Abstract
Anthropogenic activities may generate significant changes in the integrity of aquatic ecosystems, so long-term monitoring of populations that inhabit them is crucial. Counting micronucleated erythrocytes (MN) and erythrocytic nuclear abnormalities (ENA) in peripheral blood is a widely used method for detecting chromosomal damage due to chemical agents in the water. We analyzed MN and ENA frequency in blood obtained from the common toad Rhinella arenarum populations in sites with different degrees of environmental degradation. The results of this study indicate that there is an association between the frequency of micronuclei and nuclear abnormalities and the degree of environmental alteration recorded for the sites studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, several techniques have been developed to assess the pollution from human activities. Evaluation of environment quality, particularly in aquatic ecosystems, has traditionally been based on physicochemical measurements of water. Organisms in aquatic ecosystems are usually exposed to mixtures of xenobiotics in low concentrations, and it is difficult to predict adverse effects when information is obtained exclusively from chemical analyses (Quesada Garcia et al. 2013). Therefore, the development of complementary monitoring methods is a priority. The responses of populations (e.g., longevity and natality) and communities (e.g., diversity and richness) are an alternative and a complement in the evaluation of environmental quality (Lajmanovich and Peltzer 2008). In this sense, in addition to measurements of environmental variables, studies on population parameters such as recruitment, biomarkers, feeding ecology (Cabagna et al. 2005; Peltzer et al. 2008; Pollo et al. 2012; Bionda et al. 2013a, b), species richness, and diversity (Peltzer et al. 2006; Bionda et al. 2011b) are used as indicators of environmental health.
Several organisms have been proposed as indicators of environmental quality in aquatic ecosystems (Carrasco et al. 1990; Mersch et al. 1996; Djomo et al. 2000; Young et al. 2004). Amphibians are sensitive organisms appropriate for detecting genotoxic agents and, therefore, indicators of environmental health due to their permeable and moist skin and a complex life cycle that alternate aquatic and terrestrial life stages (Young et al. 2004). Rhinella arenarum has a wide distribution in South America, including coastal environments, subtropicalor tropical forests, and rural or urban areas. It has been assessed at Least Concern (LC) according to IUCN Red List of Threatened Species (IUCN 2015). Adults normally congregate in large breeding groups. Breeding occurs from mid-August until April (Bionda et al. 2011a), inhabits in a wide range of habitats. Previous studies have already reported possible negative effects about this species in areas of intensive agriculture (Bionda et al. 2011b, 2012b, 2013a; Babini et al. 2015). In addition, feeding strategy and habitat preference (Sanabria et al. 2007; Quiroga et al. 2009) as well as the demographic life-history traits have been investigated (Echeverría and Filipello 1990; Bionda et al. 2015).
Habitat loss, fragmentation and degradation as result of urbanization (Hamer and McDonnell 2008), and agricultural expansion (Peltzer et al. 2003; de Sá 2005) are some of the major threats to amphibian populations, particularly in the central region of Argentina.
In the last decade, the use of biomarkers has been of great interest in assessing the risk posed by a substance or mixture of potentially toxic chemicals. It is considered an appropriate tool to evaluate effects of exposure to xenobiotics on organisms, with the ultimate aim to relate cause and effect (McCarthy and Shugart 1990). A study with several anuran populations of Rhinella arenarum from central Argentina showed a higher biomarker frequency (micronucleus test) in populations that inhabit urban and agricultural ponds, establishing a possible association between the biomarker frequency and the degree of environmental contamination (Caraffa et al. 2013). The micronucleus test, developed by Jaylet et al. (1986) for amphibians, is one of the most commonly used tests in ecotoxicology and has been applied as an indicator of DNA damage (Machado da Rocha 2011). This test is simple, sensitive, and obtains immediate results, making it in an easy method to examine genetic damage (Junín et al. 2008). In addition, the early detection of contamination leads to prevention and/or remediation (Livingstone 1993). According to the results obtained by Caraffa et al. (2013), monitoring these populations is necessary, especially considering that studies carried out on these same populations in the last decade have revealed some deterioration of demographic, ecological, and morphological features (Bionda et al. 2011a, b, 2012a, b, 2013a, b; Caraffa et al. 2013). Furthermore, studies that include biomarkers are more reliable when the diagnosis is made from several biomarkers. During micronucleus analysis, some authors have observed the occurrence of other nuclear abnormalities such as blebbed, lobed, binucleated, and notched nuclei suggesting that they must be taken into consideration as nuclear alterations (da Silva Souza and Fontanetti 2006; Gökalp Muranli and Güner 2011). These authors consider that abnormalities are related to cell division, cell death processes and genotoxicity, and/or mutagenicity of some xenobiotics. Shimizu et al. (1998) and Baršienė et al. (2010) suggest that micronuclei and binucleated cells are related to cell division whereas the morphological nuclear abnormalities may be related to DNA amplification.
