However, the removal of the grazing factor could result in a direct decrease of urease activity in the future due to the low content of organic matter in these fields.
Populus sp. soil samples presented the highest acid phosphatase activity values and differed from all the other sites. Poplar trees form symbiotic associations with ectomycorrhizas (Conn and Dighton 2000), which secrete acid phosphatases in the rhizosphere (Lodge and Whalley 1989). Therefore, enhanced activity of the specific enzyme, contributing to the phosphorus nutrition of the trees is to be expected in these hedgerow systems. On the contrary, no significant differences were observed in the acid phosphatase activity recorded among the R. canescens hedgerows and the grazed fields. The acid phosphatase values in the grazed fields were also found to be similar to those recorded in grassland sites of other studies (Keeler et al. 2009). This could be interpreted as an indication that the P cycle has not been affected significantly by the grazing factor and the grazed fields do not present limitation on phosphorus production for plant growth. Although it is usually assumed that grazing results in decreased enzyme activity due to changes in the soil organic matter and microbial biomass content (Islam and Weil 2000; Dilly et al. 2003), the results obtained here contradict this assumption.
Soil organic C and N did not display any differentiation between the two sampling dates. Soil organic matter responds slowly to changes, and these may require many years to be detected (Srivastava 2012). The differences found among the grazed and the hedgerow sites are the result of long-term grazing of the pasture land. On the contrary, seasonal variations in soil microbial biomass and enzyme activity may be ascribed to a combination of variables such as soil temperature, root activity, and litterfall (Bardgett et al. 1999; Krämer and Green 2000). ANOVA showed that soil samples collected in May presented higher values in the majority of the estimated soil biological variables (MBC, MBN, soil respiration, urease, and β-glucosidase activity). This could be attributed mostly to the low winter temperatures of the study area that could suppress microbial growth and activity (Wardle 1992), as well as enzyme activities (Pavel et al. 2004), as most of them are of microbial origin. The only biological variable that did not present differences between the two samplings was acid phosphatase, for any of the study systems. Acid phosphatase is positively correlated with soil water availability (Krämer and Green 2000) and given the high precipitation rate of the Ioannina region, the activity of the specific enzyme was not affected by sampling time.
The decision tree classification of the soil samples could be used as a gradient of the state of soil quality in the studied management systems. Soil samples from the four different systems, no matter the sampling time, constitute four separate groups; the tree hedgerow system was classified at the right edge, the grazed fields at the left edge, and the woody R. canescens shrubs in the middle. The management system effect was much more pronounced than that of the period of sampling as the classification was based according to the management system and secondly according to the time period. Soil samples collected from the grazed fields were classified closely and were separated from the hedgerow one, indicating the similarity in their soil biochemical characteristics. However, the intensity of grazing affected significantly mostly the organic C content which is responsible for the discrimination between samples from the heavily and the lightly grazed fields. Grazing influenced the potential of organic matter decomposition triggering fast carbon cycling and resulting in low soil organic matter content. The higher the intensity of grazing, the faster the depletion of stored nutrients. Therefore, a return to a slow-cycling (C-storing) ecosystem is required to build new organic reserves (Bardgett et al. 2001), and an equilibrium between the input of plant litter and the losses to decomposition and nutrient mineralization processes has to be established (Nyssen et al. 2008). Moreover, the grazing factor affected the composition of the soil microbial community, as MBC/MBN ratio was likely the most important variable that discriminated the grazed fields from the hedgerows. Complete cessation of grazing will gradually result to a more fungal than a bacterial based energy channel in the decomposer food web (Moore and Hunt 1988; Bardgett et al. 1998). Plenty of studies have shown that microbial community of soil becomes increasingly dominant of fungi as succession proceeds (Bardgett et al. 1999; Ohtonen et al. 1999).
In terms of management, cessation of grazing alone could possibly improve the nutrient cycles without the need for active restoration. Nevertheless, untended for a long period, degraded land may recover to an extent, but it rarely recovers to its original condition. More often, degradation will get worse before it gets better and restoration action is required to reverse the trend. Our results, along with the fact that the importance analysis showed MBC/MBN ratio, MBN, and organic C variables as the most important ones for the classification of the samples, indicate that grazing, in terms of soil functionality, induced changes that are mainly expressed in soil parameters related to the C and N cycles. Management plans should aim at the improvement of variables related to these two cycles in order to favor passive reforestation from herbaceous to woody vegetation (Laskurain et al. 2013). Management plans regarding the P cycle could be applied at later stages, when woody vegetation will be well-established.
