Abstract
An atrazine-degrading enrichment culture was used to study degradation of atrazine metabolites viz. hydroxyatrazine, deethylatrazine, and deisopropylatrazine in mineral salts medium. Results suggested that the enrichment culture was able to degrade only hydroxyatrazine, and it was used as the sole source of carbon and nitrogen. Hydroxyatrazine degradation slowed down when sucrose and/or ammonium hydrogen phosphate were supplemented as the additional sources of carbon and nitrogen, respectively. The enrichment culture could degrade high concentrations of atrazine (up to 110 μg/mL) in mineral salts medium, and neutral pH was optimum for atrazine degradation. Further, except in an acidic soil, enrichment culture was able to degrade atrazine in three soil types having different physico-chemical properties. Raising the pH of acidic soil to neutral or alkaline enabled the enrichment culture to degrade atrazine suggesting that acidic pH inhibited atrazine-degrading ability. The study suggested that the enrichment culture can be successfully utilized to achieve complete degradation of atrazine and its persistent metabolite hydroxyatrazine in the contaminated soil and water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine) is a widely used selective, systemic, pre- and post-emergent herbicide for the control of broadleaf and grassy weeds. Because of its widespread use over the last 30 years, atrazine has emerged as a pollutant of environmental concern mainly due to its low biodegradability. In general, persistence of atrazine and its metabolites has been attributed to the presence of chloride and N-alkyl groups. Literature reports suggested that the half life (t1/2) of atrazine ranged from 4 to 57 weeks (Houot et al. 1998; Sparling et al. 1998). Among its major metabolites, hydroxyatrazine is highly persistent in nature with a half life of 121 days while the half-life of deethylatrazine and deisopropylatrazine ranged between 17 and 26 days (Stutz et al. 1998). Persistence of hydroxyatrazine can be attributed to its poor water solubility of 5.9 mg/L and higher sorption to soil organic carbon with organic carbon normalized partition coefficient (Koc) value of 793 L/kg (Hu et al. 2009). Due to its persistent nature, atrazine and its metabolites have high potential to contaminate surface and ground water. Herbicide is banned in Europe and has been under the scanner of environmentalists due to its endocrine disrupting properties.
Degradation of xenobiotics in soil is greatly affected by its adsorption on soil particles. Adsorption determines the availability of pesticide in soil solution for further processes such as degradation or leaching. Adsorption of atrazine in soil is mainly affected by soil pH, clay, and organic carbon content. Atrazine, a weak base (pKa-1.68) exists as neutral species at pH above its dissociation constant and adsorption decreases with increase in pH (Clay and Koskinen 1990; Liu et al. 1995). Atrazine adsorption is positively correlated to clay and organic carbon content (Swarcewicz and Skoersk 2007; Wang and Keller 2009). However, Cellis et al. (1997) showed that atrazine sorption to natural organic matter was higher than that to clay mineral. Ahmad and Rahman (2009) investigated sorption characteristics of atrazine in 101 soils of New Zealand using the batch equilibration technique. The partition coefficient (Kd) values of atrazine ranged from 0.7 to 52.1 L/kg, suggesting that atrazine was poorly sorbed in the soils. Thus, soil pH and organic carbon contents are the two important factors that may affect the atrazine degradation in soils. Soil pH has a greater effect on the rate of degradation than OM or other soil properties (Obien and Green 1969; Holford et al. 1989).
The major routes of atrazine transformation in the environment include dechlorination, dealkylation, and deamination. Some bacteria initiate atrazine degradation through hydrolytic dechlorination to hydroxyatrazine, and degradation is catalyzed by the enzyme atrazine chlorohydrolase. Hydroxyatrazine undergoes two hydrolytic deamination reactions catalyzed by amidohydrolases, and N-isopropylammelide (De Souza et al. 1998; Sadowsky et al. 1998) or N-ethylammelide (Topp et al 2000) is formed as the intermediate metabolites. These ammelides are finally converted to cyanuric acid. Another route followed for atrazine degradation is N-dealkylation of the lateral ethyl and isopropyl chains to deethylatrazine, deisopropylatrazine, and deethyldeisopropylatrazine. These dealkylated atrazine metabolites undergo hydroxylation and cyanuric acid is formed as the ultimate metabolite. Cyanuric acid, formed by either of the metabolic route, is acted upon by cyanuric acid amidohydrolase, biuret amidohydrolase, and allophanate hydrolase enzymes leading to the cleavage of the cyanuric acid to carbon dioxide and ammonia (Martinez et al. 2001). Nitrogen released from atrazine metabolism served as a nitrogen source for atrazine-degrading bacteria (Bichat et al. 1999).
