Abstract
One of the most important factors that affect the operation efficiency of sequencing batch reactor (SBR) technology is bacterial viability and biomass activity. The acute toxicity of three heavy metals to four dominant strains of sequencing batch reactor (Pseudomonas, Aeromonas, Enterobacter, and Bacillus) was investigated using a resazurin bioassay. After exposing the bacterial strains to soluble compound of Hg, Cd, and Pb, at more than five selected concentrations, the median effective concentration (EC50) and the mortality rate values were calculated. Large differences were observed in sensitivities of the four bacterial strains to the metals. Pseudomonas showed the highest sensitivity for Cd (EC50 = 0.06 μmol/L) and Hg (EC50 = 11.75 μmol/L), while Aeromonas showed the highest sensitivity for Pb (EC50 = 48.27 μmol/L). Considering the EC50 test results, it was concluded that Pseudomonas and Aeromonas are excellent and reliable bioindicators for assessing the toxicity of water and wastewaters polluted by Cd, Hg, and Pb. The rapidity (30 min) and simplicity of the resazurin bioassay procedure enable this enzymatic test to be used in toxicity assessment of small and decentralized wastewater treatment plants (WWTPs).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial wastewater, in particular, when originating from metal plating and chemical industries, usually contains very toxic compounds (Congeevaram et al. 2007). The variability of the industrial wastewaters on both composition and flow (because of the shift in operation of the plant, change of manufacturing product, washing, etc.) upsets widely the treatment process and makes it difficult to treat using conventional processes (Venkata and Sharma 2002). Sequencing batch reactor (SBR) is a kind of wastewater treatment plant (WWTP) which has been developed for periodic exposure of the bacteria to specific process conditions. These conditions are effectively achieved in a fed-batch method in which the frequency of exposure, exposure time, and amplitude of the respective concentration can be achieved independently of any inflow condition (Venkata Mohan et al. 2005). One of the most important factors that affect the operation efficiency of SBR technology is bacterial viability and biomass activity.
In small quantities, some heavy metals are nutritionally essential for microorganisms, but they become toxic when they are not excreted or metabolized by the cells. In fact, heavy metals can be toxic to most microorganisms at specific concentrations. The intensity and characteristics of damage depends on the level and nature of the metals. For example, it has been revealed that essential and non-essential metals have different biological effects on microorganisms (Irato and Piccinni 1996). Other minerals and water properties, such as organic matter content and pH, will also affect the intensity of damage. It has been reported that the heavy metal pollution in water environment leads to reduction/adaptation in the enzyme expression profile, distribution, and diversity of bacteria, which may induce adverse effects on ecosystem functioning (Jose et al. 2011).
Numerous toxicological investigations have studied the heavy metal toxicity or resistance of bacteria isolated from different media (Abou-Shanab et al. 2007; Congeevaram et al. 2007; Hassen et al. 1998; Kim et al. 2007; Sprocati et al. 2006).
Assessment of growth is one of the important factors when investigating the toxic effects of a compound. Bacterial growth inhibition can be determined in different ways: direct microscopic counts, plate counts (viable counts), turbidity measurement, dry weight, bioluminescence, absorbance, etc. (Brock et al. 1994). When several mixed strains are to be measured in one assay, it may be practicable to measure their growth within microplates. Bacterial growth in microplates is often measured as an increase in either bioluminescence or in absorbance (Gellert 2000; Gellert and Stommel 1999).
Tetrazolium-based dyes such as resazurin and resorufin have been used as bacterial growth indicators since the 1940s (Liu 1981). They detect activity of oxidative enzyme systems by acting as electron acceptors (Liu 1981; Glenner 1961). Resazurin is a colored compound (blue), and its color changes from blue to pink upon reduction via bacterial dehydrogenase enzyme. In the presence of active bacteria with dehydrogenase enzyme activity, resazurin reduces to resorufin (color changes from blue to pink). Inactive bacteria induce no change in resazurin color. So, resazurin can examine the growth and viability of microorganisms (Sreenivasan et al. 2003), but to our knowledge, it has not been used to assess the impact of heavy metals on dominant bacteria existed in SBRs. The aim of this study was to apply a resazurin assay for evaluation of toxic effect of heavy metals (Cd, Hg, and Pb) on SBR-dominant bacteria.
