Abstract
Trout farms are one of the major sources of pollution of highland streams and rivers, which are very sensitive ecosystems otherwise scarcely influenced by human activities. A trout farm with low annual production was chosen for comparative monitoring of its effects on the macrozoobenthos, epilithic diatom, and potamoplankton communities in the receiving stream. Research was conducted every 2 months during a period of 1 year (one control point and three localities downstream from the farm). The fish farm discharge induced moderate alteration of environmental factors in the receiving watercourse, viz., significant increase of the ionized and un-ionized ammonia fraction, dissolved oxygen concentration, and biological oxygen demand. Species richness and Simpson’s diversity index did not change in any of the communities. Correspondent analysis of potamoplankton community composition clearly showed trout farm effects, thereby indicating the need for reevaluation of saprobic valence values for potamoplankton species. The complete lack of influence of the trout farm discharge on the epilithic diatom community is probably due to absence of significant changes of phosphorus concentration in the receiving stream. Effects of the trout farm on the macrozoobenthos community were further characterized by 14 additional indices. The percentages of Chironomidae larvae, shredders, and total collectors; ratio of shredders to collectors; and the family biotic index are suggested as candidates for future multimetric index for measuring of trout farm influence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past decade, farming of rainbow trout has become an important economic activity. Thus, worldwide production in 2012 attained a maximum of 878,702 tons (FAO 2016), with Europe as the greatest producer. Trout production in concrete raceways is linked with mountainous regions having a well-developed drainage area because trout as a typical cold-water fish requires conditions with high content of dissolved oxygen and water free of harmful solids or gaseous waste materials (Woynarovich et al. 2011). Highland streams or upper reaches of rivers, as especially sensitive aquatic ecosystems (Allan and Castillo 2007), can be subject to considerable negative influence of trout farms. The most frequent contaminants are fish feed remains or uneaten feed and excreta (Liao 1970; Weston et al. 1996). Fish feed remains entering watercourses can lead to enrichment of the water with nutrients that is to its eutrophication, which is especially pronounced during the dry periods. A number of studies testing the chemical composition of effluent waters and that of the watercourse itself and the substrate downstream from the discharge (Boaventura et al. 1997; Bartoli et al. 2007; Koçer and Sevgili 2014) have shown that the presence of effluent waters of trout farms in the recipient watercourses most often leads to significant changes in some of the following parameters: phosphates (soluble and insoluble), nitrogen compounds, biological oxygen demand (BOD5), and total suspended solids (TSS) (Tello et al. 2010). These surveys also showed that the intensity of influence exerted by trout farms on the chemism of water of the recipient stream is highly variable. This is caused by differences in the relationship between production of the trout farm and the recipient stream’s flow (Živić et al. 2009a), the presence or absence of careful feeding and the use of correct diet, different content of phosphates in the feed, and the presence or absence of special settling ponds and systems for purification of effluent waters (Bergheim and Brinker 2003; MacMillan et al. 2003; Sindilariu et al. 2008).
The described changes in the chemism of recipient waters under the influence of trout farms can also lead to significant changes in communities of various aquatic organisms, which can thus be used as their bioindicators. Macrozoobenthos species are most frequently used as indicator organisms (Camargo 1992a, b; Kırkağaç et al. 2009; Fabrizi et al. 2010; Guilpart et al. 2012), followed by primary producers (Selong and Helfrich 1998; Villanueva et al. 2000; Daniel et al. 2005), microorganisms (Brown and Goulder 1996; Boaventura et al. 1997), and autochthonous species of fish (Oberdorff and Porcher 1994; Selong and Helfrich 1998). As far as we know, potamoplankton species were not used as indicator organisms in a single study, although many authors reported high abundance and diversity of potamoplankton in streams and rivers (Palmer 1990a, b, 1992; Schmid-Araya 1997, 1998), and it is the general opinion that changes in the composition of species of potamoplankton communities represent one of the first responses of aquatic ecosystems to stress in the environment (Schindler 1987). Hughes et al. (2000) assert that not every community responds in the same way to a certain type of stressor in the environment. The mentioned studies treating the influence of effluent waters from trout farms on the biota indicate the existence of a larger number of parameters that deviate from reference values for a given ecosystem. It is therefore necessary to include more than one community in this kind of biological monitoring and draw a conclusion as to which of them is the most sensitive to changes arising under the influence of various stressors (Resh 2008). However, it is rarely the case that researchers have simultaneously monitored the influence of trout farms on more than one community (Selong and Helfrich 1998; Camargo and Jiménez 2007; Camargo et al. 2011) and compared how they independently respond to certain types of stressors.
Most of the mentioned studies dealt with the effects of great changes of environmental factors under trout farm influence that brought about great changes in the investigated communities. Far fewer studies have attempted to answer how extremely slight changes of environmental factors under such influence affect living communities in the recipient waters (Camargo 1994; Selong and Helfrich 1998; Živić et al. 2009a, b). Thus, they give no clear answer to the question of which communities and what measures of their structure and composition are the most sensitive to changes of certain environmental factors and can as such be used as the first indicators of extremely slight changes in the environment of recipient waters under trout farm influence.
In our research, we selected a fish farm for which the ratio of annual production to the recipient stream’s water flow indicates that it should have small influence on environmental factors and tested that farm’s effects on the living communities of epilithic diatoms, macrozoobenthos, and potamoplankton. The questions we wanted answers to were the following: How great are the effects of the trout farm on environmental factors in the recipient stream? How does each community respond to trout farm influence? Which of the three communities is the most sensitive? What measures of composition and structure of the most sensitive community are the best indicators of the trout farm’s effects?
Material and methods
The study area and sampling sites
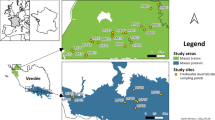
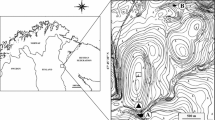
The Raška River is located in southwest Serbia and has a length of 39 km, width of 10–25 m, and depth of 0.3–1 m. Its basin has an area of 1040 km2 (Gavrilović and Dukić 2002). The Raška is a right-hand tributary of the Ibar River. The investigations were carried out in the years 2011 (April, June, August, October, December) and 2012 (March, May). Sampling of macrozoobenthos, potamoplankton, and epilithic diatoms was done at four localities on a by-channel of the Raška River that flows alongside the fish farm and has a length of 1.1 km (Fig. 1). Apart from the trout farm, there are no other significant human influences along the by-channel.
