Abstract
Concentrations of selected organochlorine pesticides (OCPs), i.e., 4,4ʹ-dichlorodiphenyltrichloroethane (p,pʹ-DDT), its metabolites (p,pʹ-DDE, p,pʹ-DDD), and hexachlorocyclohexanes (HCHs), have been determined in 100 soil samples collected from a contaminated site centered around a former storehouse in the Kyzyl Kairat village, Almaty region, Kazakhstan, which constitutes an exemplary case example. The OCPs were observed in all analyzed soil samples, with predominance of α-HCH, p,p′-DDD, p,p′-DDE, and p,p′-DDT. Total concentrations ranged from 1.38 to 11,100 μg kg−1 with an average value of 1040 μg kg−1 for DDT and its metabolites and 0.1 to 438 μg kg−1 with an average value of 24 μg kg−1 for HCHs. The observed concentrations of the OCPs were found to be in agreement with previous studies and are rationalized in terms of the possible degradation pathways of DDTs and HCHs. Spatial distribution patterns of OCPs are elucidated by contour maps. Observed concentrations of the OCPs were used to evaluate the cancer risk to humans via ingestion, dermal contact, and inhalation of soil particles. The cancer risk mainly occurs from ingestion, whereas dermal exposure contributes to a minor extent to the total cancer risk. The risk associated with inhalation was found to be negligible. The total cancer risk for the studied OCPs were found to be p,pʹ-DDT ˃ p,pʹ-DDE ˃ p,pʹ-DDD ˃ α-HCH ˃ β-HCH ˃ γ-HCH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organochlorine pesticides (OCPs) take a prominent place in the list of highly toxic chemicals covered by the Stockholm Convention (Stockholm Convention 2001). In Kazakhstan, they were legally used as insecticides till 1983, and, most probably, remaining stocks have been illegally used up to recent years (Toleubayev et al. 2011). Due to the widespread use of these chemicals and their persistence, they can even today be found in environmental and biological samples (Xu et al. 2013). OCPs are classified as PBT (persistent, bioaccumulating, toxic) or vPvB (very persistent, very bioaccumulating) compounds according to the REACH framework (EC 2006; Sailaukhanuly et al. 2013b). Accumulative properties of OCPs in living organisms and bioaccumulation in the food chain have been well studied (El-Shahawi et al. 2010). Furthermore, OCPs have raised serious public concerns in recent years due to a variety of chronic effects, such as endocrine dysfunction and potential carcinogenicity (Androutsopoulos et al. 2013; Mostafalou and Abdollahi 2013; Mrema et al. 2013).
Although the evidence regarding the carcinogenicity of DDT/DDE/DDD in humans remains inconclusive, and that available epidemiological studies on DDT’s carcinogenicity in humans do not suggest DDT and its metabolites as human carcinogens at likely dose levels, DDT and related compounds have been shown to be carcinogenic in some laboratory animals, such as rats, mice, and hamsters, which developed tumors primarily in a liver (ATSDR 2002).
Despite the lacking conclusive evidence linking DDT and related compounds to cancer in humans, both the Department of Health and Human Services and the International Agency for Research on Cancer (IARC) anticipate DDT, DDE, and DDD as potential human carcinogens (IARC 1987; NTP 2014a). The US EPA views DDT, DDE, and DDD as group B2 carcinogens (ATSDR 2002; IRIS DDT 1987; IRIS DDE 1988; IRIS DDD 1988). QSAR calculations predict 0.877, 0.916, and 0.844 probabilities that DDT, DDD, and DDE, respectively, are carcinogenic, group 2B (PASS 2015).
Technical grade HCH and α-, β-, γ-HCH have been found to be carcinogenic in mice following long-term exposure with oral exposure inducing liver tumors (ATSDR 2005). The Department of Health and Human Services (DHHS) has determined that HCH (all isomers) may reasonably be anticipated to cause cancer in humans (NTP 2014b). The International Agency for Research on Cancer (IARC) has classified HCH (all isomers) as group 2B, possibly carcinogenic to humans (IARC 1987) in accordance with QSAR calculation estimating the probability for HCH to be carcinogenic, group 2B to 0.688 (PASS 2015). The USEPA found some evidence that Lindane (γ-HCH) is carcinogenic, despite that the evidence for the time being has not been sufficient to assess its human carcinogenic potential (IRIS HCH 1987). The US EPA has analogously classified technical HCH as well as α-HCH and β-HCH as probable human carcinogens, whereas δ- and ε-HCH are not classified as to human carcinogenicity (ATSDR 2005; IRIS HCH 1987).
In the framework of the UNDP/GEF project “Initial assistance in the implementation of Kazakhstan’s obligations under the Stockholm Convention on POPs (Persistent organic pollutants)” (UNDP/GEF 2009), a preliminary inventory of pesticide storehouses for banned and obsolete pesticides was prepared in 2003–2004. As a result of surveys of former storehouses, more than 1500 t of pesticides and their mixtures were disclosed. The UNDP/GEF project surveyed more than 140 storehouses, of which only 57 were still functioning, whereas 83 of the former storehouses were found to be completely demolished (UNDP/GEF 2009). This initial inventory of POPs reported only quantities of pesticides, pesticides containers, and the condition of the storehouses. These data appeared insufficient to estimate the real danger to public health and the environment from these sites (Nurzhanova et al. 2013; Vijgen and Egenhofer 2009). Although the former storehouses were destroyed, the stored obsolete pesticides and their containers apparently were left unattended and open to the environment (Toleubayev et al. 2011). Most of bulk pesticides were released into the surrounding environment without any information to local residents about the potential danger (Toleubayev et al. 2011). People living around these former storehouses use the land for growing various crops, cattle breeding, as playing grounds for children, and as sources of construction materials (Toleubayev et al. 2011). Toleubayev et al. (2011) reported that many farmers still have little knowledge of their agro-ecosystem and the peculiarities of crop protection practices and even used the banned DDT obtained from a black market. Pesticides of an unknown nature are freely sold at village markets and farmers frequently apply pesticides without protective clothing, sometimes sprinkling them on crops with a broom. It was also stated that farmers consider pesticides as the only means to control pests and that they are unaware of the negative effects of injudicious and indiscriminate use of pesticides and thus neglect these concerns (Toleubayev et al. 2011). The contaminated areas of former storehouses have become hotspot areas of contamination that pose serious risk not only to the environment but possibly also to public health (Nurzhanova et al. 2013).
