Abstract
Groundwater is a highly important resource, especially for human consumption and agricultural production. This study offers an assessment of groundwater quality in the coastal areas of Sindh province in Pakistan. Fifty-six samples of groundwater were taken at depths ranging from 30 to 50 m. Bacteriological and physico-chemical analyses were performed using the Standard Methods for the Examination of Water and Wastewater. These were supplemented with expert interviews and observations to identify the usage of water and potential sources of pollution. The quality of the groundwater was found to be unsuitable for human consumption, despite being used for this purpose. The concentrations of sulfate and phosphate were well within the tolerance limits. Most critical were the high levels of organic and fecal pollution followed by turbidity and salinity. Metal concentrations (As, Ca, Cr, Cu, Fe, Mg, Mn, Ni, Pb, and Zn) were also determined, and Ni and Pb strongly exceeded health standards. The study stresses the need for significant improvements of the irrigation, sanitation, and sewage infrastructure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is a critical resource for Pakistan. More than 90 % of the total water withdrawal is used for the agriculture sector which contributes more than 25 % to the country’s gross domestic product (GDP) and offers employment for 45 % of the population (CIA 2014; WHO and UNICEF 2012). The demand for water will rise as the country’s population of currently about 194 million is projected to increase to 348 million in 2050 (Population Reference Bureau (PRB) 2014). At the same time, global climate change is likely to result in a more variable climate which could increase the frequency and severity of climate-related risks such as floods, storms, and droughts (McSweeney et al. 2012; Schilling et al. 2013). Today, the water situation, measured in total actual renewable water resources per capita, in Pakistan is considered “stressed” (FAO 2012). Around 2035, possibly earlier, Pakistan is likely to be a water “scarce” country (FAO 2012; PRB 2014). But especially for human and livestock consumption and agricultural production, it is not only the amount of water that matters but also its quality. According to official numbers, Pakistan has made significant progress in increasing the percentage of the population with access to improved drinking water sources from about 85 in 1990 to 91 in 2012 (CIA 2014; WHO and UNICEF 2012). But, the gap between urban and rural areas has not been closed. Access in rural areas is still lower by about 6 % (CIA 2014). Less than half of Pakistan’s population has access to sanitation facilities, and about 40 million practice open defecation (WHO and UNICEF 2012). Poor water quality is a major health risk in Pakistan. Khan et al. (2013) find that water contaminated with coliform bacteria was the main source of waterborne diseases like gastroenteritis, dysentery, diarrhea, and viral hepatitis in the Charsadda district of Khyber Pakhtunkhwa. Christoforidou et al. (2013) identify a relation between exposure to arsenic through drinking water and the occurrence and mortality of bladder cancer in Pakistan. Similarly, Kazi et al. (2009) find arsenic to cause melanosis and keratosis among villagers on the banks of Lake Manchar in Sindh province. Anwar and Rani (2013) analyze groundwater in Bahawalpur City and find arsenic to increase the risk of skin, lung, bladder, and kidney cancers in addition to possibly vascular disease, hypertension, and diabetes. It is hence important to assess the quality of groundwater in Pakistan. Nabeela et al. (2014) provide a recent review of water pollution and its impact on public health in Pakistan. Out of the 21 studies reviewed, only 7 were at least partly conducted in Sindh province and 5 of these studies focused on urban centers (Karachi and Khairpur). To address this geographical gap, the present study was conducted in rural parts of Sindh province.
The province is located in the southwest of Pakistan. On the eastern side, Sindh is bounded by the Thar desert, while the Kherthar mountains lie to its western side and the Arabian Sea to the south. The central area is dominated by fertile plains around the Indus River. Out of the 970-km-long coastline of Pakistan, Sindh’s coastline is about 270 km long (Siddiqui and Maajid 2004). The coastal belt of Sindh is highly vulnerable to climate-related risks (Schilling et al. 2013). Between 2000 and 2015, Pakistan experienced six major floods, each killing several hundreds of people and negatively impacting on groundwater through salinization (CRED 2015).
Fresh groundwater is available in only 10 % of Sindh’s land area, mostly in the form of perched aquifers in friable sandy or silty layers located under clay layers (Panhwar 2002). The depth of the water table varies between 10 and 30 m or even more. About 78 % of the irrigated land in Sindh lies on top of saline or brackish water which cannot be used for agriculture. The few groundwater reserves are overexploited, especially in coastal communities where groundwater is the only water resource available during periods of severe drought and unavailability of canal water. Most of the groundwater in the area is drawn from the left bank of the Indus (Azad 2003).
Because of the described scarcity of freshwater, the heavy reliance on groundwater, and the close connection between water quality and human health, it is important to assess the groundwater quality. This is particularly the case in the district of Thatta, located in the south of Sindh, where few studies on groundwater quality have been conducted. This paper aims to narrow this research gap by assessing the quality of groundwater in two coastal areas of Thatta. The study is important because its results help to answer questions on whether the quality of groundwater in the study area is suitable for human consumption, if humans consume the water, and what the sources of pollution are. Based on these insights, policy recommendations can be made. To arrive at these recommendations, the paper first introduces the materials and methods before the results are presented and discussed, and conclusions are drawn.
Materials and methods
The main methods of this study are bacteriological and physico-chemical analyses. These are supplemented by qualitative approaches, including observations and expert interviews. The following sections describe each method in detail as well as the study area.
Study area
Thatta is the coastal district of Sindh having an area of 17,355 km2. It is situated about 98 km east of Karachi. The district is one of the poorest and least educated in the country. With 36 %, Thatta has the lowest literacy rate in Sindh (UNDP 2012). Only a quarter of the district’s population is considered “economically active” (World Bank 2005). As there is no industry located in or nearby the study area, the majority of livelihoods is based on agriculture (Iqbal et al. 2009). The common crops are rice, wheat, and sugarcane (see also Junejo et al. 2010; Sultana et al. 2009). Owing to the scarcity of freshwater, the farmers prefer water-resistant crops.