Locality R1 represents the control locality, situated 150 m above the outlet of the “Vekoslav Vukićević” family trout farm, at an elevation of 548 m a.s.l. Locality R2 is situated 10 m below the trout farm’s outlet. At a distance of 100 m below the second locality, locality R3 is at an elevation of 535 m a.s.l. Lying at an elevation of 530 m a.s.l., locality R4 is situated just in front of the by-channel’s mouth where it empties into the main course of the Raška River at a distance of 480 m from the third locality. With an overall area of 520 m2, the trout farm is located between localities R1 and R2 (Fig. 1). It has a total of eight pools, to which water is delivered directly by an input pipe system. A common canal carries water from the fish farm and discharges it without preliminary sedimentation into the recipient stream. Annual fish production of the trout farm during the study period (2011–2012) was 28,000 kg. The fish are fed extruded feed (Royal Optima, “Skretting,” the Netherlands). Three types of feed are used depending on fish biomass: 1p, 2p, and 3p. The chemical analysis of fish feed is presented in Table 1. Inasmuch as water temperature varies between 9.9 and 12.6 °C upstream from the fish farm and a system for enrichment of the water with oxygen exists in it, production of fish is constant throughout the year and it is also possible to expect relatively constant loading of the recipient stream with organic substances originating from fish feed remains and excreta.
Analysis of physical and chemical parameters
Water temperature (t w), pH, dissolved oxygen (DO), and conductivity (EC) were measured in situ using a water field kit PCE-PHD meter (Germany). Water for chemical analysis was sampled at the same time as biological samples. Analyses of total phosphorus (P tot) and orthophosphates (PO4 3−) were preformed according to APHA protocols (APHA 1998), the content of ionized ammonia (NH4 +) was analyzed according to standard methods for testing of hygienic integrity (P-V-2/B method, Škunca-Milovanović et al. 1990), and total water hardness (WH) was determined according to EPA method 130.2 (US EPA 1979), all in a laboratory of the Institute for Chemistry, Technology and Metallurgy, Belgrade, Serbia. Un-ionized ammonia (NH3) concentration was calculated according to Newman (1995). Concentrations of the remaining anions (NO3 −, NO2 −, Cl−, and SO4 2−) were determined with a DIONEX 4000i ion chromatograph in the Water Laboratory of the Institute of General and Physical Chemistry in Belgrade using the standard EPA method (Pfaff 1993). Since the concentrations of NO2 − were below the detection limits, they were excluded from further analysis. Sample handling and analyses for biochemical oxygen demand (BOD5) were performed according to APHA protocols (1998). During the investigation, described parameters were measured seven times (once on each sampling occasion) at each study site including the fish farm outlet.
Water flow rates (Q) were calculated according to procedure described in Živić et al. (2014). Stream depth (d) and flow velocity (v) were measured three times and stream width (w) just once on each sampling occasion at each locality. Substrate composition was estimated visually and expressed in terms of percent. Substrates were relegated to one of five classes: boulder (>250 mm), cobble (100–250 mm), gravel-pebble (2–100 mm), sand (0.125–2 mm), or mud (<0.125 mm). The percentage of coverage by aquatic macrophyte Ceratophyllum demersum L. was estimated on an area of about 100 m2. Substrate composition and macrophyte coverage were estimated once on each sampling occasion at each locality (n = 7).
Macrozoobenthos, potamoplankton, and epilithic diatom sampling and analysis
Macrozoobenthos was collected using a Surber net with a catchment area of 300 cm2 and mesh size of 250 μm. Three cumulative samples were taken on each sampling occasion at each locality (n = 7), choosing riffle habitats. Macrozoobenthos samples were fixed with 96% alcohol in plastic bottles in the field, and later examined using a ZEISS Discovery V8 stereomicroscope in the laboratory of the Institute of Zoology of Belgrade University’s Faculty of Biology. Identification was done to genus or species level using relevant literature (Brinkhurst and Jamieson 1971; Rozkošny 1980; Pillot 1984a, b, Pillot 2009; Nilsson 1996; Waringer and Graf 1997; Glöer and Meier-Brook 1998; Zwick 2004; Lechthaler and Stockinger 2005; Vallenduuk and Pillot 2007; Timm 2009; Bauernfeind and Soldan 2012). The biomass (bm) of macrozoobenthos was measured using an AE 163 analytical balance (Mettler-Toledo International Inc., Switzerland).
Potamoplankton samples were taken by collecting 50 l of water at each locality using a plastic bucket, and filtering through a sieve with 23-μm mesh size (Czerniawski and Domagała 2010; Czerniawski et al. 2013). Slow-moving areas of the stream with dense vegetation were chosen. Samples were preserved using 4% formalin and examined under a Leica Galen III optical microscope with maximal magnification of ×160 in the laboratory of the Institute of Animal Sciences of Belgrade University’s Faculty of Agriculture. Potamoplankton was identified to species or genus level using common keys for identification (Flossner 1972; Koste 1978). Quantitative samples were analyzed using the subsampling technique, where the number of identified species was recalculated to a volume of 1 l.
Epilithic diatoms were brushed from the surface of five stones removed from the stream bed. The field samples were treated according to the standard laboratory method given by Kelly et al. (2001) to make permanent slides with Naphrax resin. Abundance was estimated by counting 400 valves of each taxon present on a slide. Light microscope observations and counting of the valves were performed with a Zeiss AxioImagerM.1 microscope with DIC optics. Images were taken with an AxioCam MRc5 camera and processed with AxioVision 4.8 software. Identification of epilithic diatoms was preformed with standard literature (Krammer and Lange-Bertalot 1986, 1988, 1991, 2004; Hofmann et al. 2013).
Statistical analysis
All data were expressed as the mean ± standard error. The values of physical and chemical parameters and biological metrics between localities were compared statistically using one-way ANOVA with a P < 0.05 level of significance. The Holm-Sidak test was used to make comparisons across all pairs of localities when the one-way ANOVA test was significant. The strength of association among pairs of variables was measured with the Pearson product moment correlation at a 5% level of significance (P < 0.05). The statistical tests were calculated with the aid of the Sigma Plot 11 software (Systat Software Inc., San Jose, CA, USA).
The level of organic enrichment was assessed with the saprobity index (Sap), according to Pantle and Buck (1955) calculated for macrozoobenthos and potamoplankton communities. Values of saprobity valences were taken from Wegl (1983) for potamoplankton species and from Moog (2002) for macrozoobenthos. On the basis of quantitative estimation of the representation of identified epilithic diatom taxa and their indicator values, 17 diatom indices were calculated with the aid of the OMNIDIA software package (Lecointe et al. 1993). For graphic presentation of the results of these 17 indices, the pollution sensitivity index (IPS) (Coste 1982) was selected as the most similar index used for macrozoobenthos and potamoplankton. Alpha diversity was measured for each community with the Simpson dominance index (Sim) (Simpson 1949) and the number of species (spr). Correspondence analysis (CA) was used to describe the relationship between sampling sites with respect to the abundance of taxa in each community. Only taxa with relative abundances greater than 1% at least at one study site were used in order to simplify the presentation and focus on effects on the most important species. CA was conducted with the XLSTAT software (version 7.5.2) (Addinsoft).
