Abstract
X-ray fluorescence (XRF) quantitation software programs are widely used for analyzing environmental samples due to their versatility but at the expense of accuracy. In this work, we propose an accurate, robust, and versatile technique for multielemental X-ray fluorescence analytical applications, by spiking solid matrices with standard solutions. National Institute of Standards and Technology (NIST)-certified soil standards were spiked with standard solutions, mixed well, desiccated, and analyzed by an energy dispersive XRF. Homogenous targets were produced and low error calibration curves, for the added and not added, neighboring, elements, were obtained. With the addition of few elements, the technique provides reliable multielemental analysis, even for concentrations of the order of milligram per kilogram (ppm). When results were compared to the ones obtained from XRF commercial quantitation software programs, which are widely used in environmental monitoring and assessment applications, they were found to fit certified values better. Moreover, in all examined cases, results were reliable. Hence, this technique can also be used to overcome difficulties associated with interlaboratory consistency and for cross-validating results. The technique was applied to samples with an environmental interest, collected from a ship/boat repainting area. Increased copper, zinc, and lead loads were observed (284, 270, and 688 mg/kg maximum concentrations in soil, respectively), due to vessels being paint stripped and repainted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
X-ray fluorescence (XRF) is a robust analytical tool for estimating the composition of complex solid and liquid matrices. It has numerous scientific and real life applications, ranging from NASA’s Curiosity Mars rover (NASA 2013) to in vivo studies in human organs and tissues (Tsuji et al. 2012). Its efficiency lies in minimum sample quantity, pretreatment, time, and cost for qualitative and quantitative multielemental analyses (Bero et al. 1995). In environmental monitoring and assessment applications, where time and cost is of the essence, the energy dispersive X-ray fluorescence (EDXRF) spectrometers find favorable use (Sarris et al. 2009; Meyer et al. 2012; Alyazichi et al. 2015). The reason lies in its mains strengths, which include (a) direct analyses of solid samples, therefore avoiding elaborate digestion procedures and associate contamination effects; (b) its multielemental capabilities: a vast array of elements can be simultaneously analyzed in the same spectrum; (c) its capability for detecting a wide concentration range, from milligram per kilogram to percent; (d) its ability to also measure non-targeted elements that one should not expect to exist in the sample under study; and (e) its time efficiency and overall simplicity; with a single click and within a few minutes, normally 5–15 min, EDXRF can automatically measure and display results.
However, when XRF is applied directly on solid matrices, a major advantage of the technique, results are not as accurate as the ones obtained when XRF is applied on liquid matrices. The underlying problem is the need for high purity certified standards, with similar composition and analyte concentration range, to unknown matrices. Nonetheless, the possible combinations of solid matrix compositions are practically infinite. This problem can be overcome through sample digestion, where the analyte is transferred to a liquid medium, but this approach, which requires sample pretreatment, is time-consuming and causes cross-contamination effects (Wong et al. 1997).
Limitations affecting XRF accuracy on solid samples include matrix effects, overlap interferences, difficulties in deconvolution and background fitting. In response to these, specialized methods, such as the fundamental parameter constant and the influence coefficient, as well as automatic XRF quantitation software, have been developed (Tertian and Claisse 1982; Grieken and Markowicz 1993; Lachance and Claisse 1995; Beckhoff et al. 2006). Due to their simplicity and versatility nowadays, XRF quantitation software programs are widely used in environmental monitoring and assessment applications (Kankılıç et al. 2013; Foteinis et al. 2013; Galloa et al. 2014). Nonetheless, their absolute error tends to be high while as far as minuscule concentrations, of the order of milligram per kilogram, are concerned, they are usually allowed only semiquantitative analysis. When, high analytical accuracy is needed, it can be achieved by using the XRF fusion bead method, or by other techniques, such as the atomic absorption (AAS) or inductively coupled plasma mass spectrometry (ICP-MS) (Gałuszka 2005; Yamada 2010). However, these are expensive, time-consuming, and have several sample pretreatment steps. Therefore, a simple and cost-efficient technique that can provide more accurate and reliable multielemental quantitative analysis than existing XRF quantitation software programs should be introduced.