The present study focuses on two subdistricts of Thatta, namely, Keti Bundar and Shah Bundar. Keti Bundar is located on one of the mouths of the Indus. The total area of Keti Bundar is approximately 610 km2, while that of Shah Bundar is 3074 km2. The quality of groundwater in both subdistricts is saline to brackish, indicating inland seawater progression. The groundwater table generally depends on rainwater infiltration that recharges the groundwater aquifers. Too little use of groundwater (or overirrigation from surface sources) leads to a rising water table and the risk of water logging and salinity, while overuse leads to lowering of water tables and the risk that extraction can become technically or economically infeasible.
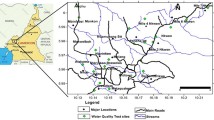
In total, 56 samples of groundwater were collected during the pre-monsoon (February to May) and monsoon (July to September) periods of 2012 and 2013. Figure 1 shows the location of the sampling sites. These samples were collected from the wells that were dug by hand to depths of only a few meters below the static water table. Many of these wells had no lining, and others were lined over only the uppermost section. The walls of several wells in the areas had collapsed, and the wells had been abandoned without backfilling. Along the river course, there were groups of several wells with separations of as little as 20 m. The dug wells are often surrounded by small dams (locally called bund) of excavated material that would restrict inflow of surface water. In some places, there was also a bush fence to prevent animals from falling into the well. Generally, however, there were no effective measures to prevent contamination of water in the dug wells.
The most common method for lifting water from a dug well is a diesel-powered centrifugal pump mounted on the ground or on a shelf within the well. Water is generally delivered through a galvanized steel pipe to a small (<1 m3) reservoir for distribution by gravity to irrigated fields via an unlined surface channel system. Water for domestic use is taken with small containers from the surface reservoir or a channel. Wells of smaller diameter were equipped with a simple rope and bucket for drawing water for domestic purposes.
Several boreholes (locally called tube wells) were also inspected in the area. These were said to have been drilled with diameters of around 300 mm to depths of around 100 m and constructed with 200 mm diameter casing and wire-wound screens. Boreholes were generally equipped with turbine pumps driven by a diesel engine. At few sites, an electric submersible pump was installed together with a diesel-electric generator set. Construction of the boreholes was suitable to prevent entry of surface water that could be a source of contamination. There are no water treatment plants present in the area.
The study area lies close to the coast where the uppermost layer (about 15 m) is made of sand, followed by clay underneath, and then the underlying bedrock that belongs to lower Goru formation of early Cretaceous period. The recharging of groundwater through rain is very low, owing to a low precipitation rate in the area which hardly exceeds 200 mm annually. The predominant source of groundwater recharge is the Indus River. However, when the flow of the Indus is low, which commonly happens, the process may be reversed leading to downstream aquifers being recharged by seawater of the Arabian Sea. This renders water unsuitable for human consumption.
Geomorphologically, a shallow aquifer system exists in the area that may have a variable thickness as it is common in neighboring district Sajawal and Jati subdistrict of district Thatta (Bablani and Soomro 2006).
The first step of sampling strategy was to divide the groundwater resource into two subunits (Keti Bundar and Shah Bundar). The sampling sites were then prioritized based on their proximity to human use and consumption. In each subunit, the sampling points were chosen at distances of 500 to 1000 m from human settlements except from a few sampling points nearby the river.
For the chemical and bacteriological analyses, the water samples were collected in pre-sterilized glass bottles to avoid possible contamination from the outside environment. Water samples were stored in a refrigerator as soon as possible after collection, but in some cases, the delay was several hours. They were transported to the Institute of Environmental Studies, University of Karachi, in insulated containers for the following analysis.
Bacteriological analysis
The public health quality of water samples was assessed using the following parameters: total aerobic count (TAC), total coliform count (TCC), total fecal coliforms (TFCs), and total fecal streptococci (TFSs). The samples were processed in laminar flow hoods using sterilized culture media. The sterility of media was checked prior to use. The TCCs were estimated using lactose broth (Merck, Germany) of single and double strengths. The positive lactose tubes were used for the determination of fecal coliforms (FCs) using EC broth (Merck, Germany). TFSs were determined by using sodium azide broth. The bacterial load of water samples was estimated by most probable number (MPN) technique as per Standard Methods for the Examination of Water and Wastewater (American Public Health Association (APHA) 2005).
Physico-chemical analysis
The samples were analyzed for pH, biological oxygen demand (BOD5), chloride, hardness as CaCO3, sulfate, nitrate, phosphate, turbidity (nephelometric turbidity unit (NTU)), total dissolved solids (TDSs), and total suspended solids (TSSs). pH and dissolved oxygen (DO) were determined on site. pH was determined by Hanna portable pH meter (HI98107), while DO was measured by DO meter (Jenway 970, England). Biological oxygen demand was measured using azide modification method as mentioned in the Standard Methods for the Examination of Water and Wastewater (APHA 2005). Chloride was estimated by argentometric method (APHA 2005). Hardness as CaCO3 of water samples was measured by EDTA titrimetric method (APHA 2005). Sulfate, TDS, and TSS in the samples were estimated by gravimetric method in accordance with Standard Methods for the Examination of Water and Wastewater (APHA 2005). Turbidity was ascertained by EUTECH meter, model no. TN-100.
