Abstract
Dead copepods (carcasses) are widespread in aquatic systems, but their scientific quantification is rare due to the difficulty in discriminating them from live ones. In this paper, we hypothesized that due to large spatial and temporal changes in hydrography in the Cochin backwaters, the percentage of copepod carcasses in the system could also change significantly on a spatial and temporal scale. In order to understand this aspect, we quantified the live and dead copepods in the Cochin backwaters under different hydrographical settings based on live and mortal staining technique. The most prominent temporal hydrographical feature during the study period was the large decline in salinity across the system, which was more pronounced downstream (15–20 units) and was caused by the large freshwater influx associated with the southwest monsoon. During the entire sampling period, copepod carcasses were pervasive all over the study area with large spatial and temporal variations in their percentage contribution (2.5–35.8 %) to the total community abundance. During all sampling, carcasses concentrated more in the downstream region, with maximum turbidity (16.5–35.8 %), than in the upstream region (2.5–14.5 %). The percentage of carcasses was the highest during the onset of the southwest monsoon (av. 23.64 ± 8.09 %), followed by the pre-southwest monsoon (av. 13.59 ± 6.72 %) and southwest monsoon (av. 8.75 ± 4.14 %). During the onset of the southwest monsoon, copepod carcasses in the downstream were contributed by ∼80 % high saline and ∼15 % low saline species, indicating a salinity shock-induced mortality. On the other hand, the cumulative effect of the long residence time of the Cochin backwaters and high partial predation rate of carnivores contributed to the high abundance of carcasses during the pre-monsoon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copepods play an important role in aquatic ecosystem, and they usually contribute >60 % to the total zooplankton abundance (Madhupratap et al. 1996; Jyothibabu et al. 2006; Calliari et al. 2008; Jagadeesan et al. 2013). It was noticed earlier that the natural samples of copepods contain a certain percentage of dead individuals (carcasses) despite the fact that routine zooplankton studies generally assume all individuals in the samples to be live (Bickel et al. 2009; Tang et al. 2009; Elliott and Tang 2011a, b; Frangoulis et al. 2011). Quantification studies without the exclusion of carcasses certainly carry a certain percentage of error, leading to biased conclusions on ecosystem/food web functioning (Hansen and Van Boekel 1991; Elliott and Tang 2011a, b). Copepod carcasses get quick colonization by bacteria, eventually leading to the diversion of secondary production to the microbial loop (Tang et al. 2006; Tang et al. 2009; Bickel and Tang 2010). It is generally considered that zooplankton carcasses channelize the carbon flux into the deep sea (Isinibilir et al. 2011); therefore, neglecting the copepod carcasses in natural samples potentially leads to an overestimation of the available carbon for higher trophic levels in surface waters and the underestimation of the organic matter available for vertical and bacterial decomposition within the water column (Ohman and wood 1995; Bickel et al. 2009; Elliott and Tang 2011a, b).
Copepods are vulnerable to rapid and drastic changes in their surroundings, and extreme changes in abiotic factors can cause their mortality (Cervetto et al. 1999; Jagadeesan et al. 2013; Martinez et al. 2013; Tang et al. 2014). Essentially, quantitative information about copepod carcasses in estuarine environment has scientific interest, because of the large and rapid hydrographical changes in such systems, especially during seasonal transition phases (Martinez et al. 2013). Even though voluminous data are available on the distribution and abundance of copepods from the tropical estuaries (Goswami and Padmavati 1996; Jyothibabu and Mandhu 2007; Madhu et al. 2007; Sooria et al. 2015), information on copepod carcasses from these estuaries is virtually absent (Martinez et al. 2013).
The Cochin backwater (CBW) is heavily influenced by the southwest monsoon rainfall (June–September) and therefore referred to as a monsoonal estuary (Vijith et al. 2009). Usually, the balance between the riverine influx on one end and marine water intrusion on the other end determines the salinity and other physicochemical characteristics of the Cochin backwaters (Madhupratap 1987; Jyothibabu et al. 2006; Balachandran et al. 2008). Since the CBW is geographically located in the tropical region, seasonal variation of temperature at the surface is about 28 °C in the summer monsoon period (June–September) and 30 °C in the pre-southwest monsoon period (pre-monsoon: March–May) (Madhupratap 1987). During the southwest monsoon, the Cochin backwater is dominated by freshwater while saline water dominates during the pre-monsoon period. The onset of the southwest monsoon is quite rapid in this part of the world, which usually occurs during the first week of June (Qasim 2003). Very heavy rainfall (40–50 cm in a few hours) occurs in the region during the peak southwest monsoon, and as a result, within a short period, salinity reaches near zero values over most parts of the CBW (Qasim 2003; Jyothibabu et al. 2006). This sudden transition from the polyhaline/mesohaline waters during the pre-monsoon to oligohaline/limnohaline waters during the southwest monsoon conditions is expected to produce physiological stress (salinity shock) to the native zooplankton community, leading to their mortality (Madhupratap 1987; Jyothibabu et al. 2006). However, estimates of such events are very few worldwide, mainly due to sampling constraints introduced by the rough weather during the period and also the short, episodic nature of such events (Martinez et al. 2013). Considering these factors, three important attributes of the CBW copepod community have been investigated in this paper: (a) the percentage of contribution of copepod carcasses to the total community abundance on a temporal and spatial scale, (b) the relative contribution of different copepod species to the carcasses in different hydrographical settings, and (c) the impact of rapid hydrographical transformation on the physiological status of copepods during the onset of the southwest monsoon.
Study area and methods
Study area and sampling
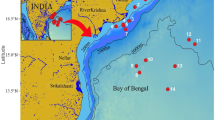
The Cochin backwater is the largest estuarine ecosystem along the west coast of India and the second largest one in the entire Indian peninsula (Fig. 1a). It extends from Azhikode (10° 10′ N, 76° 15′ E) in the north to Alappuzha (09° 30′ N, 76° 25′ E) in the south over a length of ∼80 km and covers an area of about 300 km2 (Qasim 2003; Jyothibabu et al. 2006). The depth of the estuary ranges from 3 to 10 m with an average of 2.5 m. In the present study, 14 close-interval locations were sampled along the salinity gradients in the Cochin backwaters (Fig. 1). First sampling was conducted on May 26, 2011 (pre-monsoon), to represent typical stratified/high-saline conditions of the pre-southwest monsoon. The second sampling (onset of monsoon/monsoon 1) was conducted on June 2, immediately after the onset of the southwest monsoon, to represent the sudden hydrographical transition phase. The third and fourth samplings (monsoon 2 and 3) were conducted on June 9 and June 15, respectively, to represent the actual southwest monsoon condition (Fig. 1b). In this study, we adopted the standard salinity grouping designed for estuarine conditions by McLusky (1993), based on which waters/zones were grouped as (a) euhaline (salinity >30), (b) polyhaline (salinity 25 to 32), (c) mesohaline (salinity 5 to 18), (d) oligohaline (salinity 0.5 to 5), and (e) limnetic (salinity <0.5). During the entire study, we used the terms downstream, middle stretch, and upstream to represent different regions in the Cochin backwaters sequentially from the barmouth to the river mouth.