References
Adler, P., Raff, D., & Lauenroth, W. (2001). The effect of grazing on the spatial heterogeneity of vegetation. Oecologia, 128(4), 465–479.
Aerts, R., & Chapin, F. S., III. (2000). The mineral nutrition of wild plants revisited: a reevaluation of processes and patterns. Advances in Ecological Research, 30, 1–67.
Alexander, M. (1977). Introduction to soil microbiology. New York: Wiley.
Allen, S. E. (1974). Chemical analysis of ecological materials. Oxford: Blackwell.
Allison, S. D., & Jastrow, J. D. (2006). Activities of extracellular enzymes in physically isolated fractions of restored grassland soils. Soil Biology and Biochemistry, 38(11), 3245–3256.
Altesor, A., Piñeiro, G., Lezama, F., Jackson, R. B., Sarasola, M., & Paruelo, J. M. (2006). Ecosystem changes associated with grazing in subhumid South American grasslands. Journal of Vegetation Science, 17(3), 323–332.
Bardgett, R. D., Jones, A. C., Jones, D. L., Kemmitt, S. J., Cook, R., & Hobbs, P. J. (2001). Soil microbial community patterns related to the history and intensity of grazing in sub-montane ecosystems. Soil Biology & Biochemistry, 33(12–13), 1653–1664.
Bardgett, R. D., Lovell, R. D., Hobbs, P. J., & Jarvis, S. C. (1999). Seasonal changes in soil microbial communities along a fertility gradient of temperate grasslands. Soil Biology & Biochemistry, 31(7), 1021–1030.
Bardgett, R. D., Wardle, D. A., & Yeates, G. W. (1998). Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biology & Biochemistry, 30(14), 1867–1878.
Berg, M. P., & Hemerik, L. (2004). Secondary succession of terrestrial isopod, centipede, and millipede communities in grasslands under restoration. Biology and Fertility of Soils, 40(3), 163–170.
Breiman, L., Friedman, J. H., Olshen, R. A., & Stone, C. J. (1984). Classification and regression trees. New York: Chapman and Hall.
Brookes, P. C., Landman, A., Pruden, G., & Jenkinson, D. S. (1985). Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology and Biochemistry, 17(6), 837–842.
Buka, E. (2012). Patterns of plant diversity in areas surrounding Lake Pamvotis (undergraduate thesis). University of Ioannina (In Greek, abstract in English).
Carrera, A. L., Vargas, D. N., Campanella, M. V., Bertiller, M. B., Sain, C. L., & Mazzarino, M. J. (2005). Soil nitrogen in relation to quality and decomposability of plant litter in the Patagonian Monte, Argentina. Plant Ecology, 181(1), 139–151.
Chodak, M., & Niklińska, M. (2010). Effect of texture and tree species on microbial properties of mine soils. Applied Soil Ecology, 46(2), 268–275.
Cingolani, A. M. I., Cabido, M. R., Renison, D., & Solís Neffa, V. (2003). Combined effects of environment and grazing on vegetation structure in Argentine granite grasslands. Journal of Vegetation Science, 14(2), 223–232.
Conn, C., & Dighton, J. (2000). Litter quality influences on decomposition, ectomycorrhizal community structure and mycorrhizal root surface acid phosphatase activity. Soil Biology and Biochemistry, 32(4), 489–496.
Craine, J. M., Ballantyne, F., Peel, M., Zambatis, N., Morrow, C., & Stock, W. D. (2009). Grazing and landscape controls on nitrogen availability across 330 South African savanna sites. Austral Ecology, 34, 731–740.
Deng, L., Sweeney, S., & Shangguan, Z. P. (2014). Grassland responses to grazing disturbance: plant diversity changes with grazing intensity in a desert steppe. Grass and Forage Science, 69(3), 524–533.