Materials and methods
Sampling, isolation, and molecular identification of dominant bacteria
SBR biomass samples were obtained from biological process of SBR facilities, located in Isfahan, Iran (September 2013). Effluents from this SBR were used for landscape irrigation. The operation parameters of this WWTP were as follows: flow rate, 900 m3/day; hydraulic retention time, 1.5–2.5 day; solid retention time, 8 day; dissolved oxygen, 1–3 mg/L; and temperature, 15–25 °C.
The SBR biomass was obtained by cultivation of 0.5 cm3 of SBR samples in nutrient broth. Aseptically, each single bacterial colony was transferred onto a fresh culture medium and then incubated at 35 °C for 48 h. Transferring process was repeated until a pure bacterial culture was obtained. The isolated bacteria were resuspended in 100 μL of deionized water, and genomic DNA was extracted by boiling (15 min) and centrifugation (7500 g, 5 min). The supernatant was used for PCR amplification with Eubac 27 F and 1492R primers, which amplify a ~1420-bp fragment of 16 s rDNA. All PCR reactions with a final volume of 50 μL contained 0.2 μM of each primer, 2 μL of DNA template, 0.2 mM of each dNTP, 1.25 units of Taq polymerase, and 5 μL of 10× PCR buffer. PCR was performed in a thermocycler (MJ Research, USA) under the following conditions: pre-denaturing (94 °C for 5 min) followed by 30 cycles of denaturing (94 °C for 1 min), annealing at 55 °C for 1 min, and elongation at 72 °C for 2 min and then a final extension at 72 °C for 7 min. PCR products were purified using a QIAquick PCR purification kit (Qiagen Inc., CA), and DNA sequencing of the amplified gene was performed. DNA sequences were analyzed by BLAST algorithms and databases from the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Toxicity assays
For the resazurin assays, all heavy metals of analytical grade were obtained from Sigma Chemical Co., Poole, UK. Stock solutions of mercury chloride (HgCl2), cadmium chloride (CdCl2), and lead chloride (PbCl2) were obtained by dissolving them in distilled water. The pH of the solutions was adjusted to 7.0 by adding 1 N HCl or 1 N NaOH.
For keeping the bacterial age constant during the period of the toxicity experiments, the isolated bacterial cultures were transferred every day to a fresh nutrient broth medium. A clean sample of isolated bacteria using an aseptic preparation method and the aid of centrifugation (3000 g for 10 min) was prepared for evaluation of the toxicity of metals. This step was repeated until the supernatant was clear. The pellets were then resuspended in 20 mL of sterile phosphate buffer. The optical density of the suspension was recorded at 540 nm, and serial dilutions were carried out until the optical density of 1.0 ± 0.05 was achieved.
The assay mixture (5 mL) contained 0.5 mL of nutrient broth (10×), 0.05 mL of resazurin solution at 1 g/L concentration, 0.5 mL phosphate-HCl buffer, and distilled water to a volume of 4 mL. The reaction was started by adding 1 mL of freshly harvested isolated bacteria with an optical density of 1.0 ± 0.05. In parallel, bacteria-free experiments were carried out as controls. The experiments were incubated (21 °C) in the dark on a shaker. After 0 and 30 min, a 1-mL sample was removed from the assay. After removing the bacteria by centrifugation (3 min at 5500 g), resazurin reduction was measured spectrophotometrically at 610 nm. All assays were carried out in four replicates, and the means were calculated. In the existence of active bacterial culture, the activity of dehydrogenase enzyme changes resazurin to reduced compound resorufin and the color turns from blue to pink. The results were used for calculating the median effective concentration (EC50, a statistically derived estimate of a concentration of a toxicant resulting in 50 % reduction in growth within a specified time) values.
