Abstract
Urban soil contamination is a growing concern for the potential health impact on the increasing number of people living in these areas. In this study, the concentration, the distribution, the contamination levels, and the role of land use were investigated in Erbil metropolis, the capital of Iraqi Kurdistan. A total of 74 soil samples were collected, treated, and analyzed for their physicochemical properties, and for 7 heavy metals (As, Cd, Cr, Cu, Fe, Pb, and Zn) and 16 PAH contents. High concentrations, especially of Cd, Cu Pb, and Zn, were found. The Geoaccumulation index (Igeo), along with correlation coefficients and principal component analysis (PCA) showed that Cd, Cu, Pb, and Zn have similar behaviors and spatial distribution patterns. Heavy traffic density mainly contributed to the high concentrations of these metals. The total concentration of ∑PAHs ranged from 24.26 to 6129.14 ng/g with a mean of 2296.1 ng/g. The PAH pattern was dominated by 4- and 5-ring PAHs, while diagnostic ratios and PCA indicated that the main sources of PAHs were pyrogenic. The toxic equivalent (TEQ) values ranged from 3.26 to 362.84 ng/g, with higher values in central parts of the city. A statistically significant difference in As, Cd, Cu, Pb, Zn, and ∑PAH concentrations between different land uses was observed. The highest As concentrations were found in agricultural areas while roadside, commercial, and industrial areas had the highest Cd, Cu, Pb, Zn, and ∑PAH contents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil system is a good indicator of environmental pollution, since unlike in the atmosphere or in water masses, contaminants are accumulated in soils for long periods due to their interaction with colloidal particles (Metreveli and Frimmel 2007). Industrial and economic activities are much more concentrated in urban areas, and cities have become the geographic focus of resource consumption and chemical emissions, generally associated with environmental pollution (Li and Feng 2012). Urbanization could affect soil physicochemical parameters such as their pH, texture, cation exchange capacity, and bulk density and also cause harmful substances to deposit in the soils (Liu et al. 2016). Soil contamination usually poses significant environmental hazards for terrestrial and aquatic ecosystems and health effects for biota, given the frequent involvement of potentially toxic metals, mineral oil, hydrocarbons, and persistent organic pollutants (Monaco et al. 2015). Soils could be considered as the source and sink of various pollutants that can be accumulated over long periods of time (Benhaddya et al. 2015), and changes in soil chemical and physical properties can release the adsorbed contaminants to the environment. In many developing countries, the expansion of urban areas is connected with socioeconomic growth, but it is however changing the physical, chemical, and biological composition of living environment. Hence, millions of people living in and around urban areas are exposed to an unnatural and unhealthy environment (Singh et al. 2002).
Among various types of environmental pollutants, heavy metals (HMs) are particularly hazardous (Luo et al. 2015) and their contamination is of great concern due to their tendency for bioaccumulation and toxic effect on plants, animals, and human beings (Xu et al. 2012). Heavy metals could be released to the environment from traffic, agricultural, and mining processes as well as from industrial emissions (Manta et al. 2002; Sekhar et al. 2006; Cicchella et al. 2008). These urban pollutants may be captured and attenuated in the soils (Wang et al. 2012). The excessive accumulation of HMs in urban soils may lead to the deterioration of the soil ecosystem, threaten human health, and create other environmental problems. Therefore, the contamination of HMs in soils is of increasing concern in urban environmental management.
Another important group of contaminants are polycyclic aromatic hydrocarbons (PAHs) with a small fraction from natural sources (forest fires, volcanic eruption, natural oil seeps). In general, most PAHs enter the environment via anthropogenic emissions including coal-, oil-, gas-, and wood-burning facilities; motor vehicles; cooking; waste incineration; cigarette smoke; and industrial activities (oil refining, coke, and asphalt production etc.) (Kim et al. 2013; Yang et al. 2014). The chemical properties of PAHs control the behavior of these compounds in natural environments. Their low vapor pressure and high octanol/air partition coefficients (log Kow 3–6) enable them to attach strongly onto the soil mass and persist for a long period of time. Thus, soils have been the subject of intensive studies as the sink for a variety of PAHs (Tay and Biney 2013). US Environmental Protection Agency (US EPA) and the European Community introduced 16 PAH compounds as priority pollutants (Dimashki et al. 2001); some of them are mutagenic and carcinogenic organic compounds (Lau et al. 2012) and it seems crucial to assess their levels and identify their sources in soils.
Compared to polychlorinated biphenyls and organochlorine pesticides, PAHs and HMs are closely related to urbanization development (Peng et al. 2013). To effectively decrease the risk of soil pollution in urban areas and establish reliable protection approaches, it is necessary to understand the spatial distribution patterns and assess the contamination levels of HMs and PAHs (Chen et al. 2013) as well as their potential sources in a specific site. Based on the above background, the objectives of this study are to (a) evaluate the concentration and distribution of heavy metals (As, Cd, Cr, Cu, Fe, Pb, and Zn) and PAHs in soils of Erbil metropolis, (b) determine the degree of soil contamination, and (c) explore the effect of land use on the level of contaminants.