To quantify the trout farm’s effects on the macrozoobenthos community in greater detail, several types of additional community metrics were calculated. Measures of qualitative community composition included the number of Chironomidae species (Chisp, Hayslip 1993; Barbour et al. 1996), number of Oligochaeta species (Olisp), and number of Ephemeroptera, Plecoptera, and Trichoptera species (EPTsp, Barbour et al. 1999). Measures of quantitative community composition included the percentages of Oligochaeta (Oli%, Barbour et al. 1999), Chironomidae larvae (Chi%, Barbour et al. 1999), and Ephemeroptera, Plecoptera, and Trichoptera larvae in total abundance (EPT%, MACS 1996) and the ratio of Baetidae abundance to total Ephemeroptera abundance (Bae/Eph, Živić et al. 2009a). The following biotic indices were used: the family biotic index (FBI, Hilsenhoff 1988a, b) and river longitudinal zonation index (RLZI, Moog 2002). The trout farm’s influence on trophic structure of the macrozoobenthos community was characterized by the percentages of shredders (Shr, Cummins 1988), collectors (Coll, Cummins 1988), scrapers (Scr, Cummins 1988), and predators (Pre, Kerans and Karr 1994), as well as by the ratio of shredders to collectors (Shr/Coll, Merritt and Cummins 1996). Fauna Aquatica Austriaca (Moog 2002) was used to classify macroinvertebrates according to their food intake type. Effects of the trout farm on total biomass (bm, g/m2) and abundance (ab, ind/m2) were also monitored.
Results
Environmental data
Average values of environmental parameters along the investigated localities are summarized in Tables 2 and 3. Out of water chemistry parameters (Table 2) one-way ANOVA showed that only DO, BOD5, NH4 +, and NH3 characterized statistically significant changes along investigated sampling sites. For all four parameters, the greatest difference was between the control locality and the trout farm outlet, monotonously decreasing at downstream localities which indicated the recovery from the trout farm influence. The Holm-Sidak test showed statistically significant difference between the control locality and the localities downstream from the trout farm outlet for DO, BOD5, NH4 +, and NH3 (Table 2). Additionally, values of BOD5 and DO were significantly different at locality R4 compared to those of the outlet (Table 2), further confirming fish farm discharge as the primary cause of their change. The minimal value of DO (6.7 mg/l) was recorded at R2 in October, while maximal values of NH3 (14 μg/l) and NH4 + (520 μg/l) were also recorded at R2 (in March), while that of BOD5 (3.48 mg/l) was recorded at R3 in August.
Water depth was the only hydrological parameter showing statistically significant variation along the investigated study sites due to greater values at localities R1 and R3 compared to R2 and R4 (Table 3). The composition of river bottom substrate was also fairly constant with strong domination of gravel at all four localities (Table 3). The only possible fish farm influence was a small but statistically significant increase of mud representation in the bottom substrate at the R2 compared to R1 locality (Table 3).
Comparison of sensitivity of the macrozoobenthos, potamoplankton, and epilithic diatom communities to the trout farm’s influence
In order to compare sensitivity (or the indicator potential) of the macrozoobenthos, potamoplankton, and epilithic diatom communities to fish farm influence, we first compared saprobity indices (Pantle-Buck for macrozoobenthos and potamoplankton and the pollution sensitivity index—IPS—for epilithic diatoms) as the most direct measures of sensitivity to pollution (Table 4). The trout farm’s discharge had no influence on saprobity indices of the epilithic diatom and potamoplankton communities, which both indicated oligosaprobic water quality with no statistically significant changes between study sites (Table 4). However, there was a clear effect on the macrozoobenthos community’s saprobity index, which significantly rose first at the R2 locality, rose again at the R3 locality, and only partially recovered at the R4 locality, staying significantly lower than at R3, but higher than at R1 (Table 4). Despite significant changes, saprobity index values of the macrozoobenthos community stayed within boundaries of the beta-mesosaprobic zone.
There was no effect of fish farm discharge on either aspect of alpha diversity in any of the three communities (Table 4). The greatest species richness was exhibited by epilithic diatoms, closely followed by the macrozoobenthos, with potamoplankton being by far the least species-rich community. All communities showed great and similar equitability as measured by Simpson’s index.
In order to find out if there was any measurable effect of fish farm discharge on the composition of the investigated communities, CA was used (Figs. 2 and 3; for additional information about taxa relative abundances, see Online Resource 1 for epilithic diatoms, Online Resource 2 for potamoplankton, and Online Resource 3 for macrozoobenthos). In congruence with the results of saprobity index, CA of abundances of macrozoobenthos taxa clearly separated the control site from the sites downstream from the farm outlet along the F1 axis explaining the greatest part of data variability (51.58%). If the position of taxa on the CA plane is analyzed, it is clear that inflow of the farm waste waters resulted in replacement of sensitive EPT and Coleoptera species grouped around the position of R1 locality with the tolerant Chironomidae, Simuliidae, Oligochaeta, and Hirudinea species grouped around positions of localities R2 and R3 (Fig. 2). The only exception was Nemurella picteti, sensitive species of Plecoptera most abundant at locality R3. Along the F2 axis, locality R4 is clearly separated from the rest of the localities (Fig. 2). It is similar to the localities R2 and R3 by the large representation of Chironomidae and Oligochaeta (Fig. 2), and to the locality R1 by the reappearance of Gammaridae (Fig. 2). If the Oligochaeta species composition is analyzed, it can be seen that tolerant species dominant at locality R2 and especially R3 (Limnodrillus hoffmeisteri, SI = 3.6, and Tubifex tubifex, SI = 3.7) are replaced at locality R4 with more sensitive ones (Eisniella tetraedra, SI = 2.1, and Peloscolex ferox, SI = 1.5). This, in addition to reappearance of Gammaridae, indicates partial recovery of the community at R4 as shown by the values of the saprobity index (Table 4).