a Study locations (1–14) in the Cochin backwaters. The pre-monsoon sampling was carried out on May 26, onset of southwest monsoon sampling (monsoon 1) on June 2, second and third southwest monsoon sampling on June 9 and 15, 2013, respectively. Panel b shows the daily rainfall (mm) in Ernakulum (ERN) and Alappuzha (ALP), two main revenue districts of Kerala encompassing the catchment area of the Cochin backwaters, from May 22 to June 16, 2011. The pink boxes in the x-axis represent the exact date of sampling. A motorized speed boat was used for the field sampling in the present study
Methods
Physicochemical parameters
A speed boat was used to carry out the field sampling. Vertical distributions of salinity and temperature were measured using SBE Sea-Bird 19plus CTD. Surface water samples were collected using Niskin bottles for measuring the turbidity and nutrients. Turbidity was measured by a turbidity meter following the nephelometric principle. Nutrients (nitrate, phosphate, and silicate) were analyzed based on the standard protocols of Grasshoff (1983). The rainfall data (seasonal and daily) were retrieved from the Indian Meteorological Department (IMD), Pune. Salinity stratification coefficient (SSC, m−1) was calculated as the salinity difference between the bottom and the surface layers divided by the depth difference between the layers. The horizontal salinity gradient (HSG, km−1) was estimated as the difference between the surface salinity of two consecutive stations divided by the distance between them.
Water column stratification parameter (n s ) was estimated by the formula
where δS = S bott − S surf; S′m = 1/2 (S bott + S surf); and S surf and S bott represent salinity at the surface and the bottom of the water column, respectively. Based on the n s value, the physical state of the estuary was determined. n s <0.1 represents completely mixed water column, between 0.1 to 1 represents partially mixed water column, and >1 represents stratified water column (Haralambidou et al. 2010; Shivaprasad et al. 2013).
Zooplankton sampling and staining
Zooplankton samples were collected from horizontal hauls of a WP-2 net (200 μm mesh size) attached with a digital flow meter (Hydro-Bios). The volume of the water filtered in each zooplankton net tow was estimated using flow meter readings. The zooplankton sampling consisted of two tows: one for quantitative measurements and species level identification of copepods and the other for demarcating live and dead copepods based on vital/mortal staining. This was essential to following the standard procedure for staining copepods, described in detail below (Elliott and Tang 2009). In the case of quantitative measurements and identification of zooplankton, samples were preserved in 4 % formalin. In the laboratory, collected samples were filtered through a 200-μm mesh sieve (Hydro-Bios), the excess water in the samples was removed using blotting paper, and the mesozooplankton biomass was measured as the displacement volume (Postel et al. 2000).
Samples for demarcating live and dead components were collected carefully with slow towing speed with short-duration hauls (to avoid the mortality). After the retrieval of the plankton net, the contents of the cod end were transferred to a staining jar and stained in situ for 15–30 min temperature in the dark. The nets and collection buckets were rinsed thoroughly between consecutive tows to avoid the carryover of the carcasses (Elliot and Tang 2009). We used two stains (neutral red and aniline blue) for the quantification of live and dead copepods, considering their staining efficiencies at different salinities. Aniline blue (a mortal stain) was used for quantifying copepod carcasses in oligohaline and limnohaline environments and neutral red in mesohaline and polyhaline conditions (Dubovskaya et al. 2003; Elliott and Tang 2009; Bickel et al. 2009). In the field, prior to the selection of the stain, salinity was measured using a calibrated salinity probe to determine which stain is applicable in each sampling location. After being incubated in the dark, samples were filtered through a 60-μm mesh disc and rinsed with GF/F-filtered water to remove the excess stain. These samples were then sealed in Petri dishes, transported to the laboratory in ice, and stored at −20 °C until enumeration (Elliott and Tang 2009).
Zooplankton total abundance was estimated from 50 % of the subsamples or whole samples based on the volume of the samples collected (Postel et al. 2000). Among various taxonomic groups, copepods were sorted out and identified to the species level using standard literature (Kasturirangan 1963; Sewell 1999; Conway et al. 2003). For the assessment of live and dead individuals, whole samples were thawed using filtered estuarine water. In total, 200–500 copepod specimens were imaged as soon as possible under inverted microscope using two to three subsamples from each location. Subsequently, the microscope images were examined for quantification of carcasses and species level identification. All the samples were analyzed within a month’s time from the date of collection.
Statistical analyses
Cluster/similarity profile analysis (SIMPROF) and non-metric multidimensional scaling (NMDS) were used to group the sampling locations based on their relatedness in the distribution of environmental parameters. For cluster and NMDS analysis, Euclidian distance matrix was used on the datasets normalized prior to the analysis. For comparing the difference in environmental and biological data within and between the seasons, data were initially tested for their homogeneity (Zar 1999). On parameters having homogenous distribution, parametric ANOVA was used to compare the difference, while in other cases of heterogeneous distribution, non-parametric ANOVA (Kruskal-Wallis) was performed. HSD test was performed for pairwise comparisons for parametric ANOVA, and Tukey’s and Dunn’s test were used for non-parametric ANOVA cases.
Redundancy analysis (RDA) was performed to find out the inter-relationships within and between the biological and environmental variables. RDA model was used to extract information about the extent to which the distribution of biological variables was controlled by the environmental parameters. The significances of RDA axes were tested by Monte Carlo permutations (Leps and Smilauer 2003). The result of RDA was represented in the form of a triplot, in which the environmental and biological variables were represented as solid and dotted lines and each arrow represented increasing gradients of a particular parameter in a particular direction. In the triplot, samples/observations were displayed by points. The copepod species datasets were log (1 + X) transformed prior to the analysis of RDA.
Results
Rainfall and salinity
The distribution of rainfall from May 22 to June 16, 2011, is presented in Fig. 1b, which shows the clear seasonal transition from the pre-monsoon to the southwest monsoon conditions. During the present study period, the southwest monsoon began on May 30, a few days earlier than the usual. During the onset of the monsoon, rainfall increased tremendously, reaching an excess of 50 mm. In general, earlier datasets of rainfall and runoff from rivers emptying into the Cochin backwaters showed a close positive linkage with the rainfall (Supplementary Material 1).
During the study period, temperature ranged from 27.6 to 30.4 °C, with higher values during the pre-southwest monsoon compared to the southwest monsoon period. The spatial difference in temperature during all the samplings was only marginal (Table 2). The heavy rainfall and associated runoff rapidly altered the salinity in the backwaters from a pre-monsoon surface salinity of 1.7 in the upstream regions and 26.5 in the downstream regions (SSC 0.07 in the upstream and 1.51 in the downstream) to the onset of monsoon salinity of 0.1 in the upstream region and 1.3 in the downstream region (SSC 6.34 in the upstream and <0.1 in the downstream). During the pre-monsoon to monsoon transition, the bottom salinity showed a similar trend except in the barmouth (location 1) where a decrease in the bottom salinity value was less due to a strong salt wedge even during the monsoon periods (Fig. 2; Table 1). All these essentially indicate that by the onset of the southwest monsoon, freshwater dominated in the entire Cochin backwaters with oligohaline condition close to the barmouth and limnohaline condition in the upstream.