Pollution of the environment by OCPs constitutes a serious ecological problem in Kazakhstan. Only few data on OCP concentrations in the environment, or in biological and food samples, from Kazakhstan have been reported (Erdinger et al. 2004; Hooper et al. 1997; Jensen et al. 1997; Lozowicka et al. 2014; Lutter et al. 1998; Mazhitova et al. 1998).
Nurzhanova (2010) reported that soil samples around 26 observed former storehouses contain DDTs (o,p’-DDD, p,p’-DDD, p,p’-DDT, p,p’-DDE) and HCHs (α-HCH, β-HCH, γ-HCH) exceeding the maximum allowable concentration (MAC), which according to the Kazakh legislation (Environmental code of the Republic of Kazakhstan 2007; Sizdikov 2010) is 100 μg kg−1 in soil. Thus, residue level of p,p’-DDT exceeds the MAC value 16 times (1670 ± 66 μg kg−1), p,p’-DDE 8 times (852 ± 18 μg kg−1) in Karasai district of Almaty oblast, p,p’-DDT 65 times (6584 ± 207 μg kg−1) in Talgar district, and p,p’-DDE 28 times (2097 ± 54 μg kg−1) in Eskeldinsk district; concentrations of p,p’-DDT exceeded MAC by 19 times (1955 ± 69 μg kg−1), p,p’-DDE by 28 times (2867 ± 68 μg kg−1), and β-HCH by 17 times (1731 ± 117 μg kg−1). Significant amounts of OCPs were also found in Enbekshi-Kazakh, Zhambyl, Balkhash, and Ile districts (Nurzhanova 2010). To estimate the real risk to the public health, the data on the residual level of OCPs in hotspot areas are not sufficient. Hence, the spatial distribution of the OCPs that will be elucidated in the present paper apparently plays a crucial role as well.
The Kyzyl Kairat village, located in the Almaty oblast, Kazakhstan (cf. site description below), has been studied as an exemplary case example. The study focuses on potential problems associated with former pesticide storehouses focusing on the presence and distribution of pesticide residues in the environment leading to a risk assessment emphasizing on the local population. Hence, we here report the spatial distribution and actual concentration levels of the OCPs for the studied area, and a preliminary evaluation of the risk associated with the pesticide residues due to inhalation, ingestion, and dermal exposure to the local population is presented with specific focus on the possible cancer risk.
Materials and methods
Analyses were carried out on samples taken from the area around and in the Kyzyl Kairat village. Subsequently, the spatial distribution was calculated and eventually a risk was assessed.
Site description and sampling procedure
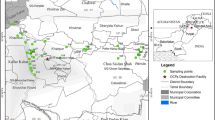
A former storehouse for pesticides located at 43° 17ʹ 58.8ʺ N, 77° 11ʹ 40.3ʺ E, at a height of 1004 m above the sea level, and 90 m to the south from the Kyzyl Kairat village of the Talgar district in Almaty oblast in Kazakhstan, was chosen as an exemplary case (Fig. 1). The village was chosen due to its close vicinity to the former storehouse, by only 90 m, as well as the slope of the landscape from the storehouse towards the village, which makes the location one of the most dangerous among the multitude of such sites in the Almaty oblast (Nurzhanova 2010). Further, a minor inhabited area is present as close as only 10 m north of the former storehouse.
The local population is engaged in agricultural activities at the surrounding area including both growing various crops as well as cattle breeding. A pasture for cattle grassing has been established directly adjacent to the former pesticide storage facility (Fig. 2).
A systematic grid approach was used as the method of soil sampling (Kenessov et al. 2012; USEPA 1992). Coordinates of sampling points were determined applying a global positioning system (GPS) using a GPS-12 (Garmin, USA) device. Taking the former storehouse as the epicenter of a possible contamination, a 30 m × 32 m regular grid was established in and around the storehouse (Fig. 2). Soil samples were collected in each point systematically from four layers: 0–10 cm, 10–20 cm, 20–30 cm, 30–40 cm. Sampling was performed in 25 grid points using a core sampler injected by hammering into soil allowing samples from 10, 20, 30, and 40 cm depths. Soil samples from every 10-cm layer were taken from the core sampler using a metal blade. Before each sampling, the core sampler was thoroughly cleaned by scraping off any smeared material to prevent cross-contamination from previous samples in order to ensure representativeness. In total, 100 samples each of approx. 50 g were collected.
Further, samples of crops (tomatoes) grown in an adjacent area and dairy milk from the cattle were collected for analyses. For comparison, similar products were collected from the central market of Almaty city.
Samples from inside the storehouse were taken under a 10-cm concrete layer crushed by hammering prior to the sampling. Blank samples were additionally collected from outside of the contaminated site at the distance of 100 m. The samples were stored in closed containers and transported to the laboratory for analyses.
Organochlorine pesticides included in the study were p,pʹ-DDT (CAS 50-29-3), p,pʹ-DDD (CAS 72-54-8), p,pʹ-DDE (CAS 72-55-9), α-HCH (CAS 319-84-6), β-HCH (CAS 319-85-7), and γ-HCH (CAS 58-88-9). OCPs included in this study were selected based on the assumption that these pesticides could be found in the samples as they had previously been detected in the area (Nurzhanova et al. 2013).
Reagents and standards
Stock solutions (100 μg mL−1) of OCPs (p,p’-DDT, p,p’-DDD, p,p’-DDE, α-HCH, β-HCH, γ-HCH) were purchased from Lezart (Almaty, Kazakhstan). All solvents applied were of analytical grade from Sigma-Aldrich (USA) and were redistilled before the use in order to achieve highest possible purities. Silica gel (70–230 mesh) was purchased from Sigma-Aldrich (USA) and was activated at 130 °C for 6 h prior to the use.
Physical and chemical parameters of soil samples
The pH was estimated by suspending soil in distilled water at 1:2 ratio using a pH150M (Aquilon, Russia) pH meter. Average pH of the soil was 7.5. Moisture content was determined to be an average of 1.72 % by gravimetric method based on drying soil at 105 °C. All data reported in the following were based on dry weight. Total organic carbon (TOC) was determined by dichromate oxidation and titration with ferrous ammonium sulfate using N-phenylanthranilic acid indicator (USEPA 2002). TOC contents were estimated to be in the range from 1.4 to 2.1 % with the mean value being 1.57 %.