Statistical analysis
Data were log transformed before the tests were set up. To determine 30-min EC50 values, probit analysis was performed using the SPSS version 16.0 software. The 95 % confidence intervals of the EC50 were also determined, presented as ± mean EC50 values. Statistical analysis of the EC50 values was performed using both the non-parametric Kruskal–Wallis test and analysis of variance (ANOVA). The Dunnett multiple comparison T3 test was used for testing the significance of the differences of EC50 among species. P values of <0.05 were considered as significant. All analyses were carried out using the SPSS version 16.0 software.
Results
The dominant bacterial species within the SBR were isolated, and the results are shown in Table 1. Table 1 shows that the dominant bacterial species in the SBR samples were Pseudomonas, Aeromonas, Enterobacter, and Bacillus.
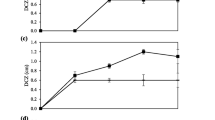
Bacterial inactivity values (±S.D.), as resazurin reduction inhibition, were measured for the four SBR-dominant bacteria after 30 min of exposure to different concentrations of heavy metal ions, and the results are shown in Fig. 1, while the 30-min EC50 values are reported in Table 2. It should be noted that no mortality was observed after 30 min in the control tests which allows us to reject possible stress conditions in bacterial cultures. Statistical analysis of the data, performed using one-way ANOVA (Table 3), demonstrated significant differences (P < 0.05) among the EC50 values of the three tests for each tested heavy metal (Cd, Hg, and Pb). These results were also confirmed using the Kruskal–Wallis test. The significance of the differences in the relative toxicity of heavy metals to each bacterial species is shown in Table 4. The results of the Dunnett multiple comparison test demonstrated the following order of sensitivity among the four bacterial species to the tested metals:
-
Cd: Pseudomonas > Enterobacter > Aeromonas > Bacillus
-
Hg: Pseudomonas > Aeromonas > Enterobacter > Bacillus
-
Pb: Aeromonas > Pseudomonas > Enterobacter > Bacillus
The Dunnett multiple comparison T3 analysis showed no statistical differences between EC50 mean values for Aeromonas and Enterobacter and for Bacillus and Enterobacter in the tests with Hg. No statistical differences were also found between the Pseudomonas and Enterobacter in the tests with Cd, which their 30-min EC50 values were 0.06 and 0.13 μmol/L, respectively. Cd was generally more toxic to bacterial species than either Hg or Pb. The 30-min EC50 values ranged from 0.06 to 2.77 μmol/L; Pseudomonas and Enterobacter showed a high sensitivity to this heavy metal. Hg and Pb showed different toxicities towards the four bacterial species. Among the tested bacterial species, Pseudomonas and Aeromonas showed the highest sensitivity, while Enterobacter and Bacillus were the most tolerant species.
Discussion
The hazardous effect of chemicals has traditionally been investigated with bioindicators such as fish and invertebrates. Due to the large inventory of chemicals, new emerging materials, and the time-consuming nature of traditional bioassays, micro-bioassays based on micro-algae, protozoa, bacteria, and enzymes have been developed for assessment of environmental toxicants. In the present study, the toxic effect of Cd, Hg, and Pb on SBR-dominant bacteria was investigated using a resazurin assay. The most dominant bacterial species in the SBR samples were Pseudomonas and Aeromonas that consisted 79.37 % of all isolated colonies. A previous study (Li et al. 2003) has also reported that Pseudomonas and Aeromonas are dominant in the SBR reactors. Concerning their existence as a dominant bacterial community in the SBR and high sensitivity in comparison with the other tested bacteria, Pseudomonas and Aeromonas bacterial species are reliable and convenient bioindicators for assessing the toxicity of wastewaters polluted by heavy metals.
Heavy metals can concentrate in the bacterial membranes and demolish their integrity. Most of the heavy metals have a very rapid effect on bacterial enzyme systems, inactivating them by binding to amino, sulfhydryl, and amino groups of enzyme protein (Albergoni and Piccinni 1983). Concerning the metals tested in this study, cadmium, at low concentrations, has a known effect on mitochondria and rough endoplasmic reticulum, while at higher concentrations, it can affect cell membranes, destroying their integrity and causing lysis (Ord and Al-Atia 1979). Wang et al. (2010) found that when environmental samples (from soil) were exposed to heavy metals, the amount of fungi and bacteria decreased with the incubation time and the total bacterial number diminished sharply. This phenomenon indicates that fungi are more tolerant, and fungus–bacterium ratio would be changed under metal stress. Changes in the bacterium-fungus ratio can inherently inhibit the treatment process, because bacterial species are the most important part of biological treatment in the WWTPs. In the present study, we found that the most dominant bacteria in the SBR are the highest-sensitive species to be studied with heavy metals. So, it can be concluded that the bacterium-fungus ratio in the SBR can be altered very fast in the case of exposure to heavy metals.