Diaz-Fierros, F., Villar, M. C., Gil, F., Carballas, M., Leiros, M. C., Carballas, T., & Cabaneiro, A. (1988). Effect of cattle slurry fractions on nitrogen mineralization in soil. Journal of Agricultural Science, 110, 491–497.
Dilly, O., Blume, H. P., & Munch, J. C. (2003). Soil microbial activities in Luvisols and Anthrosols during 9 years of region-typical tillage and fertilisation practices in northern Germany. Biogeochemistry, 65(3), 319–339.
Friedl, M. A., & Brodley, C. E. (1997). Decision tree classification of land cover from remotely sensed data. Remote Sensing of Environment, 61(3), 399–409.
Fterich, A., Mahdhi, M., & Mars, M. (2012). Impact of grazing on soil microbial communities along a chronosequence of Acacia tortilis subsp. raddiana in arid soils in Tunisia. European Journal of Soil Biology, 50, 56–63.
Gao, Y. Z., Giese, M., Lin, S., Sattelmacher, B., Zhao, Y., & Brueck, H. (2008). Belowground net primary productivity and biomass allocation of a grassland in Inner Mongolia is affected by grazing intensity. Plant and Soil, 307(1–2), 41–50.
Hellenic National Meteorological Service (2011). Ioannina—data period:1956–1997. The climate of Greece. http://www.hnms.gr/hnms/english/climatology/climatologyregiondiagramshtml?dr city=Ioannina
Hiltbrunner, D., Schulze, S., Hagedorn, F., Schmidt, M. W. I., & Zimmmermann, S. (2012). Cattle trampling alters soil properties and changes soil microbial communities in a Swiss sub-alpine pasture. Geoderma, 170, 369–377.
Islam, K. R., & Weil, R. R. (2000). Soil quality indicator properties in mid-Atlantic soils as influenced by conservation management. Journal of Soil and Water Conservation, 55(1), 69–78.
Jenkinson, D. S. (1976). The effects of biocidal treatments on metabolism in soil-IV. The decomposition of fumigated organisms in soil. Soil Biology and Biochemistry, 8(3), 203–208.
Katsalirou, E., Deng, S., Nofziger, D. L., Gerakis, A., & Fuhlendorf, S. D. (2010). European journal of soil biology spatial structure of microbial biomass and activity in prairie soil ecosystems. European Journal of Soil Biology, 46(3–4), 181–189.
Keeler, B. L., Hobbie, S. E., & Kellogg, L. E. (2009). Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems, 12(1), 1–15.
Köhler, B., Gigon, A., Edwards, P. J., Krüsi, B., Langenauer, R., Lüscher, A., & Ryser, P. (2005). Changes in the species composition and conservation value of limestone grasslands in Northern Switzerland after 22 years of contrasting managements. Perspectives in Plant Ecology, Evolution and Systematics, 7(1), 51–67.
Krämer, S., & Green, D. M. (2000). Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biology and Biochemistry, 32(2), 179–188.
Laskurain, N. A., Aldezabal, A., Olano, J. M., Loidi, J., & Escudero, A. (2013). Intensification of domestic ungulate grazing delays secondary forest succession: evidence from exclosure plots. Journal of Vegetation Science, 24(2), 320–331.
Liu, N., Kan, H. M., Yang, G. W., & Zhang, Y. J. (2015). Changes in plant, soil, and microbes in a typical steppe from simulated grazing: explaining potential change in soil C. Ecological Monographs, 85(2), 269–286.
Liu, Z., Li, Z., Nijs, I., & Bogaert, J. (2005). Fine-scale spatial pattern of Cleistogenes squarrosa population under different grazing intensities. Acta Prataculturae Sinica, 14(1), 11–17.
Liu, X., Lindemann, W., Whitford, W., et al. (2000). Microbial diversity and activity of disturbed soil in the northern Chihuahuan Desert. Biology and Fertility of Soils, 32, 243–249.
Lodge, G. M., & Whalley, R. D. B. (1989). Native and natural pastures on the tablelands of New South Wales: a review and annotated bibliography. NSW Agriculture, Technical Bulletin, 35.