Vertical distribution of salinity during a pre-monsoon, b onset/first sampling of the southwest monsoon (monsoon 1), c second sampling during the southwest monsoon (monsoon 2), and d third sampling during the southwest monsoon (monsoon 3). Salinity gradients along the study area were prominent during the pre-monsoon with polyhaline salinity in the downstream and oligohaline salinity in the upstream locations. During the onset of the southwest monsoon, freshwater dominated the entire study area with oligohaline salinity in the downstream and limnohaline salinity in the upstream locations. During the southwest monsoon sampling, salt water intrusions into the backwaters were restricted to locations very close to the barmouth. Salt wedge formation is clear in the downstream locations prominently during the pre-monsoon
Monsoonal rainfall significantly altered the stratification in the entire Cochin backwaters except in location 1 in the barmouth where a strong salt wedge was present in the subsurface waters during the southwest monsoon (Fig. 2). In general, water column was partially mixed (n s between 0.1 and 1) during the pre-monsoon/dry period and well mixed and dominated by the freshwater during the southwest monsoon (n s <0.1). However, in location 1 in the barmouth, the water column stratification parameter n s was 1.8 during the monsoon collections, which essentially indicates the influence of freshwater in the surface layer and salt wedge in the subsurface layer (Fig. 2, Table 1).
Turbidity
Turbidity of the water column significantly varied in the collected water samples. Irrespective of seasons, water column was more turbid in the downstream locations (Supplementary Material 2). During the pre-monsoon sampling, turbidity ranged from 2.76 to 11.5 NTU, which was the lowest range among all the samplings (Supplementary Material 2). During the onset of the monsoon, turbidity increased several folds (8.05–115 NTU) and then decreased in subsequent monsoon sampling (monsoon 2 and 3). Nonetheless, turbidity during the southwest monsoon was significantly higher than the pre-monsoon levels. Throughout the observations, the turbidity maxima were found in the downstream, which was very prominent during the southwest monsoon. During the monsoon 2 and 3 sampling, the turbidity maxima were pushed further seaward due to the heavy freshwater influx (Supplementary Material 2).
Nitrate, phosphate, and silicate
The distribution of nitrate showed clear temporal and spatial variations with noticeably high concentration during monsoon 3 observations. In general, nitrate was high (>10 μM) in the entire stretch of the Cochin backwaters in all collected samples (Supplementary Material 2). Irrespective of seasons, phosphate concentration was high in the downstream locations (>0.4 μM). The phosphate concentration was high in the entire stretch of the backwaters during the pre-monsoon (0.14–0.45 μM), which gradually decreased during the onset of monsoon (0.12–0.41 μM) and <0.1 μM in most of the locations (except the barmouth) during the peak monsoon period (Supplementary Material 2). Silicate concentrations were high in all study locations, ranging from 24.03 to 53.03 μM even during the least freshwater discharge period of the pre-monsoon. Silicate distribution showed an opposite trend to that of phosphate. By the onset of the southwest monsoon, silicate concentration increased twofold higher than during the pre-monsoon and ranged from 83.6 to 125.5 μM (Supplementary Material 2). During monsoon samplings 2 and 3, silicate concentrations increased significantly, especially in the upstream locations.
Segregation of locations
Based on the distribution of physicochemical parameters (salinity, turbidity, phosphate, and silicate) recorded in 14 locations, cluster/SIMPROF analysis grouped the locations into three clusters representing the pre-monsoon (Fig. 3a, b). Locations close to the barmouth (locations 1 and 2) were assembled in cluster 1, which was characterized by polyhaline waters (salinity 22.19 ± 2.29) with higher concentrations of phosphate (0.41 ± 0.09 μM) and higher turbidity (6.97 ± 3.14 NTU) compared to the rest of the locations (Table 2). Locations in the mesohaline waters (salinity 9.78 ± 3.78) with moderate levels of silicate (35.03 ± 2.83 μM) and turbidity (5.46 ± 2.03 NTU) were grouped as cluster 2 (locations 3 to 8). Locations in the oligohaline waters (2.51 ± 0.75) with the highest level of silicate (50.47 ± 1.87 μM), lesser turbidity (3.63 ± 0.78 NTU), and the lowest concentration of phosphate (0.14 ± 0.02 μM) were grouped as cluster 3 (locations 9 to 14).
Euclidian distance matrix cluster and NMDS delineated the sampling locations into three clusters based on the distribution of the physicochemical parameters (salinity, phosphate, silicate, and turbidity) representing the pre-monsoon and two clusters each representing c, d monsoon 1, e, f monsoon 2, and g, h monsoon 3. 1–14 represent the sampling locations
By the onset of the southwest monsoon (monsoon 1), freshwater dominated the entire backwaters, causing noticeable changes in the hydrography of the Cochin backwaters from that of the pre-southwest monsoon conditions. Representing this period, cluster/SIMPROF analysis demarcated the locations in the entire study area into two clusters—locations closer to the barmouth under the slight influence of saltwater intrusion from the Arabian sea formed cluster 1 (locations 1 to 6) and the remaining locations dominated by freshwater (locations 7 to 14) formed cluster 2 (Fig. 3c, d). The cluster 1 locations were oligohaline (salinity 1.98 ± 0.23), more turbid (79.38 ± 18.01 NTU), and with relatively high phosphate (0.37 ± 0.08 μM) and low silicate (98.21 ± 11.45 μM). Limnohaline waters (0.12 ± 0.04) with high silicate (120.62 ± 4.18 μM) and low phosphate (0.14 ± 0.03 μM) prevailed in cluster 2 (Table 2).
During the monsoon 2 sampling, saline water intrusion into the Cochin backwaters was confined only to the locations very close to the barmouth (Fig. 3e, f). As a result, all the sampling locations were characterized by a small cluster 1 (locations 1 and 2) and a large cluster 2 (locations 3 to 14). Cluster 1 was characterized by oligohaline conditions (salinity 1.41 ± 0.74) with high turbidity (25.15 ± 2.76 NTU). On the other hand, cluster 2 showed limnetic conditions (salinity 0.08 ± 0.06) with high silicate (119.94 ± 7.37 μM) and less turbid (13.26 ± 8.01 NTU) waters (Table 2). During monsoon 3 sampling, all the locations were grouped into two clusters (Fig. 3g, h); cluster 1 included the first three locations, and they were characterized by oligohaline conditions (salinity 1.40 ± 0.38) with high turbidity (35.3 ± 3.67 NTU). Cluster 2 locations were characterized by limnetic condition (0.10 ± 0.09) with less turbidity (22.35 ± 1.4) as compared to cluster 1 (Table 2).