A solution to this problem can be the direct addition of standard solutions into the solid matrix (Pind 1984; Mahapatra 1987; Muia and Grieken 1991; Hatzistavros and Kallithrakas-Kontos 2008). Indeed, most analytical techniques, such as AAS, ICP-MS, liquid chromatography, and anodic stripping voltammetry, rely on standard solutions in liquid matrices, usually aqueous, similar to analyzed samples. With the use of standard solutions, a simple linear calibration can be produced and quantitative results of high accuracy and precision are obtained.
Regarding XRF, liquid internal standards can be introduced into solid matrices through standard addition, a well-established calibration technique devised to overcome matrices effect that may bias measurement accuracy (Thompson 2009). Standard addition can be either conventional, where additions are performed to aliquots of the initial sample, or sequential, where additions are performed sequentially in the same part of the sample (Isoyama et al. 1990; Brown and Gillam 2012). The former is characterized by high accuracy, since errors are not cumulative, while the latter is preferably used when limited sample quantities exist, a common case in analytical chemistry applications. Standard addition has been long used in XRF analytical applications (Pind 1984; Mahapatra 1987; Muia and Grieken 1991; Hatzistavros and Kallithrakas-Kontos 2008), but a comprehensive technique for reliable multielemental analysis is still pending.
In this work, we propose a robust and versatile approach that seeks to address the problem of reliable multielemental XRF analysis. Solid matrices were spiked with standard solutions, consisting of few trace elements, and the concentrations of the added elements as well as of neighboring non-added elements were estimated. Analyses were performed with AMETEK® SPECTRO XEPOS, a versatile EDXRF spectrometer that is widely used in environmental monitoring and assessment applications (Clark et al. 2008, Meyer et al. 2012; Foteinis et al. 2013; Wolf et al. 2014; Alyazichi et al. 2015). Furthermore, results were compared to the estimations of AMETEK XRF quantitation software program TurboQuant®, which is also used in environmental applications (Kankılıç et al. 2013; Foteinis et al. 2013; Galloa et al. 2014). Sequential standard addition (SSA) was used, since, more often than not, sample mass availability tends to be a limitation in environmental and analytical chemistry applications.
Experimental
Reagents and chemicals
National Institute of Standards and Technology (NIST) reference materials, in powdered form, were utilized as solid matrices. Three NIST reference materials were utilized; specifically, the standard reference material (SRM) 1571 orchard leaves (organic origin), the SRM 1633b coal fly ash (inorganic origin, mainly aluminosilicates), and the SRM 4357 ocean sediment (inorganic origin, mainly silicates, semiquantitative trace element analysis). Standard solutions were produced using concentrated single element solutions of MERC (K, Cu, Zn), Reagecon (V, Ni, As, Se, Rb, Sr, Y, Pb), and Fluka (Fe), which were diluted in high-purity water (type I-ASTM-D1193-91, resistivity 18.0 MΩ cm) and mixed well. Dilutions and additions were performed using a 10–3-g analytical balance.
Procedure
In order to achieve high accuracy, added element quantities were of the same order as the ones of the reference materials (solid matrices). For each reference material, matching standard solutions were prepared, added to solid matrices, and manually mixed as to produce a ductile sludge. The sludge was then dried to constant weight by means of an IR lamp, as to remove water content (desiccation time ranged from 15 to 40 min depending on water content). A drying oven can also be used, but prolonged desiccation time, depending on sample water content, would be required, while microwave oven is not suitable due to sample toss. Moreover, no losses of volatile elements, such as As, were observed by IR drying, as can be seen in Table 1 and Table 2.
Dried samples were grained in a laboratory mortar as to return to their previous powdered homogenized nature. Then, 4 g of each sample was soft-pressed using a small pestle and placed in cylindrical XRF sample plastic cups (Chemplex Cat. No 1540) using 4.0-μm Prolene® film (Chemplex Cat. No. 416). After the measurements were carried out, the samples were spiked again (SSA) and the same procedure was followed.