Correspondence analysis (CA) of abundances of potamoplankton taxa that at least at one study site represented more than 1% of total abundance. Inset: Distribution of study sites obtained from CA of abundances of epilithic diatoms taxa that at least at one study site represented more than 1% of total abundance
In CA of potamoplankton community data, two factorial axes accounting for 42.54% (F1) and 33.99% (F2) of total variability were retained (Fig. 3). There was clear separation of study sites along the F1 axis, with that upstream from the trout farm placed at the positive end and those downstream from the trout farm at its negative end. Since the F1 axis explained a greater part of data variability, this indicated a strong influence of the trout farm’s discharge on the composition of the downstream potamoplankton community in contrast to the results of the saprobity index. If we take into account proximity to either group of study sites and abundance of the taxon (sее additional data given in Online Resource 2), the main effects of the trout farm’s discharge were a severe decrease in abundance of Anuraeopsis fissa, Epiphanes seneta, Lecane cornuta, Keratella quadrata, Lepadella ovallis, and Phylodina sp.; the appearance of Parencentrum lutetiae and Keratella tropica; and a great increase in abundance of Cyclops sp. and Harpacticoida. In addition, species of the genus Alona replaced Bosmina longirostris downstream from the trout farm’s outlet.
In contrast to potamoplankton, CA of abundances of epilithic diatom taxa showed no separation of the control locality from the localities downstream from the trout farm outlet along the F1 axis explaining the majority of the data variability (63%; Fig. 3, inset). This indicates the lack of trout farm influence on this community composition as already shown by the results of the IPS index.
Macrozoobenthos
The results of the saprobity index and CA clearly showed that the macrozoobenthos community was the most sensitive to the trout farm’s influence. We therefore decided to test additional commonly used measures of macrozoobenthos community composition and structure to ascertain those that are most sensitive to the trout farm’s influence and usable for fast but accurate assessment of it (Table 5, Fig. 4). As measures of the sensitivity, we took the presence of the statistically significant change between locality R1 and localities R2 or R3 and statistically significant correlation between the selected community measure and the concentration of un-ionized ammonia (NH3) as the most probable cause of eventual changes. Results of Pearson’s moment correlation showed that community measures showing statistically significant correlation with NH3 were Chisp (R = 0.995, P < 0.001), FBI (R = 0.949, P = 0.014), Olisp (R = 0.941, P = 0.017), Scr (R = −0.915, P = 0.029), Chi% (R = 0.914, P = 0.030), Coll (R = 0.905, P = 0.034), Sap (R = 0.903, P = 0.036), and RLZI (R = 0.897, P = 0.039). Of those community measures, only Olisp (Table 5) and Scr (Fig. 4) did not show statistically significant change after the trout farm’s discharge. Other community measures did not show statistically significant correlation with NH3. Among them, however, Shr, Shr/Coll, and EPT% were clearly influenced by the trout farm’s discharge, since they significantly changed between localities R1 and R2 and/or R3 (Table 5 and Fig. 4). The trout farm’s discharge induced statistically significant increase of FBI, RLZI, Chi%, Chisp, and Coll and decrease of Sch, Shr/Coll, and EPT% (Table 5 and Fig. 4). However, partial recovery of the community at R4, which could be expected from water chemistry data, in addition to Sap was indicated only by RLZI, Shr, Shr/Coll, and Coll (Table 5 and Fig. 4).
Effects of the trout farm on quantitative community composition (a) and average representation of macrozoobenthos functional feeding guilds (b) at the investigated sampling sites. Parameters with codes in the figure legend marked with an asterisk (*) showed statistically significant variation along the study sites according to one-way ANOVA. Different letters indicate significant difference among the study sites for each parameter according to the Holm-Sidak test (P < 0.05)
Discussion
For testing the influence of land-based trout farms on the quality of the recipient stream’s water, Koçer and Sevgili (2014) maintain that the minimal selection of chemical parameters for calculation of WQImin (the water quality index) can be reduced to just two parameters, viz., ammonia (NH4 +-N) and total organic nitrogen (TON). In our study, one-way ANOVA clearly emphasized NH4 +, NH3, DO, and BOD5 as the parameters subject to the greatest fish farm influence. Mean values of NH4 + (Table 2) at the outlet and localities downstream from the fish farm were somewhat greater than what is prescribed for land-based trout farms (0.25 mg/l, Laird and Needham 1988), but again, it is important to stress that they were not above the maximum of 1 mg/l prescribed and allowable for salmonid waters (TEPCD 2006). The situation is the same in the case of the un-ionized fraction, whose values, although statistically significantly higher at the indicated localities, nevertheless did not exceed that prescribed for salmonid waters (0.025 mg/l, TEPCD 2006). Mean values of dissolved oxygen concentration, although lower at the outlet itself and downstream localities, remained within the range allowable for salmonid waters (not below 6 mg/l, TEPCD 2006). If the values of BOD5 and NH4 + are analyzed as a function of saprobic levels (Junqueira et al. 2010), it is clear that the control locality is on the boundary between the oligo- and the beta-mesosaprobic zone, while water quality at the localities downstream from the fish farm is in the beta-mesosaprobic zone, that is the water is characterized by moderate organic pollution. Thus, the values of chemical parameters of the water indicate clear but moderate influence of the fish farm on the downstream localities. The results of our research indicated that the trout farm’s discharge had the greatest influence on composition and structure of the macrozoobenthos community, exerted weaker but still discernible influence on the potamoplankton community, and had no detectable influence on the community of epilithic diatoms.
Owing to the short duration of generations of epilithic diatoms (Rott 1991), their communities respond very rapidly to various kinds of changes, especially in the sense of enrichment of the water with nutrients (Hering et al. 2006), and for this reason, they are used as standard indicators of water quality. That being the case, it was expected that the trout farm’s moderate but clear effect should lead to changes in the community of epilithic diatoms. However, values of the saprobity index and indices of alpha diversity as well as the results of CA showed that such influence was completely lacking. If we compare the results of studies in which a trout farm exerted clear influence on epilithic diatom communities, we see as a common denominator a significantly increased concentration of phosphorus in the form of total phosphorus (Villanueva et al. 2000) or phosphates (Villanueva et al. 2000; Camargo and Jiménez 2007). In both of these studies, phosphorus concentrations after the fish farm were significantly greater than in our study. This was not the case with DO values, which were significantly lower in the study of Camargo and Jiménez (2007) but significantly higher in the study of Villanueva et al. (2000) than in our research. That the absence of any great changes of phosphorus concentration can be the main reason for a lack of changes in the epilithic diatom community is also indicated by studies showing that precisely phosphate content is one of the most important factors affecting the distribution and abundance of diatom taxa (Triest et al. 2001).
