Abstract
Total mercury (HgTOT), inorganic mercury (IHg), and methylmercury (MHg) were determined in dry season waters, sediments, and tailings from an active mine which has long history of gold exploitation. Although HgTOT in waters was generally low (0.03 to 19.60 ng L−1), the majority of the samples had proportions of MHg of at least 90 % of HgTOT which denotes a substantial methylation potential of the mine watersheds. Mercury was relatively high in tailing materials (up to 867 μg kg−1) and also in the mine sediments (up to 837 μg kg−1) especially in samples collected near tailing storage facilities and within a receiving water dam. Sediment profiles revealed mercury enrichment and enhanced methylation rate at deeper layers. The presence of IHg and decaying plants (organic matter) in the watersheds as well as the anoxic conditions of bulk sediments are believed to be some of the key factors favoring the mercury methylation at the site.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
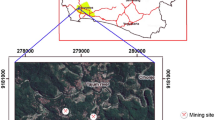
One of the most important aquifers in South Africa (SA) is the dolomitic aquifer of the West Rand and Far West Rand area which commence south of Johannesburg and pass beyond Carletonville to Orkney in the West (McCarthy and Rubidge 2005; Lusilao et al. 2014). Although gold mining industry is still considered as the basis of the economy and socio-economic development of the West Rand and Far West Rand regions, anthropogenic influences from both mining and other industrial sources are posing an increasing threat to this aquifer which risks to become a vast unexploitable groundwater reserve. Gold mining on the West Wits Line, which is found within the Far West Rand region, contains of the biggest and richest mines in the entire Witwatersrand Basin (Robb and Robb 1998). One of these West Wits (WW) gold mines is located approximately 75 km west of Johannesburg within the Gauteng Province. The mining site operation is about 7 km south of Carletonville (Fig. 1) and has neighboring towns such as Fochville and Potchefstroom situated 12 and 50 km, respectively, to the south and west of the mine (AngloGold Ashanti (AGA) 2013).
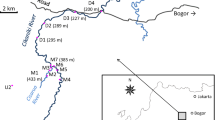
The operational area comprises a number of surface water sub-catchments (Fig. 2), which drain to streams such as the Wonderfontein Spruit and the Loopspruit which in turn are tributaries of rivers that are sources of potable water for the neighboring cities such as Potchefstroom. The land occupied by the WW mining operations is more than 4000 ha which straddle the boundary between Gauteng and the North West Provinces.
Metallurgy within the WW operations manages both the active and inactive tailing storage facilities (TSFs). The mine active TSF of interest for the purpose of this study comprises four compartments which receive the final treated pulp residue pumped from the gold plant. Part of the water from the mine complex TSF drains to a dam, which in turn flows into a stream known as Varkenslaagte. This stream does not form a tributary of any river as the water infiltrates into dolomites outside of the mine area.
During the early years of mining in the West Wits area, there were no specified environmental regulations in terms of waste management and waste materials were disposed of in borrow pits on the mine property (AGA 2009). The uncontrolled and/or poorly managed emission, release, seepage, and waste have led to a severe contamination of lands, surface water, and groundwater in the WW area. Although it is known that gold in the Wits Basin (SA) was, until about 1920, extracted using the mercury (Hg) amalgamation technique (Naicker et al. 2003), information related to Hg pollution and its speciation in the region are quite scarce (Walters et al. 2011; Lusilao et al. 2013).
The present study aimed to (i) identify the Hg “hot spots” in the WW area, (ii) characterize where possible the occurrence of the highly toxic MHg at the site, and (iii) assess the off-site impact of gold mining activities (past and/or current) in terms of Hg pollution.
Materials and methods
Sediments (surface and bulk), waters (surface), and tailings (surface) were collected from wetlands, borrow pits, dams, and TSF within the West Wits gold mining operations. In order to assess the off-site impact of WW operations, the sampling campaign was extended out of the mine site following the path of the Vaarkenslaagte canal, via the Welverdened Road, to the Wonderfontein Spruit located about 5 km to the Carltonville-Potchefstrom Road (Figs. 1 and 2). The overall distance from the initial sampling point to the Wonderfontein Spruit was approximately 20 km (Table 1).