Materials and methods
Study area and sample collection
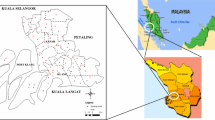
Erbil, also known as Hewlêr (in Kurdish), is the capital of Iraqi Kurdistan, located northwest of Baghdad, and its governorate has a permanent population of approximately 1.61 million as of 2011. Human settlement at Erbil can be dated back to possibly 5000 bc, and it is one of the oldest continuously inhabited areas in the world (Novácek, 2008). Erbil city, the center of Erbil Governorate, with an area of about 238 km2, lies between latitudes 36° 07′–36° 15′ N and longitudes 43° 55′–44° 07′ E. Due to a relative security and peace in the region and more economically liberal and market-oriented policies, it has a more developed economy in comparison to the rest of Iraq and its neighbor Syria. The Kurdistan Region’s economy is dominated by the oil industry, agriculture, and. The area is characterized by a semiarid continental climate with extremely hot and dry summers and cold and wet winters. The mean annual precipitation is 543 mm and annual mean temperature is 20.2 °C, while the maximum temperature often reaches nearly 50 °C in July and August. Based on the geological maps (Iraq Geological survey 2012), the study area comprises mostly quaternary deposits (silty and clayey residual soils and polygenetic deposits, locally gypsiferous or pebbly).
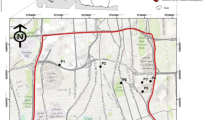
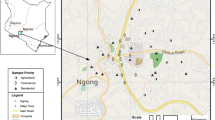
A total of 74 surface soil samples (at 0–10 cm in depth) were sampled during June 2015 (Fig. 1). The samples were collected from different land uses including agricultural area (AA), residential area (RA), industrial area (IA), public green space (PGS), commercial area (CA), and roadside (RS). At each site, six subsamples were taken and then mixed up to give a bulk sample. For HM determination, soil samples were collected using a plastic scoop, placed in polyethylene bags, and labeled. For PAH determinations, soil samples were collected using a stainless steel scoop, kept in solvent-cleaned glass jar, and stored at 4 °C until performance of instrumental analysis.
Sample preparation and analysis
In the laboratory, samples were air-dried, homogenized, and a part of them sieved through a 63-μm screen for determination of HMs, and for the rest of the physicochemical parameters, the soil was sieved to 2 mm. Soil pH and electrical conductivity (EC) were measured in aqueous suspensions (1:2.5 and 1:5 soil water ratios, respectively), and the soil textures were determined using the hydrometer method (Gee and Bauder 1986). Soil organic matter was determined following potassium dichromate wet combustion procedure, and cation exchange capacity (CEC) was measured using 0.1 M NaCl according to the ion retention method of Schofield (1949).
The 63-μm soil samples were digested by aqua regia and then the digested samples were ultrasonificated for 1 h at 70 °C and then heated for 1 h in a water bath maintained at 70 °C under a fume hood operation. The suspension was filtered through a 0.45-mm nylon syringe filter and adjusted to 30-mL volume by adding deionized water. The obtained solutions were analyzed for their As, Cd, Cr, Cu, Fe, Pb, and Zn by an inductively coupled plasma optical emission spectrometer (ICP–OES, ULTIMA 2-HORIBA SCIENTIFIC). Quality assurance and control (QA/QC) included the procedural blank, duplicate analysis, and use of standard reference materials; the recovery percentages ranged from 81 to 109 %, indicating a good agreement between the measured and the certified values.
For PAHs, all samples were freeze-dried before analysis and an ultrasonic bath (KUDOS, SK3210LHC model) was used during the extraction. Samples (5 g) were mixed with anhydrous sodium sulfate and Soxhlet extracted using a solvent mix of hexane-dichloromethane (1:1). Gas chromatography/mass spectrometry (GC–MS) with helium at a flow rate of 1.5 mL/min as carrier gas was used to determine 16 PAHs including naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Fl), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), benzo[ghi]perylene (BghiP), indeno(1,2,3 cd) pyrene (Ind), and dibenz[a,h]anthracene (DahA) (USEPA Methods 8270D and 3550C, 2007). Analytical methods were checked for precision and accuracy by analyzing the spiked blanks (standards spiked into solvent), matrix spikes, matrix blank, procedural blanks (solvent), and sample duplicates with field samples, and the PAH concentrations in the soils were corrected accordingly. The recoveries of individual PAHs ranged from 79.3 to 106.5 % with a mean value of 87.4 %. Replicate analyses of the samples gave an error between ±5 and ±15 %.
Data analysis
Geoaccumulation index of heavy metals (Igeo)
To quantify the degree of HM pollution, the geoaccumulation index (Igeo) was calculated as follows (Zhang et al. 2016):
where Cn is concentration of examined metal in soil, Bn is geochemical background or reference value of given metal, and the factor 1.5 is used to account for the possible variations in the reference values. In this study, average metal concentrations in world soils were used as reference values. The Igeo was split into 6 classes as follows (Zahra et al. 2014): Igeo ≤ 0, class 0, unpolluted; 0 < Igeo < 1, class 1, unpolluted to moderately polluted; 1 < Igeo < 2, class 2, moderately polluted; 2 < Igeo < 3, class 3, moderately to highly polluted; 3 < Igeo < 4, class 4, highly polluted; 4 < Igeo < 5, class 5, highly to very highly polluted; and 5 < Igeo < 6, class 6, very highly polluted.