It is well demonstrated that toxic effects of metals are highly selective in microorganisms; such selective targeting of specific enzymatic systems and pathways suggests that certain members of the environmental microorganisms would be more sensitive to metal exposure than others, depending on the sensitivity of their critical metabolic pathways (Fulladosa et al. 2005; Sobolev and Begonia 2008). In the present study, Pb in comparison with Cd and Hg exerted lower toxicity to the SBR-dominant bacteria. The mechanism of metal resistance takes two forms: either blockage at the level of the membrane and cell wall transportation or accumulation in the form of particular protein–metal association (Ow 1993; Tomioka et al. 1994).
The 30-min EC50 value of lead on the most sensitive bacteria (Aeromonas) is 27–80,000 orders of magnitude higher than those reported for Vibrio fischeri (Table 5) (Sillanpaa and Oikari 1996; Tsiridis et al. 2006; Fulladosa et al. 2005). Such high differences in the toxicity tests and the levels of heavy metal tolerance might be due to several factors such as the use of different microorganisms, difference in biotransformation processes, concentration of inorganic anions, differences in the media compositions, competition from other cations, and concentration of chelating agents (Sterritt and Lester 1980). Also, the toxicity of a metal can be affected by various means through bacterial incorporation and accumulation, through complex formation with organic compounds and through biological metal transformation (Kim 1985). On the other hand, an adaptation of bacteria to metals may occur in the aquatic environments (Hassen et al. 1998). In fact, bacteria originating from metal-containing environments show a greater tolerance to metals in comparison to those from uncontaminated environments.
As shown in Table 5, most of the previous toxicity studies have used bacterial bioluminescence measurement as a bioassay test. The most thoroughly investigated bioluminescent bacterium is V. fischeri (also known as Photobacterium phosphoreum), a marine bacterial strain. However, the application of this bacterium in the Microtox® test as a preventative toxicity monitoring method for WWTPs such as SBR or activated sludge is limited. The reason is that V. fischeri is excessively sensitive to many heavy metals and other toxicants, due to being a marine bacterium, and as a consequence, the response of P. phosphoreum to toxicants is different from the response of the WWTP microbial community (Kelly et al. 1999). Application of resazurin reduction assay in the toxicity assessments helps to use the same bacteria existed in the target environments. So, the laboratory data obtained with this method is more reliable and applicable.
The present study indicated that the order of toxicity of investigated metals was not different among the tested bacteria, and in all cases, the toxicity order was Cd > Hg > Pb, while the order of toxicity of heavy metals in previous studies was Pb > Cd > Cu for soil microorganisms (Wang et al. 2010) and Hg > Co > Cd for bacteria isolated from different naturally polluted environments (Hassen et al. 1998). Such variations discourage any attempt to introduce a unique assay for measuring the toxicity of heavy metals to microorganisms of different environments but point out a need for different toxicity methods with high sensitivity, for toxicity assessment of heavy metals in different media.
Conclusions
The acute toxicity of three heavy metals (Cd, Hg, and Pb) to four SBR-dominant bacteria were examined in laboratory tests. The obtained results led to the following conclusions:
-
Pseudomonas, Aeromonas, Enterobacter, and Bacillus were the dominant bacteria in the studied SBR.
-
The four tested bacteria showed large differences in heavy metal toxicity, as observed for other bacteria.
-
Considering the EC50 test results for different bacterial species, it was concluded that Pseudomonas and Aeromonas are reliable and excellent bioindicators for evaluating the toxicity of waters and wastewaters (especially SBRs) polluted by heavy metals.