Biodegradation is one of the most cost-effective natural remedial approaches to remove xenobiotic chemicals from the environment (Chatterjee et al. 2008). However, natural environment contains mixture of microorganisms which might have different enzymes required for complete degradation of xenobiotic. Therefore, mixed cultures are likely to have a greater capacity to degrade substrates by virtue of increased catabolic capability, and the rates of growth and substrate utilization are frequently higher in enriched mixed cultures than those in pure cultures isolated from the mixture (Zhang et al. 2012). Recently, author reported an enrichment culture that could degrade atrazine as the sole source of carbon and nitrogen (Dutta and Singh 2013). Atrazine-degrading bacteria appeared to be non-culturable; therefore, based on culture-independent PCR-DGGE technique, it was observed that bacteria of the genus Bacillus along with Pseudomonas and Burkholderia were enriched following repeated atrazine applications (Dutta et al. 2015). The main objective of the present study was to find out the pathway of atrazine degradation; therefore, potential of this enrichment culture to degrade major atrazine metabolites was evaluated. Effect of pH, concentration, and soil type on atrazine degradation was also studied.
Materials and methods
Chemicals
Analytical grade atrazine (95 % purity) was obtained from the Rallis India Ltd., Bangalore, India. Atrazine metabolites viz. deethylatrazine, deisopropylatrazine, and hydroxyatrazine were obtained from the Fluka Analytics (Sigma-Aldrich), NSW, Australia. Solvents and chemicals used were of analytical grade and purchased locally.
Soils
Soils used in the study included soil 1 from Hyderabad, Andhra Pradesh; soil 2 from Almora, Uttarakhand; soil 3 from Jhargram, West Bengal; and soil 4 from Pantnagar, Uttarakhand. Four soils were used to test the atrazine-degrading ability of the enrichment culture in soils having varying properties. They were collected from 0 to 15-cm depth, dried in shade, ground, sieved through a 2-mm sieve, and stored in polythene bags at room temperature. The physicochemical characteristics of the soils (Table 1) determined using standard analytical procedures were pH measured at 1:1.25 soil to water ratio (Jackson 1967), total organic carbon (TOC) content by Walkley and Black method (Black et al. 1965), and soil mechanical fractions employing the Bouyoucos hydrometer method (Jackson 1967).
Degradation of atrazine’s metabolites in mineral salt medium
Atrazine-degrading enrichment culture used in this study was earlier prepared by the selective enrichment of the atrazine-degrading microorganisms (Dutta and Singh 2013) and was used to study degradation of atrazine metabolites viz. hydroxyatrazine, deethylatrazine, and deisopropylatrazine. Degradation was studied under four different conditions where atrazine metabolites were supplemented as (i) sole source of carbon (C) and nitrogen (N) (−C,−N), (ii) additional N source added (−C,+N), (iii) additional C source supplied (+C,−N), and (iv) additional C and N source added (+C,+N). Hydroxyatrazine (100 μg), deethylatrazine, or deisopropylatrazine (200 μg) in 1 mL of acetone was individually added to pre-sterilized 100-mL Erlenmeyer flasks. After evaporation of acetone at room temperature, 20 mL of sterile mineral salts medium (MgSO4.7H2O, (0.2); K2HPO4, (0.1); FeSO4. 7H2O, (0.001); CaSO4, (0.04) g/L distilled water pH, 6.2) were dispensed into these flasks. Wherever required, sucrose (10 g/L) and ammonium hydrogen phosphate (2 g/L) were supplemented as additional source of carbon (C) and nitrogen (N), respectively. After equilibration for 4 h, one set of each treatment, in triplicate, was inoculated with 0.5 mL of enrichment culture (1 g soil + 9 mL sterile water). The enrichment culture uninoculated medium of each treatment served as control. Both uninoculated and inoculated flasks were incubated at 27 ± 1 °C and samples (0.5 mL) were removed at regular intervals for analysis.