For each matrix, different volumes were examined as to investigate if added volume impacts mixing and respectively produces homogeneity effects. For the aforementioned matrices, the following fractions (mass/volume) were examined:
-
Orchard leaves 1/3
-
Ocean sediment 1/2
-
Coal fly ash 1/0.8
Apparatus
X-ray fluorescence analyses were performed by a SPECTRO XEPOS (AMETEK) benchtop XRF spectrometer, using X-Lab Pro 4.0 quantitation software and TurboQuant screening method. Excitation was performed by means of a Palladium (Pd) anode X-ray end window tube, air cooled, with a maximum power of 50 W and a maximum voltage of 50 kV. A silicon drift detector (SDD), with Peltier cooling (−25 °C, no liquid nitrogen) and an 8-μm Moxtek Dura-Be window was utilized, with stability up to 120 k counts/s, peak to background ratio equal to 5000:1 (for Mn Kα), and detector resolution of 160 eV at 5.9 keV (Mn Kα). All measurements were performed at 40 kV and 0.9 mA, with Compton secondary/molybdenum excitation node, in ambient air using a 12-position autosampler. The irradiation time was 300 s (5 min).
Results and discussion
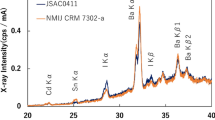
As a preamble, standard addition technique yielded quantitative results of higher analytical accuracy compared to XRF quantitative software, in this case, TurboQuant. In Fig. 1, five overlapping spectra of NIST SRM 1633b are presented. The lower one, shown in black color, was estimated without any addition, while the four consecutive spectra (green, blue, purple, and red) were obtained by performing SSA using a standard solution containing vanadium, nickel, copper, selenium, and strontium. As it can be seen, additions did not affect the yield or the spectra background. Similar results were obtained for all examined matrices (data not shown).
Direct multielemental standard addition gave low error calibration lines (coefficient of determination ≥0.99), as shown in Fig. 2 for Ni NIST SRM 4357. Similar results (data not shown) were obtained for all examined matrices and for most elements under study.
Through XRF, all elements from Na to U can be estimated, while multielemental spiking was limited to few elements. Therefore, the possibility of estimating neighboring, non-added elements by interpolating or extrapolating results was examined. For example, NIST SRM 4357 interpolation calculation is presented in Fig. 3. The standard addition slope was calculated as a function of the Kα element energy, using a second-degree polynomial. The coefficient of determination was 0.9985, which denotes that results are of high accuracy.
For all examined matrices, the same procedure was followed. The analysis was mainly focused on the elements that are presented in the matrices with low concentrations, of the order of milligram per kilogram, since in these cases, the error of commercial XRF software is high. Elemental concentrations were estimated with the multielemental spiking technique and compared to the estimations of the commercial XRF software for automatic matrix effect correction, in this case, TurboQuant. Results are presented in Tables 1, 2, and 3.
NIST SRM 1571 orchard leaves matrix was spiked with 10 elements, namely V, Fe, Ni, Cu, Zn, As, Rb, Sr, T, and Pb. Three more elements, namely Cr, Mn, and Co, were calculated through interpolation. As it is shown in Table 1, the direct elemental determination through SSA gives very accurate results, which in most cases, are more accurate than TurboQuant estimations. Moreover, elements estimated by interpolation gave also accurate results. Specifically, for added elements and for concentrations higher than 10 mg/kg, the errors of the multielemental spiking technique are significantly lower than TurboQuants’. For concentrations lower than 10 mg/kg, the multielemental spiking technique and TurboQuant gave good approximations (Table 1). Hence, TurboQuant yielded poorer results compared to the multielemental spiking technique estimations. Regarding the elements that were estimated through interpolation, the multielemental spiking technique accuracy was better than TurboQuant.
NIST SRM 1633b coal fly ash was spiked with only five elements (V, Ni, Cu, Se and Sr). Eight additional NIST-certified elements (Cr, Mn, Fe, Co, Zn, As, Br, Rb) were estimated through interpolation as to investigate if method accuracy is affected by estimating large numbers of non-added elements. Results were in tandem with certified values and again more accurate than TurboQuant estimations. It has to be noted that due to interferences with the iron Kβ line, the accuracy of cobalt concentration through SSA was affected (Table 2). Thus, even though the measurement of XRF peaks is straightforward, known elemental interference (e.g., peak overlap) of the XRF method should be taken into account in all analyses.