-
The short time (30 min) and simplicity of the resazurin reduction procedure enable this test to be used in small and decentralized WWTPs.
References
Abou-Shanab, A. I., van Berkum, P., & Angle, J. S. (2007). Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere, 68, 360–367.
Albergoni, V., Piccinni, E. (1983). Biological response to trace metals and their biochemical effects. In: Leppard, G.G. (Ed.), Trace element speciation in surface waters and its ecological implications. Plenum, New York, pp. 159–174.
Brock, T. D., Madigan, M. T., Martinko, J. M., & Parker, J. (1994). Biology of microorganisms (7th ed., pp. 169–191). Englewood Cliffs: Prentice-Hall.
Chaperon, S., & Sauve, S. (2007). Toxicity interaction of metals (Ag, Cu, Hg, Zn) to urease and dehydrogenase activities in soils. Soil Biology and Biochemistry, 39, 2329–2338.
Congeevaram, S., Dhanarani, J., Park, M., Dexilin, K., & Thamaraiselvi, K. (2007). Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. Journal of Hazardous Material, 146, 270–277.
Diaz-Ravina, M., Baath, E., & Frostegard, A. (1994). Multiple heavy metal tolerance of soil bacterial communities and its measurement by a thymidine incorporation technique. Applied Environmental Microbiology, 60, 2238–2247.
Fulladosa, E., Murat, J. C., & Villaescusa, I. (2005). Study on the toxicity of binary equitoxic mixtures of metals using the luminescent bacteria Vibrio fischeri as a biological target. Chemosphere, 58, 551–557.
Gellert, G. (2000). Sensitivity and significance of luminescent bacteria in chronic toxicity testing based on growth and bioluminescence. Ecotoxicology and Environmental Safety, 45, 87–91.
Gellert, G., & Stommel, A. (1999). Influence of microplate material on the sensitivity of growth inhibition tests with bacteria assessing toxic organic substances in water and waste water. Environmental Toxicology, 14, 424–428.
Glenner, G. G. (1961). Tetrazolium salts. In H. J. Conn (Ed.), Biological stains (pp. 230–235). Baltimore: The Williams & Wilkins Company.
Hassen, A., Saidi, N., Cherif, M., & Boudabous, A. (1998). Resistance of environmental bacteria to heavy metals. Bioresource Technology, 64, 7–15.
Hsieh, C. Y., Tsai, M. H., Ryan, D. K., & Pancorbo, O. C. (2004). Toxicity of the 13 priority pollutant metals to Vibrio fisheri in the Microtox chronic toxicity test. Science of the Total Environment, 320, 37–50.
Irato, P., & Piccinni, E. (1996). Effects of cadmium and copper on Astasia longa: metal uptake and glutathione levels. Acta Protozoologica, 35, 281–285.
Jose, J., Giridhar, R., Anas, A., Bharathi, P. A. L., & Nair, S. (2011). Heavy metal pollution exerts reduction/adaptation in the diversity and enzyme expression profile of heterotrophic bacteria in Cochin estuary, India. Environmental Pollution, 159(10), 2775–2780.
Kahru, A., Ivask, A., Kasemets, K., Po-Llumaa, L., Kurvet, I., Franc-Ois, M., & Dubourguier, H. C. (2005). Biotests and biosensors in ecotoxicological risk assessment of field soils polluted with zinc, lead, and cadmium. Environmental Toxicology and Chemistry, 24(11), 2973–2982.
Kelly, C. J., Lajoie, C. A., Layton, A. C., & Sayler, G. S. (1999). Bioluminescence reporter bacterium for toxicity monitoring in biological wastewater treatment systems. Water Environment Research, 71, 31–35.
Kim, S. J. (1985). Effect of heavy metals on natural populations of bacteria from surface microlayers and subsurface water. Marine Ecology Progress Series, 26, 203–206.
Kim, S. U., Cheong, Y. H., Seo, D. C., Hur, J. S., Heo, J. S., & Cho, J. S. (2007). Characterisation of heavy metal tolerance and biosorption capacity of bacterium strain CPB4 (Bacillus spp.). Water Science and Technology, 55, 105–111.