Sample preparation, extraction, and clean-up procedure
Soil samples
Prior to analyses, soil samples were air-dried at room temperature for 1 week and subsequently sieved through 2-mm mesh. Ten grams of soil was shaken with 20 mL dichloromethane for 1 h. Subsequently, the extract was filtered and cleaned up on silica gel columns (10 mm i.d., 200 mm length with 10 g of activated silica, initially activated with 20 mL of hexane at the rate of about 5 mL min−1). Purified extracts were evaporated to 1.0 mL under a stream of nitrogen.
Crop samples
Twenty grams of chopped and ground tomatoes was shaken with two portions of 30 mL n-hexane for 30 min. The combined extract was filtered and cleaned up on multilayer columns based on a modified silica gel (10 mm i.d., 200 mm length with 2 g of activated silica with 44 % sulfuric acid, 2 g of activated Florisil, and 2 g sodium sulfate, initially activated with 20 mL of hexane at the rate of about 5 mL min−1). Purified extracts were evaporated to 40 μL under a stream of nitrogen.
Milk samples
To extract dairy milk, 20-mL sample was placed into a 100-mL separatory funnel where 15 mL of concentrated sulfuric acid was slowly added until complete blackening of the sample. Pre-cooled in ice, the content of the funnel was shaken vigorously for 10–15 min followed by the extraction with two portions of n-hexane each having a volume of 20 mL. Subsequently, the extract was cleaned up on the multilayer column based on a modified silica gel as described above.
Analytical procedure and quality assurance
Using Combi-PAL autosampler (CTC Analytic AG, Switzerland), hexane extracts were injected into the split/splitless inlet of the 6890N (Agilent, USA) gas chromatograph (GC) equipped with a diffusion pump-based 5973N mass spectrometric (MS) detector (Agilent, USA). Injection was done at 250 °C in splitless mode, and purge was activated 1 min after injection. Separation was done using a 30 m × 0.25-mm, 0.25-μm film DB-35MS capillary column (Agilent, USA) at the constant flow of helium (purity >99.995 %, Orenburg-Tehgas, Russia) equal to 1 mL min−1. Oven temperature was programmed from 40 °С (held for 3 min) to 160 °C (3 min) at 20 °C min−1 rate followed by a 3 °C min−1 ramp to 275 °C which was kept for 20 min. Ionization was conducted applying electron impact (EI) mode at 70 eV. The detection was performed in selected ion monitoring (SIM) mode using the following specific ions (m/z, amu) for the quantification of OCPs: 235 (p,p’-DDT, p,p’-DDD); 246 (p,p’-DDE); and 181 and 183 (α-HCH, β-HCH, γ-HCH). Dwell time was 100 ms for each ion. Solvent delay was set to 10 min. Temperatures of MS detector interface, ion source, and quadrupole were 280, 230, and 150 °C, respectively. Detector was tuned using the autotune program of MSD ChemStation software ver. E.01.00 SP1 (Agilent, USA). Quantification of OCPs was performed using an external standard calibration.
Analyses of blank samples between actual analyses were carried out in order to confirm the absence of target analytes and interferences from previous analyses. Quality control was based on the analysis of blank soil samples spiked with known concentrations of OCPs. Spike recoveries were 80–120 % for all six analytes. Ranges of calibration plots were from 1 to 100 ng mL−1 for HCHs and 5 to 100 ng mL−1 for DDTs. Limits of detection ranged from 0.05 ng g−1 for α-HCH to 0.25 ng g−1 for p,p’-DDT corresponding to a signal to noise ratio 3:1 for samples with minimal concentrations of analytes.
At elevated temperatures (T ≥200 °C), p,p’-DDT may be degraded in the GC injector port to p,p’-DDD and p,p’-DDE (Foreman and Gates 1997). To prevent such degradation of p,p’-DDT, a deactivated 4-mm dual taper splitless liner (Agilent, USA) was used for sample injection. Possible decomposition of p,p’-DDT in the inlet was periodically checked by analyzing stock solution of p,p’-DDT (1 μg mL−1), subsequently identifying possible signals from p,p’-DDD and p,p’-DDE.
Concentration profiles
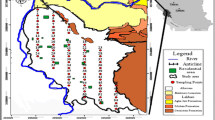
The obtained data for soil samples were for the single points of the sampling grid (Fig. 3) averaged for four depths, as systematic vertical variations in the OCP concentrations were not detected. This lack of vertical concentration profiles can apparently be associated to the typical management of the soil, e.g., plowing, leaving the upper 40 cm as mixed topsoil. Hence, the averaging procedure was chosen in order to weaken the errors due to the possible inaccuracy of sampling over the depth and, as such, it was believed to represent the topsoil concentration corresponding to a specific sampling point (cf. Fig. 3). Subsequently, averaged concentration profiles for the single pesticides were obtained by an interpolation procedure leading to contour plots of distribution over the sampled area. The interpolation process was carried out using the GRI program package (GRI 2014) applying the Barnes procedure to construct contour plots and carry out the two-dimensional interpolation procedure (Barnes 1964).
Risk assessment methodology
The present study comprises a preliminary risk assessment to elucidate the possible health risk to the humans due to direct contact, i.e., dust and contaminated soil by dermal contact and inhalation of chemicals from the soil with residues of the OCPs, the primary focus being the cancer risk. According to the Agency of Toxic Substances and Disease Registry (ATSDR 2004), the cancer risk is defined as a theoretical risk for getting cancer if exposed to a substance every day for 70 years (a lifetime exposure).
Assessment of cancer risks was based on the USEPA risk assessment methodology (USEPA 2009, 2011). Hence, cancer risks of contaminant via soil ingestion, dermal contact, and inhalation can be estimated according to Eqs. 1, 2, and 3, respectively.
where CRingest is the cancer risk via accidental ingestion of soil; Csoil is the contaminant concentration in soil (mg kg−1); IngR is the ingestion rate of contaminated soil (mg day−1), 100 mg day−1 for adults and 200 mg day−1 for children; EF is the exposure frequency (days year−1); ED is the exposure duration (year); CF is the conversion factor (1 × 10−6 kg mg−1); SF is the oral slope factor (mg kg−1 day−1)−1); BW is the body weight (kg); and AT is the averaging time (days).
where CRdermal is the cancer risk via dermal contact of soil; SA is the surface area of skin that contacts the soil (cm2); AFsoil is the skin adherence factor for soil (mg cm−2); ABS is the dermal absorption factor (chemical specific); and GIABS is the gastrointestinal absorption factor (unitless).
where CRinhale is the the cancer risk via inhalation of soil; InhR is the inhalation rate (m3 days−1); AFinh is the absorption factor for the lungs (unitless); IUR is the inhalation unit risk (mg m3)−1); and PEF is the inhalation of pollutants absorbed to inhaled particles (1.36 × 109 m3 kg−1).