As far as we know, the present study is the first in which anyone has investigated the influence of a trout farm on potamoplankton communities in the recipient stream. CA clearly showed that the trout farm causes significant changes in composition of the potamoplankton community (Fig. 3). The investigated part of the Raška River’s course was especially suitable for such studies because of the significant presence of macrophytes and low-flow habitats, so-called dead zones (Robertson et al. 1995). To be specific, it has been demonstrated that communities of potamoplankton can be persistent and subject to monitoring from season to season even in the upper reaches of rivers, especially in the eventual presence of macrophytes, which promote the formation of low-flow habitats and refugia, where the life cycle of potamoplanktonic organisms can proceed and resting eggs can be deposited (Robertson et al. 1995; Basu et al. 2000). Their community’s persistence follows from the presence of the same species in dead zones and in main-channel habitats, which indicates that potamoplankton can “migrate” (circulate) through dead zones (Robertson et al. 1995). In view of the fact that Rotatoria constitute the dominant community in the Raška, which was to be expected as far as potamoplankton is concerned, the good indicatory properties of this group (Sládeček 1983) justify its use in a biomonitoring study dealing with the effects of a trout farm, as in the case of our research. However, changes in composition of the potamoplankton community were not accompanied by changes of the saprobity index (Fig. 3). Since effects of the trout farm are indisputable, as is clearly shown both by chemical analyses and increase of the saprobity index in the case of the macrozoobenthos community, this disagreement indicates the necessity of carrying out reiterated autoecological investigations and stresses the need to modify values of saprobic valences for species that are frequently encountered in lotic systems, above all Rotatoria.
Analysis of macrozoobenthos community metrics indicated that indices of alpha diversity exhibited the least sensitivity to effects of the trout farm. This was somewhat unexpected because alpha diversity in certain surveys was reported to be a good measure of trout farm impact (Camargo 1992b; Živić et al. 2009a, b). However, the influence of a trout farm on water chemistry in the first of these studies (Camargo 1992b) was significantly greater than in the Raška River. In the Trešnjica River, on the other hand, Chironomidae and Oligochaeta were not determined (Živić et al. 2009a, b), which could have led to falsely positive results. This is indicated by the fact that if the determined species of Chironomidae and Oligochaeta in the Raška River were not taken into account, statistically significant changes of both components of alpha diversity would occur under the fish farm’s influence (unpublished data), just as in the case of the Trešnjica River. It can be concluded that the indices of alpha diversity of macrozoobenthos communities are little sensitive to the trout farm’s effects since they respond only to great changes of environmental factors, and that their values can be considered reliable only if detailed determination of the whole community is conducted to the species level. This conclusion is supported by results of the investigations of Guilpart et al. (2012), who stated that fish farm wastes induced shifts of dominance among macrozoobenthos taxa rather than lowering overall diversity.
The saprobity index very precisely reflected effects of the fish farm on the macrozoobenthos community, placing localities on the same saprobic levels as those obtained on the basis of values of chemical parameters (BOD5, DO, and NH4 +, Junqueira et al. 2010). In addition to this, the saprobity index together with RLZI also succeeded in quantifying a mild recovery of the community at locality R4, which could be expected on the basis of changes in chemical parameters. Although these indices are very sensitive to effects of the trout farm, they (like diversity indices) demand very detailed determination to the species level in order to be reliable. For this reason, they are not suitable for rapid estimation of a trout farm’s effects. Of metrics that require determination only to the level of higher taxonomic categories, the most sensitive to effects of the trout farm in our study were FBI, Chi%, and EPT%. As an index used in rapid bioassessment protocols or RBP (Barbour et al. 1999), Chi% exhibited considerable sensitivity to various kinds of pollution (Rawer-Jost et al. 2000). In regard to the effect of trout farms, apart from the present study, a significant increase of Chi% was also recorded in the case of a trout farm on the Trešnjica River (Živić et al. 2009a, b). In the study of Camargo et al. (2011) and Guilpart et al. (2012), high sensitivity to trout farm effects was exhibited by a similar index, the percentage of Oligochaeta and Chironomidae in total abundance (OC%). These results indicate that Chi%, independently or in combination with Oli%, could be an index of choice for rapid estimation of the effects of trout farms. In our survey, EPT% in relation to Chi% exhibited slightly lower but still significant sensitivity to effects of the trout farm, but in other studies (Camargo et al. 2011, Guilpart et al. 2012), it proved to be just as sensitive as Chi%, which makes it a relatively reliable measure of such effects. The value of FBI was very sensitive to the trout farm’s effects in our survey, as in a study of Imanpour Namin et al. (2013). As far as we know, these are the only studies where the effects of a trout farm on FBI were examined, and in view of the simplicity of determining this index, it would be expedient to test its sensitivity on additional trout farms so as to determine more fully its reliability.
Although in the present study valence values determined for individual species were used to calculate indices based on functional feeding groups (Moog 2002), they differ from the saprobity index and RLZI in being generally uniform throughout genera and often families (e.g., Simuliidae and Rhyacophilidae) and even classes (Oligochaeta), which makes it possible for indices based on functional feeding groups to be reliably obtained without determination to the species level. The first attempts have already been made to define functional feeding groups using indicator values on the family level (Guilpart et al. 2012). In the Raška River, the greatest sensitivity to the fish farm’s effects was exhibited by the representation of collectors (Fig. 4). Increase of Coll (collectors) apparently is a very reliable measure of a trout farm’s effects, since it exhibited significant sensitivity in all studies where it was followed (Camargo 1992a, 1994; Loch et al. 1996; Camargo and Gonzalo 2007; Živić et al. 2009a; Guilpart et al. 2012). Although owing to complete recovery at the R4 locality there was no high correlation with changes in the concentration of NH3, the representation of shredders declined drastically after the inflow of waters from the fish farm. With only one exception, where no statistically significant difference was recorded between the reference locality and the locality directly below the investigated trout farm (Loch et al. 1996), the representation of shredders clearly declined under the influence of trout farms in previous studies (Camargo 1992a, 1994; Camargo and Gonzalo 2007; Živić et al. 2009a; Guilpart et al. 2012; Rawer-Jost et al. 2000). In view of uniformity of the response of these two functional groups, it was expected that the index based on their ratio Shr/Coll would exhibit high sensitivity, which proved to be the case in the present study and in a previous study of ours (Živić et al. 2009a), where values of the ratio downstream from the trout farm dropped suddenly to less than 0.25, which is considered to be the lower limit for normal summer and autumn communities of shredders (Merritt and Cummins 1996). Although not explicitly ascertained, in view of results obtained for the individual groups comprising this index, it could be expected that the ratio in question would respond in a similar manner under the influence of the majority of other trout farms.
Considerably more varied results are characteristic of changes in the participation of predators and scrapers. These two indices showed no sensitivity to trout farm influence in the present study, in a previous investigation of ours on the Trešnjica River (Živić et al. 2009a), in the study of Rawer-Jost et al. (2000) in the case of scrapers, or in that of Loch et al. (1996) in the case of predators. On the other hand, the expected decrease in representation of scrapers downstream from fish farms was detected in a series of other studies (Camargo 1992a, 1994; Camargo and Gonzalo 2007; Guilpart et al. 2012; Loch et al. 1996), as was an increase in the representation of predators (Camargo 1992a, 1994; Camargo and Gonzalo 2007). It should be added that in watercourses of southern Germany, scrapers were considerably more sensitive to acidification of the water, while predators were sensitive to all of the tested types of pollution (Rawer-Jost et al. 2000). The potential indicatory value of predators is also supported by the fact that they are the only functional feeding group retained in the multimeric B-IBI index (Karr and Chu 1999).