Therefore, the aim of our study is to analyze Rhinella arenarum populations from sites with different degrees of environmental alteration under the observation of two biomarkers, micronucleus test, and nuclear abnormalities.
Materials and methods
Study areas
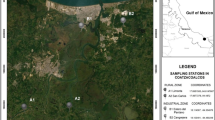
The study area is located in the southwest region of the province of Córdoba, Argentina (Fig. 1). Rhinella arenarum populations were sampled in four sites with different degrees of environmental change. The sampling sites were selected based on previous studies on changes in population features of different amphibian species (Bionda et al. 2011a, b, 2012a, b, 2013a, b) and fish (Pollo et al. 2012), possibly because of environmental alteration produced by agricultural and anthropogenic activities.
The first site, Villa Dalcar Lake (VD: 33° 05′ S, 64° 26′ W, 467 m a.s.l.), is an urban aquatic system located in Río Cuarto city, which serves as an important location for fishing and recreation (Mancini et al. 2012). Several studies had been conducted in this sampling site, due to multiple fish mass mortality events recorded in this lake (Mancini et al. 2000), as well as unusual algae growth (Novoa et al. 2006).
The second site was an agricultural land (AG: 9 ha: 33° 05′ S, 64° 26′ W, 465 m a.s.l.). This site is sparsely vegetated with low growing plants (heights <0.5 m) and permanent and temporary ponds used intensively for cattle grazing. During the study period, these ponds were surrounded by soybean crop.
The third site was a pond called Charca de las Brujas (CB: 33° 06′ S, 64° 25′ W, 465 m a.s.l.), not affected by crops or livestock, near to a protected natural area, the native forest El Espinal, located in Universidad Nacional de Río Cuarto Campus, considered a semi-modified environment. This is an area with permanent and temporary ponds, and a small strip of forest surrounding the ponds with grasslands and forest formations of native and non-native trees (Doffo 1989).
The fourth site, Alpa Corral (AC: 32° 42′ S, 64° 42′ W, 880 m a.s.l.), is a mountain area, Sierra de Comechingones, dominated by vegetation characteristic of the highland forest and not affected by crops or livestock.
We therefore chose sites along a gradient, with Villa Dalcar Lake (VD) and the agricultural site (AG) serving as areas with high levels of degradation due to urbanization, grazing, and cropland, respectively, and Charca de las Brujas (CB) and Alpa Corral (AC) as sites with low levels of alteration.
Data collection
We sampled all four sites in October 2011. In each site, 15–20 adult individuals were captured by hand. To each individual were recorded: snout-vent length (SVL) and weight (W) using a digital caliper Mahr 16 (0.01 mm) and an Ohaus digital balance (0.01 g). The sex was determined according to external secondary sexual characters such as vocal sacs, nuptial pads, and coloration, as males are brownish or greenish on the back and females a grayish or light brown (Duellman and Trueb 1994).
The blood sample was obtained by angularis vein puncture without sacrificing specimens (Nöller 1959). Peripheral blood smears for each individual were prepared on clean slides, fixed and stained by the May Grünwald-Giemsa method (Dacie and Lewis 1995).
A total of about 2000 erythrocytes were examined per animal under the light microscope Zeiss Axiophot-Axiolab with ×100 objective oil immersion. The criteria for identification of micronuclei (MN) were chosen according to Fenech (2000), and for erythrocytic nuclear abnormalities (ENA) following da Silva Souza and Fontanetti (2006) and Pollo et al. (2012). MN and ENA frequency were calculated as follows:
On each visit, we recorded different habitat variables: water temperature (with standard thermometer; uC), water pH, electrical conductivity, total dissolved solids and salinity, with digital equipment 35-Series 35425–10 Multiparameter Tests.