Li, J., Xing, X. H., & Wang, B. Z. (2003). Characteristics of phosphorus removal from wastewater by biofilm sequencing batch reactor (SBR). Biochemical Engineering Journal, 16, 279–285.
Liu, D. (1981). A rapid biochemical test for measuring chemical toxicity. Bulletin of Environmental Contamination and Toxicology, 26, 145–149.
Ord, M.J., Al-Atia, G.R. (1979). The intracellular effects of cadmium: an experimental study using Amoeba proteus as a single cell model. In: Webb, M. (Ed.), The chemistry, biochemistry and biology of cadmium. Elsevier/North-Holland Biomedical, pp. 141–173.
Ow, D. (1993). Phytochelatin-mediated cadmium tolerance in Schizosaccharomyces pombe. In Vitro Cellular & Developmental Biology, 29, 213–219.
Ren, S. (2003). Frymier, P.D. Kinetics of the toxicity of metals to luminescent bacteria. Advances in Environmental Research, 7, 537–547.
Sillanpaa, M., & Oikari, A. (1996). Assessing the impact of complexation by EDTA and DTPA on heavy metal toxicity using Microtox bioassay. Chemosphere, 32(8), 1485–1497.
Sobolev, D., & Begonia, M. F. T. (2008). Effects of heavy metal contamination upon soil microbes: lead-induced changes in general and denitrifying microbial communities as evidenced by molecular markers. International Journal of Environmental Research and Public Health, 5, 450–456.
Sprocati, A. R., Alisi, C., Segre, L., Tasso, F., Galletti, M., & Cremisini, C. (2006). Investigating heavy metal resistance, bioaccumulation and metabolic profile of a metallophile microbial consortium native to an abandoned mine. Science of the Total Environment, 366, 649–658.
Sreenivasan, P. K., Tambs, G., Gittins, E., Nabi, N., & Gaffar, A. (2003). A rapid procedure to ascertain the antimicrobial efficacy of oral care formulations. Oral Microbiology and Immunology, 18, 371–378.
Sterritt, R. M., & Lester, J. N. (1980). Interactions of heavy metals with bacteria. Science of the Total Environment, 14, 5–17.
Tomioka, N., Uchiyama, H., & Yagi, O. (1994). Cesium accumulation and growth characteristics of Rhodococcus erythropolis CS98 and Rhodococcus sp. strain CS40Z. Applied and Environmental Microbiology, 60, 2227–2231.
Tsiridis, V., Petala, M., Samaras, P., Hadjispyrou, S., Sakellaropoulos, G., & Kungolos, A. (2006). Interactive toxic effects of heavy metals and humic acids on Vibrio fischeri. Ecotoxicology and Environmental Safety, 63, 158–167.
Venkata Mohan, S., Chandrashekara Rao, N., Krishna Prasad, K., Madhavi, B. T. V., & Sharma, P. N. (2005). Treatment of complex chemical wastewater in a sequencing batch reactor (SBR) with an aerobic suspended growth configuration. Process Biochemistry, 40(5), 1501–1508.
Venkata, M. S., & Sharma, P. N. (2002). Pharmaceutical wastewater and treatment technologies. Pharma Bio World, 11(1), 93–100.
Wang, F., Yao, J., Si, Y., Chen, H., Russel, M., Chen, K., Qian, Y., Zaray, G., & Bramanti, E. (2010). Short-time effect of heavy metals upon microbial community activity. Journal of Hazardous Material, 173, 510–516.
Acknowledgments
This study has been performed with financial support from the Environment Research Center, Isfahan University of Medical Sciences, Isfahan, Iran, Project No. 2282, and the Department of Environmental Health Engineering, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran. This study was conducted by the first author as part of the requirement to attain a Ph.D. degree, Isfahan University of Medical Sciences, Isfahan, Iran.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zare, MR., Amin, MM., Nikaeen, M. et al. Acute toxicity of Hg, Cd, and Pb towards dominant bacterial strains of sequencing batch reactor (SBR). Environ Monit Assess 187, 263 (2015). https://doi.org/10.1007/s10661-015-4457-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4457-y