NIST SRM 4357 ocean sediment was spiked with four elements (V, Ni, AS and Sr), while seven elements (Cr, Mn, Fe, Co, Zn, Ga and Se) were calculated by interpolation and one (Mo) was calculated by extrapolation. Again, it is shown that the direct elemental estimation through the multielemental spiking technique yielded results similar to NIST values, while the estimation of non-added elements was very accurate and was not affected by the low number of added elements. What is more, in most of the cases, the multielemental spiking technique errors, for elements estimated by interpolation (extrapolation for Mo), are lower than the ones of TurboQuant (Table 3).
Finally, all matrix mass to solution volume fractions gave similar results, with low fraction values, i.e., 1/3, to require higher desiccation time and higher fractions, i.e., 1/0.8, to be more difficult to mix. Hence, optimum mixing and desiccation time could be achieved by using a mean value of mass/volume fraction between 1/2 and 1/1.
Standard addition yielded reliable results, for the added and non-added elements, even for low concentrations, of the order of milligram per kilogram. Therefore, if other analytical techniques, such as AAS and IPC-MS, are not available, the standard addition technique can be used for cross-validating results and to overcome difficulties associated with interlaboratory consistency (Trejos et al. 2013).
What is more, standard addition results were more accurate than the ones estimated by TurboQuant. Specifically, for all NIST matrices, results were reliable, while for IEAE matrix, results were not as accurate, which may be attributed to error accumulation. Figure 4 compares standard values, given the y = x line, with the multielemental spiking technique and TurboQuant estimations for all matrices. As can be observed, data obtained by the standard addition technique better fits the certified values, since these data are closer to the y = x line, which is not the case for TurboQuant. Specifically, as far as concentrations from 0 to 1400 mg/kg are concerned, standard addition technique better fits certified values, with a regression equation of y = 1.081x (R 2 = 0.982), while the regression equation for TurboQuant is y = 1.212x (R 2 = 0.847). When lower concentrations, up to 200 mg/kg, are concerned, the regression equation of standard addition is y = 0.907x (R 2 = 0.919), while for TurboQuant, the regression equation is y = 1.435x (R 2 = 0.814). What is more, TurboQuant has the tendency to overestimate certified values, since the majority of the TurboQuant measurements are above the y = x line (Fig. 4).
Since the multielemental spiking technique was found to produce more accurate estimations than the commercial XRF software, it is proposed that future software programs can include its utilization as to further improve their capabilities and overall accuracy.
Application in real environmental samples—a case study of heavy metal pollution from vessel repainting activities
The applicability of the multielemental spiking technique was assessed in real soil samples. As a case study, the port and beach of Nea Chora (Western Crete, Greece) were used. It is known that substandard maintenance activities (i.e., vessel paint stripping and repainting) taking place in many Greek ports may lead to pollution by heavy metals. Elevated copper, zinc, and lead concentrations have been noticed in one sample from the specific port (Foteinis et al. 2013). Thus, this location was used as a case study and the multielemental spiking technique was applied in order to assess its applicability for environmental monitoring as well as the current loads of copper, zinc, and lead. Sediment samples were collected from the seaward side of the port’s breakwater, where ship and boat repainting works take place, as well as from the adjacent beach (Fig. 5).
The samples (soil and fine beach sediments) were taken to the laboratory, air dried, and passed through a 2-mm sieve, as to secure uniform particle size. Then, they were directly spiked with standard solutions containing the elements under study, analyzed by XRF and assessed by the multielemental spiking technique. The sampling points and their corresponding heavy metal loads are shown in Table 4 and Fig. 5 respectively. In beach sediments, the mean zinc, copper, and lead loads were 56, 22, and 22 mg/kg (ppm), respectively, as estimated from samples 9 to 11. The concentrations of these elements in sediments collected from the windward breakwater were significantly higher (compared to beach sediment). Specifically, in sample no. 3, which was taken from the spot where vessel stripping and repainting take place, the loads of copper, zinc, and lead were about 5, 12, and 31 times higher than the respective mean loads of beach sediments. Sample no. 4 was taken from the northeastern side of the breakwater (Fig. 5), which is heavily affected by waves. In this sample, copper, zinc, and lead loads, about two, four, and three times higher than the beach sediment respectively, were observed (Table 4). Samples no. 1 and no. 2 were collected from the westernmost part of the breakwater; their relatively low metal loads are probably attributed to the exposure of this breakwater part to wave overtopping during storms. Samples no. 5 and 6 were taken from the upper western part of the port, and slightly elevated zinc and lead were observed. Finally, samples no. 7 and no. 8 were taken from the accreted sediment of the eastern breakwater, which bounds the beach. Elevated zinc loads were observed in sample no. 7, about three times higher than beach sediment.