Data analysis
Significant differences between sites for SVL and weight were analyzed using ANOVA. A posteriori paired comparison test between sites was performed. The non-parametric Kruskal-Wallis statistical test (Zar 1999) was used to assess differences of MN and ENA frequency between sites, and a Mann–Whitney test to compare sexes. In addition, a simple regression analysis between MN and ENA frequency versus sex was applied to each site (independent variable: sex; dependent variable: frequency of MN and ENA). InfoStat software was used for data analysis (Di Rienzo et al. 2012). The criterion for significance was p < 0.05.
Results
Mature erythrocytes of R. arenarum have an oval shape with a centric nucleus. The nucleus is clearly structured and has a well-defined boundary, which facilitates the identification of fragments in their cytoplasm (Fig. 2). We identified the maximum micronuclei per cell and cells with nuclear abnormalities such as binucleate cells, or notched, bud and lobed nucleus (Fig. 2).
Using the Mann–Whitney test, it was observed that MN frequency showed no significant differences between sexes in any of the sampling sites (p > 0.05 in all sites). Consequently, for the subsequent analyses, we combined males and females per site. It is important to note that males in all sites, except AC showed a higher mean MN frequency than females (VD: male = 0.65 ± 0.82, female = 0.34 ± 0.41; AG: male = 0.49 ± 0.4, female = 0.39 ± 0.42; CB: male = 0.25 ± 0.35, female = 0.08 ± 0.2; AC: male = 0.03 ± 0.06, female = 0.14 ± 0.24).
Table 1 shows means and standard deviations recorded for MN frequency and ENA in each of the study sites. Significant differences in MN frequency among sites were found (Kruskal-Wallis, p = 0.0227), with the lowest frequency recorded for AC, followed by CB. AG and VD record the highest micronucleus frequencies.
The ENA frequency showed no statistically significant difference between sexes for sites studied (p > 0.05). Analyzing the complete sample, AC showed the lowest average frequency for all registered nuclear abnormalities (Table 1).
A posteriori test was conducted to identify differences between sites for MN and ENA frequency. AC differed from other sites for bud and notched abnormalities, while CB and AC differed for micronuclei number (Table 1).
The simple regression analysis between micronuclei and abnormalities versus sex was not significant (p > 0.05, in all cases).
Average values in the SVL and weight of males and females are shown in Table 2. Females not showed significant differences between sites for SVL (F 3,24 = 2.3, p = 0.10), whiles males if (F 3,38 = 11.14, p < 0.001). Weight females (F 3,24 = 4.27, p < 0.05) and males (F 3,38 = 6.61, p < 0.01) were different among sites.
Table 3 shows values of the habitat variables. AC site presents the lowest values for salinity, electrical conductivity, and solids dissolved, while the highest values were recorded in VD.
Discussion
Our results showed that there is an association between MN and ENA frequency and degree of environmental alteration recorded for the sites studied. Populations of amphibians that inhabit disturbed ecosystems (VD and AG) exhibited higher MN and ENA frequency than those from less disturbed habitats (CB and AC), and those proposed as basal value for the species R. arenarum (0.30 ± 0.09) (Bosch et al. 2011).
The origin of micronuclei has been extensively studied, and two possible causes have been recognized: (1) a genotoxic agent generates DNA lesions (Fenech 2000; Zalacain et al. 2005; Prieto García et al. 2007) or (2) excess material causes genomic instability, and is then removed from the main nucleus through the MN formation (Prieto García et al. 2007). However, the mechanisms underlying ENA formation have not been fully explained yet (Çavaş and Ergene-Gözükara 2003; da Silva Souza and Fontanetti 2006). Several authors have considered using nuclear abnormalities as indicators of genotoxic damage (Ayylon and Garcia-Vazquez 2000; Bombail et al. 2001; Çavaş and Ergene-Gözükara 2003; da Silva Souza and Fontanetti 2006; Prieto García et al. 2007), while others consider them to be results of cytotoxic damage (Çavaş and Ergene-Gözükara 2003; Lajmanovich et al. 2014). da Silva Souza and Fontanetti (2006) and Lajmanovich et al. (2014) indicated that ENA are directly associated with genomic instability and represent a way to remove any amplified genetic material in the cell.