Mastrogianni, A., Papatheodorou, E. M., Monokrousos, N., Menkissoglu-Spiroudi, U., & Stamou, G. P. (2014). Reclamation of lignite mine areas with Triticum aestivum: the dynamics of soil functions and microbial communities. Applied Soil Ecology, 80, 51–59.
Matthews, A. (2013). Greening agricultural payments in the EU’s Common Agricultural Policy. Bio-based and Applied Economics, 2(1), 1–27.
McLean, E. O. (1982). Soil pH and lime requirement. In A. L. Page, R. H. Miller, & D. R. Kenney (Eds.), Methods of soil analysis, part 2 (pp. 199–224). American Society of Agronomy: Madison.
Monokrousos, N., Boutsis, G., & Diamantopoulos, J. D. (2014). Development of soil chemical and biological properties in the initial stages of post-mining deposition sites. Environmental Monitoring and Assessment, 186(12), 9065–9074.
Moore, J. C., & Hunt, H. W. (1988). Resource compartmentation and the stability of real ecosystems. Nature, 333, 261–263.
Morgan, J. A., Pataki, D. E., Körner, C., Clark, H., Del Grosso, S. J., Grünzweig, J. M., Knapp, A. K., Mosier, A. R., Newton, P. C. D., Niklaus, P. A., Nippert, J. B., Nowak, R. S., Parton, W. J., Polley, H. W., & Shaw, M. R. (2004). Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia, 140(1), 11–25.
Nautiyal, C. S., Chauhan, P. S., & Bhatia, C. R. (2010). Changes in soil physico-chemical properties and microbial functional diversity due to 14 years of conversion of grassland to organic agriculture in semi-arid agroecosystem. Soil and Tillage Research, 109(2), 55–60.
Nyssen, J., Temesgen, H., Lemenih, M., Zenebe, A., Haregeweyn, N., & Haile, M. (2008). Spatial and temporal variation of soil organic carbon stocks in a lake retreat area of the Ethiopian Rift Valley. Geoderma, 146(1–2), 261–268.
Ohtonen, R., Fritze, H., Pennanen, T., Jumpponen, A., & Trappe, J. (1999). Ecosystem properties and microbial community changes in primary succession on a glacier forefront. Oecologia, 119(2), 239–246.
Papatheodorou, E. M. (2008). Responses of soil microbial communities to climatic and human impacts in Mediterranean regions. In T. X. Liu (Ed.), Soil ecology research developments (pp. 63–87). New York: Nova Science Publishers.
Papatheodorou, E. M., Kapagianni, P., Georgila, E. D., Monokrousos, N., & Stamou, G. P. (2013). Predictability of soil succession patterns under different agricultural land use practices: continual conventional cultivation versus transformation to organic cultivation or fallow periods. Pedobiologia, 56(4–6), 233–239.
Pavel, R., Doyle, J., & Steinberger, Y. (2004). Seasonal pattern of cellulase concentration in desert soil. Soil Biology and Biochemistry, 36, 549–554.
Peco, B., Carmona, C. P., de Pablos, I., & Azcárate, F. M. (2012). Effects of grazing abandonment on functional and taxonomic diversity of Mediterranean grasslands. Agriculture, Ecosystems and Environment, 152, 27–32.
Peco, B., Sánchez, A. M., & Azcárate, F. M. (2006). Abandonment in grazing systems: consequences for vegetation and soil. Agriculture, Ecosystems and Environment, 113(1–4), 284–294.
Pineiro, G., Paruelo, J. M., Jobbágy, E. G., Jackson, R. B., & Oesterheld, M. (2009). Grazing effects on belowground C and N stocks along a network of cattle exclosures in temperate and subtropical grasslands of South America. Global Biogeochemical Cycles, 23(2).
Prieto, L. H., Bertiller, M. B., Carrera, A. L., & Olivera, N. L. (2011). Soil enzyme and microbial activities in a grazing ecosystem of Patagonian Monte, Argentina. Geoderma, 162(3–4), 281–287.
Ross, D. J. (1990). Influence of soil mineral nitrogen content on soil respiratory activity and measurements of microbial carbon and nitrogen by fumigation-incubation procedures. Australian Journal of Soil Research, 28, 311–321.