Effect of pH and concentration on atrazine degradation
To evaluate the ability of enrichment culture to withstand high concentrations of atrazine, degradation of herbicide at 10, 55, and 110 μg/mL levels was studied in mineral salts medium. Atrazine in 1-mL acetone was aseptically added to sterile 100-mL Erlenmeyer flasks. After evaporation of acetone, 20-mL mineral salts medium was added to each flask and equilibrated for 4 h. Final concentrations of atrazine in medium were 10, 50, and 110 μg/mL. One set of three flasks of each concentration was inoculated with enrichment culture as mentioned in the previous section. One set (three flasks) of each concentration was kept as uninoculated control. Flasks were incubated at 27 ± 1 °C and samples were withdrawn for atrazine analysis.
Effect of pH on the atrazine-degrading ability of enrichment culture was studied in mineral salts medium. Atrazine (10 μg/mL) containing mineral salts medium of pH 5, 6, 7, and 8 was inoculated with enrichment culture, and study was continued as mentioned above.
Degradation of atrazine in soils
Degradation of atrazine was studied in four different soils having varying pH and organic carbon content. Two sets for each soil (100 g) in sterilized 500-mL Erlenmeyer flasks were supplemented with 10 μg/g of atrazine by fortifying 5-g portion of the soil with the required amount of atrazine in 0.1-mL acetone and after evaporation of acetone, mixing it with the remaining soil. This was done to minimize the effect of acetone on native soil microflora. Soils were supplemented with sterile distilled water to maintain moisture at 60 % of water holding capacity (WHC). One set of each soil was inoculated with 1-mL suspension of atrazine-degrading enrichment culture (1-g enriched soil mixed with 9 mL of sterile distilled water), while the other set was maintained as the respective control. The study was performed in triplicate. Samples were incubated at 27 ± 1 °C and, at regular intervals samples (triplicate), were removed under sterile conditions for extraction and analysis of atrazine.
Further, to confirm the role of pH on the atrazine-degrading ability of the enrichment culture, pH of soil 3 was raised to near neutral or alkaline using CaCO3. Soil 3 (50 g) in sterile Erlenmeyer flasks was thoroughly mixed with 31.25 mg (T1) and 62.5 mg (T2) CaCO3. Soil samples were supplemented with sterile distilled water to maintain 60 % WHC and were incubated for 10 days at 27 ± 1 °C. After 10 days of incubation, one set of three flasks of each treatment was inoculated with enrichment culture while uninoculated flasks of each treatment were maintained as control. Study was continued as mentioned above.
Adsorption of atrazine in soils
Adsorption of atrazine in soils was studied by batch sorption method. Soil (5 g) and 10 mL aqueous solution of atrazine (10 μg/mL) in glass test tubes (50 mL) were equilibrated on an end-over-end shaker at room temperature for 4 h. Blank (without soil) were kept as control. Three replicates were maintained for each treatment. After equilibration, soil suspension was centrifuged at 3500 rpm for 20 min and concentration of atrazine in the supernatant was quantified. The amount of atrazine adsorbed by the soils was calculated from the difference of initial and final concentration of herbicide in the supernatant.
Extraction and analysis
Atrazine residues from soil (10 g) samples were extracted using ethyl acetate (10 mL) and anhydrous sodium sulfate (1 g) by equilibrating on a rotary shaker for 2 h. The ethyl acetate layer (5 mL) was separated and evaporated to dryness at room temperature. The residue thus obtained was re-dissolved in 5 mL of acetonitrile (HPLC grade) for analysis. Aqueous samples containing atrazine or atrazine metabolites namely deethylatrazine, deisopropylatrazine, and hydroxyatrazine were injected directly after filtration through a 0.45-μm filter.