Tables 1 and 2 represent the exposure parameters for the cancer risk calculations and input parameters for the studied chemicals.
The estimation of cancer risk via ingestion, dermal contact, and inhalation was based on the following human lifespan: infant 0 to 2 years; child 2 to 16 years; and adult 16 to 70 years.
The total cancer risk was calculated by adding different exposure pathways as cancer risks via ingestion, dermal contact, and inhalation neglecting possible synergistic and/or antagonistic effects. With reference to the ATSDR (2004), the qualitative ranking of cancer risk estimates was used to rank from very low to very high: very low (value ≤10−6); low (10−6 ˂ value ≤ 10−4); moderate (10−4 ˂ value ≤ 10−3); high (10−3 ≤ value ˂ 10−1); and very high (value ≥ 10−1).
Results and discussion
Total concentrations of DDTs and HCHs in soil
The OCPs p,p’-DDT, p,p’-DDD, p,p’-DDE, α-HCH, β-HCH, and γ-HCH were detected in all soil samples from the site of a demolished former storehouse close to Kyzyl Kairat. In Table 3, concentration ranges for the OCPs are summarized. It should be noted, as described above (cf.“Concentrations profiles” section), that no vertical trends in concentrations were observed and, as such, the averaged samples for the four depths are considered as representing the topsoil concentrations. ∑DDTs (sum of p,p’-DDT, p,p’-DDD, and p,p’-DDE) in soil ranged from 1.38 to 11,100 μg kg−1 which appeared much higher than the ∑HCH (sum of α-HCH, β-HCH, and γ-HCH) content, which ranged from 0.1 to 438 μg kg−1. Average values of ∑DDTs and ∑HCHs were 1040 and 24 μg kg−1, respectively. The differences in values for these compounds can be explained by difference of their respective degradation rates. The highest concentrations of p,p’-DDT, p,p’-DDD, and p,p’-DDE were 6660, 5240, and 4580 μg kg−1, respectively. These concentrations are slightly higher than residual levels at this site previously reported by Nurzhanova (2010) being 6584, 1899, and 2097 μg kg−1 for p,p’-DDT, p,p’-DDD, and p,p’-DDE, respectively. This can be explained by a relatively slow degradation of OCPs and their persistency. Average concentrations of p,p’-DDT, p,p’-DDD, and p,p’-DDE were 253, 430, and 357 μg kg−1, respectively.
The OCPs can be expected to have been involved in a variety of physical, chemical, and biological processes such as biodegradation, photolysis, leaching, adsorption, volatilization, etc. (Heberer and Dünnbier 1999; Man et al. 2011).
Technical grade p,p’-DDT usually consists of approx. 75 % p,p’-DDT, 15 % o,p’-DDT, 5 % p,p’-DDE, and <5 % others (Yang et al. 2009). In the environment, the relative amount of p,p’-DDT metabolites will gradually increase, and the contents of p,p’-DDT will simultaneously decrease if further technical p,p’-DDT is not administered (Ge et al. 2013). p,p’-DDT can aerobically degrade to p,p’-DDD (direct degradation) or anaerobically to p,p’-DDE (indirect degradation) (Heberer and Dünnbier 1999). Thus, the ratio of p,p’-DDT / (p,p’-DDD + p,p’-DDE) indicates whether there is a degradation of p,p’-DDT in soil. The ratio <1 indicates a relatively fast p,p’-DDT degradation, whereas the ratio >1 may be associated with a slow or even lacking degradation of p,p’-DDT (Man et al. 2011; Zhang et al. 2006). It should be noted that the value 1 is a rather conservative approach and can, as such, be taken as a “safe” indication. In our case, the ratio p,p’-DDT / (p,p’-DDD + p,p’-DDE) was 0.32 based on the averaged values (Table 3), i.e., clearly <1 thus indicating a relatively fast degradation of p,p’-DDT. Looking at the single sampling points, we found that only for four sampling points, the ratio was higher than 1. High ratio for these sampling points can apparently be explained by a simultaneous relatively high level of TOC, which possibly leads to a relative slow degradation of p,p’-DDT to its metabolites and a limited bioavailability (Man et al. 2011) possibly due to a complexation between the of p,p’-DDT and the organic carbon in the soil.
Construction materials, such as concrete basement and concrete blocks of storehouse, could a priori constitute a source of new inputs of the OPCs since this material could be contaminated by the OPCs. New inputs of the OPCs from old barrels can, on the other hand, be excluded, as the old barrels were transported to burial grounds in Kostanay and disposed of in accordance with the requirements of the Stockholm Convention on POPs (Stockholm Convention 2001).
Technical HCH usually consist of 55–80 % α-HCH, 5–14 % β-HCH, and 8–15 % γ-HCH and δ-HCH, while Lindane consists of more than 98 % of γ-HCH (Ge et al. 2013). The residue level of β-HCH (76.5 %) was the highest among isomers of HCH while α-HCH and γ-HCH were 21.5 and 2 %, respectively.
Highest concentrations of HCH isomers were 163 μg kg–1 (α-HCH), 347 μg kg–1, (β-HCH), and 20.7 μg kg–1 (γ-HCH). Such concentration of α-HCH was much lower than the residue level of α-HCH (1239 μg kg–1) in the surface layer (0–20 cm) for this site previously reported by Nurzhanova (2010). This can be explained by stronger abilities of α-HCH and γ-HCH to penetrate downwards compared to β-HCH and δ-HCH (Yang et al. 2009). However, as we studied only samples that apparently can be associated to topsoil, i.e., to a depth of 40 cm, this was not pursued further. Additionally, possible isomerization of α-HCH to stable β-HCH, which is energetically more favorable (Wu et al. 1997), may play a role. The relative low concentration of the gamma isomer (Lindane) can be explained by the degradation of γ-HCH to β-HCH via α-HCH (Wu et al. 1997). The degradation of γ-HCH depends on environmental factors such as temperature, moisture, pH, redox potential, organic carbon content, and soil type (Abhilash and Singh 2008). Further, γ-HCH may be lost due to volatilization and plant absorption (Waliszewski 1993).