Overall, it was observed that vessel stripping and repainting works, taking place at the breakwaters of Nea Chora port, result to elevated copper, zinc, and lead concentrations in the area, thus posing stresses to sediment-dwelling organisms and the ecosystem. These loads are attributed to the dyes, pigments, and other chemicals, especially those that are applied in the hulls of the vessels, used to enhance antifouling capabilities. Heavy metals are scattered in the area by aeolian forces, waves, and precipitations, until they reach sediments, where they are redistributed throughout the beach profile. Finally, the observed heavy metal loads were found to be in agreement with the measurements taken in the same area a few years before (Foteinis et al. 2013), indicating that this is a long-term problem that has not been addressed yet.
Conclusions
A versatile and robust approach to address the problem of reliable multielemental XRF analysis was studied. Standard solutions consisting of few trace elements were directly added into solid matrices. The concentrations of added as well as of non-added elements were estimated. Results were compared to the estimations of XRF quantitation software program TurboQuant, which is used for environmental applications
It was found that the direct addition of liquid standards into solid matrices of unknown composition can be a major asset, when samples are analyzed by means of XRF. The technique was applied in various solid matrices and elements could be measured down to the milligram per kilogram level. Non-added element concentrations were also calculated by interpolating or extrapolating (for neighboring elements) calibration equations. Results were reproducible and fitted very well with certified values. Hence, standard addition technique can be a useful tool for cross-validating results.
Standard addition to solid technique achieved more accurate results, yielding lower absolute errors than the widely used commercial XRF software, namely TurboQuant. Therefore, it is proposed that future XRF software applications include standard addition mode in order to further improve their accuracy.
The technique was successfully applied in solid samples with environmental interest, where high metal concentration variations are observed. It was found that ship and boat stripping and repainting works are responsible for elevated copper, zinc, and lead loads in the local environment, thus posing stresses to sediment-dwelling organisms and the ecosystem.
References
Alyazichi, Y. M., Jones, B. G., & McLean, E. (2015). Source identification and assessment of sediment contamination of trace metals in Kogarah Bay, NSW, Australia. Environmental Monitoring and Assessment, 187, 20.
Beckhoff, B., Kanngießer, B., Langhoff, N., Wedell, R., & Wolf, H. (2006). Handbook of practical X-ray fluorescence analysis. Berlin: Springer.
Bero, B. N., Braun, M. C., Knowles, C. R., & Hammel, J. E. (1995). Further studies using X-ray fluorescence to sample lead contaminated carpeted surfaces. Environmental Monitoring and Assessment, 36(2), 123–138.
Brown, R. J. C., & Gillam, T. P. S. (2012). On the generalised case of sequential standard addition calibration. Chemometrics and Intelligent Laboratory Systems, 110, 97–101.
Clark, H. F., Hausladen, D. M., & Brabander, D. J. (2008). Urban gardens: lead exposure, recontamination mechanisms, and implications for remediation design. Environmental Research, 107, 312–319.
Foteinis, S., Kallithrakas-Kontos, N.G., & Synolakis, C. (2013). Heavy metal distribution in opportunistic beach nourishment: a case study in Greece. The Scientific World Journal, 2013, 5 pages, Article ID 472149, doi: 10.1155/2013/472149.
Galloa, L., Corapia, A., Loppib, S., & Lucadamo, L. (2014). Element concentrations in the lichen Pseudevernia furfuracea (L.) Zopf transplanted around a cement factory (S Italy). Ecological Indicators, 46, 566–574.
Gałuszka, A. (2005). The chemistry of soils, rocks and plant bioindicators in three ecosystems of the holy cross mountains, Poland. Environmental Monitoring and Assessment, 110(1–3), 55–70.
Grieken, R. E., & Markowicz, A. A. (1993). Handbook of X-ray spectrometry: methods and techniques. New York: Marcel Dekker.
Hatzistavros, V. S., & Kallithrakas-Kontos, N. G. (2008). Trace element ink spiking for signature authentication. Journal of Radioanalytical and Nuclear Chemistry, 277(2), 399–404.