Some authors attribute development of nuclear buds to DNA amplification or polyploidization; the abnormality removes excess DNA with a subsequent MN formation (Fenech and Crott 2002; Prieto García et al. 2007). Lajmanovich et al. (2014) suggested that formation of erythrocytes with bilobed nuclei and anucleated to increase in situations of stress (e.g., diet alterations, pathology, and metabolic damage). However, the exact mechanism of formation of other abnormalities such as notched and lobed nuclei is unknown.
Often, it is difficult to have basal values of MN and ENA frequencies for wildlife. Therefore, simultaneously as controls, samples from different species are collected at nature reserves or sites not impacted by human activity. Furthermore, the incorporation of reference sites is due to the fact that results obtained under laboratory conditions do not always coincide with those obtained in natural conditions. Consequently, in situ studies provide a more realistic understanding, as many toxic compounds interact with environmental factors to generate additive or synergistic, or alter the conditions generating indirect effects in the ecosystem (Tsui and Chu 2003; Chen et al. 2004; Agostini 2013). Moreover, in situ studies consider environmental phenomena such as bioavailability or bioaccumulation (Djomo et al. 2000). It is worth mentioning that the reference sites (AC and CB) recorded the lowest frequency of micronuclei and nuclear abnormalities, even lower than the basal values considered for the species.
VD is an urban lake surrounded by residences, with frequent problems of eutrophication. Its watershed sump has historically supported varied agricultural activities. Furthermore, the presence of several car salvage yards near the lake represents potential revenue of toxic substances. Studies in the last decade indicate a high degree of environmental degradation of this urban lake, raising serious questions about management and use as a recreational area and fishing site. Among these records, algal blooms are evidence of excessive addition of nutrients (Novoa et al. 2006); more than ten cases of fish kills have been documented in recent years (Mancini et al. 2012); a high frequency of morphological abnormalities in R. arenarum is present (Bionda et al. 2012a); and genotoxic damage in populations of R. arenarum (Caraffa et al. 2013) and fishes (Pollo et al. 2012) are evident.
The AG site was another environment which recorded high values of micronuclei. A recent study by Caraffa et al. (2013) with these same populations also showed a high frequency of micronuclei, attributed to the degree of environmental damage at this site because of its immersion in a cropland matrix. Other studies conducted in populations of R. arenarum and other anuran species that inhabit this site have indicated modifications or alterations to demographic and ecological features, possibly attributed to the degree of environmental alteration in this agricultural area (Bionda et al. 2011b, 2012a, b, 2013a, b). In our study, in addition to MN frequency, ENA were high for this site. Agriculture and livestock practice is the main commercial activity in the region (Pengue 2005; Rossi 2006), and therefore considered one of the main causes of deterioration and loss of natural habitat. In this context, some studies have indicated that agroecosystems could represent inhospitable habitats for anuran populations (Carey and Bryant 1995; Taylor et al. 2005; Peltzer et al. 2008; Bionda et al. 2013a, b; Babini et al. 2015) particularly those with intensive practice characteristic of soybean crops in the central region of Argentina.
Among the environmental variables analyzed for this study, values for conductivity, salinity, and dissolved solids were higher for VD and AG sites than other sites. Consequently, pH differed among sites, with VD showing the most basic values, followed by AG. The toxicity levels of many pollutants depend primarily on pH, in addition to the temperature (Cairns et al. 1975; Boyd 1984; Hoffman et al. 2003). Therefore, acid and alkaline stress—values outside the normal pH range of 6.3 to 7.7 (García and Fontúrbel 2003)—can cause a genetic disorder in some species of amphibian (Pough and Wilson 1977). Water quality of the ponds was lower at VD and AG; these results are consistent with expectations based on modification levels previously proposed for these sampling sites.