Prevalence of β-HCH among other isomers of HCH is consistent with a relatively low vapor pressure (3.6 × 10−7 mmHg, 200 °C) and a relatively high Kow (log Kow = 3.78), the latter promoting an increased binding to soil organic matter (SOM) that will reduce the degradation rate relative to other isomers of HCH (Man et al. 2011).
The α-HCH/γ-HCH ratio has been used to identify the source of HCH. Thus, a α-HCH/γ-HCH ratio <1 or close to zero is characteristic of Lindane source, whereas a ratio >3 is indicative for the source being technical HCH (Ge et al. 2013). In this study, the α-HCH/γ-HCH ratio was found to be 10.6 (Table 3), which strongly indicates technical HCH as the source.
The levels of DDTs and HCHs in soil in the present study were compared to the data previously reported from a variety of locations. Thus, we found residual levels of DDTs to be higher than reported for North-Eastern Romania (4.4–79 μg kg−1) (Tarcau et al. 2013), Midway Atoll (1–642 μg kg−1) (Ge et al. 2013), urban and outskirt soils in Beijing, China (0.77–2178.56 μg kg−1) (Li et al. 2006), and in two districts of Assam, India (166–2288 μg kg−1) (Mishra et al. 2012), and to be significantly lower than reported for soils from dumping ground in Hyderabad, Pakistan (77–212200 μg kg−1) (Alamdar et al. 2014), industrial soils in China (13.20–67.43 mg kg−1) (Yang et al. 2009), and outdoor soils from Chiapas, Mexico (not detected–26.98 mg kg−1) (Martınez-Salinas et al. 2011).
Similarly, residual levels of HCHs were found to be higher than HCH levels found in North-Eastern Romania (1.1–9.8 μg kg−1) (Tarcau et al. 2013), in urban and outskirt soils in Beijing, China (1.36–48.83 μg kg−1) (Li et al. 2006), and at the Midway Atoll (not detected–127 μg kg−1) (Ge et al. 2013) and to be significantly lower than in soils from dumping ground in Hyderabad, Pakistan (43–4090 mg kg−1) (Alamdar et al. 2014), industrial soils in China (3.02–67.43 mg kg−1) (Yang et al. 2009), and in two districts of Assam, India (98–1945 μg kg−1) (Mishra et al. 2012).
Spatial distribution of OCPs
Concentration profiles of the OCPs in the topsoil are presented in Fig. 3. The high concentrations of OCPs at a former storehouse and its surroundings can obviously be associated to previous agricultural activities such as growing various crops and the storage of obsolete pesticides in the location. Epicenters of contamination are immediately identified. Apparently, in the case of the HCHs, single epicenters are observed (Fig. 3), whereas in the case of the DDT group, several epicenters can be seen (Fig. 3) with some of the centers coinciding.
The distribution patterns indicate that in addition to the high OCP concentrations, more or less in the center of the investigated area, relatively higher concentrations of DDTs were found in the Northeast part of the investigated site. These high concentrations coincided with the locations where the OCPs were stored for a longer time as well as with the main gate of the former storehouse located in the Northeast part (Figs. 2 and 3).
Residual levels of p,p’-DDT were apparently higher than corresponding levels of p,p’-DDD and p,p’-DDE (Fig. 3). This is in accordance with a slow degradation of p,pʹ-DDT to its metabolites in soil under both aerobic as well as anaerobic conditions (Heberer and Dünnbier 1999; Man et al. 2011).
It should be noted that the actual contaminated area may be larger than that studied in this work. Concentration profiles depicting concentrations for the p,p’-DDT group increasing to the Northeast direction clearly indicated that. Taking into consideration a possible both vertical and horizontal migration of p,p’-DDTs in soil, the possible contamination of groundwater and thus drinking water supplies cannot be ruled out. This was, however, not investigated in the present study.
Total concentration of OCPs in food products
In food samples, only the DDT metabolites p,p’-DDE and p,p’-DDD were detected. Highest concentrations were found in samples of dairy milk (1.07 ng g−1 DDE and 2.46 ng g−1 DDD) and tomatoes (0.14 ng g−1 DDE and 0.01 ng g−1 DDD) taken from Kyzyl Kairat village. For comparison, the corresponding concentrations of p,p’-DDE and p,p’-DDD in tomatoes taken from market Almaty were 0.131 ng g−1 DDE and 0.005 ng g−1 DDD. OCPs were not detected in dairy products taken from market of Almaty (Sailaukhanuly et al. 2013a). It was noted that concentrations of p,p’-DDE and p,p’-DDD in food products from the contaminated site were only slightly higher than those found in the products from commercial Almaty shops.
Risk assessment
In order to disclose a potential risk associated with OCP residues in the terrestrial environment, the obtained concentrations of OCPs from soil samples from the studied site were compared with Kazakhstan target values. MAC values for both DDTs and HCHs in Kazakh soils are 100 μg kg−1 (Environmental code of the Republic of Kazakhstan 2007). MAC values for total DDTs in dairy products and vegetables are 50 and 100 ng g−1, respectively (Environmental code of the Republic of Kazakhstan 2007; Sizdikov 2010). Human risk was assessed looking at different exposure pathways, i.e., ingestion, dermal contact, and inhalation, applying the US EPA methodology described in the “Materials and methods” section (USEPA 2009, 2011).
Concentrations of p,p’-DDT, p,p’-DDD, and p,p’-DDE in the studied soil samples exceeded the Kazakh MAC value by factors of 66, 52, and 45, respectively (cf. Table 3). In the case of HCHs, it was noted that the concentration of γ-HCH (Fig. 3) was lower than concentrations of its two isomers and did apparently not exceed the MAC value, whereas both α-HCH and β-HCH exceeded the MAC value by factors of 1.6 and 3.4, respectively (cf. Table 3).
For the food samples, concentrations of the OCPs here studied did not exceed the MAC values prevailing in Kazakhstan. However, it has to be emphasized that these MAC limits may well be outdated as they were adopted during Soviet time. Further, it should be noted that these MAC values are significantly higher than the maximum residue levels defined for food samples in the EU regulations (EC 2008). Hence, results of this study indicate that the OCPs levels in soils a priori should be regarded as high enough to pose an ecological risk and may as such potentially constitute a threat to living organisms including humans. Therefore, it can be concluded that the soil here studied is not suitable for agricultural use, with reference to OCP concentrations. Consequently, it must be concluded that the soils may constitute a health hazard to the residents in the village and especially to the farmers. It should be noted that the local population to a major extent is engaged in agricultural activities at the surrounding area including both growing various crops as well as cattle breeding.