All reagents used for sample preparation and analysis were of analytical grade except for those used during mercury species determination where ultrapure reagents were mandatory. All vessels used for sample collection and treatment were acid cleaned following the cleaning protocol, adapted after Monperrus et al. (2005). The different sampling and analytical methodologies used for the determination of total elements, HgTOT, IHg, and MHg, in both solid and liquid samples were described in details in our previous papers (Lusilao-Makiese et al. 2013, 2014).
Briefly, inductively coupled plasma (ICP)-OES (Spectro Genesis, Germany) was used for total element determination, except for HgTOT in sediments which was determined by an approved EPA and ASTM direct solid introduction technique using an automated Hg analyzer, AMA 254 (Leco, USA). IHg and MHg species were analyzed by the isotope dilution gas chromatography ICP mass spectrometry (ID-GC-ICP-MS) method using the X-Series 2 GC-ICP-MS (Thermo Scientific, USA). Samples were analyzed after being spiked with appropriate amounts of isotopically enriched solutions of Hg2+ (Hg199) and CH3Hg+ (CH3Hg201) and derivatized using either sodium tetraethylborate (NaBEt4; Sigma Aldrich) or sodium tetrapropylborate (NaBPr4; Sigma Aldrich). The validation was performed using Certified Reference Materials (CRMs) of sediments certified for HgTOT (RTC 015–050) and IHg/MHg (e.g., IAEA 405). Satisfactory precision and accuracy were obtained for both HgTOT (recovery 99.7 ± 2.5 % relative standard deviation (RSD), n = 3) and IHg/MHg (recovery 104.3 ± 3.0 % RSD IHg and 99.9 ± 7.3 % RSD MHg, n = 3) analyses of CRMs.
Result and discussion
Hg in waters
The pH of the WW waters (Table 2) ranged from the near-neutral to slightly alkaline values (i.e., 5.9 to 8.9).
This range was similar to what was observed in waters from the Vaal River (VR) gold mine which is located about 100 km south of the WW mine (Lusilao et al. 2014). Redox conditions were moderately anoxic with the highest ORP (E h ) value recorded at 0.48 V. The electrical conductivity (E c ) was at all points, with the exception of the Wonderfontein Spruit, within the mS cm−1 range denoting a considerable presence of dissolved ions probably due to acid mine drainage (AMD).
Except for water sample SW3, collected from a water dam near TSF, which showed HgTOT (i.e., IHg + MHg) beyond the US Environmental Protection Agency (USEPA) threshold effect level (TEL) of 12 ng L−1 (USEPA 1992), mercury values were generally low (Fig. 3a) and also similar to values obtained in VR waters (Lusilao et al. 2014). This could be anticipated, as WW waters were collected during dry season which was the same for the VR waters. In addition, the obtained HgTOT were within the range of <0.02 to 26.65 ± 3.53 ng Hg L−1 reported for the 19 designated Water Management Areas (WMAs) of SA (Walters et al. 2011).
On another hand, Fig. 3b shows that only three of the analyzed waters (i.e., 30 % overall), all collected along the Welverdened Road (i.e., off-site), had MHg concentrations below 10 % of HgTOT (range 0 to 7 %), which is the range of values commonly seen in relatively uncontaminated surface waters with normal methylation potential (Kelly et al. 1995).
Proportions of MHg within the mine were high in dams (SW3 to SW5 33 to 99 %) and at the mine canal (e.g., SW1 and SW2 99 and 96 %, respectively). Note that high %MHg were also observed from the water near the Carltonville-Potchefstrom Road (SW9 99 %) and also in the water from the Wonderfontein Spruit (SW10 90 %). These high MHg proportions are particularly significant, as it shows that conditions within the WW sampling area may favor methylation of Hg. It is important to note that none of the 19 WMAs were reported to have MHg beyond 1 ng L−1 (Walters et al. 2011). The relatively high MHg values obtained in the current study (i.e., up to 7.83 ng L−1) are a clear indication of a substantial methylation process occurring in the WW watersheds.
It has to be mentioned that the vegetation in the area is characterized by a consistent number of grasslands, wetlands, and woodlands with an important presence of decaying plants (i.e., high organic matter content and microbial activity) providing ideal conditions for Hg methylation (Shao et al. 2012).
Some of the GC-ICP-MS chromatograms of WW waters revealed a remarkable presence of unknown Hg peaks (Fig. 4), similar to those observed in our study on the speciation of Hg in Highveld coals (Lusilao et al. 2012) which were attributed by extrapolation to ethylmercury (EtHg). This could be an evidence of the occurrence of EtHg at the site, although little is found in the literature to discuss the natural occurrence of this species in water systems (Mao et al. 2008).