Finally, considering that R. arenarum is widely distributed in South America and common in all ecosystems, natural or altered, the results obtained in this work are readily transferable to other regions or environments. Since MN and ENA frequency was higher in sites with greater environmental perturbation, we confirmed that these biomarkers are reliable indicators of the environmental health and R. arenarum is a true environmental sentinel. Finally, further studies, including other parameters or biomarkers can demonstrate the effects of exposure to anthropogenic activity in these organisms.
References
Agostini, M.G. (2013). Ecotoxicología de anfíbios en agroecosistemas del noreste de la region pampeana. Tesis de Ph.D. UNLP.
Ayylon, F., & Garcia-Vazquez, E. (2000). Induction of micronuclei and other nuclear abnormalities in european minow Phoxinus phoxinus and mollie Poecilia latipinna: an assessment of the fish micronucleus test. Mutation Research Genetic Toxicology and Environmental Mutagenesis, 467(2), 177–186.
Babini, M. S., Bionda, C. L., Salas, N. E., & Martino, A. L. (2015). Health status of tadpoles and metamorphs of Rhinella arenarum (Anura, Bufonidae) that inhabit agroecosystems and its implications for land use. Ecotoxicology and Environmental Safety, 118, 118–125.
Baršienė, J., Bjornstad, A., Rybakovas, A., Šyvokienė, J., & Andreikėnaitė, L. (2010). Environmental genotoxicity and cytotoxicity studies in mussels and fish inhabiting northern Atlantic zones impacted by aluminum industry. Ekologija, 56(3), 116–123.
Bionda, C. L., di Tada, I. E., & Lajmanovich, R. C. (2011a). Composition of amphibian assemblages in agroecosystems from the central region of Argentina. Russian Journal of Herpetology, 18, 93–98.
Bionda, C. L., Lajmanovich, R. C., Salas, N. E., Martino, A. L., & di Tada, I. E. (2011b). Reproductive ecology of the common South American toad Rhinella arenarum [Anura: Bufonidae]: reproductive effort, clutch size, fecundity, and mate selection. Journal of Herpetology, 45(2), 261–264.
Bionda, C. L., Gari, N., Luque, E., Salas, N. E., Lajmanovich, R. C., & Martino, A. L. (2012a). Ecología trófica en larvas de Rhinella arenarum [Anura: Bufonidae] en agroecosistemas y sus posibles implicaciones para la conservación. Revista de Biología Tropical, 60(2), 771–779.
Bionda, C. L., Salas, N. E., Caraffa, E., Baraquet, M., & Martino, A. L. (2012b). On abnormalities recorded in an urban population of Rhinella arenarum from central Argentina. Herpetology Notes, 5, 237–241.
Bionda, C. L., Lajmanovich, R. C., Salas, N. E., Martino, A. L., & di Tada, I. E. (2013a). Demografía poblacional de Rhinella arenarum [Anura: Bufonidae] y physalaemus biligonigerus [Anura: Leiuperidae] en agroecosistemas de la provincia de córdoba, argentina. Revista de Biología Tropical, 61(3), 1389–1400.
Bionda, C. L., Luque, E., Gari, N., Salas, N. E., Lajmanovich, R. C., & Martino, A. L. (2013b). Diet of tadpoles of Physalaemus biligonigerus [Leiuperidae] from agricultural ponds in the central region of Argentina. Acta Herpetologica, 8(2), 141–146.
Bionda, C. L., Kost, S., Salas, N. E., Lajmanovich, R. C., Sinsch, U., & Martino, A. L. (2015). Age structure, growth and longevity in the common toad, Rhinella arenarum, from Argentina. Acta Herpetologica, 10(1), 55–62.
Bombail, V., Aw, D., Gordon, E., & Batty, J. (2001). Application of the comet and micronucleus assays to butterfish [Pholis gunnelus] erythrocytes from the Firth of Forth, Scotland. Chemosphere, 44(3), 283–392.
Bosch, B., Mañas, F., Gorla, N., & Aiassa, D. (2011). Micronucleus test in post metamorphic Odontophrynus cordobae and Rhinella arenarum [Amphibia: Anura] for environmental monitoring. Journal of Toxicology and Environmental Health Sciences, 3(6), 155–163.
Boyd, C. (1984). Water quality management for pond fish culture. Amsterdam, Netherlands: Elsevier.