Rutigliano, F. A., D’Ascoli, R., & Virzo De Santo, A. (2004). Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biology and Biochemistry, 36(11), 1719–1729.
Shand, C. A., Williams, B. L., Dawson, L. A., Smith, S., & Young, M. E. (2002). Sheep urine affects soil solution nutrient composition and roots: differences between field and sward box soils and the effects of synthetic and natural sheep urine. Soil Biology and Biochemistry, 34(2), 163–171.
Singh, B. K., Millard, P., Whiteley, A. S., & Murrell, J. C. (2004). Unravelling rhizosphere-microbial interactions: opportunities and limitations. Trends in Microbiology, 12(8), 386–393.
Sinsabaugh, R. L., Reynolds, H., & Long, T. M. (2000). Rapid assay for amidohydrolase (urease) activity in environmental samples. Soil Biology and Biochemistry, 32(14), 2095–2097.
Somda, I., Harkous, S., & Brun, H. (1997). Bipolar heterothallism in B-group isolates of Leptosphaeria maculans. Plant Pathology, 46(6), 890–896.
Srivastava, A. K. (2012). Advances in citrus nutrition. Maharashtra: National Research Centre for Citrus Nagpur.
Stephens, S. E., Walker, J. A., Blunck, D. R., Jayaraman, A., Naugle, D. E., Ringelman, J. K., & Smith, A. J. (2008). Predicting risk of habitat conversion in native temperate grasslands. Conservation Biology, 22(5), 1320–1330.
Su, Y. Z., Li, Y. L., Cui, J. Y., & Zhao, W. Z. (2005). Influences of continuous grazing and livestock exclusion on soil properties in a degraded sandy grassland, Inner Mongolia, northern China. Catena, 59(3), 267–278.
Su, Y. Z., Zhao, H. L., Zhang, T. H., & Zhao, X. Y. (2004). Soil properties following cultivation and non-grazing of a semi-arid sandy grassland in northern China. Soil & Tillage Research, 75, 27–36.
Thornes, J. B. (2007). Modelling soil erosion by grazing: recent developments and new approaches. Geographical Research, 45(1), 13–26.
Trasar-Cepeda, C., Leirós, M. C., & Gil-Sotres, F. (2008). Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biology and Biochemistry, 40, 2146–2155.
Tzialla, C. E., Veresoglou, D. S., Papakosta, D., & Mamolos, A. P. (2006). Changes in soil characteristics and plant species composition along a moisture gradient in a Mediterranean pasture. Journal of Environmental Management, 80(1), 90–98.
Wade, J. (1997). A study of vegetation composition and soil chemistry on five year non-rotational set-aside under four management regimes, (Ph.D dissertation), Essex, UK.
Wan, Y., Bao, Y., & Zhou, Q. (2010). Simultaneous adsorption and desorption of cadmium and tetracycline on cinnamon soil. Chemosphere, 80(7), 807–812.
Wang, L., Wang, D., Liu, J., Huang, Y., & Hodgkinson, K. C. (2011). Diet selection variation of a large herbivore in a feeding experiment with increasing species numbers and different plant functional group combinations. Acta Oecologica, 37(3), 263–268.
Wang, D., Wu, G. L., Zhu, Y. J., & Shi, Z. H. (2014). Grazing exclusion effects on above- and below-ground C and N pools of typical grassland on the Loess Plateau (China). Catena, 123, 113–120.
Wardle, D. A. (1992). A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biological Reviews, 67(3), 321–358.
Wu, G. L., Du, G. Z., Liu, Z. H., & Thirgood, S. (2009). Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai-Tibetan Plateau. Plant and Soil, 319, 115–126.
Zhu, G. Y., Deng, L., Zhang, X. B., & Shangguan, Z. P. (2016). Effects of grazing exclusion on plant community and soil physicochemical properties in a desert steppe on the Loess Plateau, China. Ecological Engineering, 90, 372–381.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panayiotou, E., Dimou, M. & Monokrousos, N. The effects of grazing intensity on soil processes in a Mediterranean protected area. Environ Monit Assess 189, 441 (2017). https://doi.org/10.1007/s10661-017-6161-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6161-6