Table 4 summarizes the results of cancer risk for different exposure pathways for the six studied OCPs. It can be noted that the calculated cancer risk for p,pʹ-DDT, p,pʹ-DDD, and p,pʹ-DDE are higher than the generally acceptable lifetime health risk or threshold value, which is 10−6 (ATSDR 2004), whereas the calculated cancer risks for HCHs apparently are well below the 10−6 limit. It was noticed that in general, very low cancer risks prevail for all OCPs except for cancer risk via ingestion of DDTs (Table 4). Hence, the most prominent cancer risk can be associated with exposure due to ingestion, which probably can be assigned to small dust particles that are widespread not only in contaminated areas but also in households. Thus, the cancer risk via ingestion intake for all OCPs contributes from 65.5 to 99.9 % of overall risk, whereas the cancer risk via dermal contact for all OCPs contributes from 0.1 to 34.5 % of health risk.
The total cancer risk for the studied OCPs is ranked as follows: p,pʹ-DDT ˃ p,pʹ-DDE ˃ p,pʹ-DDD ˃ α-HCH ˃ β-HCH ˃ γ-HCH (Table 4) and ranges from low (p,p’-DDT, p,p’-DDE, p,p’-DDD) to very low (HCH isomers) according to ATSDR (cf. “Materials and methods” section). The increasing cancer risk values for all OCPs were as follows: inhalation ˂ dermal contact ˂ ingestion. Thus, cancer risk mainly occurs from ingestion and dermal contact of soil, whereas inhalation exposure contributes to a minor extent to the total cancer risk (Mostafalou and Abdollahi 2013; Rogan and Chen 2005).
Calculated cancer risk for p,pʹ-DDE and p,pʹ-DDD for tomatoes taken from the contaminated site of Kyzyl Kairat village were 1.64 × 10−8 and 8.25 × 10−10, respectively. Corresponding values for p,pʹ-DDE and p,pʹ-DDD for tomatoes from market of Almaty were 1.53 × 10−8 and 4.29 × 10−10, respectively. Cancer risks for p,pʹ-DDE and p,pʹ-DDD for dairy milk taken from the contaminated site of Kyzyl Kairat village were 1.4 × 10−6 and 2.27 × 10−6, respectively.
The calculated cancer risks for p,pʹ-DDT and its metabolites for tomatoes are lower than the generally acceptable lifetime health risk or threshold value, which is 10−6 (ATSDR 2004) for tomatoes but somewhat higher for dairy milk. It was noticed that, in general, very low cancer risks prevail for all OCPs in tomatoes except for cancer risk via ingestion of DDTs from dairy milk (USEPA 2011). Although calculated cancer risk for p,pʹ-DDT and its metabolites for tomatoes is lower, these compounds may still pose a serious risk due to their accumulative properties in living organisms (EC 2006; Sailaukhanuly et al. 2013b).
Conclusions and Outlook
This study was focused on the hotspot area adjacent to the Kyzyl Kairat village. One hundred soil samples were analyzed for their content of p,p’-DDT, p,p’-DDD, and p,p’-DEE as well of α-HCH, β-HCH, and γ-HCH. Obtained data were subsequently used for the construction of concentration profiles for the six main OCPs. Eventually, a preliminary risk assessment was performed disclosing potential cancer risks for the village residents and especially farmers using the area for their agricultural activities because residual concentrations in general exceeded the maximum allowed concentrations. In the case of the p,p’-DDT group, concentration levels significantly exceeded the MAC values whereas in the case of the HCH, only minor contravention was noted. Concentrations of OCPs in food samples did not exceed MAC values.
Apparently, the cancer risk is mainly due to ingestion and dermal contact of soil, whereas inhalation exposure contributes to a minor extent to the total cancer risk. Studies of food chains as an exposure pathway of OCPs have shown that cancer risk is associated with ingestion of food samples, posing serious health problems to local inhabitants. Based on the present study, it is estimated that an area of approx. 750 m2 is contaminated with OCPs in concentrations exceeding the accepted MAC values. Obviously, appropriate remediation activities should be imposed for reducing the unwanted negative health impact as revealed by the preliminary risk assessment.
Taking into account the above described data, there is a potential risk for human health and it is necessary to pay close attention to this contaminated site of former storehouses. Furthermore, results of this study can be used as an example to solve environmental problems related to OCPs and to determine whether other former demolished storehouses in Kazakhstan pose a serious ecological hazard and possibly a health hazard to the local population because the areas of these former storehouses have become hot spots of contamination.
References
Abhilash, P., & Singh, N. (2008). Distribution of hexachlorocyclohexane isomers in soil samples from a small scale industrial area of Lucknow, North India, associated with Lindane production. Chemosphere, 73, 1011–1015. doi:10.1016/j.chemosphere.2008.07.037.
Alamdar, A., Syed, J., Malik, R., Katsoyiannis, A., Liu, J., & Li, J. (2014). Organochlorine pesticides in surface soils from obsolete pesticide dumping ground in Hyderabad City, Pakistan: contamination levels and their potential for air-soil exchange. Science of Total Environment, 470–471, 733–741. doi:10.1016/j.scitotenv.2013.09.053.
Androutsopoulos, V., Hernandez, A., Liesivuori, J., & Tsatsakis, A. (2013). A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology, 307, 89–94. doi:10.1016/j.tox.2012.09.
ATSDR. (2002). Toxicological profile for DDT, DDE, and DDD. http://www.atsdr.cdc.gov/toxprofiles/tp35.pdf. Accessed 18 October 2015.
ATSDR. (2004). Public health assessment guidance manual (Update). http://www.atsdr.cdc.gov/hac/PHAManual/PDFs/PHAGM_final1-27-05.pdf Accessed January 2015).
ATSDR. (2005). Toxicological profile for alpha-, beta-, gamma-, and delta-hexachlorocyclohexane. http://www.atsdr.cdc.gov/toxprofiles/tp43.pdf Accessed 18 October 2015.
Barnes, S. (1964). Mesoscale objective analysis using weighted time-series observations. NOAA Technical Memorandum. National Severe Storms laboratory.
EC. (2006). Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:136:0003:0280:en.pdf. Accessed 18 October 2015.