Atrazine and its metabolites were analyzed using HPLC (Varian, Prostar) equipped with a quaternary pump, a UV detector, and a Rheodyne injection system using Lichrospher C-18 stainless steel column (250 mm × 4 mm (i.d.)), acetonitrile/0.1 % aqueous o-phosphoric acid (55:45) as a mobile phase at a flow rate of 0.8 mL/min at a wave length of 222 nm. Under these conditions, the retention time (min) of different compounds was as follows: deethylatrazine—2.41; deisopropylatrazine—2.24; hydroxyatrazine—4.66; atrazine—6.52. The recovery of atrazine from soils or water was studied at fortification levels of 0.1, 1, and 10 μg/g or μg/mL by fortifying the 10-g soil/water with the required amount of atrazine in 0.1 mL of acetone. The atrazine recoveries ranged from 90.2 ± 1.1 to 94.2 ± 1.0 %. The recovery studies of deethylatrazine, deisopropylatrazine, and hydroxyatrazine from water were studied at fortification levels of 0.1, 1, and 5 μg/mL and it ranged from 93.2 to 95 % for deethylatrazine, 94.2–95.6 % for deisopropylatrazine, and 92.5–94.8 % for hydroxyatrazine.
Calculation
Data for atrazine/metabolite degradation was fitted to the first-order kinetic equation:
where C o is the initial concentration of the substrate (μg/mL or µg/g), C is its concentration (μg/mL or µg/g) after time t (days), and K obs is the rate constant of the reaction. The half-life (t1/2) values were calculated from the k obs values using the following formula:
Results and discussion
Degradation of atrazine’s metabolites
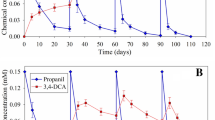
Degradation of atrazine metabolites namely deethylatrazine, deisopropylatrazine, and hydroxyatrazine was studied using the atrazine-degrading enrichment culture. Results indicated that out of the three atrazine metabolites, the enrichment culture was able to degrade only hydroxyatrazine, while no degradation of deethylatrazine and deisopropylatrazine was observed. Further, degradation of hydroxyatrazine was influenced by the medium composition (Fig. 1). Fastest hydroxyatrazine degradation was observed in the −C,−N medium where no additional source of carbon (C) and nitrogen (N) was added, and hydroxyatrazine concentration reached below non-detectable levels on the sixth day. This suggested that the enrichment culture used hydroxyatrazine as the sole source of carbon and nitrogen. It took 10 days for complete hydroxyatrazine dissipation in +C,−N medium (medium containing sucrose as additional C source) and 15 days in −C,+N medium (medium containing ammonium hydrogen phosphate as additional N source). These results suggested that the enrichment culture used hydroxyatrazine preferably as nitrogen source than as carbon source. No hydroxyatrazine degradation was observed in the +C,+N medium even after 20 days of incubation. No hydroxyatrazine degradation was observed in the uninoculated controls suggesting that the degradation of hydroxyatrazine was microbial and not chemical in nature.
Table 2 shows the degradation rate constant and the half-life (t1/2) values of hydroxyatrazine in different media. The rate of hydroxyatrazine degradation followed the order −C,−N > +C,−N > −C,+N with respective t1/2 values of 0.9, 1.5, and 2.6 days. These results are in accordance with the previous findings which suggested that atrazine-degrading microorganisms used triazine compounds primarily as nitrogen source (Govantes et al. 2009), and presence of additional nitrogenous compounds slowed down the degradation of triazines (Clausen et al. 2002; Garcia-Gonzalez et al. 2003). The HPLC chromatograms (Fig. 2) of the enrichment culture-inoculated samples showed additional peaks at retention time (Rt) of 2.36 (metabolite 1) and 3.83 min (metabolite 2). The peak at 2.36 min corresponded to biuret. Earlier studies (Dutta and Singh 2013) suggested as well that biuret was detected as the only metabolite of atrazine. The peak at 3.83 min could not be identified due to unavailability of authentic hydroxyatrazine metabolites. Further, formation of hydroxyatrazine metabolites depended on the medium composition. Results in Table 3 suggested that even on the third day, both metabolites were detected in the −C,−N and +C,−N media. However, in −C,+N medium, metabolite 2 was detected on the sixth day while metabolite 1 appeared on the tenth day. Further, metabolite 1 was formed in higher amounts in −C,−N and −C,+N media while lower amounts were detected in the +C,−N medium. Metabolite 2 did not accumulate in the −C,−N and −C,+N media and was further degraded. This suggested that medium composition greatly affected hydroxyatrazine/metabolite degradation.