However, studies have shown that derivatization processes (especially the ethylation) of water samples and the extraction of Hg species can be affected by elevated chloride content (Stoichev et al. 2006; Yin et al. 2010) and an important presence of humic acid (Yin et al. 2010).
As mentioned previously, high E c values were measured at the site waters indicating high salinity. This reveals an important ion activity in the watersheds probably from AMD generated in the Vaarkenslaagte canal by the oxidized pyrites from TSFs. This could affect the derivatization of Hg species and lead to a wrong estimation of their occurrence. Nevertheless, Jia et al. (2012) have studied the effects of common coexisting ions (K+, Ca2+, Mg2+, Cl−, SO4 2−, etc.) in real seawater samples on the recoveries of IHg, MHg, and EtHg and obtained recoveries in the range of 89.7–103.6 % for the three species. The authors concluded that the interference from coexisting ions was negligible and could, therefore, be ignored. In addition, in order to minimize the matrix related concern of the ethylation, all the water samples were derivatized with NaBPr4, which is reported to provide low detection limit for organomercurials (Geerdink 2007). However, aqueous NaBPr4 solution is also unstable and the propylation reaction suffers from matrix effects when dealing with seawater (Schubert et al. 2000; Bravo-Sanchez et al. 2004). Depending on the purity of the derivatization agent used, artifact formation of EtHg during aqueous propylation is sometimes a problem. For example, a previous study reported a conversion of up to 2.9 % Hg2+ to EtHg during the propylation reaction (Huang 2005). Although the NaBPr4 used in the present study was of high purity (99.999 %), a quality control analysis with appropriate Hg standards is still of importance to confirm the occurrence of EtHg and its magnitude in WW waters.
Elevated sulfur concentrations were also measured in WW waters, especially in the sample collected from the Vaarkenslaagte canal (SW1 4210 mg L−1) where the evidence of pyrite oxidation was observed (Table 2).
The pH measured at this point was the lowest (pH 5.9) of all the studied WW watersheds and with elevated Fe and Mn contents (28 and 44 mg L−1, respectively). Oxidizing pyrites in nearby TSF are the likely source of Fe, as well as S (or SO4 2−) in the mine canal which is a historic tributary of the Wonderfontein Spruit. This could explain the considerable S value (608 mg L−1) measured in sample SW10 collected from the Wonderfontein stream.
Hg in tailings and sediments
Concentrations of triplicate analyses of collected surface tailings and sediments for both total and speciation analyses of Hg are presented in Table 3.
From the obtained results, the background Hg concentration in the area was estimated to be below 50 μg kg−1. This is in agreement with background Hg values reported by Dixon (1997), which ranged between <11 and 300 μg kg−1 with a mean value below 36 ng kg−1. HgTOT in surface sediments within the WW mine varied between 125 and 837 μg kg−1 (mean 413 μg kg−1) with 18 out of the 21 analyzed samples (i.e., 86 %) showing values above the USEPA sediment TEL value of 174 μg kg−1 (MacDonald et al. 2000). High Hg concentrations were recorded in sediments collected near TSF (S1 and S2) and from the dam (S6). Note that sediments from the Vaarkenslaagte canal (S3 and S4) also exhibited relatively high Hg concentrations which clearly show that this canal is being affected by the pollution occurring at the site. The lowest concentration measured on the mine sediments was from sample S7 collected at the boundaries of the mine lease.
The trend obtained when comparing HgTOT in surface sediments collected from and out of the mine sites (Fig. 5) agreed perfectly with what is reported in the literature (Pestan et al. 2000; Santos-Francés et al. 2011; Lusilao et al. 2013), where patterns of highly contaminated sediments near gold mine waste and lower concentrations away from these sites were observed. This confirms that mining operations are the main source of pollution at the WW area. No particular indication of contamination was observed out of the mining site as all the analyzed off-site surface sediments had Hg at background level. However, the above observation has to be taken with caution since this trend might change during wet season with intensive rainfalls as it was observed elsewhere (e.g., Lacerda and Salomons 1998; Kortatsi 2006; Lusilao et al. 2014).