Cabagna, M., Lajmanovich, R. C., Stringhini, G., Sanchez Hernandez, J. C., & Peltzer, P. M. (2005). Hematological parameters of health status in the common toad Rhinella arenarum in agroecosystems of Santa Fe province, Argentina. Applied Herpetology, 2, 373–380.
Cairns, J., Heath, A. G., & Parker, B. C. (1975). The effects of temperature upon the toxicity of chemicals to aquatic organisms. Hydrobiologia, 47(1), 135–171.
Caraffa, E., Bionda, C. L., Pollo, F. E., Salas, N. E., & Martino, A. L. (2013). Determinación de la frecuencia de micronúcleos en eritrocitos de Bufo arenarum que habitan ambientes urbanizados. Acta toxicológica Argentina, 21(2), 78–84.
Carey, C., & Bryant, C. J. (1995). Possible interrelations among environmental toxicants, amphibian development, and decline of amphibian populations. Environmental Health Perspectives, 103(suppl.4), 13–17.
Carrasco, K. R., Tilbury, K. L., & Myers, M. S. (1990). Assessment of the piscine micronucleus test as an in situ biological indicator of chemical contaminant effects. Canadian Journal of Fisheries and Aquatic Sciences, 47(11), 2123–2136.
Çavaş, T., & Ergene-Gözükara, S. (2003). Micronuclei, nuclear lesions and interphase silver-stained nucleolar organizer regions [agnors] as cyto-genotoxicity indicators in Oreochromis niloticus exposed to textile mill effluent. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 538(1), 81–91.
Chen, C., Hathaway, K., & Folt, C. (2004). Multiple stress effects of Vision® herbicide, ph, and food on zooplankton and larval amphibian species from forest wetlands. Environmental Toxicology and Chemistry, 23(4), 823–831.
da Silva Souza, T., & Fontanetti, C. (2006). Micronucleus test and observation of nuclear alteration in erythrocytes of Nile tilapia exposed to waters affected by refinery effluent. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 605(1), 87–93.
Dacie, J. V., & Lewis, S. M. (1995). Practical hematology (8th ed.). Livingstone: Churchill.
de Sá, R. O. (2005). Crisis global de biodiversidad: importancia de la diversidad genética y la extinción de anfibios. Agrociencia, 1–2, 513–522.
Di Rienzo, J.A., Casanoves, F., Balzarini, M.G., Gonzalez, L., Tablada, M. & Robledo, C.W. (2012). InfoStat versión 2012. Grupo InfoStat, FCA. Argentina: Universidad Nacional de Córdoba. URL http://www.infostat.com.ar
Djomo, J. E., Ferrier, V., & Békaert, C. (2000). Amphibian micronucleus test in vivo [Jaylet test] to evaluate the genotoxicity of petrochemical waste waters. Bulletin of Environmental Contamination and Toxicology, 65(2), 168–174.
Doffo, N. (1989). Geomorfología del área urbana de la ciudad de Río cuarto y de la cuenca del arroyo El bañado, algunas consideraciones aplicadas al manejo del medio natural. Trabajo final. Río Cuarto: Universidad Nacional de Río Cuarto.
Duellman, W. E., & Trueb, L. (1994). Biology of amphibians. Baltimore: John Hopkins University Press.
Echeverría, D. D., & Filipello, A. M. (1990). Edad y crecimiento en Bufo arenarum (Anura: Bufonidae). Cuadernos de Herpetología, 5, 25–31.
Fenech, M. (2000). The in vitro micronucleus technique. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 455(1), 81–95.
Fenech, M., & Crott, J. W. (2002). Micronuclei, nucleoplasmic bridges and nuclear buds induced in folic acid deficient human lymphocytes-evidence for breakage–fusion-bridge cycles in the cytokinesis-block micronucleus assay. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 504(1), 131–136.
García, G., & Fontúrbel, F. (2003) Propuestas para un desarrollo sostenible. Lago Titikaka por estrategas K. Ed. Publicaciones Integrales. La Paz.
Gökalp Muranli, F. D., & Güner, U. (2011). Induction of micronuclei and nuclear abnormalities in erythrocytes of mosquito fish [Gambusia affinis] following exposure to the pyrethroid insecticide lambda-cyhalothrin. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 726(2), 104–108.