The ability of the enrichment culture to degrade only hydroxyatrazine, not deethylatrazine and deisopropylatrazine, suggested that degradation of atrazine followed via dechloination and not via dealkylation. Hydroxyatrazine can be further metabolized to cyanuric acid via the formation of N-isopropylammelide or N-ethyammelide (Fig. 3). In the present study, we could not confirm the identity of metabolite 2 detected during hydroxyatrazine degradation; we therefore could not ascertain whether hydroxyatrazine degradation proceeded via the formation of N-isopropylammelide or N-ethyammelide. Cyanuric acid formed from hydroxyatrazine degradation did not accumulate in detectable amounts and was immediately converted to biuret. Earlier, Dutta and Singh (2013) reported that cyanuric acid was not detected during atrazine degradation while study by Dutta et al. (2015) suggested that atrazine-degrading enrichment culture was capable of degrading cyanuric acid and formation of biuret and an unidentified metabolite, other than urea, was observed. Based on the result obtained in the present study and results reported earlier (Dutta and Singh 2013; Dutta et al. 2015) the following pathway for atrazine degradation can be suggested: Atrazine–hydroxyatrazine–cyanuric acid–biuret–CO2 and ammonia (Fig. 3). Earlier, Pseudomonas sp. ADP has been reported as the best-characterized bacterial strain capable of degrading atrazine and the catabolic pathway in this bacterium contained all six enzymes required for complete mineralization of atrazine (De Souza et al. 1998). Bacillus subtilis, isolated from the industrial wastewater, degraded atrazine via the formation of hydroxyatrazine and cyanuric acid (Wang et al. 2014). However, they were utilized as nitrogen source and required additional carbon source for growth and mineralization of s-triazine ring. The enrichment culture used in the present study degraded atrazine and hydroxyatrazine and did not require additional carbon or nitrogen source, and none of their intermediate metabolite accumulated in the medium.
Degradation of atrazine
Effect of concentration on atrazine degradation was studied in the mineral salts medium at initial concentrations of 10, 55, and 110 μg/mL (Table 4). Results indicated that the enrichment culture was capable of degrading atrazine up to 110 μg/mL concentration, though an initial lag period was observed as atrazine concentration in the medium increased. On the tenth day, no atrazine was detected in the medium where initial concentration of atrazine was 10 μg/mL, while during the period, only 85 and 70 % of initially added atrazine in media fortified with 55 and 110 μg/mL of atrazine was degraded. But, in both these treatments, atrazine concentration reached below detectable levels on the 15th day. These results suggested that microorganisms were tolerant to high levels of atrazine, and enrichment culture can be successfully used for atrazine degradation in contaminated water. Recently, Wang et al. (2014) reported that B. subtilis isolated from industrial wastewater was able to degrade atrazine up to 500 μg/mL concentrations, but degradation rate slowed down after 100 μg/mL concentration.
Results of atrazine degradation study at different pH suggested that till the sixth day, no atrazine degradation was observed in the mineral salts medium at pH 5 (Table 5). It further confirmed our previous observation that acidic pH inhibited the activity of enrichment culture (Dutta and Singh 2013). Results in Table 5 suggested that pH range of 6–8 suited well to the microorganisms, and neutral pH was optimum for maximum atrazine degradation. Earlier, Solomon et al. (2013) and Dehghani et al. (2013) also reported that three atrazine-degrading microbes grew well at pH 6–8, and pH 4 and 5 inhibited their growth.