The nutrient parameters such as nitrate and phosphate were determined by brucine method and ascorbic acid method, respectively, as outlined in Standard Methods for the Examination of Water and Wastewater (APHA 2005). The samples were also analyzed for Ca and Mg and for heavy metals including As, Cr, Cu, Fe, Pb, Ni, and Zn using appropriate kits on a Merck NOVA 60, Germany.
The data were statistically analyzed using STATISTICA software. Descriptive statistics including mean, median, minimum and maximum values, quartile range, standard deviation, standard error, and skewness were computed for each of the variables. Principal component analysis and cluster analysis were performed using the appropriate software mentioned above. For cluster analysis, Ward’s method was employed. Principal component analysis was applied on the normalized data sets of physical, chemical, and microbiological characteristics of groundwater.
Observations and interviews
Participating and non-participating observations were conducted in the research area focusing on how the water was extracted by the community members and for what it was used (e.g., direct consumption or agriculture). A total of 198 interviews were conducted with local farmers, government officials, and representative of non-governmental organizations (NGOs) to gain qualitative insights into usage, pollution, and further issues related to water quality in Keti Bundar and Shah Bundar.
Results
Bacteriological and physico-chemical analyses
The results of bacteriological and physico-chemical analyses are reported in Table 1. Values for pH of all the samples range between 7.0 and 7.9, while the mean of all the samples is 7.32. Only one water sample shows a high pH of 7.9 (S-4). This sample was collected from a dug well in Hashim Mallah Goth. The pH of the samples is more towards alkaline.
The main indices generally used to determine the organic pollution load in an aqueous system are biochemical oxygen demand (BOD), chemical oxygen demand (COD), and total organic carbon (TOC). BOD represents the biodegradable portion of the organic pollutants (Liu et al. 2005). BOD values ranged between 10 and 76 mg/l with an average of 28.91 mg/l.
The concentration of chloride and hardness are higher as compared to the National Standards for Drinking Water Quality (NSDWQ). The former ranges from 100 to 1050 mg/l, while hardness is in the range of 336 to 1220 mg/l. Samples taken near the coast have exceptionally higher levels of chloride and hardness (see Figs. 1 and 2).
The concentrations of sulfate and nitrate are well within the limit of NSDWQ, indicating no risk to human health. The mean value of phosphate is 1.51 mg/l. No standard value of phosphate is given in NSDWQ.
All the water samples are reportedly turbid and have crossed the turbidity limit as reported in NSDWQ. The concentration of TDS ranged between 291 and 5230 mg/l with an average of 1099 mg/l. TSS concentration ranged between 80 and 680 mg/l. The mean value of all the samples is 150.3 mg/l.
Arsenic concentrations were well below critical thresholds (Table 1). Maximum permissible limit of Ca and Mg is not reported in NSDWQ. Concentration of Ca ranged between 20.1 and 90.5 with an average of 44.74 mg/l. The concentration of Mg was relatively higher. It ranged between 265 and 761 with an average of 512.1 mg/l. Both Ca and Mg are responsible for imparting hardness in water. It is interesting to note that the samples also contain Cr while there is no industrial activity in and around the area. Moreover, no tannery is located in the vicinity. The concentration of Cr ranged between 0.01 and 0.17 with an average of 0.04 mg/l. Its maximum permissible limit according to NSDWQ is 0.05 mg/l.
The maximum permissible limit of Cu is 2.0 mg/l as per NSDWQ. Table 1 shows that the concentration of Cu is relatively low with an average of 0.022 mg/l. A limit of Fe is not given in NSDWQ. As such, it will not be responsible for causing any serious health problem. The concentration of Mn in all the samples ranged between 0.21 and 0.96 mg/l, which is relatively higher as compared to NSDWQ (<0.05 mg/l). Similarly, the concentration of Ni on an average is 1.38 mg/l of the water samples, whereas the limit as per NSDWQ is only <0.02 mg/l.
The average Pb concentration of 0.204 mg/l was four times higher than the NSDWQ value. In contrast, the concentration of Zn is not a matter of concern from a human health perspective. It was found to be ranging between 0.54 and 1.69 mg/l. The mean concentrations of the heavy metals were found to be in the following order: Ni > Zn > Mn > Fe > Pb > Cr > Cu > As.
It may be noted that none of the well water samples met the WHO (2011) guidelines with respect to public health quality as mentioned in Table 1. The TAC, TCC, and TFC were exceptionally higher as compared to WHO guidelines. The number of fecal streptococci was relatively low in all samples. Since the numbers of fecal coliforms and fecal streptococci discharged by human beings and animals are significantly different, it is suggested that the ratio of FCs to fecal streptococci (FSs) count in a given sample can be used to detect whether the suspected streptococci are derived from human or from animal wastes. The FC/FS ratio in water of animal origin is generally considered to be less than 1.0, while it is more than 4.0 for human beings. If the ratio is within 1 to 2, the interpretation is uncertain. This ratio is helpful in determining the source of pollution (Tchobanoglous and Burton 1991).
Groundwater levels
In many areas, there were groups of wells with a separation distance of about 50 m. Local communities reported that when one well was pumped, the water levels fell in nearby wells. It has been observed that the groundwater is overexploited, which has resulted in lowering of the water table. Depletion of groundwater is mostly observed in those areas where surface water is not available and the crop cultivation is largely dependent on tube well irrigation. Groundwater pumping can lead to strong lowering of the groundwater table. Because tapping of groundwater resources is often not registered or metered, it is not known how much groundwater is withdrawn and where the extractions occur. Due to a climatologic water deficit, there is an overexploitation of groundwater mainly from the farmers in the form of private tube wells.