EC. (2008). Regulation (EC) No 149/2008 of the European Parliament and of the Council of 29 January 2008 amending Regulation (EC) No 396/2005 of the European Parliament and of the Council by establishing Annexes II, III and IV setting maximum residue levels for products covered by Annex I there to http://www.uzb.minpolj.gov.rs/attachments/117_149%202008.pdf. Accessed 18 October 2015
El-Shahawi, M., Hamza, A., Bashammakh, A., & Al-Saggaf, W. (2010). An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta, 80, 1587–1597. doi:10.1016/j.talanta.2009.09.055.
Environmental code of the Republic of Kazakhstan. (2007). http://online.zakon.kz/Document/?doc_id=30085593 Accessed 18 October 2015.
Erdinger, L., Eckl, P., Ingel, F., Khussainova, S., Utegenova, E., & Mann, V. (2004). The Aral Sea disaster human biomonitoring of Hg, As, HCB, DDE, and PCBs in children living in Aralsk and Akchi, Kazakhstan. International Journal of Hygiene and Environmental Health, 207, 541–547. doi:10.1078/1438-4639-00325.
Foreman, W., & Gates, P. (1997). Matrix-enhanced degradation of p, p′-DDT during gas chromatographic analysis: a consideration. Evironmental Science and Technology, 31, 905–910. doi:10.1021/es960671q.
Ge, J., Woodward, L., Li, Q., & Wang, J. (2013). Composition, distribution and risk assessment of organochlorine pesticides in soils from the Midway Atoll, North Pacific Ocean. Science of Total Environment, 452–453, 421–426. doi:10.1016/j.scitotenv.2013.03.015.
GRI online software. (2014). http://gri.sourceforge.net. Accessed 23 August 2015.
Heberer, T., & Dünnbier, U. (1999). DDT metabolite bis(chlorophenyl)acetic acid: the neglected environmental contaminant. Evironmental Science and Technology, 33, 2346–2351. doi:10.1021/es9812711.
Hooper, K., Petreas, M., She, J., Visita, P., Winkler, J., & McKinney, M. (1997). Analysis of breast Mmlk to assess exposure to chlorinated contaminants in Kazakstan: PCBs and organochlorine pesticides in Southern Kazakstan. Environmental Health Perspectives, 105, 1250–1254. doi:10.2307/3433905.
IARC. (1987). IARC monographs programme on the evaluation of carcinogenic risks to humans.Vol.1-42.Occupational exposures in insecticide application, and some pesticides. http://monographs.iarc.fr/ENG/Monographs/vol53/mono53.pdf. Accessed 20 November 2015.
IRIS DDD. (1988). Integrated risk information system.US EPA. http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0347_summary.pdf. Accessed 20 November 2015.
IRIS DDE. (1988). Integrated Risk Information System. U.S. Environmental Protection Agency. http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0328_summary.pdf. Accessed 20 November 2015.
IRIS DDT. (1987). Integrated Risk Information System. U.S. Environmental Protection Agency. http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0147_summary.pdf. Accessed 20 November 2015.
IRIS HCH. (1987). Integrated Risk Information System. U.S. Environmental Protection Agency. http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0065_summary.pdf. Accessed 20 November 2015.
Jensen, S., Mazhitova, Z., & Zetterström, R. (1997). Environmental pollution and child health in the Aral sea region in Kazakhstan. Science of Total Environment, 206, 187–193. doi:10.1016/S0048-9697(97)00225-8.
Kenessov, B., Alimzhanova, M., Sailaukhanuly, Y., Baimatova, N., Abilev, M., Batyrbekova, S., Carlsen, L., Tulegenov, A., & Nauryzbayev, M. (2012). Transformation products of 1,1-dimethylhydrazine and their distribution in soils of fall places of rocket carriers in Central Kazakhstan. Science of Total Environment, 427–428, 78–85. doi:10.1016/j.scitotenv.2012.04.017.
Li, X., Zhu, Y., Liu, X., Fu, S., Xu, X., & Cheng, H. (2006). Distribution of HCHs and DDTs in soils from Beijing City, China. Archives Of Environmental Contamination And Toxicology, 51, 329–336. doi:10.1007/s00244-005-0016-9.
Lozowicka, B., Kaczynski, P., Paritova, A., Kuzembekova, G., Abzhalieva, A., & Sarsembayeva, N. (2014). Pesticide residues in grain from Kazakhstan and potential health risks associated with exposure to detected pesticides. Food and Chemical Toxicology, 64, 238–248. doi:10.1016/j.fct.2013.11.038.
Lutter, C., Iyenga, V., Barnes, R., Chuvakova, T., Kazbekova, G., & Sharmanov, T. (1998). Breast milk contamination in Kazakhstan: implications for infant feeding. Chemosphere, 31, 1761–1772. doi:10.1016/S0045-6535(98)00241-0.
Man, Y., Chow, K., Wang, H., Lau, K., Sun, X., & Wu, S. (2011). Health risk assessment of organochlorine pesticides with emphasis on DDTs and HCHs in abandoned agricultural soils. Journal of Environmental Monitoring, 13, 2250–2259. doi:10.1039/c1em10168d.
Martınez-Salinas, R., Dıaz-Barriga, F., Batres-Esquivel, L., & Perez-Maldonado, I. (2011). Assessment of the levels of DDT and its metabolites in soil and dust samples from Chiapas, Mexico. Bulletin of Environmental Contamination and Toxicology, 86, 33–37. doi:10.1007/s00128-010-0174-y.
Mazhitova, Z., Jensen, S., Ritzen, M., & Zetterström, R. (1998). Chlorinated contaminants, growth and thyroid function in schoolchildren from the Aral Sea region in Kazakhstan. Acta Paediatrica, 87, 991–995. doi:10.1111/j.1651-2227.1998.tb01771.x.
Mishra, K., Sharma, R., & Kumar, S. (2012). Contamination levels and spatial distribution of organochlorine pesticides in soils from India. Ecotoxicology and Environmental Safety, 76, 215–225. doi:10.1016/j.ecoenv.2011.09.014.
Mostafalou, S., & Abdollahi, M. (2013). Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicology and Applied Pharmacology, 268, 157–177. doi:10.1016/j.taap.2013.01.025.
Mrema, E., Rubino, F., Brambilla, G., Moretto, A., Tsatsakis, A., & Colosio, C. (2013). Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology, 307, 74–88. doi:10.1016/j.tox.2012.11.015.