The atrazine-degrading enrichment culture was evaluated to degrade atrazine in different soil types (Fig. 4). Results suggested that out of the four soils used in the study, the enrichment culture was able to degrade atrazine in soil 1, soil 2, and soil 4, while no atrazine degradation was observed in the soil 3. Among the four soils used, soil 3 was acidic in nature (pH 5.2) while the remaining three soils had pH near neutral (6.5–7.8), with soil 1 having the maximum pH. Fastest atrazine degradation was observed in soil 1, where after 6 days of incubation, nearly 95 % of atrazine was degraded while only 70 and 86 % atrazine degradation was observed in soil 2 and soil 4, respectively. The half life of atrazine in soils calculated using first-order rate equation was 1.6, 2.3, and 2.4 days in soil 1, soil 2, and soil 4, respectively (Table 6). Results of adsorption study suggested that 10.3, 29.1, 13.8, and 34.6 % of atrazine adsorption was observed in soil 1, soil 2, soil 3, and soil 4, respectively. Thus, except soil 3 where no atrazine degradation was observed, the atrazine-degrading ability of the enrichment culture was inversely related to atrazine adsorption in soils. Further, among these three soils, soil 4 had highest organic carbon (OC—1.2 %) content than soil 2 (OC—0.63 %) and soil 1 (OC—0.55 %). Thus, atrazine adsorption in soils was directly proportional to their organic carbon content. These findings were in agreement with the previous results which suggested that soil organic carbon content affected atrazine adsorption (Swarcewicz and Skoersk 2007; Wang and Keller 2009). Further, atrazine degradation by enrichment culture in soils was inversely proportional to its adsorption as adsorption limits the bioavailability of pesticide in soil solution (Guo et al. 2000). Thus, fastest atrazine degradation in soil 1 having maximum pH and minimum atrazine sorption suggested that both pH and organic carbon content affected atrazine degradation in soils. Even though soil 3 had the lowest OC content and showed moderate atrazine adsorption, inability of enrichment culture to degrade atrazine clearly suggested that the acidic pH inhibited the activity of the enrichment culture (Dutta and Singh 2013). To confirm this hypothesis, pH of soil 3 was raised by liming and results of the degradation study are shown in Fig. 5. Following CaCO3 amendment, the enrichment culture was able to degrade atrazine as it raised pH from 5.2 (T0) to 6.75 (T1) and 8.3 (T2). Compared to T1 treatment, atrazine degraded at a faster rate in T2 treatment. This can be attributed to partial chemical degradation of atrazine under alkaline conditions as nearly 20 % atrazine degradation was observed in the uninoculated control soil. However, atrazine degradation in T1 treatment was mediated by the enrichment culture. On the sixth day, compared to 1–2 % atrazine loss in control samples, nearly 80 % of atrazine was degraded in the inoculated samples. Further, increasing the soil pH from 5.2 (T0) to 8.3 (T2) did not significantly change the herbicide adsorption (T0—13.8 %, T2—11.5 %). These results suggested that it was the acidic pH of soil which inhibited the atrazine-degrading ability of the enrichment culture. This finding further confirmed the results obtained in mineral salts medium study which suggested no atrazine degradation in pH 5 medium. Earlier, Guox et al. (2003) suggested that acidic pH inhibited atrazine degradation in soils inoculated with atrazine-degrading microbes. Further, ability of enrichment culture to degrade atrazine in non-sterile soils suggested that atrazine degrading consortia was able to survive and proliferate even in the presence of native soil microflora.
Conclusion
The results of the present study suggested that the enrichment culture can be utilized to degrade atrazine in different soil types, although acidic pH hindered the atrazine degradation. The enrichment culture was able to sustain and degrade high levels of atrazine. The ability of enrichment culture to degrade only hydroxyatrazine, not deethylatrazine and deisopropylatrazine, suggested that mechanism of atrazine degradation involved its dechlorination to hydroxyatrazine, which was subsequently degraded to biuret via the formation of cyanuric acid. Ability of the enrichment culture to completely degrade atrazine/hydroxyatrazine in medium and diverse soil types can be successfully exploited to degrade these persistent triazines in the contaminated soil-water environments.