Principal component analysis
Principal component analysis (PCA) was applied on the normalized data set of physical, chemical, and microbiological characteristics of water samples (Table 2). The first three components together explained 38.26 % of the total variance. The first component that explained 17.21 % of the total variance was largely a function of chemical and microbiological variates including nitrate, Mn, TAC, and TDSs. The second component explaining 11.93 % is chiefly regulated by phosphate, Cu, Zn, and total aerobic count. The third component retaining 9.11 % of the overall variability is principally governed by pH, phosphate, TFSs, and TCC. Apparently, each component is amalgam of chemical and microbiological factors.
Cluster analysis of chemical characteristics (Fig. 2) shows two main groups. Group 1 includes only one sample that is S-12. Group 2 includes 55 samples which can be divided into two subgroups. Group 2A includes 45 samples, while group 2B consists of 10 samples. The members of group 2A show relatively higher concentration of chloride, hardness, TDS, and turbidity, while group 2B shows relatively lower values of these characteristics.
The dendogram derived from the cluster analysis of metals indicates three main groups (Fig. 3). Group 1 further subdivided into 1A and 1B. Group 1 comprises of 16 samples, while group 1B consists of 17 samples. Group 1A showed relatively higher values of all the metals. Samples belonging to group 1A present higher values of Ca and Mg and were generally close to the coast. Group 1B represented sample of relatively lower values of Ca and Mg. Group 2 which consists of seven samples is generally high in Ni. Group 3 which comprises of 16 samples is characterized by high levels of Zn.
The dendogram derived from microbiological analysis of water samples shows two main groups (Fig. 4). Comparison of group 1 and group 2 derived from cluster analysis shows that the samples of group 2 are away from the coastline while those of group 1 belongs to samples collected near the coast. Group 1 is characterized by high values of TAC, TCC, TFC, and TFS, while those of group 1 shows relatively lower values of TFS. However, in both groups, the water samples are heavily polluted with organic load.
Discussion
The quality of almost all groundwater samples taken is not suitable as potable water with respect to NSDWQ and WHO guidelines. However, the interviews and observations indicate that humans drink from the analyzed water sources.
The mean pH value of all the samples was 7.32, which means that it is pH neutral and well within the higher desirable limit (HDL). The value is at the lower end of values reported by Memon et al. (2011), ranging from 7.1 to 9.9.
The high average BOD concentration (28.91 mg/l) can be attributed to high suspended solid concentration, which is an indication of organic pollution. As the groundwater is also used for drinking purposes, the BOD concentration reflect high organic pollution load. The European Environment Agency (EEA 2015) reports that over 80 % of rivers in northern Europe have a biochemical oxygen demand of 2.0 mg/l, which indicates a relatively clean river. Given this figure, all water samples are heavily polluted with organic load. High BOD values in the underground water samples clearly show that most of these wells were unprotected. Covering of the well using lids is uncommon in the area. However, these wells do not have concrete linings. In general, these wells serve as public utilities. The possible sources of high BOD values could be the seepage of domestic wastewater as the study area has poor sewerage infrastructure and the use of open spaces for defecation is quiet common. Another possible source of BOD could be the indiscriminate disposal of solid waste, which undergoes microbial decomposition and thus increases organic load. In a study on Nigeria, Adekunle et al. (2007) reported BOD values of groundwater ranging from 1.20 to 3.59 mg/l. However, the authors concluded that the BOD value decreased with an increasing distance to dump sites. A high BOD level tends to reduce dissolved oxygen concentration because oxygen is consumed by bacteria creating anoxic conditions. High BOD could also be attributed to high suspended solid concentration.
The concentration of chloride and hardness are higher compared to the NSDWQ. The former ranges from 100 to 1050 mg/l, while hardness is in the range of 336 to 1220 mg/l. Samples taken near the coast have exceptionally higher levels of chloride and hardness (see Figs. 1 and 2). High concentrations of chloride and hardness are an indication of seawater intrusion that is responsible for groundwater aquifer contamination. Bablani et al. (2006) reported that seawater intrusion is responsible for the pollution of all the groundwater systems in Shah Bundar area even up to the shallow depth, since surface water resources mainly in the form of River Indus and its canal system are highly unpredictable. People mostly rely on groundwater resources which have tremendously increased the rate of withdrawal of groundwater. Excessive extraction of groundwater thus allows vertical and lateral movement of seawater into freshwater aquifers. High concentrations of chlorides are attributed to the movement of seawater inland. Memon et al. (2011) reported hardness values as high as 6720 mg/l from the dug wells of the neighboring district Thar. The authors also reported high hardness values in the underground water of Thatta and Badin districts. Bablani et al. (2006) found aquifer resistivities that ranged from 0.27 to 0.66 Ωm. This confirms the progression of seawater inland, which in turn is responsible for increased groundwater salinity. These results are in line with the findings of present research which finds a very high value of Cl concentration (on average 635 mg/l) in the groundwater samples. In fact, Cl is a predominant ion of seawater. Further, high concentrations of chloride and hardness are associated with water logging and increased salinity. These issues together with already existing brackish groundwater in the study area are compromising the sustainability of agriculture.
Water logging and salinity are likely to be caused by poor irrigation systems (Chandio et al. 2012). The situation is further aggravated by the topography of the area, seepage from unlined canals, and the absence of adequate drainage systems. Azad (2003) suggested that the increase in irrigated area is responsible for rise in the salinity of soil in Sindh. Other studies found that the salinity problem along the coastline of Sindh is mainly attributed to capillary rise in groundwater, seawater intrusion, and flooding (Khan et al. 2008; Schilling et al. 2013). Qureshi et al. (2008) point to the poor operation and maintenance of drainage systems in contributing to salinization. Chaniho et al. (2010) evaluated the extent of soil and groundwater salinities in the coastal district Badin, Sindh. The authors reported that the soils of the area are affected in nature by salty groundwater. Salinity and water logging remain serious problems in irrigated areas of Sindh, particularly in the study area where much of the groundwater is naturally saline (of marine origin) and thus unsuitable for irrigation.