Zooplankton biomass and density distribution
Zooplankton biomass and abundance during the present time series sampling is presented in Fig. 4. Irrespective of collections, zooplankton biomass and abundances were spatially high in the downstream and middle reaches of the backwaters as compared to the upstream region. Seasonally, zooplankton biomass (ml m−3) and density (No. m−3) were high during the pre-monsoon (0.01 to 0.13 ml m−3; 36.6 to 636.21 No. m−3 in density), whereas the biomass and density decreased in the entire stretch of the backwaters during the onset of the monsoon (0.01 to 0.08 ml m−3; 13.2–285.85 No. m−3). This decline was more prominent in the downstream and middle reaches of the backwaters (Fig. 4). The biomass and density values decreased further during the subsequent monsoon sampling (monsoon 2 and 3). The NMDS plots visualized the spatial differences of the zooplankton biomass and abundances in relation to the spatial and temporal variations of the salinity (Figs. 5 and 6). Zooplankton abundance during the pre-monsoon was significantly higher than the monsoon water samples (ANOVA, P < 0.05). On the other hand, differences in zooplankton abundance between the monsoon samples were insignificant (ANOVA, P > 0.05). Copepods contributed 40–80 % of the total zooplankton density, and the highest contribution of copepods to the total density was found in the downstream and middle reaches during the pre-monsoon period. During the southwest monsoon, copepod contribution to the total community abundance decreased, whereas during this period, the cladocera contribution increased. During the pre-monsoon, carnivorous zooplankton (hydromedusae, siphonophores, chaetognaths) contributed 7.62 % to the total zooplankton density in the downstream locations and 1.12 % in the middle reaches of backwaters, whereas they were absent in the upstream locations. During monsoon sampling, carnivorous zooplankton were absent in the entire stretch of the backwaters (Table 3).
Spatial and temporal changes in the distribution of zooplankton biomass (green bars), density (blue bars), and copepod density (white diamonds). Zooplankton biomass (ml m−3) and density (No. m−3) were high during the pre-monsoon, which declined abruptly by the onset of the southwest monsoon (monsoon 1). Zooplankton biomass and density further decreased during subsequent monsoon sampling (monsoon 2 and monsoon 3). Copepod density distribution closely followed the total zooplankton abundance pattern
Bubbles overlaid NMDS presenting the distribution of salinity, zooplankton biomass, and density on spatially assembled locations representing a–d pre-monsoon and e–h monsoon 1. Green, brown, blue, and orange circles represent polyhaline, mesohaline, oligohaline, and limnohaline salinity, respectively. Station positions of the bubbles in b–d are the same as in panel a representing the pre-monsoon. Similarly, station positions in panels f–h are the same as in panel e representing monsoon 1. The value in each station is relative to the reference bubbles in each panel
Bubbles overlaid NMDS showing the distribution of salinity, zooplankton biomass, and density on spatially assembled locations representing a–d monsoon 2 and e–h monsoon 3. Blue and orange circles represent oligohaline and limnohaline salinity, respectively. Station positions of the bubbles in b–d are same as in panel a representing the monsoon 2. Similarly, station positions in panels f–h are the same as in panel e representing monsoon 3. The value in each station is relative to the reference bubbles in each panel
Carcasses of copepods
The stained organisms showed three different color patterns during analysis under the microscope: fully stained, without stain, and partial or patchy stained. When stained with neutral red, partially and fully stained individuals were considered live and the animals without stains were considered dead (Fig. 7). In the case of aniline blue, the stained individuals were considered as dead and the partially patchy stained/without stain was considered as live (Fig. 8). The occurrence of carcasses was widespread in the entire stretch of the backwaters, and their percentage contribution to the total abundance ranged from 2.5 to 35.8 % (Fig. 9). Irrespective of seasons, carcasses were higher in the downstream region (16.5–35.8 %) as compared to the upstream region (2.5–14.5 %). During the pre-monsoon, the copepod carcasses ranged from 4 to 24.1 % of the total abundance, whereas during the onset of the southwest monsoon, the percentage of carcasses increased in the entire stretch of the backwaters and found 6.4 to 35.8 %. Later, during the monsoon 2 and 3 sampling, the percentage of copepod carcasses decreased (3 to 17.5 %) and was lesser than the respective values in the pre-monsoon (Fig. 9).
The quantification of live and dead copepods using neutral red (vital stain). Blue arrow represents the different stain absorption patterns. SP-1 indicates stain on the entire body, SP-II indicates individuals without stain, and SP-III indicates stain only on the metasome. Individuals with stain on the entire body were live and others either with partial stain or without stain were dead. The black arrow represents an aged copepod carcass. Panel f indicate the dead (unstained) individuals with wounds from partial predation (pink arrows)
The quantification of live and dead copepods using aniline blue (mortal stain). Red arrows represent different stain absorption patterns. SP-1 indicates stain on the entire body, SP-II stain only on appendages, SP-III stain on some parts of metasome, and SP-IV without stain. Individuals without stain were live and all others were dead. The black arrows represent aged copepod carcasses
Percentage contributions of copepod carcasses to the total community abundance during the four field samplings carried out in the present study (pre-monsoon, monsoon 1, monsoon 2, and monsoon 3). Throughout the observations, copepod carcasses were high in the downstream compared to the upstream locations. During the onset of the southwest monsoon (monsoon 1), copepod carcasses remarkably increased in the entire stretch of the Cochin backwaters. However, in later collections in monsoon (monsoon 2 and 3), copepod carcasses decreased to a level lower than the pre-monsoon
Copepod community structure and carcasses
During the pre-monsoon, altogether 21 copepod species were recorded from the study area. Their details and the percentage contribution to the total copepod density are presented in Table 4. In general, carcasses were high in Calanoid copepods, followed by the Poecilostomatoids and least in Cyclopoids and Harpacticoids. During the pre-monsoon period, 14 species were reported in the downstream locations (cluster 1) where carcasses varied from 3.32 to 38.27 % to the particular species density (Table 5). The carcasses were found >25 % of the density of Acrocalanus gracilis, Paracalanus parvus, Acartia erythraea, Acartia danae, Corycaeus danae, and Corycaeus catus in the downstream locations. In the upstream locations, carcasses of seven species of copepods (Allodiaptomus mirabilipes, Heliodiaptomus cinctus, Acartiella gravely, Limnocalanus macrurus, Mesocyclops sp., Thermocyclops sp., and Microcyclops sp.,) varied from 4.77 to 8.61 % of their total abundance (Table 5). In the middle reaches, 20 copepod species were found, which include 14 downstream and 6 upstream species. In the middle reaches, Pseudodiaptomus annandalei was found as the most dominant form contributing 53.57 % of the total community abundance and 4.45 % of the carcass abundance. Carcasses of Calanoid and Poecilostomatoid were high in the middle reaches of the estuary compared to either downstream or upstream.
During the onset of monsoon (monsoon 1), the copepod community in the downstream locations varied from that existing during the pre-monsoon. Totally, 19 species were found in the downstream locations, which include many species of the pre-monsoon community as well as newcomers from the freshwater region. In the entire stretch of the backwaters, carcass percentage was noticeably higher during the onset of monsoon (monsoon 1) than the rest of the sampling (Tables 4 and 5). In the downstream locations, the contribution of Paracalanus parvus, Acrocalanus gracilis, and Acartia danae to the total density was less compared to the pre-monsoon. But carcasses of these three species were >80 % of their total abundance. During the onset of the monsoon (monsoon 1 sampling), copepod species composition in the upstream locations was almost similar to the pre-monsoon condition but the carcass percentage increased to become ∼15 % of their total abundance (Tables 4 and 5).
During the monsoon 2 observation, Allodiaptomus mirabilipes, Heliodiaptomus cinctus, Acartiella gravely, and Limnocalanus macrurus dominated the total abundance, with a few individuals of Acartia sp., Paracalanus parvus, Acrocalanus gracilis, Centropages orsini, Corycaeus danae, and Corycaeus catus of which >90 % of the individuals were dead (Table 5). During the monsoon 2 and 3 observations, the carcass percentages of freshwater forms significantly decreased as compared to the onset of the monsoon (monsoon 1). During the monsoon 3 observation, high-saline copepods were absent in the downstream locations and the entire estuary was dominated by freshwater forms (Table 4).