Potential ecological risk
The potential ecological risk index (RI) was introduced by Hakanson (1980) to assess the ecological risk degree of HMs in soil or sediments. RI is calculated using the following equations:
where RI is the sum of all risk factors for heavy metals in soils, \( {E}_r^i \) is the ecological risk factor, \( {T}_r^i \) is toxic response factor for the analyzed metals (Zn = 1 < Cr = 2 < Cu = Pb = 5 < As = 10 < Cd = 30), \( {C}_r^{\mathrm{i}} \) is the contamination coefficient, \( {C}_{\mathrm{s}}^{\mathrm{i}} \) represents metal content in soil, and \( {C}_{\mathrm{n}}^{\mathrm{i}} \) is the reference value for heavy metals. Based on Hakanson (1980), the ecological risk factors are classified as low (Er < 40), moderate (40 < Er < 80), considerable (80 < Er < 160), high (160 < Er < 320), and very high (Er ≥ 320). Consequently, the potential ecological risk is low (RI < 150), moderate (150 > RI < 300), considerable (300 > RI < 600), or very high (RI ≥ 600).
PAH toxicity assessment
The toxicities of PAHs in the soil samples were evaluated using the relative toxicity value of each PAH compound, separately, and based on the set of toxicity equivalency factors (TEFs) in which BaP is the reference chemical (Nisbet and LaGoy 1992). Toxic equivalents (TEQs) of PAHs were calculated using the following equation:
where Cn is the concentration of PAHs, and TEFn is the toxic equivalency factor for PAHs. TEFi for Nap, Ace, Acy, Fl, Phe, Ant, Flu, Pyr, BaA, Chr, BbF, BkF, BaP, Ind, DahA, and BghiP are 0.001, 0.001, 0.001, 0.001, 0.001, 0.01, 0.001, 0.001, 0.1, 0.01, 0.1, 0.1, 1, 0.1, 1, and 0.01, respectively.
Statistical analysis and GIS
Statistical data treatment was performed using SPSS 19.0 software package for Windows. Descriptive statistics were examined to study the distributions of the variables, and the normality of variables was assessed using Kolmogorov–Smirnov normality test. The analysis of existing correlations between HM concentrations and principal component analysis (PCA) were performed for analyzing relationships among the observed variables. Also, the Kruskal–Wallis H test was used to assess the influence of land uses on the accumulation of HMs and total PAHs in Erbil soils. In order to visualize the spatial distribution of pollutants in soils, a spatial interpolation with inverse distance weighting (IDW), the simplest and most practical interpolation method (Wang 2006), was used.
Results and discussion
Physicochemical parameters and HM contents of Erbil soils
The descriptive statistics of physicochemical parameters and heavy metal concentrations of soils are shown in Table 1. The soil pH ranged from 6.98 to 8.48, suggesting typically alkaline nature for the studied soils. Based on Craul (1992), the elevated pH value of urban soils is commonly related to the presence of building rubble containing brick, cement, plaster, mortar, and concrete. The alkaline substrates tend to be weathered, releasing calcium, and in dry conditions, this is responsible for cementation at soil surface, creating an impermeable crust. In such alkaline soils, most HMs are likely to be in a less mobile form (Skrbic and Miljevic 2002). The organic matter (OM) averaged 3.12 % with a range of 0.94–7.22 % and EC averaged 6236.84 μS/cm with a range of 620.73–26,814.12 μS/cm. The maximum OM contents were observed in agricultural and public green space areas. Cation exchange capacity (CEC) of the soil samples ranged from 3.12 to 52.64 meq/100 g with an average value of 24.29 meq/100 g. Mean sand, silt, and clay contents in Erbil soils were 30.57, 39.34, and 30.09 %, respectively, showing the relative dominance of fine grain size in the study area.
The ranges of As, Cd, Cr, Cu, Fe, Pb, and Zn concentrations in soil samples were 2.60–14 mg/kg, 0.10–3.25 mg/kg, 33.65–54.30 mg/kg, 14.20–432.70 mg/kg, 1.20–3.15 %, 22.15–842.20 mg/kg, and 76–1201.65 mg/kg, respectively. The mean values of HM concentrations in Erbil soils were higher than the reference values (world average), except As, Cr, and Fe. However, 33.78 % of As concentrations exceeded their corresponding average contents in world soils. In addition, 82.43 % of Cd, 89.18 % of Cu, 90.54 % of Pb, and 91.89 % of Zn concentrations were higher than the world soil concentrations, while no Fe and Cr concentrations of the soil samples exceeded the world soil values.