References
Ahmad, R., & Rahman, A. (2009). Sorption characteristics of atrazine and imazethapyr in soils of New Zealand: importance of independently determined sorption data. Journal of Agricultural and Food Chemistry, 57, 10866–10875.
Bichat, F., Sims, G. K., & Mulvaney, R. L. (1999). Microbial utilization of heterocyclic nitrogen from atrazine. Soil Science Society of America Journal, 63, 100–110.
Black, C. A., Evans, D. D., White, J. L., Ensminger, L. E., & Clark, F. E. (1965). Methods of soil analysis. 2nd ed. Agronomy Monograph 9, Agronomy Society of America and Soil Science Society of America, Madison.
Cellis, R., Cornjeo, J., Hermosin, A., & Koskinen, W. C. (1997). Sorption-desorption of atrazine and simazine by model soil colloidal components. Soil Science Society of America Journal, 61, 436–443.
Chatterjee, S., Chattopadhyay, P., Roy, S., & Sen, S. K. (2008). Bioremediation: a tool for cleaning up polluted environments. Journal of Applied Biosciences, 11, 594–601.
Clausen, G. B., Larsen, L., Johnsen, K., Lipthay, J. R., & Aamand, J. (2002). Quantification of the atrazine degrading Pseudomonas sp. strain ADP in aquifer sediment by quantitative competitive polymerase chain reaction. FEMS Microbiology Ecology, 41, 211–229.
Clay, S. A., & Koskinen, W. C. (1990). Adsorption and desorption of atrazine, hydroxyatrazine, and s-glutathione atrazine in two soils. Weed Science, 38, 262–266.
De Souza, M., Seffernick, J., Mmartinez, B., Sadowsky, M. J., & Wackett, L. P. (1998). The atrazine catabolism genes atzABC are widespread and highly conserved. Journal of Bacteriology, 180, 1951–1954.
Dehghani, M., Nasseri, S., & Hashemi, H. (2013). Study of the bioremediation of atrazine under variable carbon and nitrogen sources by mixed bacterial consortium isolated from corn field soil in Fars province of Iran. Journal of Environmental and Public Health, 2013, 973165. doi:10.1155/2013/973165.
Dutta, A., & Singh, N. (2013). Degradation of atrazine in mineral salts medium and soil using enrichment culture. Journal of Environmental Science and Health. Part. B, 48, 860–868.
Dutta, A., Vasudevan, V., Nain, L., & Singh, N. (2015). Characterization of bacterial diversity in an atrazine degrading enrichment culture and degradation of atrazine, cyanuric acid and biuret in industrial wastewater. Journal of Environmental Science and Health. Part. B, 51, 24–34.
Garcia-Gonzalez, V., Govantes, F., Shaw, L. J., Burns, R. G., & Santero, E. (2003). Nitrogen control of atrazine utilization in Pseudomonas sp. strain ADP. Applied and Environmental Microbiology, 69, 6987–6993.
Govantes, F., Porrúa, O., García-González, V., & Santero, E. (2009). Atrazine biodegradation in the lab and in the field: enzymatic activities and gene regulation. Microbial Biotechnology, 2, 178–185.
Guo, L., Jury, W. A., Wagenet, R. J., & Flury, M. (2000). Dependence of pesticide degradation on sorption: nonequilibrium model and application to soil reactors. Journal of Contaminant Hydrology, 43, 45–62.
Guox, S., Shapir, N., Fantroussi, N., Lelong, S., Agathos, S. N., & Pussemier, L. (2003). Long-term maintenance of rapid atrazine degradation in soils inoculated with atrazine degraders. Water, Air, & Soil Pollution: Focus, 3, 131–142.
Holford, I. C. R., Haigh, B. M., & Ferris, I. G. (1989). Atrazine persistence and phytotoxicity on wheat as affected by nitrogen and rotation-induced changes in soil properties. Australian Journal of Agricultural Research, 40, 1143–1153.