Relationship between environmental and biological parameters
The RDA triplot visualized the relationships between the environmental and biological parameter distribution and their inter-relationships during the time series sampling in CBW. The environmental parameters altogether explained 72.7 % variance of zooplankton biomass, zooplankton density, copepod abundance, and percentage of copepod carcasses. The environmental parameters, nitrate and silicate, were oriented on the left side of the plot, and they showed a close positive relationship with one another and an inverse relationship with salinity and phosphate (Fig. 10a). The salinity axis was orientated toward the observations of the pre-monsoon and the nitrate and silicate axes toward the monsoon 2 and 3 sampling locations. The water column stratification and the SSC axes were oriented toward the downstream locations of the monsoon sampling, indicating the dominance of the freshwater in the surface and salt wedge structure in the subsurface. The carcass axis was oriented on the right side and opposite to monsoon 2 and 3 observations, indicating their low values (<10 % except the downstream) during those periods. The carcass percent axis was oriented toward the bottom right side with close association to SSC, n s , and turbidity axes indicating high percent carcasses in the downstream locations (Fig. 10b).
RDA Triplot explains the distribution and inter-relationship between the environmental and biological variables. Panel a represents the basic RDA plot showing the distribution of various parameters and their inter-relationships. Panel b represents the plot of carcasses overlaid (contours), clearly evidencing the gradients in carcass distribution and their relation with other parameters. Red dots (P1–P14) represent pre-monsoon, pink dots (M1.1–M1.14) represent monsoon 1, green dots (M2.1–M2.14) represent monsoon 2, and gray dots (M3.1–M3.14) represent monsoon 3
Discussion
Physicochemical setting
The study domain experiences tropical climatic conditions with intense rainfall during the southwest monsoon, which usually begins in the first week of June (Qasim 2003). In the present study period, the onset of the monsoon rainfall occurred on May 30, which was a few days earlier than the normal year. The study clearly showed low rainfall (<2 mm) during the pre-monsoon period. But the rainfall increased several folds higher by the onset of the southwest monsoon as observed earlier (Qasim 2003; Jyothibabu et al. 2006; Sooria et al. 2015). Heavy rainfall during the first week of June significantly transformed the saltwater-dominant pre-monsoon condition to a freshwater-dominant monsoon condition (Qasim 2003; Jyothibabu et al. 2006; Revichandran et al. 2012).
During the pre-monsoon, the river runoff into the Cochin backwaters was very low (1.4 % of total runoff) and therefore salinity intrusion dominated, which resulted in euhaline conditions in the downstream region and oligohaline in the upstream region (Jyothibabu et al. 2006). Less river runoff during the pre-monsoon also leads to longer flushing time (14.7 days; Shivaprasad et al. 2013). On the other hand, heavy rainfall and freshwater influx into the Cochin backwaters during the southwest monsoon lead to shorter flushing time (1 to 2 days), causing oligohaline salinity in the barmouth area and limnohaline salinity in the rest of the region (Shivaprasad et al. 2013; Sooria et al. 2015). During the pre-monsoon period, a clear gradient of salinity (HSC found) was evident from the barmouth to the river mouth. The stratification parameters n s showed that the entire Cochin backwaters were partially mixed during the pre-monsoon (SSC 0.2 to 2.2). By the onset of the southwest monsoon, saltwater intrusion to the Cochin backwaters was limited only to the locations closer to the barmouth and the stratification parameters showed that the entire Cochin backwaters were well mixed in the barmouth region due to the subsurface salt wedge formation (n s >1) in the region. The turbidity of the estuarine water column depends upon various factors like runoff, tidal activity, morphology of the estuary, and estuarine bed sediment characteristics (Acharyya et al. 2012). It was observed that water column was less turbid during the pre-monsoon, whereas by the onset of the monsoon (monsoon 1), turbidity increased several folds than the pre-monsoon due to initial flooding which carry high amounts of suspended particles from the watershed and re-suspended particles from the river and estuarine bed (Howarth et al. 2000; Acharyya et al. 2012). The nutrient distribution in the Cochin backwaters observed in the present study showed significant spatial and temporal variations as noticed in a few earlier observations (Balachandran et al. 2008; Thottathil et al. 2008; Madhu et al. 2010; Martin et al. 2013). Irrespective of seasons, phosphate was high in the downstream locations and the silicate concentration in the upstream locations indicating the source of these nutrients (Martin et al. 2013).
Zooplankton and copepod carcasses
Zooplankton biomass and density distribution in tropical estuaries show significant spatial and temporal variations (Madhupratap and Haridas 1975; Madhupratap 1979, 1980, 1987; Goswami and Padmavati 1996; Jyothibabu et al. 2006; Madhu et al. 2007; Sooria et al. 2015), and a similar trend was observed in the present study as well. In the present study, zooplankton biomass and density were generally high during pre-monsoon, which declined significantly during the southwest monsoon especially in the downstream locations. It was observed that high copepod abundance was located in the downstream and middle stretches of the Cochin backwaters as compared to the environmental setting in the upstream oligohaline condition. The high zooplankton biomass and abundance during the pre-southwest monsoon period was related to intrusions of coastal marine waters into the upstream locations of the Cochin backwaters (Madhupratap 1987; Goswami and Padmavati 1996).
The survival and proliferation of organisms in an estuary depend upon their adaptation capacity and tolerance limits toward varying environmental conditions, especially salinity (Cervetto et al. 1999; Calliari et al. 2006, 2008). Copepods are mostly osmoconformers and they are able to cope up with certain changes in salinity, but this ability varies between the species, even within members of the same genus (Cervetto et al. 1999; Calliari et al. 2008). Sudden fluctuations in salinity can affect the physiological status of all copepod species but with varying intensity, and the secondary productivity pattern varies with salinity tolerance level of copepods. Even though copepods can survive in suboptimum salinity levels, their production in such condition is significantly lower than when they are in optimum conditions (Chinnery and Williams 2004; Chen et al. 2006).
In the Cochin backwaters, the presence of carnivorous zooplankton (siphonophores, hydromedusae, and chaetognaths) was noticed only during the pre-southwest monsoon. Similar observations were made earlier as well (Madhupratap 1987). The predatory action of the larger carnivores causes the predatory mortality to copepods (Haury et al. 1995; Ohman and Wood 1995). In the present study, we considered predatory and non-predatory carcasses together. During the pre-monsoon, incidence of wounds on the copepod dead bodies was high in the middle and lower reaches of the Cochin backwaters (Fig. 9f) indicating the effect of partial predation of copepods by carnivorous zooplankton. Carcasses with the wounds in exoskeleton, internal organ expulsions, and body part remains are the result of predatory mortality (Genin et al. 1995; Haury et al. 1995; Ohman and Wood 1995), and the carcasses without any signs of the predatory actions are considered as non-predatory/non-consumptive mortality (Elliott and Tang 2009; Elliott and Tang 2011a, b). The non-predatory mortality can be caused by various factors such as senescence, temperature change, injury, starvation, environmental stresses, diseases, parasitism, and harmful algal blooms (Cervetto et al. 1999; Kimmerer and McKinnon 1990; Bickel et al. 2011; Kirillin et al. 2012) .