There are numerous reports on metal contamination in urban soils around the world. Comparison of HM concentrations in Erbil city with some of the published data (Table 2) showed that median concentration of As in the study area is low compared with several cities around the world except for Ibadan. The concentration of Cd in Erbil urban soils is below that of Novi Sad, Moscow, and Baltimore but higher than Palermo, Shanghai, Cosenza-Rende, Changchun, and Ibadan. Also, mean or median concentration of Cr in the study area is lower than that in the other cities presented in Table 2, except Palermo and Novi Sad, and average Fe concentration is lower than that in Cosenza-Rende and higher than in Novi Sad. Moreover, the average Pb concentration in urban soils of Erbil is higher than that of the other cities except for Palermo and Baltimore. On the other hand, concentrations of Cu and Zn in the study area are higher than the concentrations reported at other sites with an exception in Shanghai (for Zn).
Contamination assessment and source determination of HMs
In this study, Igeo and RI were calculated to assess the degree of HM contamination. The average Igeo values of As, Cd, Cr, Cu, Fe, Pb, and Zn were −0.76, 1.16, −1.41, 0.96, −1.22, 1.01, and 1.30, respectively, and could be ranked as follows: Zn > Cd > Pb > Cu > As > Fe > Cr (Fig. 2). Based on mean Igeo values, Cd, Pb, and Zn were placed in “Class 2,” indicating moderate pollution of these metals in soils of Erbil metropolis. Also, Cu with an average Igeo of 0.96 was placed in “Class 1” showing an unpolluted to moderately polluted situation. On the other hand, the maximum Igeo of Cd, Cu, Pb, and Zn were 3.2, 3.27, 4, and 3.74, respectively, which all occurred in Barzani Namr Street (H70) revealing high pollution of this sampling station. Furthermore, the mean Igeo values for As, Cr, and Fe were placed in “Class 0,” which indicates no pollution and suggests they were not highly affected by anthropogenic sources. Overall, the percentage of cases of class 0, 1, 2, 3, and 4 in Erbil soil samples was as follows: 17.56, 20.27, 39.19, 20.27, and 2.71 % for Cd; 16.21, 32.44, 28.37, 20.27, and 2.71 % for Cu; 13.52, 44.6, 22.97, 14.86, and 4.05 % for Pb; and 8.1, 35.11, 31.06, 22.94, and 2.7 % for Zn, respectively. Also, in none of the samples, Cr and Fe had Igeo greater than zero, whereas As indicated unpolluted to moderately polluted situations (0 < Igeo < 1) in 12.6 % of the samples.
Boxplot of geoaccumulation index for metals in Erbil soils. Numbers correspond to the pollution classes: 1, unpolluted to moderately polluted; 2, moderately polluted; 3, moderately to highly polluted; 4, highly polluted (Zahra et al. 2014)
The calculated ecological risk factors for HMs (As, Cd, Cr, Cu, Pb and Zn) and the potential ecological risk index in Erbil soils revealed that the highest ecological risk factor (Er) is associated to Cd, with an average of 87.97 (considerable risk). Also, Er values for As, Cr, and Zn in all sampling stations were below 40, indicating that these metals bear a low ecological risk in the study area. The values of Er for Pb and Cu ranged from 2.37 to 72.11 (low-moderate risk) and from 3.16 to 120.32 (low-considerable risk), respectively, showing that ecological risk of these metals varied widely in different locations of the study area. Based on calculated RI of heavy metals in the soil samples, 66.21 % of the samples had RI values below 150, showing low potential ecological risk, while 27.03 and 6.76 % of the samples revealed RI values between 150 and 300 (moderate risk) and between 300 and 600 (considerable risk), respectively. The highest RI values were observed in Barzani Namr Street (H70), Barzani Namr and Sultan Muzaffar Intersection (H71), Shoresh Bridge (H73), Barzani Namr and Iskan Intersection (H58), and Peshawa Qazi Beltway (H53), which are all located in the center and east of the city.
To identify the relationship among HMs, and between HMs and physicochemical parameters in Erbil soils, a correlation coefficient analysis was performed. Prior to statistical analysis, the normality of all the parameters was assessed using the Kolmogorov–Smirnov normality test, and due to the non-normal distribution of the data, the Spearman method was used (Table 3). Results showed that there are strong correlations (r > 0.8; p < <0.01) between 4 urban metals (Cd, Cu, Pb, and Zn) which are clearly affected by anthropogenic sources of contamination such as traffic and industries. Significant, although weaker, correlations are also observed between these HMs and some soil parameters (CEC, OM, clay content), suggesting that these may be mostly retained by the fine soil particles. The medium correlation(r = 0.53; p < <0.01) between As and OM could be due to their sources, because the maximum values for both parameters are observed in the samples collected from agricultural areas. Also, the strong correlation of CEC with clay and organic matter contents of the soil samples showed that these two parameters had the main role determining the cation exchange capacity of soil particles. The metal concentrations were also analyzed using PCA, as interelement relationships can provide helpful information on element sources and pathways (Zhao et al. 2014). The results of the PCA for metal concentrations in the soil samples are tabulated in Table 4. Three principal components were extracted accounting for over 82 % of the total variance. Cd, Cu, Pb, and Zn showed strong positive loadings in the first component explaining 47.9 % of the total variance. Cr and Fe are mainly distributed with the second component, which explained 19.9 % of the total variance. As confirmed by Igeo and Er, these metals were believed to be contributed by the lithogenic source. The third principal component includes As and explained 14.9 % of the total variance. It seems that this element is mostly geogenic in the study area with an exception in agricultural areas in which As had higher Igeo and Er values.