The present study clearly showed that of the three compared communities, the macrozoobenthos community has the best indicatory properties as far as effects of the trout farm are concerned. It is demonstrated that Chi%, FBI, Coll, Shr, and Shr/Coll are extremely sensitive to even very moderate trout farm influence and are therefore possible candidates for formation of a future multimetric index for rapid estimation of water quality in the recipient stream. Further research is needed to confirm the sensitivity of certain other indices (Pre and Scr), and their incorporation into a combined index will be a basic task in the future.
References
Allan, J. D., & Castillo, M. M. (2007). Stream ecology: structure and function of running waters. Dordrecht: Springer.
APHA. (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington, DC: American Public Health Association.
Barbour, M., Gerritsen, J., Griffith, G., Frydenborg, R., McCarron, E., White, J., et al. (1996). A framework for biological criteria for Florida streams using benthic macroinvertebrates. Journal of the North American Benthological Society, 15(2), 185–211.
Barbour, M. T., Gerritsen, J., Snyder, B., & Stribling, J. (1999). Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish, second edition. Washington D.C: U.S. Environmental Protection Agency, Office of Water.
Bartoli, M., Nizzoli, D., Longhi, D., Laini, A., & Viaroli, P. (2007). Impact of a trout farm on the water quality of an Apennine creek from daily budgets of nutrients. Chemistry and Ecology, 23(1), 1–11.
Basu, B. K., Kalff, J., & Pinel-Alloul, B. (2000). The influence of macrophyte beds on plankton communities and their export from fluvial lakes in the St Lawrence River. Freshwater Biology, 45(4), 373–382.
Bauernfeind, E., & Soldan, T. (2012). The mayflies of Europe (Ephemeroptera). Ollerup: Apollo Books.
Bergheim, A., & Brinker, A. (2003). Effluent treatment for flow through systems and European environmental regulations. Aquacultural Engineering, 27(1), 61–77.
Boaventura, R., Pedro, A. M., Coimbra, J., & Lencastre, E. (1997). Trout farm effluents: characterization and impact on the receiving streams. Environmental Pollution, 95(3), 379–387.
Brinkhurst, R. O., & Jamieson, B. G. (1971). Aquatic Oligochaeta of the world. Edinburg: Oliver-Boyd.
Brown, S., & Goulder, R. (1996). Extracellular-enzyme activity in trout-farm effluents and a recipient river. Aquaculture Research, 27(12), 895–901.
Camargo, J. A. (1992a). Structural and trophic alterations in macrobenthic communities downstream from a fish farm outlet. Hydrobiologia, 242(1), 41–49.
Camargo, J. A. (1992b). Temporal and spatial variations in dominance, diversity and biotic indices along a limestone stream receiving a trout farm effluent. Water, Air, and Soil Pollution, 63(3), 343–359.
Camargo, J. A. (1994). The importance of biological monitoring for the ecological risk assessment of freshwater pollution: a case study. Environment International, 20(2), 229–238.
Camargo, J. A., & Gonzalo, C. (2007). Physicochemical and biological changes downstream from a trout farm outlet: Comparing 1986 and 2006 sampling surveys. Limnetica 26(2), 405–414
Camargo, J. A., & Jiménez, A. (2007). Ecological responses of epilithic diatoms and aquatic macrophytes to fish farm pollution in a Spanish river. Anales del Jardín Botánico de Madrid, 64, 213–219.
Camargo, J. A., Gonzalo, C., & Alonso, Á. (2011). Assessing trout farm pollution by biological metrics and indices based on aquatic macrophytes and benthic macroinvertebrates: a case study. Ecological Indicators, 11(3), 911–917.
Coste, M. (1982). Étude des méthodes biologiques d’appréciation quantitative de la qualité des eaux. Rapport Cemagref QE Lyon-AF Bassin Rhône Méditerranée Corse. France, Bordeaux.
Cummins, K. (1988). Rapid bioassessment using functional analysis of running water invertebrates. In T. Simon, L. Holst, & L. Shepard (Eds.), Proceedings of the First National Workshop on Biological Criteria. EPA-905/9-89/003 (pp. 49–54). Chicago: US Environmental Protection Agency.
Czerniawski, R., & Domagała, J. (2010). Similarities in zooplankton community between River Drawa and its two tributaries (Polish part of River Odra). Hydrobiologia, 638(1), 137–149.
Czerniawski, R., Pilecka-Rapacz, M., & Domagała, J. (2013). Zooplankton communities of inter-connected sections of lower River Oder (NW Poland). Central European Journal of Biology, 8(1), 18–29.
Daniel, H., Bernez, I., Haury, J., & Le Coeur, D. (2005). The ability of aquatic macrophytes to assess fish farm pollution in two salmon rivers. Hydrobiologia, 551(1), 183–191.
Fabrizi, A., Goretti, E., Compin, A., & Céréghino, R. (2010). Influence of fish farming on the spatial patterns and biological traits of river invertebrates in an Appenine stream system (Italy). International Review of Hydrobiology, 95, 410–427.
FAO (2016). Global aquaculture for Oncorhynchus mykiss 1950-2013 (online query). Fisheriesand Aquaculture Department, Food and Agriculture Organisation of the United Nations. http://www.fao.org/fishery/culturedspecies/Oncorhynchus_mykiss/en. Downloaded on 25 January 2016.
Flossner, D. (1972). Krebstiere, Crustacea, Kiemen-und Blattfusser, Branchiopoda, Fischlause, Branchiura. Die tierwelt deutschlands. Jena: VEB Gustav Fischer Verlag.
Gavrilović, L., & Dukić, D. (2002). River of Serbia. Belgrade: Institute for textbook publishing and teaching aids (In Serbian).
Glöer, P., & Meier-Brook, C. (1998). Süsswassermollusken: ein Bestimmungssschlüssel für die Bundesrepublik Deutschland. Hamburg: Deutscher Jugendbuch für Naturbeobachtung.
Guilpart, A., Roussel, J.-M., Aubin, J., Caquet, T., Marle, M., & Le Bris, H. (2012). The use of benthic invertebrate community and water quality analyses to assess ecological consequences of fish farm effluents in rivers. Ecological Indicators, 23, 356–365.