Hamer, A. J., & Mcdonnell, M. J. (2008). Amphibian ecology and conservation in the urbanising world: a review. Biological Conservation, 141(10), 2432–2449.
Hoffman, D. J., Rattner, B. A., Burton, G. A., & Cairns, J. (2003). Handbook of ecotoxicology (2nd ed.). Boca Raton: Lewis Publishers.
IUCN (2015). Conservation international, and NatureServe. An analysis of amphibians on the 2008 IUCN red list. http://www.iucnredlist.org/amphibians. Accessed 6 July 2015.
Jaylet, A., Deparis, P., Ferrier, V., Grinfeld, S., & Siboulet, R. (1986). A new micronucleus test using peripheral blood erythrocytes of the newt Pleurodels walt to detect mutagens in fresh-water. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 164, 245–257.
Junín, M., Rodríguez Mendoza, N., Heras, M., & Braga, L. (2008). Valoración preliminar de la utilización de bioindicadores de contaminación en algunas especies de peces del delta del río Paraná, argentina. Ciencias Ambientales, 1, 17–24.
Lajmanovich, R.C., & Peltzer, P.M. (2008). Plan de monitoreo ambiental para el estudio del impacto de cultivos extensivos de arroz sobre el macrosistema Iberá. Cátedra de Ecotoxicología. Facultad de Bioquímica y Ciencias Biológicas, Universidad Nacional del Litoral-CONICET.
Lajmanovich, R. C., Cabagna-Zenklusen, M. C., Attademo, A. M., Junges, C. M., Peltzer, P. M., Bassó, A., & Lorenzatti, E. (2014). Induction of micronuclei and nuclear abnormalities in tadpoles of the common toad (rhinella arenarum) treated with the herbicidesLiberty®and glufosinate-ammonium. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 769, 7–12.
Livingstone, D. (1993). Biotechnology and pollution monitoring: use of molecular biomarkers in the aquatic environment. Journal of Chemical Technology and Biotechnology, 57(3), 195–211.
Machado da Rocha, C. (2011). The micronucleus test in erythrocytes of amphibian larvae as tool for xenobiotic exposure risk assessment: a brief review and an example using lithobates catesbeianus exposed to copper sulphate. Middle-East Journal of Scientific Research, 8(1), 23–29.
Mancini, M., Rodriguez, C., Finola, M., Basualdo, C., & Prosperi, C. (2000). Mortandad de peces en un lago recreacional del Sur de Córdoba, Argentina. Revista Aquatic, 11, 1–7.
Mancini, M., Crichigno, S., Ortiz, M., & Haro, J. G. (2012). Lagos urbanos: importancia, dinamismo y multiplicidad de usos. El caso del lago Villa Dálcar (Córdoba, Argentina). Biología acuática, 27, 175–189.
McCarthy, J., & Shugart, L. (1990). Biological markers of environmental contamination. In J. McCarthyand & L. Shugart (Eds.), Biomarkers of environmental contamination (pp. 3–14). Boca Raton: Lewis.
Mersch, J., Beauvais, M. N., & Nagel, P. (1996). Induction of micronuclei in haemocytes and gill cells of zebra mussels, Dreissena polymorpha, exposed to clastogens. Mutation Research/Genetic Toxicology, 371(1), 47–55.
Nöller, H. G. (1959). Eine einfache technik der blutentnahme beim frosch. Pflügers Archiv Physiology, 269, 98–100.
Novoa, M., Luque, E., Lombardo, D., & de Fabricius, A. (2006). Estudio ficológico de lagos urbanos artificiales del sur de la provincia de córdoba. Boletín de la Sociedad Argentina de Botánica, 41(3–4), 203–231.
Peltzer, P. M., Lajmanovich, R. C., & Beltzer, A. H. (2003). The effects of habitat fragmentation on amphibian species richness in the floodplain of the middle Parana river, Argentina. Herpetological Journal, 13(2), 95–98.
Peltzer, P. M., Lajmanovich, R. C., Attademo, A. M., & Beltzer, A. H. (2006). Diversity of anuran across agricultural ponds in Argentina. Biodiversity and Conservation, 15(11), 3499–3513.