Comparison of HM concentrations in different land uses
To investigate the role of different land uses on HM concentrations in the soils of the study area, soil samples were grouped and Kruskal–Wallis H test was performed (Table 5). This test is the non-parametric test equivalent to the one-way ANOVA and an extension of the Mann–Whitney U test to allow the comparison of more than two independent groups (6 land uses in this study). The results showed that the p values for As, Cd, Cu, Pb, and Zn were lower than 0.05, which suggested that land uses had significant effects on the distribution of these metals. However, Fe and Cr showed p values higher than 0.05, indicating no significant differences in their concentrations between different land uses. For better understanding, mean HM concentrations of each land use were calculated and related column charts were provided (Fig. 3). The Cu, Pb, and Zn concentrations in different land uses followed the similar sequence: RS > CA > IA > RA > PGS > AA. Also, Cd had relatively the same trend (RS > CA > IA >PGS > RA > AA) with an exception in PGS and RA. The highest mean concentration of As (9.73 mg/kg) was observed in agricultural areas, likely due to the use of As-containing pesticides in farm lands (WHO 2001). Very slight differences of mean Fe and Cr concentrations between the considered land uses in this study were observed.
Figure 4 shows the HM distribution in soils of the study area. Soils of southern and western parts of the city, with agricultural lands, had the highest As contents, while the highest concentrations of the urban metals (Cd, Cu, Pb, and Zn) were observed in the city center. This result is in accordance with Wang et al. (2012), who concluded, for Beijing city, that the longer the duration of urbanization, the greater was the accumulation of HMs, especially Cd, Cu, Pb, and Zn. Also, the central part of Erbil experiences the highest traffic loads. Only slight changes in Fe and Cr concentrations were indicated.
PAH contamination and the potential sources in Erbil soils
The descriptive statistics of PAH concentrations in soils of the Erbil metropolis is presented in Table 6. Total PAH concentrations, low-molecular-weight PAH (2–3 ring), and high-molecular-weight PAH (4–6 ring) of all the 74 samples ranged from 24.25 to 6129.15 ng/g (with an average value of 2296.1 ng/g), from 4.20 to 1401.75 ng/g (with an average value of 610.18 ng/g), and from 9.05 to 4821.10 ng/g (with an average of 1685.81 ng/g), respectively. Based on Maliszewska-Kordybach (1996) classification system, 18.1 % of the samples in the study area are heavily contaminated, 24.9 % are contaminated, 31 % are weakly contaminated, and 26 % are uncontaminated. Figure 5 shows the distribution map of ∑PAH concentration in the soil samples. The highest total PAH concentrations occurred at Barzani Namr Street (H70) with high traffic load. Other highly contaminated sampling stations include Shoresh Bridge (H73), west industrial area (H15), Erbil diesel power plant (H17), north industrial area (H16), Ankawa Street (H26), Peshawa Qazi Beltway (H53), Barzani Namr and Iskan Intersection (H58), and Barzani Namr and Sultan Muzaffar Intersection (H71). Total concentrations of ∑non-carcinogenic PAHs (Np, Acy, Ace, Fl, Phe, Ant, Flu, Pyr, and BgPer) and ∑carcinogenic PAHs (BaA, Chr, BaP BbF, BkF, IP, DiBA) ranged from 10.60 to 3194.65 ng/g and from 2.65 to 2836.50 ng/g, respectively. The concentration of BaP, the most carcinogenic compound among PAHs, was in the range below detection limit to 52.35 ng/g, with an average of 45.3 μg/kg. The results indicate high carcinogenic potential of soils in the study area. To better characterize the carcinogenic properties of PAH mixtures, toxic equivalents (TEQs) of PAHs were calculated. TEQ of the soil samples ranged from 3.25 to 362.85 ng/g with a mean value of 174.32 ng/g in the study area. Higher TEQ values were calculated for soils from the central parts of the city where commercial and roadside land uses are dominant.
PAHs in the present study were classified based on the number of aromatic rings, 2-ring including Np; 3-ring including Acy, Ace, Fl, Phe and Ant; 4-ring including Flu, Pyr, BaA, and Chr; 5-ring including BbF, BkF, BaP, and DiBA; and 6-ring including BgPer and IndPy (Fig. 6). Results showed that 4-ring PAHs were dominant components in Erbil soils. Aromatic diagnostic criteria could be used in the interpretation of PAH sources. Some PAH pair ratios, such as Flu/(Flu + Pyr), BaA/(BaA + Chr), Ant/(Ant + Phe) as well as low molecular weight (LMW)/high molecular weight (HMW) were used to infer the possible sources of PAHs in the soil samples (Qiao et al. 2006; Yunker et al. 2002; Pies et al. 2008). Overall, the petrogenic sources are rich in LMW PAHs and the pyrogenic sources contain a greater percentage of HMW (Yu et al. 2014). In this study, LMW/HMW ratios in most of the sampling stations were less than 1. Also, the mean values of Flu/(Flu + Pyr), BaA/(BaA + Chr), and Ant/(Ant + Phe) were 0.84, 0.51, and 0.21, respectively. These results reveal the dominance of the pyrogenic sources of the PAHs in the soil samples of Erbil metropolis.