Hayslip, G. A. (1993). EPA Region 10 in-stream biological monitoring handbook (for wadablestreams inthe Pacific Northwest).U. S. Environmental Protection Agency-Region 10, Environmental Services Division, Seattle, Washington. EPA-910-9-92-013.
Hering, D., Johnson, R. K., Kramm, S., Schmutz, S., Szoszkiewicz, K., & Verdonschot, P. F. (2006). Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: a comparative metric-based analysis of organism response to stress. Freshwater Biology, 51(9), 1757–1785.
Hilsenhoff, W. (1988a). Seasonal correction factors for the biotic index. Great Lakes Entomologist, 21(1), 9–13.
Hilsenhoff, W. L. (1988b). Rapid field assessment of organic pollution with a family-level biotic index. Journal of the North American Benthological Society, 7(1), 65–68.
Hofmann, G., Werum, M., & Lange-Bertalot, H. (2013). Diatomeen im Süßwasser-Benthos von Mitteleuropa: Bestimmungsflora Kieselalgen für die ökologische Praxis: über 700 der häufigsten Arten und ihre Ökologie. Königstein: Koeltz Scientific Books.
Hughes, R., Paulsen, S., & Stoddard, J. (2000). EMAP-surface waters: a multiassemblage, probability survey of ecological integrity in the USA. Hydrobiologia, 422(423), 429–443.
Imanpour Namin, J., Sharifinia, M., & Bozorgi Makrani, A. (2013). Assessment of fish farm effluents on macroinvertebrates based on biological indices in Tajan River (north Iran). Caspian Journal of Environmental Sciences, 11(1), 29–39.
Junqueira, M. V., Friedrich, G., & Pereira de Araujo, P. R. (2010). A saprobic index for biological assessment of river water quality in Brazil (Minas Gerais and Rio de Janeiro states). Environmental Monitoring and Assessment, 163(1), 545–554.
Karr, J. R., & Chu, E. W. (1999). Restoring life in running waters: better biological monitoring. Washington, D.C.: Island Press.
Kelly, M., Adams, C., & Graves, A. (2001). The trophic diatom index: a user’s manual (Revised ed.). Bristol: Environment Agency.
Kerans, B., & Karr, J. R. (1994). A benthic index of biotic integrity (B-IBI) for rivers of the Tennessee Valley. Ecological Applications, 4(4), 768–785.
Kırkağaç, M. U., Pulatsu, S., & Topcu, A. (2009). Trout farm effluent effects on water sediment quality and benthos. CLEAN – Soil, Air, Water, 37(4–5), 386–391.
Koçer, M. A. T., & Sevgili, H. (2014). Parameters selection for water quality index in the assessment of the environmental impacts of land-based trout farms. Ecological Indicators, 36, 672–681.
Koste, W. (1978). Rotatoria die Radertiere Mitteleuropas. Überorderung Monogononta. Berlin: Gerbruder Brontraeger.
Krammer, K., & Bertalot, L. H. (1988). Bacilariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süßwasserflora von Mitteleuropa 2/2 (pp. 1–596). Stuttgart: G. Fischer Verlag.
Krammer, K., & Bertalot, L. H. (1991). Bacillariophyceae. 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. In H. Ettl, G. Gartner, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süßwasserflora von Mitteleuropa 2/4 (pp. 1–437). Stuttgart: G. Fischer Verlag.
Krammer, K., & Lange-Bertalot, H. (1986). Bacillariophyceae, 1 Teil: Naviculaceae. In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süßwasserflora von Mitteleuropa, Band 2/1 (pp. 1–876). Jena: G. Fischer Verlag.
Krammer, K., & Lange-Bertalot, H. (2004). Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süßwasserflora von Mitteleuropa. 2/3 (pp. 1–598). Munchen: Elsevier GmbH.
Laird, L. M., & Needham, T. (1988). Salmon and trout farming. UK: Ellis Horwood Limited.
Lechthaler, W., & Stockinger, W. (2005). Trichoptera—key to larvae from Central Europe. (CD-Rom-Edition ed.). Vienna: Eutaxa - Technisches Büro für Biologie.
Lecointe, C., Coste, M., & Prygiel, J. (1993). “Omnidia”: Software for taxonomy, calculation of diatom indices and inventories management. Hydrobiologia, 269(1), 509–513.
Liao, P. B. (1970). Pollution potential of salmonid fish hatcheries. Water & Sewage Works, 117, 291–297.
Loch, D. D., West, J. L., & Perlmutter, D. G. (1996). The effect of trout farm effluent on the taxa richness of benthic macroinvertebrates. Aquaculture, 147(1–2), 37–55.
MacMillan, J. R., Huddleston, T., Woolley, M., & Fothergill, K. (2003). Best management practice development to minimize environmental impact from large flow-through trout farms. Aquaculture, 226(1), 91–99.
MACS (Mid-Atlantic Coastal Streams Workgroup). (1996). Standard operating procedures and technical basis: macroinvertebrate collection and habitat assessment for low-gradient nontidal streams. Dover: Delaware Department of Natural Resources and Environmental Conservation.
Merritt, R., & Cummins, K. (1996). Trophic relations of macroinvertebrates. In F. R. Hauer & G. A. Lamberti (Eds.), Methods in stream ecology (pp. 453–474). San Diego: Academic Press Inc..
Moog, O. (2002). Fauna Aquatica Austriaca. 2nd edition. Wassewirtschaftskataster, Bundesministerium für Land-und Forstwirtschaft, Umwelt und Wasserwirtschaft. Wien.
Newman, M. (1995). Quantitative methods in aquatic ecotoxicology. Advances in trace substances research. Boca Raton: Lewis Publishers.
Nilsson, A. N. (1996). Aquatic insects of North Europe: a taxonomic handbook. Volume 1: Ephemeroptera, Plecoptera, Heteroptera, Neuroptera, Megaloptera, Coleoptera, Trichoptera, Lepidoptera. Denmark: Apollo Books.
Oberdorff, T., & Porcher, J. P. (1994). An index of biotic integrity to assess biological impacts of salmonid farm effluents on receiving waters. Aquaculture, 119(2), 219–235.
Palmer, M. (1990a). Temporal and spatial dynamics of meiofauna within the hyporheic zone of Goose Creek, Virginia. Journal of the North American Benthological Society, 9, 17–25.
Palmer, M. (1990b). Understanding the movement dynamics of a stream-dwelling meiofauna community using marine analogs. Stygologia, 5, 67–74.
Palmer, M. (1992). Incorporating lotic meiofauna into our understanding of faunal transport processes. Limnology and Oceanography, 37(2), 329–341.
Pantle, R., & Buck, H. (1955). Die biologische Überwachung der Gewässer und die Darstellung der Ergebnisse. Gas-und Wasserfach, 96, 604.