Earlier researches (Terazaki and wada 1988; Hirst and Kiroboe 2002) differentiated the predatory carcasses through their morphological appearances (empty exoskeleton, partially broken specimens, animals with damage, etc.) with the aid of microscopes which is practically not feasible in the case of extensive studies because of the special skill and labor required. Using traditional methods, quantification of non-consumptive mortality is difficult, though it contributes significant proportions (1/4 to 1/3) to the total mortality (Hirst and Kiroboe 2002) because of their morphological resemblances with the live individuals (Tang et al. 2006; Elliott and Tang 2009; Bickel et al. 2009; Martinez et al. 2013).
The high abundance of Paracalanus parvus, Acartia danae, Acartia erythraea, Centropages orsini, Corycaeus danae, and Corycaeus catus in the barmouth region and Allodiaptomus mirabilipes, Acartiella gravely, and Heliodiaptomus cinctus in the upstream locations indicated that their optimum level of salinity prevailed in the respective regions (Madhupratap and Haridas 1986, 1990; Madhupratap et al. 1990, 1992). In the middle reaches, a mixed community of both upstream and downstream copepods was found but less in abundance than in their preferred environments (Madhupratap and Haridas 1975; Madhupratap 1979, 1987; Jyothibabu et al. 2008). Carcasses were found in the entire stretch of the Cochin backwaters with percentage contributions to the total abundance varying from 2.5 to 35.8 %. Irrespective of sampling, carcass percentage was clearly high in the downstream as compared to the upstream (Fig. 10) which corroborates the observations made in previous work (Elliott et al. 2010; Elliott and Tang 2011a, b; Martinez et al. 2013).
During the onset of the southwest monsoon, salinity changed from mesohaline/polyhaline to oligohaline levels in the downstream locations, during which a mixed copepod community was found consisting of the newcomers from the upstream locations; the pre-monsoon also formed a copepod community but with less abundance. Noticeably, >80 % of coastal/estuarine species like Paracalanus parvus, Acrocalanus gracilis, Acartia danae, Acartia erythraea, and Centropages orsini were found dead during the onset of monsoon (monsoon 1) due to the sudden salinity change during the seasonal transition. Similar observations of rapid salinity changes associated with mortality of copepods were noticed in fjord environments (Kaartvedt and Aksnes 1992) and in the Westerschelde estuary (Soetaert and Herman 1994). It was noticed earlier in laboratory experimental studies that copepods can tolerate gradual decrease/increase of salinity from their optimum levels, but any large change in salinity leads to mass mortality even in their suboptimum salinity levels (Kaartvedt and Aksnes 1992; Soetaert and Herman 1994; Cervetto et al. 1999; Chinnery and Williams 2004; Calliari et al. 2006, 2008; Chen et al. 2006; Hubareva et al. 2008).
The residence time of carcasses in estuaries depends upon their sinking rate, bacterial decomposition, surface flow transportation, stratification, and turbulent mixing. Carcasses reside in surface layers from a few hours to a few days, whereas their sinking rate was less in turbulent mixing conditions like in estuarine barmouth (Tang et al. 2006; Elliott et al. 2010). The high percentage of the carcasses in the barmouth locations in the present study was associated with high turbidity (Fig. 10), considered as the result of suspension of the settled/partially settled carcasses to the surface associated with their dynamic nature and turbulent mixing (Kaartvedt and Aksnes 1992; Tang et al. 2006; Elliott et al. 2010; Martinez et al. 2013).
The presence of mesohaline and polyhaline copepod carcasses in the barmouth during the monsoon 2 and 3 sampling indicated their possible immigration from adjacent regions (Aksnes et al. 1997; Martinez et al. 2013) to the estuary. The overall carcass percentage was minimum during the monsoon 2 and 3 collections, which could be linked to the dominance of a well-adapted oligohaline/mesohaline community in the estuary and the short residence time (faster flushing) of the Cochin backwaters (Shivaprasad et al. 2013). On the other hand, longer residence time and increased abundance of carnivorous plankton (predatory mortality) of the Cochin backwaters could be contributing to the second highest percentage abundance of copepods during the pre-monsoon period. The increased predatory mortality of copepods (carcasses) during the pre-monsoon was supported by high incidence of wounds on the carcasses during the period (Figs. 9 and 10).
Conclusion
Copepod carcasses in a tropical monsoonal estuary in relation to the hydrographical setting is presented in this study. The rapidity of the monsoon alters salinity distribution and the status of the estuary. During the onset of the southwest monsoon, downstream locations are characterized with oligohaline salinity and the upstream locations with limnohaline salinity. The occurrences of the copepod carcasses were found widespread in the estuary which varied from 2.5 to 35.8 % of the total copepod abundance. The copepod community structure varied spatially and seasonally which associated with salinity distribution. Irrespective of the season, copepod carcasses were high in the downstream locations. During the onset of the southwest monsoon, copepod carcasses increased significantly in the entire stretch of the Cochin backwaters. During the onset of the monsoon period, >80 % of the pre-monsoon copepods and 11–19 % of the newcomers (copepods) from the oligohaline conditions were found dead in the downstream due to salinity shock associated with the rapid hydrographical transformation. The minimum carcass abundance during late southwest monsoon sampling was associated with the dominance of a well-adapted oligohaline/mesohaline community in the estuary and the short residence time (faster flushing) of the Cochin backwaters. On the other hand, longer residence time and increased abundance of carnivorous plankton in the backwaters have caused the second highest percentage of carcass abundance during the pre-monsoon period.
References
Acharyya, T., Sarma, V. V. S. S., Sridevi, B., Venkataramana, V., Bharathi, M. D., Naidu, S. A., Kumar, B. S. K., Prasad, V. R., Bandyopadhyay, D., Reddy, N. P. C., & Kumar, M. D. (2012). Reduced river discharge intensifies phytoplankton bloom in Godavari estuary, India. Marine Chemistry, 132-133, 15–22.
Aksnes, D. L., Miller, C. B., Ohman, M. D., & Wood, S. N. (1997). Estimation techniques used in studies of copepod population dynamics—a review of underlying assumptions. Sarsia, 82, 279–296.
Balachandran, K., Reddy, G., Revichandran, C., Srinivas, K., Vijayan, P., & Thottam, T. (2008). Modelling of tidal hydrodynamics for a tropical ecosystem with implications for pollutant dispersion (Cohin estuary, Southwest India). Ocean Dynamics, 58, 259–273.
Bickel, S., & Tang, K. (2010). Microbial decomposition of proteins and lipids in copepod versus rotifer carcasses. Marine Biology, 157, 1613–1624.
Bickel, S. L., Tang, K. W., & Grossart, H. P. (2009). Use of aniline blue to distinguish live and dead crustacean zooplankton composition in freshwaters. Freshwater Biology, 54, 971–981.
Bickel, S. L., Malloy Hammond, J. D., & Tang, K. W. (2011). Boat-generated turbulence as a potential source of mortality among copepods. Journal of Experimental Marine Biology and Ecology, 401, 105–109.
Calliari, D., Christian Marc, A., Peter, T., Elena, G., & Peter, T. (2006). Salinity modulates the energy balance and reproductive success of co-occurring copepods Acartia tonsa and A. clausi in different ways. Marine Ecology Progress Series, 312, 177–188.