To improve the accuracy of the source identification, PCA was performed. Kolmogorov–Smirnov normality test indicated that the data were not normally distributed; therefore, all PAH data were log-transformed prior to performing PCA. Varimax rotation which minimizes the number of variables which have high loadings on each component was used in this analysis. Table 7 shows the individual PAHs in the principal component matrix in the soil samples. Three components with eigenvalues >1 explain 87.41 % of the total variation. Factor 1 explaining 52.18 % of the total variance is highly loaded by Phe, Flu, Pyr, BaA, BbF, BkF, and DiBa and moderately weighted by Chr and BaP. This component is an indicator of pyrogenic sources such as diesel-powered vehicles (Park et al. 2002; Fang et al. 2004; Suman et al. 2016). Factor 2 contributing 28.36 % of total variance is highly weighted by 2- and 3-ring PAHs (Np, Acy, Ace, Fl and Ant) related to petrogenic sources since Np is the signal for incomplete combustion-related sources (Jiang et al. 2009; Dong and Lee 2009). The third factor makes only 6.87 % of the total variance and is highly weighted in BgPer and IndPy (6-ring PAHs) and moderately loaded on Chr and BaP, indicating pyrogenic sources. Thus, it can be inferred from PCA that vehicular emissions are the main source of PAH contamination in the soils of the study area.
Comparison of PAHs concentrations for different land uses
Differences in PAH concentrations between land uses were tested by means of the non-parametric Kruskal–Wallis rank test. This method was used because, based on Skewness and Kolmogorov–Smirnov normality test, the data were non-normally distributed. The null hypothesis was that the true mean value was the same in each of the land uses (p > 0.05). The p value for ∑PAHs was 0.001, lower than 0.05 (Table 8). Therefore, the difference of ∑PAH concentrations in AA, IA, CA, PGS, RA, and RS was statistically significant, which suggested that land uses had significant effects on the distribution of ∑PAHs in soils.
Mean concentrations of ∑PAHs in the soil samples of studied land uses are illustrated in Fig. 7. ∑PAH concentrations in the soils showed a wide variation, depending on the location of the sampling point. Mean ∑PAH concentrations of soils followed the decreasing order: RS > CA > IA > RA > PGS > AA. Mean concentrations in the soils of RS (3967.1 ng/g) and CA (3283.94 ng/g) were significantly higher than those at other sites (P < 0.05). This indicates that the surface soil in areas of high traffic load may have the greatest ∑PAH pollution potential.
Conclusion
The concentrations of 7 metals (As, Cd, Cr, Cu, Fe, Pb and Zn ) and 16 PAHs were measured in 74 soil samples collected from different land uses in Erbil metropolis, Kurdistan Region, northeast Iraq. The results showed that among the studied metals, Cd, Cu, Pb, and Zn are highly affected by anthropogenic sources including industries and traffic, so these metals are severely enriched in most sampling stations. Also, based on isomeric ratios, the pyrogenic sources of the PAHs are dominant in the soil samples, while locally petrogenic sources were observed. These results were confirmed by PCA by which diesel-powered vehicles and incomplete combustion-related sources were determined as PAH sources in the study area. Kruskal–Wallis H test suggested that land uses had significant effects on the distribution of As, Cd, Cu, Pb, Zn, and ∑PAHs in soils of Erbil metropolis, while Fe and Cr showed no significant differences in their concentrations between different land uses. Roadside, commercial, and industrial areas were the most contaminated land uses by Cd, Cu, Pb, Zn, and PAHs. Also, the highest As concentrations were observed in agricultural areas. Erbil, the most important city in the Kurdistan Region due to its strategic location, has a heavy traffic of motor vehicles including passenger cars and light and heavy commercial vehicles, which import various goods into the region and export mainly crude oil. Results evidence the need to implement an air quality monitoring program in the urban area and to apply a comprehensive approach in transport planning (such as adopting emission standards, enhancing traffic culture, improving urban infrastructures, etc.) in order to control and decrease major sources of urban soil pollution in the study area.
References
Adriano, D. C. (2001). Trace elements in terrestrial environments, biogeochemistry, bioavailability and risks of metals. New York: Springer.
Benhaddya, M. L., Boukhelkhal, A., Halis, Y., & Hadjel, M. (2015). Human health risks associated with metals from urban soil and road dust in an oilfield area southeastern Algeria. Archives of Environmental Contamination and Toxicology. doi:10.1007/s00244-015-0244-6.