The mean concentration found in tailings was about 466 μg kg−1 (samples T1 to T3). Although the relationship between Hg concentrations in tailings and grain size has not been characterized in this study, it was reported that the finest tailing particles (<2 mm), such as silt-clay (which is the case in the present study), can be enriched in Hg and exhibit concentrations around 200 μg kg−1 (Ashley et al. 2002). The relatively high Hg values of the WW tailings can be attributed to either old metallurgical procedures that used Hg amalgamation to extract gold (Naicker et al. 2003; McCarthy and Venter 2006) or to the naturally occurring Hg in the mined ore (Erasmus et al. 1982).
Total analysis of samples from a TSF and its adjacent soil (Fig. 6) revealed extremely high elemental concentrations, especially for Fe, Mn, Cu, Ni, and S, among others. These elements are well known for their contribution in AMD generation (Hammarstrom et al. 2005) and could explain the high Fe, Mn, and S loads in waters near TSFs (e.g., SW1). The similarity in concentrations obtained between both samples suggests that the TSF is likely the main source of metal release to the surroundings. Furthermore, the Hg trend in this sediment profile (S1) shows an enrichment in deeper layers (40–50 and 60–70 cm depths), which could indicate a historic pollution of the site (Yan et al. 2008) since the surface contamination tends to slowly affect deeper layers through water infiltration leading to metal accumulation in bulk sediment. Bulk sediments are usually oxygen-depleted zone with a considerable presence of sulfate-reducing bacteria (SRB) which are key factors for Hg methylation (Benoit et al. 2003; Hines et al. 2004; Sonke et al. 2013; Bełdowski et al. 2015). Although MHg was not identified at deeper layers for this particular sample, probably because of its low organic matter content, the Hg accumulation in deeper layers of WW sediments is, nevertheless, a reason of concern and needs to be considered with care.
On another hand, MHg in tailings ranged between 1.9 and 6.6 μg kg−1, indicating that there are conditions favorable for Hg methylation in the tailing dumps. It has been reported that MHg production can be correlated with different factors such as HgTOT or IHg concentrations; organic content; in situ abundance; and activities of SRB, pH, E h , SO4 −2, dissolved sulfide, and other geochemical variables (Lusilao-Makiese et al. 2013, 2014). Although some of these parameters have not been characterized in the present study, interesting positive correlations were, however, obtained between HgTOT in tailings and total sulfur (0.09–0.43 %; mean 0.22 %). These correlations ranged between R 2 values of 0.460 and 0.900. Since approximately 99 % of HgTOT in tailings were in the form of IHg (Table 3), it can be assumed that these correlations also apply for IHg which also exhibited a strong positive correlation with MHg in tailings (R 2 0.874). Winch et al. (2008) reported that although SRBs would be neither abundant nor highly active in tailings due to low sulfate levels, they would still lead to significant accumulation of MHg, given the high concentrations of HgTOT. Thus, the TSFs at the WW site presented environmental conditions that would favor MHg production and accumulation, because not only they contained high HgTOT (and IHg) but they were also revegetated and, therefore, featured an organic-rich layer at the surface. Note that the organic matter (OM) content in WW tailings ranged between 0.62 and 1.02 % (mean 0.85 %). In addition, since Hg is mostly deposited in tailings as Hg0, the presence of MHg in WW tailings is likely due to in situ Hg methylation, and because abiotic methylation is quite scarce in natural environments (Berman and Bartha 1996), the biotic process through SRB remains the likely cause of Hg methylation in WW tailings.
MHg also was identified in the majority of the mine surface sediments with concentrations ranging from 0.8 to 9.2 μg kg−1, corresponding to 0.6 to 1.7 % of HgTOT. As it was the case with water samples, high %MHg in sediment profiles were found in samples collected from the water dam. For example, sample S6 (Fig. 7) exhibited high proportions of MHg in bulk sediments with an enrichment observed at 20–30 cm depth (11 μg kg−1) corresponding to a MHg proportion of 2 % of HgTOT. But, the highest MHg proportion occurred at deeper layer (40–50 cm) with more than 5 % of HgTOT in the form of MHg. This suggests a considerable methylation activity occurring within the dam. It still needs to be recalled that the overall methylation rate observed in WW sediments falls into the average value of 1 to 3 % reported in the literature (Harris et al. 2007). While no particular correlation could be drawn between IHg and MHg in the WW waters, remarkably strong positive correlations were observed between IHg/MHg (R 2 0.904) and IHg/%MHg (R 2 0.933) in WW sediments, which indicates that the abundance of IHg is an important factor controlling the methylation rate at the site sediment (Harris et al. 2007; Bełdowski et al. 2015).