Isoyama, H., Okuyama, S., Uchida, T., Takeuchi, M., Iida, C., & Nakagawa, G. (1990). In-furnace standard addition method for the determination of trace metals in biological samples by electrothermal vaporization-inductively coupled plasma atomic emission spectrometry. Analytical Sciences, 6(4), 555–561.
Kankılıç, G. B., Tüzün, İ., & Kadıoğlu, Y. K. (2013). Assessment of heavy metal levels in sediment samples of Kapulukaya Dam Lake (Kirikkale) and lower catchment area. Environmental Monitoring and Assessment, 185(8), 6739–6750.
Lachance, G. R., & Claisse, F. (1995). Quantitative X-ray fluorescence analysis. Chichester: Wiley.
Mahapatra, N. S. (1987). An approach to the solution of matrix problems in the XRF analysis of lead–zinc ore by the use of an internal standard and mathematical corrections. X-Ray Spectrometry, 16(4), 171–176.
Meyer, M.C., Spötl, C., Mangini, A., & Tessadri R. (2012). Speleothem deposition at the glaciation threshold—an attempt to constrain the age and paleoenvironmental significance of a detrital-rich flowstone sequence from Entrische Kirche Cave (Austria). Palaeogeography, Palaeoclimatology, Palaeoecology, (319–320), 93–106.
Muia, L. M., & Grieken, R. (1991). Energy-dispersive x-ray fluorescence analysis of geological materials in borax beads using Tertian’s binary coefficient approach combined with internal standard addition. X-Ray Spectrometry, 20(4), 179–183.
NASA—National Aeronautics and Space Administration, Mars Science Laboratory/Curiosity, prepared by Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California, 07/2013.
Pind, N. (1984). Standard-addition procedure for the determination of traces of lead in solid samples by x-ray fluorescence spectrometry. Talanta, 31(12), 1118–20.
Sarris, A., Kokinou, E., Aidona, E., Kallithrakas-Kontos, N., Koulouridakis, P., Kakoulaki, G., Droulia, K., & Damianovits, O. (2009). Environmental study for pollution in the area of megalopolis power plant (Peloponnesos Greece). Environmental Geology, 58, 1769–1783.
Tertian, R., & Claisse, F. (1982). Principles of quantitative X-ray fluorescence analysis. London: Heyden.
Thompson, M., (2009). Standard additions: myth and reality. Amc technical briefs, Analytical Methods Committee, AMCTB No 37, ISSN 1757- 5958.
Trejos, T., Koons, R., Becker, S., Berman, T., Buscaglia, J., Duecking, M., Eckert-Lumsdon, T., Ernst, T., Hanlon, C., Heydon, A., Mooney, K., Nelson, R., Olsson, K., Palenik, C., Pollock, E. C., Rudell, D., Ryland, S., Tarifa, A., Valadez, M., Weis, P., & Almirall, J. (2013). Cross-validation and evaluation of the performance of methods for the elemental analysis of forensic glass by μ-XRF, ICP-MS, and LA-ICP-MS. Analytical and Bioanalytical Chemistry, 405(16), 5393–409.
Tsuji, K., Nakano, K., Takahashi, Y., Hayashi, K., & Ro, C. U. (2012). X-ray spectrometry. Analytical Chemistry, 84(2), 636–68.
Wolf, M., Lehndorff, E., Mrowald, M., Eckmeier, E., Kehl, M., Frechen, M., & Wulf, A. (2014). Black carbon: fire fingerprints in Pleistocene loess–palaeosol archives in Germany. Organic Geochemistry, 70, 44–52.
Wong, M. K., Gu, W., & Ng, T. L. (1997). Sample preparation using microwave assisted digestion or extraction techniques. Analytical Sciences, 13, 97–102.
Yamada, Y. (2010). X-ray fluorescence analysis by fused bead method for ores and rocks. The Rigaku Journal, 26(2), 15–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 488 kb)
Rights and permissions
About this article
Cite this article
Kallithrakas-Kontos, N., Foteinis, S., Paigniotaki, K. et al. A robust X-ray fluorescence technique for multielemental analysis of solid samples. Environ Monit Assess 188, 120 (2016). https://doi.org/10.1007/s10661-016-5127-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5127-4