The mean sulfate concentration of the samples was 151.2 mg/l, which means that it does not pose a health risk. Sulfate concentrations in groundwater are not a concern in Pakistan in general groundwater (see Iqbal et al. 2009; Ullah et al. 2009).
The mean value of nitrate in the groundwater samples was 0.24 mg/l. In Pakistan, groundwater generally contains low levels of nitrate. However, higher values may be attained due to agriculture runoff (Pakistan Environmental Protection Agency 2005). Tahir and Rasheed (2008) already pointed out that out of 747 samples, which they have collected from all over the country, only 19 % of the samples had nitrate concentrations beyond the standard limits. They further pointed out that both Sindh and Punjab generally have higher levels of nitrate concentration compared to other parts of the country. It may be noted that concentration of nitrate is usually higher when the sowing seasons start and when the farmers indiscriminately apply inorganic fertilizers. Other sources of nitrate could be the manure of biological origin. Water contaminated with the nitrate is responsible for “methemoglobinemia” commonly known as the “blue baby syndrome.” A maximum permissible limit of phosphate is not available in NSDWQ. The possible source of phosphate could be the inorganic fertilizers which are indiscriminately used in the research area.
The high turbidity found in this study is similar to levels found in Sindh by Memon et al. (2011). The high turbidity is mainly due to the presence of high suspended solids. These suspended solids may include a wide variety of materials, such as silt, decaying plant, and animal matter that can increase the turbidity of water. If they are of biological origin, they would tend to increase the biological oxygen demand. Mahmood et al. (2014) reported turbidity values of surface water samples in the area ranging from 5.1 to 5.5 NTU in pre-monsoon period and 6.9 to 8.7 NTU in post-monsoon period.
The water samples having TDS concentration depreciate the aesthetic quality of water which may change the taste. Water above 1500 mg/l of TDS is generally considered brackish. In the present study, TDS values range between 291 and 5230 mg/l. High TDS values are reported in the samples close to the coast. In general, the quality of water is saline to brackish. Ullah et al. (2009) already reported that because of limited availability of freshwater in Sindh, people have no choice except to drink brackish water. This further confirms the findings that the sea is progressing towards land. Memon et al. (2011) also reported very high values (440 to 8300 mg/l) in dug wells of neighboring Thar district. The present study is in line with the findings of Rafique et al. (2008).
The concentration of TSS is relatively higher ranging from 80 to 680 mg/l, which indicates organic pollution mainly of anthropogenic origin. This is critical as the interviews and observations suggest that most of the water sources analyzed are used for human consumption.
Hardness in underground water samples is mainly due to the presence of Ca and Mg ions originated from the limestone that are present in abundance in the sedimentary rocks available in the area. The mean values of Ca and Mg of the samples were 44.74 and 512 mg/l, respectively. Farah et al. (2002) also reported similar results of underground water samples of Faisalabad district in Punjab.
Ahmed et al. (2004) reported that approximately 16 to 36 % of the population in Sindh consumes water containing As with the concentration ranging between 10 and 50 μg/l. Like other South Asian countries, Pakistan is also facing severe public health adversities due to arsenic-contaminated water. Ahmad et al. (2004) reported that in Sindh, around 16 to 36 % of the population is exposed to high arsenic levels in groundwater. Baig et al. (2009) reported As values in the groundwater of Jamshoro district Sindh well within the limits. The mean As value of the sample was 0.005 mg/l, which is well within the limits as per NSDWQ.
The mean Cr concentration in the samples is below the maximum permissible limit of Cr (0.05 mg/l) as per NSDWQ. The low Cr concentrations reflect the absence of industry in the study area. The Pakistan Council for Research in Water Resources (2005) documented that only 1 % of groundwater samples in Pakistan contain Cr higher than the safe limit.
The mean Cu concentration in the sample was 0.022 mg/l, which is well within the limits as per NSDWQ. Cu is generally found in low concentration in both surface water and groundwater resources in Pakistan (Azizullah et al. 2011).
Iron is often considered as one of the major pollutants of drinking water resources in the country (Azizullah et al. 2011). The mean value of Fe in the sample was 0.347 mg/l, which is slightly above the WHO maximum permissible limit of 0.3 mg/l. The Pakistan Council for Research in Water Resources (2005) found elevated concentration of Fe in 28 % of groundwater samples across Pakistan.
In Pakistan, as such Mn does not pose a significant problem (Azizullah et al. 2011). In the present study, average Mn concentration is 0.45 mg/l, which is below the NSDWQ limit (0.5 mg/l). The highest Mn of 0.96 mg/l was found in the village Allah Bachio, Shah Bundar (see S-7 in Fig. 1). There are no industrial sites in the vicinity of Allah Bachio. It is therefore likely that the high Mn concentration is of natural, rather than anthropogenic origin. But, more research is needed to identify the specific source of Mn pollution. Even higher levels of Mn were reported by Midrar-Ul-Haq et al. (2005), who found a concentration of 2.56 mg/l in groundwater from Khyber Pakhtoonkhwa. Similarly, Mahmood and Maqbool (2006) reported 1.06 mg/l in water samples from Faisalabad. In these locations, however, the source of Mn is likely to be caused by industrial activity.