It appears from the results obtained in this study that constructed dams had the highest measured values for both HgTOT and MHg in waters as well as in sediments. Vorosmarty et al. (2003) reported that significant amounts of the basin-scale sediment fluxes in regulated basins are potentially trapped in artificial impoundments and that a significant proportion of the Hg associated with river sediments and transported downstream by rivers is trapped by these impoundments.
Even more importantly, these impoundments cause increases in MHg concentrations (in water, sediment, and biota) by creating organic-rich anoxic deposits conducive to Hg methylation (Hines et al. 2000, and references therein). The same mechanism might explain the observed trend in the present study. Mercury in the WW site, which is mainly transported from TSFs by the site canal and creeks, seems to accumulate in constructed dams which are organic rich due to a massive presence of decaying plants. These dams in turn become important sources of MHg production under anoxic condition characteristic of bottom sediment.
Conclusion
The assessment of mercury contamination and its speciation in a West Wits active gold mine were investigated.
Although the mercury found at the site waters was in general low, elevated concentrations, beyond reported threshold levels, were measured in the majority of the mine sediments, especially those collected near TSFs. Tailing materials also had high mercury values. Indications of a long-term pollution were observed in bulk sediments enriched with mercury. The study was carried out during the dry season only, and therefore, no particular conclusion could be drawn concerning seasonal load and off-site transportation of mercury. Nevertheless, the decrease of mercury level from the mine site to areas away from mining operations suggests that mining activities, especially TSFs, are the main sources of mercury contamination in the area.
Elevated concentrations of other elements such as Fe, Mn, and S were also measured in tailings as well as in some waters and sediments, suggesting an impact of AMD due to pyrite oxidation in tailings.
Constructed dams at the site appear to be the main points of mercury accumulation and are also believed to favor the methylation process through anoxic conditions and high presence of organic matter. The occurrence of methylmercury at the site is of course of concern due to its mobility and risk of accumulation in biota.
The results obtained in this study can be used in developing a remedial action plan for the West Wits mine.
References
AGA. (2009). AngloGold Ashanti West Wits Environmental Management Programme Report. Johannesburg: WSP Environmental.
AGA (2013). AngloGold Ashanti West Wits Operations, Social and Labour Plan Report: Half Year 2013. http://www.anglogoldashanti.com/en/sustainability/MiningCharter/Social%20and%20Labour%20Plans/2013HalfYearSocialandLabourPlanReportWestWits.pdf. Accessed 07 July 2015.
Ashley, R.P., Rytuba, J.J., Rogers. R., Kotlyar, B.B. & Lawler, D. (2002). Preliminary report on mercury geochemistry of placer gold dredge tailings, sediments, bedrock, and waters in the clear creek restoration area, Shasta County, California. U.S. Geological Survey Open-File Report 02–401, pp47.
Bełdowski, J., Miotk, M., & Pempkowiak, J. (2015). Methylation index as means of quantification of the compliance of sedimentary mercury to be methylated. Environmental Monitoring and Assessment, 187, 498–510.
Benoit, J., Gilmour, C.C., Heyes, A., Mason, R.P. & Miller, C. (2003). Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. In Y. Chai and O.C. Braids (Eds.), Biogeochemistry of Environmentally Important Trace Elements (pp. 262–297). Washington, DC: ACS Symposium Series 835, American Chemical Society.
Berman, M., & Bartha, R. (1996). Levels of chemical vs. biological methylation of mercury in sediments. Bulletin of Environmental Contamination and Toxicology, 36, 401–404.
Bravo-Sanchez, L. R., Ruiz Encinar, J., Fidalgo Martinez, J. I., & Sanz-Medel, A. (2004). Mercury speciation analysis in sea water by solid phase microextraction-gas chromatography-inductively coupled plasma mass spectrometry using ethyl and propyl derivatization. Matrix effects evaluation. Spectrochimic Acta. Part B: Atomic. Spectroscopy, 59B, 59–66.
Dixon, K.L. (1997). Background concentrations of metals in wetland soils on and near the Savannah River site. WSRC-MS-97-00692, Rev 1, pp. 19.