Calliari, D., Andersen Borg, M. C., Thor, P., Gorokhova, E., & Tiselius, P. (2008). Instantaneous salinity reductions affect the survival and feeding rates of the co-occurring copepods Acartia tonsa Dana and A. clausi Giesbrecht differently. Journal of Experimental Marine Biology and Ecology, 362, 18–25.
Cervetto, G., Gaudy, R., & Pagano, M. (1999). Influence of salinity on the distribution of Acartia tonsa (Copepoda, Calanoida). Journal of Experimental Marine Biology and Ecology, 239, 33–45.
Chen, Q., Sheng, J., Lin, Q., Gao, Y., & Lv, J. (2006). Effect of salinity on reproduction and survival of the copepod Pseudodiaptomus annandalei Sewell, 1919. Aquaculture, 258, 575–582.
Chinnery, F. E., & Williams, J. A. (2004). The influence of temperature and salinity on Acartia (Copepoda: Calanoida) nauplii survival. Marine Biology, 145, 733–738.
Conway, D.V.P., White, R.G., Hugues-Dit-Ciles, J., Gallienne, C.P., & Robins, D.B. (2003). Guide to the coastal and surface zooplankton of the south western Indian Ocean. Occasional publication no 15 Marine Biological Association of the United Kingdom, 354.
Dubovskaya, O., Gladyshev, M., Gubanov, V., & Makhutova, O. (2003). Study of non-consumptive mortality of Crustacean zooplankton in a Siberian reservoir using staining for live/dead sorting and sediment traps. Hydrobiologia, 504, 223–227.
Elliott, D. T., & Tang, K. W. (2009). Simple staining method for differentiating live and dead marine zooplankton in field samples. Limnology and Oceanography: Methods, 7, 585–594.
Elliott, D., & Tang, K. (2011a). Spatial and temporal distributions of live and dead copepods in the lower Chesapeake Bay (Virginia, USA). Estuaries and Coasts, 34, 1039–1048.
Elliott, D. T., & Tang, K. W. (2011b). Influence of carcass abundance on estimates of mortality and assessment of population dynamics in Acartia tonsa. Marine Ecology Progress Series, 427, 1–12.
Elliott, D. T., Harris, C. K., & Tang, K. W. (2010). Dead in the water: the fate of copepod carcasses in the York River estuary, Virginia. Limnology and Oceanography, 55, 1821–1834.
Frangoulis, C., Skliris, N., Lepoint, G., Elkalay, K., Goffart, A., Pinnegar, J. K., & Hecq, J. H. (2011). Importance of copepod carcasses versus faecal pellets in the upper water column of an oligotrophic area. Estuarine, Coastal and Shelf Science, 92, 456–463.
Genin, A., Gal, G., & Haury, L. (1995). Copepod carcasses in the ocean. II. Near coral reefs. Marine Ecology Progress Series, 123, 65–71.
Goswami, S. C., & Padmavati, G. (1996). Zooplankton production, composition and diversity in the coastal waters of Goa. Indian Journal of Marine science, 25, 91–97.
Grasshoff, K. (1983). Methods of seawater analysis. In: K. Grasshoff, Ehrhardt, M., Kremling, K., (eds.) (pp. 89–224): Weinheim, Verlag Chemie.
Hansen, F. C., & Van Boekel, W. H. M. (1991). Grazing pressure of the calanoid copepod Temora longicornis on a Phaeocystis dominated spring bloom in a Dutch tidal inlet. Marine Ecology Progress Series, 78, 123–129.
Haralambidou, K., Sylaios, G., & Tsihrintzis, V. A. (2010). Salt-Wedge propagation in Mediterranean microtidal river mouth. Estuarine Coastal Shelf Science, 90, 174–184.
Haury, L., Fey, C., Gal, G., Hobday, A., & Genin, A. (1995). Copepod carcasses in the ocean. I. Over seamounts. Marine Ecology Progress Series, 123, 57–63.
Hirst, A. G., & Kiroboe, T. (2002). Mortality of marine planktonic copepods: global rates and patterns. Marine Ecology Progress Series, 230, 195–209.
Howarth, R. W., Swaney, D. P., Butler, T. J., & Marino, R. (2000). Rapid communication: climatic control on eutrophication of the Hudson River Estuary. Ecosystems, 3, 210–215.
Hubareva, E., Svetlichny, L., Kideys, A., & Isinibilir, M. (2008). Fate of the black sea Acartia clausi and Acartia tonsa (Copepoda) penetrating into the Marmara Sea through the Bosphorus. Estuarine, Coastal and Shelf Science, 76, 131–140.
Isinibilir, M., Svetlichny, L., Hubareva, E., Yilmaz, I. N., Ustun, F., Belmonte, G., & Toklu-Alicli, B. (2011). Adaptability and vulnerability of zooplankton species in the adjacent regions of the Black and Marmara seas. Journal of Marine Systems, 84, 18–27.
Jagadeesan, L., Jyothibabu, R., Anjusha, A., Mohan, A. P., Madhu, N. V., Muraleedharan, K. R., & Sudheesh, K. (2013). Ocean currents structuring the mesozooplankton in the Gulf of Mannar and the Palk Bay, southeast coast of India. Progress in Oceanography, 110, 27–48.
Jyothibabu, R., & Mandhu, N.V. (2007). Zooplankton in the Mandovi and Zuari estuary. In: Mandovi and Zuari estuaries eds.(SR.Shyte, M.Dileep kumar and D.Shankar). 83–90.
Jyothibabu, R., Madhu, N. V., Jayalakshmi, K. V., Balachandran, K. K., Shiyas, C. A., Martin, G. D., & Nair, K. K. C. (2006). Impact of large river influx on microzooplankton and its implications on the food web of tropical estuary (Cochin backwaters—India). Estuarine, Coastal Shelf Science, 69, 505–518.
Jyothibabu, R., Asha Devi, C. R., Madhu, N. V., Sabu, P., Jayalakshmy, K. V., Jacob, J., Habeebrehman, H., Prabhakaran, M. P., Balasubramanian, T., & Nair, K. K. C. (2008). The response of microzooplankton (20–200 mm) to coastal upwelling and summer stratification in the southeastern Arabian Sea. Continental Shelf Research, 28, 653–671.
Kaartvedt, S., & Aksnes, D. L. (1992). Does freshwater discharge cause mortality of fjord-living zooplankton? Estuarine, Coastal and Shelf Science, 34, 305–313.
Kasturirangan, L.R. (1963). A key for the identification of the more common planktonic Copepoda of the Indian coastal waters,Publication No.2. Indian National Committee on Oceanic Research, 1–87.
Kimmerer, W. J., & McKinnon, A. D. (1990). High mortality in a copepod population caused by a parasitic dinoflagellate. Marine Biology, 107, 449–452.
Kirillin, G., Grossart, H. P., & Tang, K. W. (2012). Modeling sinking rate of zooplankton carcasses: effects of stratification and mixing. Limnology and Oceanography, 57, 881–894.
Leps, J., & Smilauer, P. S. (2003). Multivariate analysis of ecological data using CANOCA. Cambridge University Press, Cambridge, United Kingdom, p. 269.