Pfaff, J. D. (1993). Determination of inorganic anions by ion chromatography. EPA method 300.0, Environmental Monitoring Systems Laboratory Office of Research and Development, U.S. Cincinnati: Environmental Protection Agency.
Pillot, H. (1984a). De larven der Nederlandse Chironomiae (Diptera). 1B: Orthocladiinae sensu lato (Vol. 1, Vol. B). Leiden: St. EIS Nederland.
Pillot, H. (1984b). De larven der Nederlandse Chironomidae (Diptera). 1A: Inleiding, Tanypodinae & Chironomini (Vol. a). Leiden: St. EIS Nederland.
Pillot, H. (2009). Chironomidae larvae. In Biology and ecology of the Chironomini (Environment international). Zeist: KNNV Publishing.
Rawer-Jost, C., Böhmer, J., Blank, J., & Rahmann, H. (2000). Macroinvertebrate functional feeding group methods in ecological assessment. Hydrobiologia, 422, 225–232.
Resh, V. H. (2008). Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Environmental Monitoring and Assessment, 138(1), 131–138.
Robertson, A. L., Lancaster, J., & Hildrew, A. G. (1995). Stream hydraulics and the distribution of microcrustacea: a role for refugia? Freshwater Biology, 33(3), 469–484.
Rott, E. (1991). Methodological aspects and perspectives in the use of periphyton for monitoring and protecting rivers. Paper presented at the Use of algae for monitoring rivers, Institut für Botanik, University of Innsbruck, Austria,
Rozkošny, R. (1980). Key for determination of larvae of water insects. Praha: Ceskoslovenska Akademie Ved.
Schindler, D. W. (1987). Detecting ecosystem responses to anthropogenic stress. Canadian Journal of Fisheries and Aquatic Sciences, 44(Suppl.1), 6–25.
Schmid-Araya, J. (1997). Temporal and spatial dynamics of meiofaunal assemblages in the hyporheic interstitial of a gravel stream. In J. Gilbert, J. Mathieu, & F. Fournier (Eds.), Groundwater/ surface water ecotones: biological and hydrological interactions options (pp. 29–36). Cambridge University Press.
Schmid-Araya, J. (1998). Small-sized invertebrates in a gravel stream: community structure and variability of benthic rotifers. Freshwater Biology, 39(1), 25–39.
Selong, J. H., & Helfrich, L. A. (1998). Impacts of trout culture effluent on water quality and biotic communities in Virginia headwater streams. The Progressive Fish-Culturist, 60(4), 247–262.
Simpson, E. (1949). Measurement of diversity. Nature, 163, 688.
Sindilariu, P. D., Wolter, C., & Reiter, R. (2008). Constructed wetlands as a treatment method for effluents from intensive trout farms. Aquaculture, 277(3–4), 179–184.
Škunca-Milovanović, S., Feliks, R., Đurović, B., & Poček, B. (1990). Drinking water: standard methods for testing hygienic safety. The Federal Institute for Health Protection NIP “Economic review”. (In Serbian).
Sládeček, V. (1983). Rotifers as indicators of water quality. Hydrobiologia, 100(1), 169–201.
Tello, A., Corner, R. A., & Telfer, T. C. (2010). How do land-based salmonid farms affect stream ecology? Environmental Pollution, 158(5), 1147–1158.
The European Parliament and the Council Directive 2006/44/EC of 6 September 2006, on the quality of fresh waters needing protection or improvement in order to support fish life. Official Journal of the European Union, L 264/20, 25.9.2006.
Timm, T. (2009). A guide to the freshwater Oligochaeta and Polychaeta of Northern and Central Europe. Lauterbornia, 66, 1–235.
Triest, L., Kaur, P., Heylen, S., & De Pauw, N. (2001). Comparative monitoring of diatoms, macroinvertebrates and macrophytes in the Woluwe River (Brussels, Belgium). Aquatic Ecology, 35(2), 183–194.
US EPA,1979. EPA methods for chemical analysis of water and wastes. EPA-600/4-79-020, Cincinnati.
Vallenduuk, H. J., & Pillot, H. K. (2007). Chironomidae larvae of the Netherlands and adjacent lowlands: general ecology and Tanypodinae. Zeist: KNNV Publishing.
Villanueva, D., Queimaliños, C., Modenutti, B., & Ayala, J. (2000). Effects of fish farm effluents on the periphyton of an Andean stream. Archive of Fishery and Marine Research/Archiv fur Fischerei und Meeresforschung, 48(3), 283–294.
Waringer, J., & Graf, W. (1997). Atlas der österreichischen Köcherfliegenlarven: unter Einschluss der angrenzenden Gebiete. Wien: Facultas Universitätsverlag.
Wegl, R. 1983. Index für Limnosaprobität. Wasser und Abwasser. Aus Beitrage zur Gewasserforschung XIII, Bd. 26, Wien.
Weston, D. P., Phillips, M. J., & Kelly, L. A. (1996). Environmental impacts of salmonid culture. Developments in Aquaculture and Fisheries Science, 29, 919–967.
Woynarovich, A., Hoitsy, G., & Moth-Poulsen, T. (2011). Small-scale rainbow trout farming: Food and Agriculture Organization of the United Nations.
Živić, I., Marković, Z., Filipović-Rojka, Z., & Živić, M. (2009a). Influence of a trout farm on water quality and macrozoobenthos communities of the receiving stream (Trešnjica River, Serbia). International Review of Hydrobiology, 94(6), 673–687.
Živić, I., Marković, Z., & Živić, M. (2009b). Influence of a trout farm on macrozoobenthos communities of the Trešnjica River, Serbia. Archives of Biological Sciences, 61(3), 483–492.
Živić, I., Živić, M., Bjelanović, K., Milošević, D., Stanojlović, S., Daljević, R., & Marković, Z. (2014). Global warming effects on benthic macroinvertebrates: a model case study from a small geothermal stream. Hydrobiologia, 732(1), 147–159.
Zwick, P. (2004). Key to the West Palaearctic genera of stoneflies (Plecoptera) in the larval stage. Limnologica-Ecology and Management of Inland Waters, 34(4), 315–348.
Acknowledgements
The study was supported by the Serbian Ministry of Education, Science and Technological Development (TR 31075). We would like to express our gratitude to the farmers who supported this research. Many thanks to Dalibor and Ivan Stojnović for their invaluable help with field work and Nikola Vesović for help with art work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Stojanović, K., Živić, M., Dulić, Z. et al. Comparative study of the effects of a small-scale trout farm on the macrozoobenthos, potamoplankton, and epilithic diatom communities. Environ Monit Assess 189, 403 (2017). https://doi.org/10.1007/s10661-017-6114-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6114-0