Erasmus, C.S., Sellschop, J.P.F. & Hallbauer, D.K. (1982). Major amount of mercury in native gold from upper Witwatersrand sediments. Mintek Analytical Chemistry Division Report, M54, pp23
Geerdink, R. B., Breidenbach, R., & Epema, O. J. (2007). Optimization of headspace solid-phase microextraction gas chromatography-atomic emission detection analysis of monomethylmercury. Journal of Chromatography A, 1174, 7–12.
Hammarstrom, J. M., Seal, R. R., II, Meier, A. L., & Kornfeld, J. M. (2005). Secondary sulfate minerals associated with acid drainage in the eastern US: recycling of metals and acidity in surficial environments. Chemical Geology, 215, 407–431.
Harris, R., Krabbenhoft, D. P., Mason, R., Murray, M. V., Reash, R., & Saltman, T. (2007). Ecosystem responses to mercury contamination, indicators of change. New-York: SETAC.
Hines, M. E., Horvatt, M., Fagneli, J., Bonzongo, J. C. J., Batkay, T., Major, E. B., Scott, K. J., Bailey, E. A., Warwick, J. J., & Lyons, W. B. (2000). Mercury biogeochemistry in the Idrija River, Slovenia, from the above the mine into the Gulf of Trieste. Environmental Research, 83, 129–139.
Hines, N. A., Brezonik, P. L., & Engstrom, D. R. (2004). Sediment and porewater profiles and fluxes of mercury and methylmercury in a small seepage lake in northern Minnesota. Environmental Science & Technology, 38, 6610–6617.
Huang, J. H. (2005). Artifact formation of methyl- and ethyl-mercury compounds from inorganic mercury during derivatization using sodium tetra(n-propyl)borate. Analytica Chimica Acta, 532, 113–120.
Jia, X. Y., Gong, D. R., Han, Y., Wei, C., Duan, T. C., & Chen, H. T. (2012). Fast speciation of mercury in seawater by short-column high-performance liquid chromatography hyphenated to inductively coupled plasma spectrometry after on-line cation exchange column preconcentration. Talanta, 88, 724–729.
Kelly, C. A., Rudd, J. W. M., St. Louis, V. L., & Heyes, A. (1995). Is total mercury concentration a good predictor of methyl mercury concentration in aquatic systems? Water, Air, and Soil Pollution, 80, 715–724.
Kortatsi, B.K. (2006). Concentration of trace metals in boreholes in the Ankobra Basin, Ghana. West African Journal of Applied Ecology. www.ajol.info/index.php/wajae/article/view/45706/29185. Accessed 29 November 2011.
Lacerda, L. D., & Salomons, W. (1998). Mercury from gold and silver mining: a chemical time bomb? New-York: Springer.
Lusilao-Makiese, J., Tessier, E., Amouroux, D., Tutu, H., Chimuka, L., & Cukrowska, E. M. (2012). Speciation of mercury in South African coals. Toxicological & Environmental Chemistry, 94(9), 1688–1706.
Lusilao-Makiese, J. G., Cukrowska, E. M., Tessier, E., Amouroux, D., & Weiersbye, I. (2013). The impact of post gold mining on mercury pollution in the West Rand region, Gauteng, South Africa. Journal of Geochemical Exploration, 134, 111–119.
Lusilao-Makiese, J. G., Tessier, E., Amouroux, D., Tutu, H., Chimuka, L., Weiersbye, I., & Cukrowska, E. M. (2014). Seasonal distribution and speciation of mercury in a gold mining area, North West province, South Africa. Toxicological & Environmental Chemistry, 96(3), 387–402.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31.
Mao, Y., Liu, G., Meichel, G., Cai, Y., & Jiang, G. (2008). Simultaneous speciation of monomethylmercury and monoethylmercury by aqueous phenylation and purge-and-trap preconcentration followed by atomic spectrometry detection. Analytical Chemistry, 80(18), 7163–7168.
McCarthy, T. S., & Rubidge, B. (2005). The story of Earth and life: a southern African perspective on a 4.6 billion-year journey. Cape Town: Struik Publishers.
McCarthy, T. S., & Venter, J. S. (2006). Increasing pollution levels on the Witwatersrand recorded in the peat deposits on the Klip River wetland. South African Journal of Science, 102, 27–34.
Monperrus, M., Tessier, E., Veschambre, S., Amouroux, D., & Donard, O. (2005). Simultaneous speciation of mercury and butyltin compounds in natural waters and snow by propylation and species-specific isotope dilution mass spectrometry analysis. Analytical and Bioanalytical Chemistry, 381, 854–862.