Madhu, N. V., Jyothibabu, R., Balachandran, K. K., Honey, U. K., Martin, G. D., Vijay, J. G., Shiyas, C. A., Gupta, G. V. M., & Achuthankutty, C. T. (2007). Monsoonal impact on planktonic standing stock and abundance in a tropical estuary (Cochin backwaters—India). Estuarine, Coastal and Shelf Science, 73, 54–64.
Madhu, N. V., Jyothibabu, R., & Balachandran, K. K. (2010). Monsoon-induced changes in the size-fractionated phytoplankton biomass and production rate in the estuarine and coastal waters of southwest coast of India. Environmental Monitoring and Assessment, 166, 521–528.
Madhupratap, M. (1979). Distribution, community structure and species succession of copepods from Cochin backwaters. Indian Journal of Marine Science, 8, 1–8.
Madhupratap, M. (1980). Ecology of the coexisting copepod species in cochin backwaters. Mahasagar, 13, 45–52.
Madhupratap, M. (1987). Status and strategy zooplankton of tropical Indian estuaries: a review. Bulletin of Plankton Society of Japan, 65–81.
Madhupratap, M., & Haridas, P. (1975). Omposition and variations in zooplankton abundance in the backwaters from Cochin to Alleppey. Indian Journal of Marine Science, 4, 77–85.
Madhupratap, M., & Haridas, P. (1986). Epipelagic calanoid copepods of the Northern Indian Ocean. Oceanologia Acta, 9, 105–117.
Madhupratap, M., & Haridas, P. (1990). Zooplankton, especially calanoid copepods, in the upper 1000 m of the south east Arabian Sea. Journal of Plankton Research, 12, 305–321.
Madhupratap, M., Sreekumaran Nair, S. R., Haridas, P., & Padmavati, G. (1990). Response of zooplankton to physical changes in the environment: coastal upwelling along the central west coast of India. Journal of Coastal Research, 6, 413–426.
Madhupratap, M., Haridas, P., Ramaiah, N., & Achuthankutty, C. T. (1992). Zooplankton of the southwest coast of India: abundance, composition,temporal and spatial variability in 1987. In B. N. Desai (Ed.), Oceanography of the Indian Ocean (pp. 99–112). New Delhi: Oxford & IBH.
Madhupratap, M., Gopalakrishnan, T. C., Haridas, P., Nair, K. K. C., Aravindakshan, P. N., Padmavati, G., & Paul, S. (1996). Lack of seasonal and geographic variation in mesozooplankton biomass in the Arabian Sea and its structure in the mixed layer. Current Science, 71, 863–868.
Martin, G. D., Jyothibabu, R., Madhu, N. V., Balachandran, K. K., Nair, M., Muraleedharan, K. R., Arun, P. K., Haridevi, C. K., & Revichandran, C. (2013). Impact of eutrophication on the occurrence of Trichodesmium in the Cochin backwaters, the largest estuary along the west coast of India. Environmental Monitoring and Assessment, 185, 1237–1253.
Martinez, M., Espinosa, N., & Calliari, D. (2013). Incidence of dead copepods and factors associated with non-predatory mortality in the RÃo de la Plata estuary. Journal of Plankton Research, 36, 265–270.
McLusky, D. (1993). Marine and estuarine gradients “an overview. Netherland Journal of Aquatic Ecology, 27, 489–493.
Ohman, M. D., & Wood, S. N. (1995). The inevitability of mortality. ICES Journal of Marine Science: Journal du Conseil, 52, 517–522.
Postel, L., Fock, H., & Hagen, W. (2000). Biomass and abundance, ICES Zooplankton Methodology manual. In: Harris R.P., Wiebe. P.H., Leiz. J., Skjoldal et al. (eds.) Academic Press, 193–213.
Qasim, S.Z. (2003). Indian estuaries (Allied publication Pvt. Ltd. Heriedia Marg, Ballard estate, Mumbai). Pp. 259.
Revichandran, C., Srinivas, K., Muraleedharan, K. R., Rafeeq, M., Amaravayal, S., Vijayakumar, K., & Jayalakshmy, K. V. (2012). Environmental set-up and tidal propagation in a tropical estuary with dual connection to the sea (SW Coast of India). Environmental Earth Sciences, 66, 1031–1042.
Sewell, R. (1999). The copepoda of Indian seas (p. 407). Delhi: Biotech Books.
Shivaprasad, A., Vinita, J., Revichandran, C., Reny, P. D., Deepak, M. P., Muraleedharan, K. R., & Naveen Kumar, K. R. (2013). Seasonal stratification and property distributions in a tropical estuary (Cochin estuary, west coast, India). Hydrology and Earth System Science, 17, 187–199.
Soetaert, K., & Herman, P. M. J. (1994). One foot in the grave: zooplankton drift into the Westerschelde estuary (the Netherlands). Marine Ecology Progress Series, 105, 19–29.
Sooria, P. M., Jyothibabu, R., Anjusha, A., Vineetha, G., Vinita, J., Lallu, K. R., Paul, M., & Jagadeesan, L. (2015). Plankton food web and its seasonal dynamics in a large monsoonal estuary (Cochin backwaters, India)—significance of mesohaline region. Environmental Monitoring and Assessment, 187(C7), 427 .1-22
Tang, K. W., Freund, C. S., & Schweitzer, C. L. (2006). Occurrence of copepod carcasses in the lower Chesapeake Bay and their decomposition by ambient microbes. Estuarine, Coastal and Shelf Science, 68, 499–508.
Tang, K. W., Bickel, S. L., Dziallas, C., & Grossart, H. P. (2009). Microbial activities accompanying decomposition of cladoceran and copepod carcasses under different environmental conditions. Aquatic Microbial Ecology, 57, 89–100.
Tang, K. W., Gladyshev, M. I., Dubovskaya, O. P., Kirillin, G., & Grossart, H. P. (2014). Zooplankton carcasses and non-predatory mortality in freshwater and inland sea environments. Journal of Plankton Research, 36, 597–612.
Terazaki, M., & Wada, M. (1988). Occurrence of large numbers of carcasses of the large, grazing copepod Calanus cristatus from the Japan Sea. Marine Biology, 97, 177–183.
Thottathil, S. D., Balachandran, K. K., Gupta, G. V. M., Madhu, N. V., & Nair, S. (2008). Influence of allochthonous input on autotrophic heterotrophic switch-over in shallow waters of a tropical estuary (Cochin Estuary), India. Estuarine, Coastal and Shelf Science, 78, 551–562.
Vijith, V., Sundar, D., & Shetye, S. R. (2009). Time-dependence of salinity in monsoonal estuaries. Estuarine, Coastal and Shelf Science, 85, 601–608.
Zar, J. H. (1999). Biostatistical analysis (4th ed.). Upper Saddle River: Prentice-Hall, Inc..
Acknowledgments
The authors thank the Director, CSIR-National Institute of Oceanography (NIO), India, for facilities. The authors thank the Scientist-in-Charge, CSIR NIO RC Kochi for encouragement. The author L. Jagadeesan thanks CSIR for SRF funding. This is NIO contribution 5940.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jyothibabu, R., Jagadeesan, L. & Lallu, K.R. Copepod carcasses in a tropical estuary during different hydrographical settings. Environ Monit Assess 188, 559 (2016). https://doi.org/10.1007/s10661-016-5572-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5572-0