Naturally occurring Ni is present at low levels in water where it is found as a divalent cation with pH ranging from 5 to 9. The maximum allowable concentration of Ni as per WHO guidelines is 0.02 mg/l, while that of NSDWQ reported a limit of <0.02 mg/l in drinking water. In Pakistan, Ni concentration varies from 0 to 3.66 mg/l in groundwater (Azizullah et al. 2011). The highest concentration in the country was reported in the water samples from Khyber Pakhtoonkhwa (Midrar-Ul-Haq et al. 2005). The mean Ni value of the samples was 1.38 mg/l, which is exceptionally higher as compared to WHO guidelines and NSDWQ. Since there is no industrial setup in the area, the possible source of Ni is likely to be natural rather than anthropogenic. Sedimentary rocks could be one source. People consuming water with high concentration of Ni may be exposed to heart and lung diseases, respiratory cancer, and kidney problems (Seilkop and Oller 2003).
The mean Pb concentration in the groundwater samples was 0.204 mg/l, which is exceptionally higher as compared to NSDWQ (0.05 mg/l). Across Pakistan, Pb concentration in groundwater varies from 0.001 to 2.0 mg/l (Azizullah et al. 2011). Ullah et al. (2009) reported that all of the water samples from district Sialkot have higher Pb values than the critical level of 0.01 mg/l. The possible sources of Pb in the study area could be the household paint and emissions from vehicle (Nadeem-ul-Haq et al. 2009). Overexposure to Pb may cause malfunctioning of nervous, cardiovascular, digestive, reproductive, and immunological systems (Riess and Halm 2007; Gidlow 2004).
In the present study, the mean Zn concentration was 1.22 mg/l, which is well below the maximum permissible limit as reported by WHO (2011).
With respect to public health quality of drinking water, the most serious problem is bacteriological contamination, which is responsible for severe health problems (Pakistan Council for Research in Water Resources 2005). Even in cities, the problem is of acute nature (Faruque and Nai 2008; Shar et al. 2008; Mumtaz et al. 2011). In Islamabad, the capital of Pakistan, the people are at risk of consuming contaminated water (Mashiatullah et al. 2010). Underground water contamination is likely to occur mainly due to surface runoff, leakage from wastewater disposal systems, and indiscriminate disposal of wastewater (Pakistan Council for Research in Water Resources 2005). Bacterial contamination in rural areas is usually expected to be higher than in urban areas (Azizullah et al. 2011). Bacteriological contamination of groundwater may lead to a number of waterborne diseases like diarrhea, typhoid, dysentery, and gastroenteritis. As reported earlier, all water samples were heavily polluted with organisms of public health concern. It may be however noted that TCC is not alone associated with ailments (Barrell et al. 2000), but the presence of coliform in water is an indication of pathogenic organisms (Aziz 2005). As such, fecal contamination indicates the contamination of drinking water by human and animal wastes (Faruque and Nai 2008). According to WHO (2011), the TCC and TFC should be zero per 100 ml of water, but in the present study, the water quality is of an alarming poor level. A high percentage of the population is suffering from waterborne diseases as they have no choice except to consume contaminated water.
The reason for the fecal contamination could be the anthropogenic activities near the reservoir. Aziz (2005) reported that most of the drinking water supplies in Pakistan are contaminated by fecal coliforms, which results in high incidence of waterborne diseases. In general, during the rainfall and flood conditions, the microbial load of the flowing water increases, which in turn decreases the water quality.
Conclusion
The aim of this paper was to assess the quality of groundwater in the coastal areas of Sindh. Bacteriological and physico-chemical analyses show that almost all samples were not suitable for human consumption. However, interviews and observations indicate that the polluted water sources are used by humans as drinking water. This is critical as previous studies have pointed to the health risks associated with the consumption of unsafe water (Khan et al. 2013; Nabeela et al. 2014). Organic and fecal pollution followed by turbidity and salinity are the central concerns, while levels of Ni and Pb also strongly exceed health standards. It is hence important to stop the local population from consuming polluted water and to provide safe alternatives. Groundwater pollution by organic and fecal materials can only be addressed with significant improvements of the irrigation, sanitation, and sewage infrastructure. This paper points to some of the locations where such infrastructure is needed. Further research should determine where exactly the infrastructure, including water treatment plants, would be best placed. Research into how farmers can be incentivized to refrain from overexploitation of groundwater is promising to reduce salinization. This is particularly important in the coastal areas where global climate change is likely to increase the frequency of floods and storms.
References
Adekunle, I., Adetunji, M., Gbadebo, A., & Banjoko, O. (2007). Assessment of groundwater quality in a typical rural settlement in southwest Nigeria. International Journal of Environmental Research and Public Health, 4(4), 307.
Ahmed, R., Viqar-Un-Nisa, Hussain, M., Tanwir, R., & Qureshi, S. A. (2004). Monitoring of fluoride and iodide levels in drinking water using ion selective electrodes. Nucleus, 41(1–4), 51–58.
Anwar, M. M., & Rani, M. (2013). Ground drinking water and its consequences on health of residents; a case study of selected areas in Bahawalpur City. [Article]. Sindh University Research Journal-Science Series, 45(3), 524–528.
APHA. (2005). Standard methods for the examination of water and wastewater. Washington DC: American Public Health Association.
Azad, A. (2003). Sindh water resources management: issues and options. Rome: FAO.
Aziz, J. A. (2005). Management of source and drinking water quality in Pakistan. Eastern Mediterranean Health Journal, 11(5–6), 1087–1098.
Azizullah, A, Khattak, MNK., Richter, P, & Häder, D-P. (2011). Water pollution in Pakistan and its impact on public health—a review. Environment International, 37(2), 479–497. doi:http://dx.doi.org/10.1016/j.envint.2010.10.007.