Naicker, K., Cukrowska, E., & McCarthy, T. S. (2003). Acid mine drainage arising from gold mining activity in Johannesburg, South Africa and environs. Environmental Pollution, 22, 29–40.
Pestan, M. H. D., Lechler, P., Formoso, M. L. L., & Miller, J. (2000). Mercury in sediments from gold and copper exploitation areas in the CamaquaÄ River Basin, Southern Brazil. Journal of South American Earth Sciences, 13, 537–547.
Robb, L.J. & Robb, V.M. (1998). Gold in the Witwatersrand basin. In: M.G.C. Wilson and C.R. Anhaeusser (Eds), The Mineral Resources of South Africa (pp. 294–349), Council for Geoscience, South Africa, Handbook 16.
Santos-Francés, F., García-Sánchez, A., Alonso-Rojo, P., Contreras, F., & Adams, M. (2011). Distribution and mobility of mercury in soils of a gold mining region, Cuyuni river basin, Venezuela. Journal of Environmental Management, 92(4), 1268–1276.
Schubert, P., Rosenberg, E., & Grasserbauer, M. (2000). Comparison of sodium tetraethylborate and sodium tetra(n-propyl)borate as derivatization reagent for the speciation of organotin and organolead compounds in water samples. Fresenius Journal of Analytical Chemistry, 366, 356–360.
Shao, D., Kang, Y., Wu, S., & Wong, M. H. (2012). Effects of sulfate reducing bacteria and sulfate concentrations on mercury methylation in freshwater sediments. Science of the Total Environment, 424, 331–336.
Sonke, J. E., Heimburger, L.-E., & Dommergue, A. (2013). Mercury biogeochemistry: paradigm shifts, outstanding issues and research needs. Comptes Rendus Geoscience, 345, 213–224.
Stoichev, T., Amouroux, D., Rodriguez Martin-Doimeadios, R. C., Monperrus, M., Donard, O. F. X., & Tsalev, D. L. (2006). Speciation analysis of mercury in aquatic environment. Applied Spectroscopy Reviews, 41, 591–619.
USEPA. (1992). Water quality standards, establishment of numeric criteria for priority toxic pollutants. States’ compliance, final rule. Federal Register, 40 CFR Part, 131(246), 847–860.
Vorosmarty, C. J., Meybeck, M., Fekete, B., Sharma, K., Green, P., & Syvitski, J. P. M. (2003). Anthropogenic sediment retention: major global impact from registered river impoundments. Global and Planetary Change, 39, 169–190.
Walters, C. R., Somerset, V. S., Leaner, J. J., & Nel, J. M. (2011). A review of mercury pollution in South Africa: current status. Journal of Environmental Science and Health, Part A, 46, 1129–1137.
Winch, S., Fortin, D., Lean, D. R. S., & Parsons, M. (2008). Factors affecting methylmercury levels in surficial tailings from historical Nova Scotia gold mines. Geomicrobiology Journal, 25(2), 112–129.
Yan, H., Feng, X., Shang, L., Qiu, G., Dai, Q., Wang, S., & Hou, Y. (2008). The variations of mercury in sediment profiles from a historically mercury-contaminated reservoir, Guizhou province, China. Science of the Total Environment, 407, 497–506.
Yin, Y. G., Chen, M., Peng, J. F., Liu, J. F., & Jiang, G. B. (2010). Dithizone-functionalized solid phase extraction–displacement elution-high performance liquid chromatography–inductively coupled plasma mass spectrometry for mercury speciation in water samples. Talanta, 81, 1788–1792.
Acknowledgments
The authors would like to express their gratitude to the French National Centre for Scientific Research (CNRS) and the Laboratory of Analytical, Bio-Inorganic and Environmental Chemistry (LCABIE, France) for the financial support and for the access to their facilities and The National Research Foundation-Technology and Human Resources for Industry Programme (NRF-THRIP, SA) and the Wits University Research Committee (URC, SA) for funding our research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lusilao-Makiese, J.G., Tessier, E., Amouroux, D. et al. Mercury speciation and dispersion from an active gold mine at the West Wits area, South Africa. Environ Monit Assess 188, 47 (2016). https://doi.org/10.1007/s10661-015-5059-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-5059-4