NTP. (2014a). Report on Carcinogens. DDT. Eighth report on carcinogens. National Toxicology Program. http://ntp.niehs.nih.gov/ntp/roc/content/profiles/dichlorodiphenyltrichloroethane.pdf. Accessed 20 November 2015.
NTP. (2014b). Report on Carcinogens. Linadane, hexachlorocyclohaxane (technical grade), and other hexachlorocyclohaxane isomers. National Toxicology Program. http://ntp.niehs.nih.gov/ntp/roc/content/profiles/lindane.pdf. Accessed 20 November 2015.
Nurzhanova, A. K. (2010). Obsolete pesticides pollution and phytoremediation of contaminated soil in Kazakhstan. In NATO Science for Peace and Security Series C: Environmental Security (pp. 87–111). Berlin: Springer. doi:10.1007/978-90-481-3592-9_6.
Nurzhanova, A., Kalugin, S., & Zhambakin, K. (2013). Obsolete pesticides and application of colonizing plant species for remediation of contaminated soil in Kazakhstan. Environmental Science and Pollution Research, 20, 87–111. doi:10.1007/s11356-012-1111-x.
PASS. (2015). Way2Drug, predictive services. http://www.way2drug.com/PASSOnline/Accessed 18 April 2016.
Rogan, W., & Chen, A. (2005). Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). Lancet, 366, 763–773. doi:10.1016/S0140-6736(05)67182-6.
Sailaukhanuly, Y., Kenessov, B., & Amutova, F. (2013a). Concentrations of organochlorine pesticides in food samples taken from markets in Kazakhstanby optmized GC-MS method.Abstracts of 245 th ACS National Meeting. New Orleans, AGFD-206.
Sailaukhanuly, Y., Zhakupbekova, A., Amutova, F., & Carlsen, L. (2013b). On the ranking of chemicals based on their PBT characteristics. Comparison of different ranking methodologies using selected POPs as an illustrative example. Chemosphere, 90, 112–117. doi:10.1016/j.chemosphere.2012.08.015.
Sizdikov, O. (2010). Preparation of the first national report on Persistent Organic Pollutants Secretariat of the Stockholm Convention on POPs: research report. http://aarhus.kz/Accessed 12 January 2015.
Stockholm Convention. (2001). http://chm.pops.int/Home/tabid/2121/mctl/ViewDetails/EventModID/870/EventID/543/xmid/6921/Default.aspx. Accessed 12 January 2015
Tarcau, D., Cucu-Man, S., Boruvkova, J., Klanova, J., & Covaci, A. (2013). Organochlorine pesticides in soil, moss and tree-bark from North-Eastern Romania. Science of Total Environment, 456–457, 317–324. doi:10.1016/j.scitotenv.2013.03.103.
Toleubayev, K., Jansen, K., & Van Huis, A. (2011). From integrated pest management to indiscriminate pesticide use in Kazakhstan. Journal of Sustainable Agriculture, 35, 350–375. doi:10.1080/10440046.2011.562036.
UNDP/GEF. (2009). National implementation plan of the Republic of Kazakhstan on the obligation of under the Stockholm Convention on persistent organic pollutants approved by the decree of the government of the Republic of Kazakhstan from 8 December 2009 N 261. Astana. http://chm.pops.int/Portals/0/download.aspx?d = UNEP-POPS-NIP-Kazakhstan-1.English.pdf. Accessed 20 November 2015
USEPA. (1992). Preparation of soil sampling protocols: sampling techniques and strategies. http://www.epa.gov/OUST/cat/mason.pdf. Accessed 12 January 2015.
USEPA. (2002). Method for determination of total organic carbon (TOC) in soils and sediments method, NCEA-C- 1282 EMASC-001. http://epa.gov/esd/cmb/research/papers/bs116.pdf. Accessed 12 January 2015.
USEPA. (2009). Risk assessment guidance for superfund. Vol. I: human health evaluation manual (F, supplemental guidance for inhalation risk assessment) EPA/540/R/070/002. http://www.epa.gov/swerrims/riskassessment/ragsf/pdf/partf_200901_final.pdf. Accessed 12 January 2015.
USEPA. (2011). Exposure factors handbook. EPA/600/R-090/052F. http://www.epa.gov/ncea/efh/pdfs/efh-complete.pdf. Accessed 12 January 2015.
Vijgen, J., & Egenhofer, C. (2009). Obsolete pesticides. A ticking time bomb and why we have to act. now.www.ceps.eu/system/files/book/1841.pdf. Accessed 12 January 2015.
Waliszewski, S. (1993). Residues of lindane, HCH isomers and HCB in the soil after lindane application. Environmetal Pollution, 3, 289–293. doi:10.1016/0269-7491(93)90131-7.
Wu, W., Xu, Y., Schramm, K., & Kettrup, A. (1997). Study of sorption, biodegradation and isomerization of HCH in stimulated sediment/water system. Chemosphere, 35, 1887–1894. doi:10.1016/S0045-6535(97)00266-X.
Xu, W., Wang, X., & Cai, Z. (2013). Analytical chemistry of the persistent organic pollutants identified in the Stockholm Convention: A review. Analytica Chimica Acta, 790, 1–13. doi.org/10.1016/j.aca.2013.04.026.
Yang, W., Wang, R., Zhou, C., & Li, F. (2009). Distribution and health risk assessment of organochlorine pesticides (OCPs) in industrial site soils: a case study of urban renewal in Beijing, China. Journal of Environmental Sciences, 21, 366–372. doi:10.1016/S1001-0742(08)62278-0.
Zhang, H., Luo, Y., Zhao, Q., Wong, M., & Zhang, G. (2006). Residues of organochlorine pesticides in Hong Kong soils. Chemosphere, 63, 633–641. doi:10.1016/j.chemosphere.2005.08.006.
Acknowledgments
This work was performed as part of the PhD project of Yerbolat Sailaukhanuly and the project “Development of the base of methodological approaches to solution of food safety problems based on physical chemical and biological studies” (No. 659/GF1) both funded by the Ministry of Education and Science of the Republic of Kazakhstan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sailaukhanuly, Y., Carlsen, L., Tulegenov, A. et al. Distribution and risk assessment of selected organochlorine pesticides in Kyzyl Kairat village from Kazakhstan. Environ Monit Assess 188, 358 (2016). https://doi.org/10.1007/s10661-016-5353-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5353-9