Peltzer, P. M., Lajmanovich, R. C., Sánchez Hernández, J. C., Cabagna, M., Attademo, A. M., & Bassó, A. (2008). Effects of agricultural pond eutrophication on survival and health status of Scinax nasicus tadpoles. Ecotoxicology and Environmental Safety, 70(1), 185–197.
Pengue, W. A. (2005). Transgenic crops in Argentina: the ecological and social debt. Bulletin of Science, Technology & Society, 25(4), 314–322.
Pollo, F. E., Salas, N. E., Mancini, M. A., & Martino, A. L. (2012). Estudio comparativo de la frecuencia de micronúcleos y anormalidades nucleares en eritrocitos de tres especies ícticas. Acta toxicológica argentina, 20(2), 62–67.
Pough, F. H., & Wilson, R. E. (1977). Acid precipitation and reproductive success of Ambystoma salamanders. Water Air Soil Pollution, 7(3), 307–316.
Prieto García, F., Baez Ramirez, O. A., Scout, W., Gaytán Oyarzún, J. C., & Zúñiga Estrada, A. (2007). Toxicidad y teratogénesis por arsénico en el pez cebra [Danio rerio]. Revista de Toxicología, 24(1), 18–22.
Quesada Garcia, A., Valdehita, A., Torrent, F., Villarroel, M., Hernando, M. D., & Navas, J. M. (2013). Use of fish farms to assess river contamination: combining biomarker responses, active biomonitoring, and chemical analysis. Aquatic Toxicology, 140, 439–448.
Quiroga, L. B., Sanabria, E. A., & Acosta, J. C. (2009). Size-and sex-dependent variation in diet of Rhinella arenarum (Anura: Bufonidae) in a wetland of San Juan, Argentina. Journal of Herpetology, 43(2), 311–317.
Rossi, R. L. (2006). Impactos recientes de la soja en la Argentina. Panorama productivo del cultivo. Agromercado, 129, 4–7.
Sanabria, E. A., Quiroga, L. B., & Acosta, J. C. (2007). Sitios de oviposición y esfuerzo reproductivo en Chaunus arenarum (Anura: Bufonidae) en el desierto del Monte, Argentina. Revista española de herpetología, 21, 49–53.
Shimizu, N., Itoh, N., Utiyama, H., & Wahl, G. M. (1998). Selective entrapment of extrachromosomally amplified DNA by nuclear budding and micronucleation during S phase. The Journal of cell biology, 140(6), 1307–1320.
Taylor, B., Skelly, D., Demarchis, L. K., Slade, M. D., Galusha, D., & Rabinowitz, P. M. (2005). Proximity to pollution sources and risk of amphibian limb malformation. Environmental Health Perspectives, 113, 1497–1501.
Tsui, M. T. K., & Chu, L. M. (2003). Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere, 52(7), 1189–1197.
Young, B. E., Stuart, S. N., Chanson, J. S., Cox, N. A., & Boucher, T. M. (2004). Joyas que están desapareciendo: El estado de los anfibios en el nuevo mundo. Arlington: Narture Serve.
Zalacain, M., Sierrasesúmaga, A., & Patiño, A. (2005). El ensayo de micronúcleos como medida de inestabilidad genética inducida por agentes genotóxicos. Anales del Sistema Sanitario Navarra, 28, 227–236.
Zar, J. H. (1999). Biostatistical analysis. New Jersey: Prentice-Hall.
Acknowledgments
Financial support was provided by SECyT-UNRC (Grant PPI 18/C416) and FONCyT (Grant PICT 2012–0932). We thank the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET)-Argentina for fellowship granted and Audrey Wilson for English Editing Service. The investigation was conducted according to the state law “Protection and Conservation of Wild Fauna” (Argentina National Law No. 22.421). Our study was authorized by the Agencia Córdoba Ambiente (A.C.A.S.E.) and Secretaría de Ambiente del Gobierno de Córdoba.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pollo, F.E., Bionda, C.L., Salinas, Z.A. et al. Common toad Rhinella arenarum (Hensel, 1867) and its importance in assessing environmental health: test of micronuclei and nuclear abnormalities in erythrocytes. Environ Monit Assess 187, 581 (2015). https://doi.org/10.1007/s10661-015-4802-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4802-1