Chen, X., Lu, X., Li, L. Y., & Yang, G. (2013). Spatial distribution and contamination assessment of heavy metals in urban topsoil from inside the Xi’an second ring road, NW China. Environment and Earth Science, 68, 1979–1988.
Cicchella, D., De Vivo, B., Lima, A., Albanese, S., & Fedele, L. (2008). Urban geochemical mapping in Campania region, Italy. Geochemistry: Exploration, Environment, Analysis, 8, 19–29.
Craul, P. J. (1992). Urban soil in landscape design. New York: Wiley.
Dimashki, M., Lim, L. H., Harrison, R. M., & Harrad, S. (2001). Temporal trends, temperature dependence, and relative reactivity of atmospheric polycyclic aromatic hydrocarbons. Environmental Science & Technology, 35, 2264–2267.
Dong, T. T. T., & Lee, B. K. (2009). Characteristics, toxicity, and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in road dust of Ulsan, Korea. Chemosphere, 74, 1245–1253.
Fang, G. C., Chang, C. N., Wu, Y. S., Fu, P. P. C., Yang, I. L., & Chen, M. H. (2004). Characterization, identification of ambient air and road dust polycyclic aromatic hydrocarbons in central Taiwan, Taichung. The Science of the Total Environment, 327, 135–146.
Gee, G.W., Bauder, J.W. (1986). Particle-size analysis. In: Klute A (ed.), Methods of soil analysis. Part 1. 2nd ed. (p. 383–411) Agron. Monogr. 9. ASA and SSSA, Madison, WI.
Guagliardi, I., Cicchella, D., & De Rosa, R. (2012). A geostatistical approach to assess concentration and spatial distribution of heavy metals in urban soils. Water, Air, and Soil Pollution, 223, 5983–5998.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control, a sedimentological approach. Water Research, 14(8), 975–1001.
Iraq Geological Survey (2012). Maps of Iraq. Baghdad: Ministry of Industry and Minerals.
Jiang, Y. F., Wang, X. T., Wang, F., Jia, Y., Wu, M. H., Sheng, G. Y., et al. (2009). Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in urban soil of Shanghai, China. Chemosphere, 75, 1112–1118.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. Berlin Heidelberg: Springer.
Kim, K. H., Jahan, S. A., Kabir, E., & Brown, R. J. C. (2013). A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environment International, 60, 71–80.
Lau, E. V., Gan, S., & Ng, H. K. (2012). Distribution and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface soils from five different locations in Klang Valley, Malaysia. Bulletin of Environmental Contamination and Toxicology, 88, 741–746.
Li, X., & Feng, L. (2012). Geostatistical analyses and fractionation of heavy metals in urban soil from industrial district in Weinan, NW China. Environment and Earth Science, 67, 2129–2140.
Liu, R., Wang, M., Chen, W., & Peng, C. (2016). Spatial pattern of heavy metals accumulation risk in urban soils of Beijing and its influencing factors. Environmental Pollution, 210, 174–181.
Luo, X. S., Xue, Y., Wang, Y. L., Cang, L., Xu, B., & Ding, J. (2015). Source identification and apportionment of heavy metals in urban soil profiles. Chemosphere, 127, 152–157.
Maliszewska-Kordybach, B. (1996). Polycyclic aromatic hydrocarbons in agricultural soils in Poland: preliminary proposals for criteria to evaluate the level of soil contamination. Appl Geochemy, 11, 121–127.
Manta, D., Angelone, M., Bellanca, A., Neri, R., & Sprovieri, M. (2002). Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy. The Science of the Total Environment, 300, 229–243.
Metreveli, G., & Frimmel, F. H. (2007). Influence of Na-bentonite colloids on the transport of heavy metals in porous media. In F. H. Frimmel, F. Frank, & F. Hans-Curt (Eds.), Colloid transport in porous media (pp. 29–53). Berlin: Springer.
Monaco, D., Riccio, A., Chianese, E., Adamo, P., Di Rosa, S., & Fagnano, M. (2015). Chemical characterization and spatial distribution of PAHs and heavy hydrocarbons in rural sites of Campania region, south Italy. Environmental Science and Pollution Research, 22, 14993–15003.
Nisbet, I. C. T., & LaGoy, P. K. (1992). Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs. Regulatory Toxicology and Pharmacology, 16(3), 290–300.
Novácek, K. (2008). Research of the Arbil Citadel, Iraq, First Season. PAMÁTKY ARCHEOLOGICKÉ XCIX: 259–302.
Odewande, A. A., & Abimbola, A. F. (2008). Contamination indices and heavy metal concentrations in urban soil of Ibadan metropolis, southwestern Nigeria. Environmental Geochemistry and Health, 30, 243–254.
Park, S. S., Kim, Y. J., & Kang, C. H. (2002). Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmospheric Environment, 36, 2917–2924.
Peng, C., Ouyang, Z., Wang, M., Chen, W., Li, X., & Crittenden, J. C. (2013). Assessing the combined risks of PAHs and metals in urban soils by urbanization indicators. Environmental Pollution, 178, 426–432.