Houot, S., Barriuso, E., & Bergheaud, V. (1998). Modifications to atrazine degradation pathways in a loamy soil after addition of organic amendments. Soil Biology and Biochemistry, 30, 2147–2157.
Hu, D., Henderson, K., & Coats, J. (2009). Fate of transformation products of synthetic chemicals. In: Boxall, A. B. A. (ed), Transformation products of synthetic chemicals in the environment. The Handbook of Environmental Chemistry, vol. 2, Part P. Berlin (pp. 103–120). Germany: Springer-Verlag.
Jackson, M. L. (1967). Soil chemical analysis. New Delhi: Prentice Hall Inc.
Liu, Z., Clay, S. A., Clay, D. E., & Harper, S. S. (1995). Ammonia fertilizers affect atrazine adsorption–desorption characteristics. Journal of Agricultural and Food Chemistry, 43, 815–819.
Martinez, B., Tomkins, J., Wackett, L. P., Wing, R., & Sadowsky, M. J. (2001). Complete nucleotide sequence and organization of atrazine catabolic plasmid pADP-1 from Pseudomonas sp. strain ADP. Journal of Bacteriology, 183, 5684–5697.
Obien, S. R., & Green, R. E. (1969). Degradation of atrazine in four Hawaiian soils. Weed Science, 17, 509–514.
Sadowsky, M. J. Z., Tong, M. L., de Souza, M., & Wackett, L. P. (1998). AtzC is a new member of the amidohydrolase protein superfamily and is homologous to other atrazine-metabolizing enzymes. Journal of Bacteriology, 180, 152–158.
Solomon, R. D. J., Kumar, A., & Satheeja, S. V. (2013). Atrazine biodegradation efficiency, metabolite detection, and trzD gene expression by enrichment bacterial cultures from agricultural soil. Journal of Zhejiang University. Science, B4, 1162–1172.
Sparling, G., Dragten, R., Aislabie, J., & Fraser, R. (1998). Atrazine mineralization in New Zealand topsoils and subsoils: influence of edaphic factors and numbers of atrazine-degrading microbes. Australian Journal of Soil Research, 36, 557–570.
Stutz, H., Pittertschatscher, K., & Malissa, H. (1998). Capillary zone electrophoretic determination of hydroxymetabolites of atrazine in potable water using solid-phase extraction with Amberchrom resins. Mikrochimica Acta, 128, 107–117.
Swarcewicz, M., & Skoersk, E. (2007). Adsorption of atrazine by soils varying in organic carbon content in the presence of the adjuvant atpolan. Bulletin of Environmental Contamination and Toxicology, 78, 231–234.
Topp, E., Mulbry, W. M., Zhu, H., Nour, S. M., & Cuppels, D. (2000). Characterization of s-triazine herbicide metabolism by a Nocardioides sp. isolated from agricultural soils. Applied and Environmental Microbiology, 66, 3134–3141.
Wang, P., & Keller, A. A. (2009). Sorption and desorption of atrazine and diuron onto water dispersible soil primary size fractions. Water Research, 43, 1448–1456.
Wang, J., Zhu, L., Wang, Q., Wang, J., & Xie, H. (2014). Isolation and characterization of atrazine mineralizing Bacillus subtilis strain HB-6. PLoS ONE, 9, e107270. doi:10.1371/journal.pone.0107270.
Zhang, Y., Cao, B., Jiang, Z., Dong, X., Hu, M., & Wang, Z. (2012). Metabolic ability and individual characteristics of an atrazine-degrading consortium DNC5. Journal of Hazardous Materials, 237–238, 376–381.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. Consent to publish the work from co-author and the responsible authority of institution, where work was carried out, has been obtained. Research does not involve humans or animals.
Rights and permissions
About this article
Cite this article
Kumar, A., Singh, N. Atrazine and its metabolites degradation in mineral salts medium and soil using an enrichment culture. Environ Monit Assess 188, 142 (2016). https://doi.org/10.1007/s10661-016-5144-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5144-3