Bablani, S. A., & Soomro, S. A. (2006). Evaluation of seawater intrusions in left bank sediments of coastal district Thatta, Sindh, Pakistan. Paper presented at the 1st SWIM-SWICA Joint Saltwater Intrusion Conference, 24–29 September 2006
Baig, JA, Kazi, TG, Arain, MB, Afridi, HI, Kandhro, GA, Sarfraz, RA, et al. (2009). Evaluation of arsenic and other physico-chemical parameters of surface and ground water of Jamshoro, Pakistan. Journal of Hazardous Materials, 166(2–3), 662–669, doi:http://dx.doi.org/10.1016/j.jhazmat.2008.11.069.
Barrell, R. A., Hunter, P. R., & Nichols, G. C. D. P. H. (2000). Microbiological standards for water and their relationship to health risk. Communicable Disease and Public Health, 3(1), 8–13.
Chandio, AS, Lee, TS, & Mirjat, MS. (2012). The extent of waterlogging in the lower Indus basin (Pakistan)—a modeling study of groundwater levels. Journal of Hydrology, 426–427(0), 103–111, doi:http://dx.doi.org/10.1016/j.jhydrol.2012.01.017.
Chaniho, H. B., Rajpar, I., Talpur, U., Sial, N., & Zia-ul-Hassan. (2010). Evaluating soil and groundwater salinity in Taluka Tando Bago, Sindh. Journal of Agriculture Engineering and veterinary Sciences, 26(2), 19–26.
Christoforidou, E. P., Riza, E., Kales, S. N., Hadjistavrou, K., Stoltidi, M., Kastania, A. N., et al. (2013). Bladder cancer and arsenic through drinking water: a systematic review of epidemiologic evidence. Journal of Environmental Science and Health Part a-Toxic/Hazardous Substances & Environmental Engineering, 48(14), 1764–1775. doi:10.1080/10934529.2013.823329.
CIA (2014). The world factbook—Pakistan. https://www.cia.gov/library/publications/the-world-factbook/geos/pk.html. Accessed 2 December 2014.
CRED (2015). Country profile Pakistan. www.emdat.be/result-country-profile. Accessed 2 February 2015.
EEA (2015). Oxygen consuming substances in rivers. http://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/oxygen-consuming-substances-in-rivers-7. Accessed 15 February 2015.
FAO (2012). Aquastat—Pakistan. http://www.fao.org/nr/water/aquastat/countries_regions/pak/index.stm. Accessed 2 December 2014.
Farah, N., Zia, M. A., Khalil-ur-Rehman, & Sheikh, M. A. (2002). Quality characteristics and treatment of drinking water of Faisalabad City. International Journal of Agriculture & Biology, 4(3), 347–349.
Faruque, S. M., & Nai, G. B. (Eds.). (2008). Vibrio cholerae: genomics and molecular biology. Norfolk: Caister Academic Press.
Gidlow, D. A. (2004). Lead toxicity. Occupational Medicine, 54(2), 76–81. doi:10.1093/occmed/kqh019.
Iqbal, U., Qasim, H., Khan, A. K., Rashid, R., Nasreen, S., Mahmood, Q., et al. (2009). Surface and ground water quality risk assessment in district Attock Pakistan. World Applied Sciences Journal, 7(8), 1029–1036.
Junejo, S., Kaloi, G. M., Panhwar, R. N., Chohan, M., Junejo, A. A., & Soomro, A. F. (2010). Performance of some newly developed sugarcane genotypes for some quantitative and qualitative traits under Thatta conditions. Journal of Animal & Plant Sciences, 20(1), 40–43.
Kazi, T. G., Arain, M. B., Baig, J. A., Jamali, M. K., Afridi, H. I., Jalbani, N., et al. (2009). The correlation of arsenic levels in drinking water with the biological samples of skin disorders. [Article]. Science of the Total Environment, 407(3), 1019–1026. doi:10.1016/j.scitotenv.2008.10.013.
Khan, S., Rana, T., Gabriel, H., & Ullah, M. (2008). Hydrogeologic assessment of escalating groundwater exploitation in the Indus basin, Pakistan. [Article]. Hydrogeology Journal, 16(8), 1635–1654. doi:10.1007/s10040-008-0336-8.
Khan, S., Shahnaz, M., Jehan, N., Rehman, S., Shah, M. T., & Din, I. (2013). Drinking water quality and human health risk in Charsadda District, Pakistan. [Article]. Journal of Cleaner Production, 60, 93–101. doi:10.1016/j.jclepro.2012.02.016.
Liu, L., Ji, H., Liu, Y., & Xin, H. (2005). Chemical oxygen demand of seawater determined with a microwave heating method. Journal of Ocean University of China, 4(2), 152–156. doi:10.1007/s11802-005-0009-3.
Mahmood, S., & Maqbool, A. (2006). Impacts of wastewater irrigation on water quality and on the health of local community in Faisalabad. Pakistan Journal of Water Resources, 10(2), 19–22.
Mahmood, K., Alamgir, A., & Khan, M. A. (2014). Seasonal variation in water quality of lower Sindh, Pakistan. FUUAST Journal of Biology, 4(2), 147–156.
Mashiatullah, A., Chaudhary, M. Z., Khan, M. S., Javed, T., & Qureshi, R. M. (2010). Coliform bacterial pollution in Rawal Lake, Islamabad and its feeding streams/river. Nucleus, 47(1), 35–40.
McSweeney, C, New, M, & Lizcano, G. (2012). UNDP climate change country profiles—Pakistan. http://www.geog.ox.ac.uk/research/climate/projects/undp-cp/index.html?country=Pakistan&d1=Reports. Accessed 2 December 2014.