Pies, C., Hoffmann, B., Petrowsky, J., Yang, Y., Ternes, T. A., & Hofmann, T. (2008). Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils. Chemosphere, 72, 1594–1601.
Plyaskina, O. V., & Ladonin, D. V. (2009). Heavy metal pollution of urban soils. Eurasian Soil Science, 42, 816–823.
Qiao, M., Wang, C., Huang, S., Wang, D., & Wang, Z. (2006). Composition, sources, and potential toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake, China. Environment International, 32(1), 28–33.
Schofield, R. K. (1949). Effect of pH on electric charges carried by clay particles. Journal of Soil Science, 1, 1–8.
Sekhar, K. C., Chary, N. S., Kamala, C. T., Vairamani, M., Anjaneyulu, Y., Balaram, V., & Sorlie, J. E. (2006). Risk communications: around the world-environmental risk assessment studies of heavy metal contamination in the industrial area of Kattedan, India a case study. Human and Ecological Risk Assessment, 12, 408–422.
Shi, G. T., Chen, Z. L., Xu, S. Y., Zhang, J., Wang, L., Bi, C. J., & Teng, J. Y. (2008). Potentially toxic metal contamination of urban soils and roadside dust in Shanghai, China. Environmental Pollution, 156, 251–260.
Singh, M., Müller, G., & Singh, I. B. (2002). Heavy metals in freshly deposited stream sediments of rivers associated with urbanization of the Ganga Plain, India. Water, Air, and Soil Pollution, 141, 35–54.
Skrbic, B., & Durisic-Mladenovic, N. (2002). An evaluation of residues at an oil refinery site following fires. Journal of Environmental Science and Health, Part A, 37(6), 1029–1039.
Suman, S., Sinha, A., & Tarafdar, A. (2016). Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad, India. The Science of the Total Environment, 545–546, 353–360.
Tay, C. K., & Biney, C. A. (2013). Levels and sources of polycyclic aromatic hydrocarbons (PAHs) in selected irrigated urban agricultural soils in Accra, Ghana. Environment and Earth Science, 68, 1773–1782.
Wang, F. H. (2006). Quantitative methods and applications in GIS. New York: Taylor & Francis.
Wang, M., Bai, Y., Chen, W., Markert, B., Peng, C., & Ouyang, Z. (2012). A GIS technology based potential eco-risk assessment of metals in urban soils in Beijing, China. Environmental Pollution, 161, 235–242.
World Health Organization (WHO), (2001). Arsenic and Arsenic compounds (Environmental Health Criteria 224), 2nd ed. Geneva: World Health Organization International Programme on Chemical Safety.
Xu, P., Zeng, G. M., Huang, D. L., Feng, C. L., Hu, S., Zhao, M. H., Lai, C., et al. (2012). Use of iron oxide nanomaterials in wastewater treatment: a review. The Science of the Total Environment, 424, 1–10.
Yang, W., Lang, Y., & Li, G. (2014). Cancer risk of polycyclic aromatic hydrocarbons (PAHs) in the soils from Jiaozhou Bay wetland. Chemosphere, 112, 289–295.
Yang, Z., Lu, W., Long, Y., Bao, X., & Qingchun, Y. (2011). Assessment of heavy metals contamination in urban topsoil from Changchun City, China. Journal of Geochemical Exploration, 108, 27–38.
Yesilonis, I. D., Pouyat, R. V., & Neerchal, N. K. (2008). Spatial distribution of metals in soils in Baltimore, Maryland: role of native parent material, proximity to major roads, housing age and screening guidelines. Environmental Pollution, 156, 723–731.
Yu, B., Xie, X., Ma, L. Q., Kan, H., & Zhou, Q. (2014). Source, distribution, and health risk assessment of polycyclic aromatic hydrocarbons in urban street dust from Tianjin, China. Environmental Science and Pollution Research, 21(4), 2817-2825.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33, 489–515.
Zahra, A., Hashmi, M. Z., Malik, R. N., & Ahmed, Z. (2014). Enrichment and geoaccumulation of heavy metals and risk assessment of sediments of the Kurang Nallah—feeding tributary of the Rawal Lake reservoir, Pakistan. The Science of the Total Environment, 470, 925–933.
Zhang, Z., Juying, L., Mamat, Z., & QingFu, Y. (2016). Sources identification and pollution evaluation of heavy metals in the surface sediments of Bortala River, Northwest China. Ecotoxicology and Environmental Safety, 126, 94–101.
Zhao, L., Xu, Y., Hou, H., Shangguan, Y., & Li, F. (2014). Source identification and health risk assessment of metals in urban soils around the Tanggu chemical industrial district, Tianjin, China. The Science of the Total Environment, 68–469, 654–662.
Acknowledgments
The authors would like to express their gratitude to Ms. Estelle Toerien for the English correction.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amjadian, K., Sacchi, E. & Rastegari Mehr, M. Heavy metals (HMs) and polycyclic aromatic hydrocarbons (PAHs) in soils of different land uses in Erbil metropolis, Kurdistan Region, Iraq. Environ Monit Assess 188, 605 (2016). https://doi.org/10.1007/s10661-016-5623-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5623-6