Memon, M., Soomro, M. S., Akhtar, M. S., & Memon, K. S. (2011). Drinking water quality assessment in southern Sindh (Pakistan). [Article]. Environmental Monitoring and Assessment, 177(1–4), 39–50. doi:10.1007/s10661-010-1616-z.
Midrar-Ul-Haq, Khattak, R. A., Puno, H. K., Saif, M. S., & Memon, K. S. (2005). Surface and groundwater contamination in NWFP and Sindh provinces with respect to trace elements. International Journal of Agriculture & Biology, 7(2), 214–217.
Mumtaz, M. W., Adnan, A., Mukhtar, H., Nawaz, K., Raza, A., & Ahmad, Z. (2011). Estimation of bacteriological levels in surface water samples to evaluate their contamination profile. Environmental Monitoring and Assessment, 172(1–4), 581–587. doi:10.1007/s10661-010-1357-z.
Nabeela, F., Azizullah, A., Bibi, R., Uzma, S., Murad, W., Shakir, S. K., et al. (2014). Microbial contamination of drinking water in Pakistan—a review. Environmental Science and Pollution Research, 21(24), 13929–13942. doi:10.1007/s11356-014-3348-z.
Nadeem-ul-Haq, A. M., Haque, Z., Badar, N., & Mughal, N. J. (2009). Drinking water contamination by chromium and lead in industrial lands of Karachi. Journal of Pakistan Medical Association, 59(5), 270–274.
Pakistan Council for Research in Water Resources. (2005). Water quality report 2003–2004. Islamabad: Pakistan Council for Research in Water Resources.
Pakistan Environmental Protection Agency. (2005). State of environment report 2005. Islamabad: Government of Pakistan.
Panhwar, M. H. (2002). Water requirements of riverine areas of Sindh. Hyderabad: Sindh Educational Trust.
PRB (2014). 2014 world population data sheet. http://www.prb.org/Publications/Datasheets/2014/2014-world-population-data-sheet/data-sheet.aspx. Accessed 3 December 2014.
Qureshi, A. S., McCornick, P. G., Qadir, M., & Aslam, Z. (2008). Managing salinity and waterlogging in the Indus basin of Pakistan. [Review]. Agricultural Water Management, 95(1), 1–10. doi:10.1016/j.agwat.2007.09.014.
Rafique, T., Naseem, S., Bhanger, M., & Usmani, T. (2008). Fluoride ion contamination in the groundwater of Mithi sub-district, the Thar desert, Pakistan. Environmental Geology, 56(2), 317–326. doi:10.1007/s00254-007-1167-y.
Riess, M. L., & Halm, J. K. (2007). Lead poisoning in an adult: lead mobilization by pregnancy? Journal of General Internal Medicine, 22(8), 1212–1215. doi:10.1007/s11606-007-0253-x.
Schilling, J., Vivekananda, J., Nisha, P., & Khan, M. A. (2013). Vulnerability to environmental risks and effects on community resilience in mid-west Nepal and south-east Pakistan. Environment and Natural Resources Research, 3(4), 1–19.
Seilkop, SK, & Oller, AR. (2003). Respiratory cancer risks associated with low-level nickel exposure: an integrated assessment based on animal, epidemiological, and mechanistic data. Regulatory Toxicology and Pharmacology, 37(2), 173–190, doi:http://dx.doi.org/10.1016/S0273-2300(02)00029-6.
Shar, A. H., Kazi, Y., Zardari, M., & Soomro, I. H. (2008). Enumeration of total and fecal coliform bacteria in drinking water of Khairpur Sindh. Pakistan Journal of Medical Research, 47(1), 9–10.
Siddiqui, MN, & Maajid, S. (2004). Monitoring of geomorphological changes for planning reclamation work in coastal area of Karachi, Pakistan. Advances in Space Research, 33(7), 1200–1205, doi:http://dx.doi.org/10.1016/S0273-1177(03)00373-9.
Sultana, H., Ali, N., Iqbal, M. M., & Khan, A. (2009). Vulnerability and adaptability of wheat production in different climatic zones of Pakistan under climate change scenarios. Climatic Change, 94(1–2), 123–142. doi:10.1007/s10584-009-9559-5.
Tahir, M. A., & Rasheed, H. (2008). Distribution of nitrate in the water resources of Pakistan. African Journal of Science and Technology, 2(11), 397–403.
Tchobanoglous, G., & Burton, F. L. (1991). Wastewater engineering: treatment disposal reuse. New York: McGraw-Hill.
Ullah, R., Malik, R. N., & Qadir, A. (2009). Assessment of groundwater contamination in an industrial city, Sialkot, Pakistan. African Journal of Science and Technology, 3(4), 429–446.
UNDP. (2012). Report on the status of millennium development goals, Sindh. Islamabad: UNDP.
WHO. (2011). Guidelines for drinking-water quality. Geneva: WHO.
WHO & UNICEF. (2012). Progress on drinking water and sanitation. New York: WHO, UNICEF.
World Bank. (2005). Socioeconomic study and proposal for livelihood improvements: Badin and Thatta districts, Sindh, Pakistan. Islamabad: World Bank.
Acknowledgments
The authors thank the reviewers for their helpful comments. This work was conducted under the auspices of the Higher Education Commission of Pakistan. The authors gratefully acknowledge the financial support provided by the Higher Education Commission of Pakistan. The work has also partly been supported through the Cluster of Excellence “Integrated Climate System Analysis and Prediction—CliSAP,” Universität Hamburg, funded by the German Science Foundation (DFG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Alamgir, A., Khan, M.A., Schilling, J. et al. Assessment of groundwater quality in the coastal area of Sindh province, Pakistan. Environ Monit Assess 188, 78 (2016). https://doi.org/10.1007/s10661-015-5061-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-5061-x